Abstract
Background
Nighttime activity, a common occurrence in persons with dementia, increases the risk for injury and unattended home exits, and impairs the sleep patterns of caregivers. Technology is needed that will alert caregivers of nighttime activity in persons with dementia to help prevent injuries and unattended exits.
Methods
As part of a product development grant, a randomized pilot study was conducted to test the effectiveness of a new night monitoring system designed for informal caregivers to use in the home. Data from 53 subjects were collected at 9 points in time over a 12-month period regarding injuries and unattended home exits that occurred while the caregiver slept. Nighttime activity frequently resulted in nursing home placement.
Results
The night monitoring system proved a reliable adjunct to assist caregivers in managing nighttime activity. A total of 9 events (injuries or unattended home exits) occurred during the study with 6 events occurring in the control group. Using intent-to-treat analysis, there was no difference between the groups. However, in a secondary analysis based on use of the intervention, experimental subjects were 85% less likely to sustain an event than control subjects.
Conclusion
When nighttime activity occurred, it resulted in severe injuries sometimes associated with subsequent nursing home placement. The night monitoring system represents a new technology that caregivers can use to assist them in preventing nighttime injuries and unattended home exits in care recipients with dementia.
Keywords: Alzheimer’s disease, dementia, nighttime activity, sleep, caregivers, injury, technology, monitoring system
1. Introduction
Currently in the U.S., approximately 5.2 million individuals have Alzheimer’s disease, a number projected to reach 10 million by midcentury.1 More than 70% of these individuals live at home and receive care from family members.2 The choice of placing a loved one in an institutional setting for care is generally a last resort because it takes a tremendous emotional toll on both caregivers and the person with dementia (PWD), and is a significant financial encumbrance on families and on private and government insurance programs.3,4 Although delaying nursing home placement was rated as an extremely important issue for dementia caregivers,5 approximately 70% of caregivers report the predominant reason they opt for nursing home placement is the ongoing sleep disruption they suffer as a result of PWDs’ erratic nighttime activity.6–8 Profound changes occur in the sleep-wake cycle of PWDs, including increases in time awake and number of awakenings during the night.9–11 In a large sample study, Craig (2005) found that sleep disturbance occurred in 54% of a community-dwelling sample, and caregivers reported this as one of the most stressful neuropsychiatric symptoms.12 In a recent review, sleep disturbances in PWDs are reported to occur in 19–54% of PWDs and in 53–68% of caregivers.13
1.1. Consequences of nighttime activity
In addition to an increased risk of nursing home placement, PWDs are at risk for injury or unattended home exits when active during the night.14 In a study on injuries resulting in an emergency room visit that occurred in PWDs living at home, 40% of injuries happened during the nighttime hours and all injuries occurred as a result of a fall.15 In 70% of cases, the primary type of injury was fracture/dislocation, most often involving the hip (40%), with injuries occurring throughout the house. Eight percent of nighttime injuries occurred outside after the PWD left the home. A sobering finding was that most individuals (62%) were not able to return home after hospitalization and required nursing home placement.13
In another study of PWDs who had become lost in the community, researchers found that almost one quarter of these individuals left their homes or care settings during the evening and nighttime.16 Moreover, 39% of PWDs who died after becoming lost in the community left during nighttime hours, indicating that lack of supervision during night hours can have devastating consequences.17
1.2. Solutions to assist in prevention of untoward effects of nighttime activity
Interventions directed at improving sleep in PWDs have successfully reduced, but not eliminated nighttime awakenings.11,18 Thus these strategies by themselves will not eliminate the need for caregiver supervision during the night. Gaugler and colleagues (2000) found that institutional placement was less likely to occur when family members provided night respite for the primary caregiver.19 This lends support to the importance of developing strategies that can assist caregivers in managing nighttime activity.
Currently there are no specific systems or devices to assist caregivers in preventing untoward events (injuries or unattended exits) during the night hours. Potentially, caregivers could use electronic alerts designed for home security or other purposes. For instance, an intercom system designed to assist parents hear an infant cry can be used to hear the activity of a PWD sleeping in a separate room. However, the sounds of a PWD rising from a bed may be too quiet to awaken the caregiver. Another option is to use free-standing door alarms (that sound when a door is opened) or home security systems. These alarms are widely available, but the sound is generally loud and may cause agitation or distress in the PWD. Furthermore, they do little to prevent injuries that may occur since the caregiver is not alerted until a door is actually opened.
1.3. Study purpose
The purpose of the study was to develop a system specifically designed to alert caregivers when care recipients left their beds and then track them as the recipients moved about the house. After product development and a reliability study, a controlled pilot study was conducted to test the system’s effectiveness with caregivers during the night (or during other periods when caregivers sleep). Details about the night monitoring system (NMS) can be accessed elsewhere.20 In the controlled pilot study, the research questions were:
Are caregivers satisfied with the NMS?
Are untoward events (injuries and unattended home exits) decreased when the NMS is used during caregiver sleep as compared to a control group?
2. Methods
2.1. Design
A pretest-posttest control group repeated measures design was used. Data were collected at baseline and then in months 2, 3, 4, 5, 6, 8, 10, and 12. Both PWDs and caregivers were recruited into the study, with caregivers providing most of the information. Once recruited, caregivers were randomly assigned to receive the NMS (experimental group) or to the control group. The control group received payment ($15.00) each time data were collected, assistance with registration to the SafeReturn program, and some education material on topics not related to the intervention, such as coping with holidays and understanding the diagnosis of dementia. (The Safe Return program is administered by the Alzheimer’s Association to assist in recovering individuals who have become lost in the community.)
Briefly, the NMS uses a home security system platform plus a bed occupancy sensor to provide information to the caregiver regarding the whereabouts of the PWD.20 A text, voice and alarm sound are played when the PWD leaves the bed; then location announcements are made as PWD moves through the home. An emergency alarm sounds if an outside door is opened. When the PWD returns to bed, an announcement is made and the system goes into hibernate mode until the individual arises again.
2.2. Participants
Fifty-three dyads (caregivers and PWDs) were recruited, 26 dyads in the experimental group and 27 in the control group. The inclusion/exclusion criteria for caregivers were:
primary caregiver in the home without provisions for professional care at night.
care for an individual with a medical diagnosis of Alzheimer’s disease or other dementia as reported by the caregiver.
21 years of age or older.
had expressed concern about or reported nighttime activity in the PWD.
had no physical impairments that would prevent caregiver from providing rapid assistance when alarms sounded (e.g., able to walk through the home without assistance).
not undergoing active treatment for sleep disorders (e.g., using prescription sleeping medications on a nightly basis).
able to speak and read English.
no cognitive impairments (Mini-Mental State Exam 21 score > 27).
The inclusion criteria for PWDs were a diagnosis of dementia (as reported by the caregiver), an MMSE score <23, and a history of regular nighttime awakenings.
Subjects were recruited through newspaper and newsletter advertisements, during presentations about the study at caregiver conferences and support groups, and through care providers at day care centers and home health agencies. Interested subjects contacted the researcher. A brief prescreening was conducted by phone to determine if the first seven inclusion/exclusion criteria were met. A home visit was then scheduled and data were collected on the remaining criterion. One potential caregiver subject scored too low on the MMSE and was not recruited into the study. In order to reduce the complexity of the informed consent document, consent was obtained after randomization to group. One potential subject signed a consent but did not participate in any data collection due to family concerns about study participation; no other subjects declined to participate after the introductory visit. One subject was lost to follow-up for unknown reasons and another subject was lost due to a household move. Of subjects who signed consents and had at least one data collection point, subsequent subject loss was due to institutional placement or death of the PWD. This is detailed in Table 1.
Table 1.
Group Comparison Demographic and Clinical Variables at Baseline
| Experimental | Control | Test statistic | |
|---|---|---|---|
| Age in years (person with dementia) |
78.45 | 80.75 | 0.32* |
| Age in years (caregiver) | 61.35 | 63.37 | 0.54* |
| Female (person with dementia) | 42.0% | 52.0% | 0.49† |
| Female (caregiver) | 73.0% | 85.0% | 0.28† |
| Caregiver relationship to care recipient | 0.17† | ||
| Wife | 46.2% | 33.3% | |
| Husband | 11.5% | 11.1% | |
| Daughter | 23.1% | 51.9% | |
| Son | 15.4% | 3.7% | |
| Granddaughter | 3.8% | 0.0% | |
| Race/Ethnicity | 0.29† | ||
| White, not Hispanic | 73.0% | 85.0% | |
| African-American | 19.0% | 15.0% | |
| Hispanic | 8.0% | 0.0% | |
| Caregiver burden score | 2.65 | 3.03 | 0.06* |
| Average time caregiver awake (minutes) | 31–60 | 31–60 | 0.41* |
| Caregiver sleeping location | 1.54† | ||
| Same room | 60% | 40% | |
| Different room | 40% | 60% | |
| MMSE score (person with dementia) | 13.67 | 14.00 | 0.91* |
| Measures to manage nighttime activity (overall) | 74.1% | 71.4% | 0.04† |
| Increased surveillance | 37.0% | 14.3% | |
| Changed door locks | 14.8% | 23.8% | |
| Other monitors (baby monitors, security system) | 18.5% | 33.3% | |
| Respite | 3.7% | 0.0% | |
| Changes to home environment | 18.5% | 19.0% |
Key. Test statistic for group differences -
Independent samples t- test.
Chi-Square test; all test statistics were not significant at α = 0.05.
Because a major focus of the study was development of new technology, over-recruitment of minorities was attempted to ensure that all races/ethnicities could easily use the NMS. Unfortunately, in our geographic area we were unable to oversample the Hispanic population primarily because of the requirement that the caregiver read and speak English. We were, however, able to oversample the African-American population.
2.3. Measures
System reliability
Because any false negative (e.g., the event occurred but the system did not alarm) of the bed or exit door sensors could have significant consequences, subjects were instructed to call the researchers within 24 hours if this ever occurred, and these data were recorded. In addition, at each data collection point, the researchers asked the caregiver if any false negatives had occurred. For these events, data would be collected from the NMS automated log (system and sensor activation codes for last 100 events) and on the circumstances about the event (time, presence and location of caregiver, location of care recipient, and how caregiver identified false negative event). Researchers were to visit the home within 24 hours of a false negative to collect these data.
Satisfaction with the NMS
Satisfaction (usability, ease of use, effectiveness) with the technology was measured only in the experimental group using an adapted Quebec User Evaluation of Satisfaction with Assistive Technology questionnaire.22 This questionnaire measures satisfaction with a technological device, and its validity has been previously reported.22 The items were adapted to be more specific to the NMS and scored on a Likert-type scale with “0” indicating “not satisfied at all” and “5” indicating “very satisfied.” This scale was administered at the 6- and 12-month data collection points. The alpha Cronbach of the 13-item scale was 0.91 at the 6-month point.
Nighttime injuries
At baseline, caregivers were asked to recall any injuries that occurred to the PWD during the previous 6 months. At subsequent data collections, caregivers were asked about any injuries that occurred since the last data collection point. Injuries were coded according to the American National Standards method of recording injuries.23 The following data were collected: the nature of injury; the part of the body affected; the object, substance, exposure, or bodily motion that caused the injury; the event that directly resulted in the injury; and the time and place of the injury’s occurrence.24,25 An injury was considered nighttime if the caregiver reported being asleep at the time the injury occurred.
Unattended exits from the home
An unattended exit was defined as the PWD walking completely through an outside door of the home. If the caregiver was notified of the exit by an alarm (either the NMS or pre-existing home security system), it was considered an interrupted exit. Caregivers were asked at baseline if there had been any unattended exits in the previous 6 months, then at each data collection if there had been an exit since the last data collection point. Data collected included time of exit, circumstances before the exit, whereabouts of the caregiver, amount of time gone, and information about any injuries sustained. Only nighttime exits were included in this analysis (i.e., those that occurred while the caregiver was asleep).
Mechanisms to manage nighttime activity
At baseline, caregivers were asked to describe measures they were currently using to ensure a safe environment for the PWD during the night. A checklist included the following items: changed door locks, changed where caregiver sleeps, used a monitor, and used respite care. Data were also collected on any additional measures used.
Baseline variables
Demographic variables of age, gender, and race/ethnicity were collected about both the caregiver and PWD. Data were also collected on caregiver education, occupation, employment status, and relationship to PWD. Caregivers also reported on the average number of nights per week and times per night they arose to supervise the PWD.
The shortened version of the Zarit Burden Interview was used to measure the burden/strain that caregivers feel in five dimensions—time-dependence, personal development, physical symptoms, social impact, and emotional burden.26 The instrument consists of 12 items to be answered using a Likert-scale format. Reliability and validity have been supported in previous studies.26 The alpha Cronbach coefficient for this sample at baseline was 0.89.
The Neuropsychiatric Inventory – Questionnaire (NPI-Q) was used to measure the severity of behavioral symptoms of dementia.27 The questionnaire was completed by the caregiver at baseline, and the score indicating the overall severity of behaviors was used.
2.4. Procedures
Assignment was managed by a college staff member not on the research team. When a caregiver was recruited, the researcher called that staff member who drew a slip of paper from an envelope indicating assignment to experimental or control group and then informed the researcher. Forty-five subjects were thus randomly assigned.
Four participants from a previously conducted prototype reliability study, who met inclusion/exclusion criteria, were invited to participate in this study. All consented and were assigned to the experimental group since they already had an NMS. Baseline data on these subjects were collected prior to any system being installed.
Two participants were initially assigned to the experimental group, but chose to participate only if they were in the control group. Because of the difficulty in recruiting this population, we allowed these two participants to opt into the control group. Two subjects who were originally assigned to the experimental group had a home/sleeping configuration that was not compatible with the NMS. In one case, the PWD and caregiver slept in a variety of locations in the house, and in the other, the PWD slept on a couch and the caregiver in a reclining chair. Both of these subjects were asked to participate as control subjects and both agreed. In summary, 45 subjects were randomly assigned, and 8 subjects (4 to each group) were assigned by preference or participation in a previous reliability study. Approval for the study was received from the Institutional Review Board of the primary investigator’s institution. All data collection was done in subjects’ homes at a prescheduled time convenient for them.
Since the NMS was developed specifically for this study, it was critical to ensure its reliability after it was installed in each experimental home. This procedure has been detailed elsewhere.20 Briefly, sensor and system activation data were collected continuously for a 2–3 week period, and the caregiver was educated on system operation at weekly visits. When a set of reliability proofs were met then, the reliability period ended. Proofs of feasibility were met for two consecutive weeks: no false negatives on bed or home exits, less than 10% false negatives on movement notifications through the home, and less than 10% false positives for any alarm. Four weeks after this point, the first post-test data collection was done. The NMS was successfully installed in all experimental homes, and all caregivers easily learned system operation. There were no system failures during the study.
2.5. Statistical Analysis
Univariate and bivariate statistics were used to describe the sample and determine whether there were significant differences between the groups. An alpha level of 0.05 was used for all statistics.
To determine if there was a significant group difference in the amount of time to first untoward event (either night injury or exit) between experimental and control groups, data were analyzed using the Kaplan-Meier estimate and displayed using a survival plot. Failures were defined as any time a subject had a nighttime injury or exit; cases were censored at the point they completed the study or left the study for an unrelated event. Days to failure (censor) was calculated as the number of days from the planned data collection date immediately following the event (or to final date of data collection) from baseline data collection date. A Cox proportional-hazards regression was done to determine relative risk and importance of covariates. SPSS 15.0 and NCSS 2001 were used to analyze the data.
3. Results
3.1. Baseline characteristics
Table 1 displays the demographic characteristics of the 53 subjects. The average age of the PWD was 79.62 years and 62.37 years for the caregiver. There were 53% male PWDs; 80% of caregivers were female. Both PWDs and caregivers (all dyads matched) were 79% White (not Hispanic), 17% African-American, and 4% Hispanic. Thirty-two percent of caregivers were employed outside the home. Most caregivers (83%) had at least some college education.
At baseline, caregivers most frequently reported being awakened 1–2 times/night (66%), with 9% reporting being awakened >4 times/night. Awakenings as a result of PWD activity occurred nightly for 57% of caregivers. At baseline, a variety of techniques were employed to assist the caregiver in knowing when the PWD arose. These are detailed in Table 1, with the majority of subjects employing at least one measure. Of interest was that 7 caregivers (16%) actually changed their normal sleeping location in order to provide increased surveillance, highlighting how disruptive nighttime activity can be to caregivers’ normal sleep routines.
Using a 7-day sleep diary, caregivers’ baseline mean total sleep time per night was 380 (± 96) minutes with an average total wake time of 105 (± 80) minutes. Caregivers retired on average at 11:08 p.m. and arose at 7:22 a.m. Using independent t-tests, there were no significant differences in sleep diary variables between groups.
PWDs generally had moderate levels of dementia with a mean MMSE score of 13.83 (range 2–26). All but one was ambulatory at baseline. One subject required use of a wheelchair, but frequently attempted to exit the bed on his own accord. The average NPI-Q score was 11.73 (±7.06) with a range of .00 to 27.00. There was no significant difference between the experimental and control groups (t = 0.91, p = 0.37).
There were no significant differences between the control and experimental groups on age, gender or race. There were proportionally more daughter caregivers in the control group although the difference did not reach statistical significance. Groups were not significantly different on MMSE scores of PWDs and did not differ on the average amount of time caregivers reported being up at night to provide care for PWDs. Furthermore, there were no significant differences at baseline between groups on the item from the NPI-Q that asks specifically about nighttime behaviors in terms of incidence, severity or caregiver distress rating.
At baseline, the average caregiver burden score was 2.79 on a scale of 1–5, indicating moderate levels of perceived burden; the control group had a significantly higher baseline burden scale than the experimental group. Caregiver burden did not increase with increased average number of caregiver night awakenings (categorized as 0, 1–2, 3–4, or >4) (F2,50=0.80, p=0.92).
3.2. Retention and Adherence
Approximately 60% of both experimental and control subjects completed the study. There were no withdrawals related to improper use or difficulty using the NMS, or because of problems adhering to study requirements. In the experimental group, the causes of study withdrawal were (# of subjects/study month exited): night event caused NHP (n=1/month 6 ); NHP related to dementia (n=2/months 4,8 ); NHP unrelated to dementia (n=2/months 2,8); died unrelated to study event (n=4/months 2,2,5,6); or, lost to follow-up (n=1/month 6) In the control group, the causes of study withdrawal were: night event caused NHP (n=2/months 2,5 ); NHP related to dementia (n=0); NHP unrelated to dementia (n=5/months 3,3,5,5,6); died unrelated to study event (n=2/months 4,4); or, lost to follow-up (n=1/month 3).
All experimental caregivers used the system on a nightly basis throughout the trial. In one experimental home, a temporary caregiver was required after the primary caregiver became hospitalized. The temporary caregiver disabled the NMS by unplugging it and disconnecting the back-up battery (confirmed by researchers with visual inspection and reviewing events recorded in system log at home visit). The first night the system was disabled, the temporary caregiver slept through nighttime activity of the PWD who was subsequently injured, resulting in a permanent nursing home placement.
3.3. System reliability
There were no reported false negatives of either the bed sensor or home exit sensors during the pilot study. When researchers viewed system logs during monthly visits, no evidence of false negatives was apparent. No subjects had any untoward event (injury or home exit) because the system failed to notify the caregiver that the PWD had left the bed/home.
3.4. Satisfaction with the NMS
Means and standard deviations for the items on the Quebec User Evaluation of Satisfaction with Assistive Technology questionnaire at month 12 are reported in Table 2. The overall means (SD) for the “0” to “5”-scale at months 6 and 12 were 4.72 (±0.41) and 4.79 (±0.30), respectively, indicating that subjects were very satisfied with the NMS. All experimental subjects completing the pilot study chose to keep the NMS at the study’s conclusion.
Table 2.
Satisfaction Scale Means/Standard Deviations
| Item | Meana | SD |
|---|---|---|
| Activating NMS for night monitoring | 4.92 | 0.27 |
| Individualizing the alarms to meet your needs | 4.69 | 0.48 |
| Notification of exits from bed | 4.83 | 0.38 |
| Notification of your relative’s movement in the home | 4.58 | 0.66 |
| Notification of your relative’s movement out of the home | 5.00 | 0.00 |
| Easy to use | 4.83 | 0.57 |
| Preventing injuries in your relative | 4.91 | 0.28 |
| Preventing your relative from exiting the home unattended | 5.00 | 0.00 |
| Improving the quality of your sleep | 4.50 | 0.67 |
| Improving your ability to cope with your relative | 4.83 | 0.38 |
Key. PWD – person with dementia;
Likert scale – 0=not satisfied at all, 5=very satisfied.
3.5. Injuries and exits
Data were collected by caregiver report at baseline on injuries and exits that occurred in the previous six months. Four caregivers reported that there were unattended home exits of their care recipient during the night, but all were found easily on home property. Two night injuries were reported; both injuries were caused by falls, one resulting in a joint dislocation and bone fracture.
During the study, nine subjects had at least one untoward event; six were related to falls and three were home exits (see Table 3). Seven of these events occurred while the caregiver was asleep, six in control subjects and one in an experimental subject when the NMS was disabled by a temporary caregiver. Two events in the experimental group occurred at night after the caregiver was awakened by the NMS. Four of these nine subjects required unplanned nursing home placement as a result of the untoward event.
Table 3.
Description of Untoward Events in Survival Analysis
| Group | Caregiver | Untoward Event |
|---|---|---|
| Experimental | A | Fell in bathroom sustaining superficial skin injuries (assisted by caregiver) |
| Experimental | S | Fell in guest bedroom sustaining deep lacerations* |
| Control | S | Fell in bathroom fracturing collarbone |
| Control | S | Fell in hallway sustaining superficial skin injuries |
| Control | S | Fell in another bedroom fracturing hip* |
| Control | S | Fell in bathroom injuring shoulder and hip* |
| Experimental | A | Exited home while caregiver was monitoring night activity from her bed |
| Control | S | Exited home while caregiver asleep |
| Control | S | Exited home frequently throughout the day/early morning* |
Key. A= awake; S=sleeping;
event resulted in permanent nursing home placement
3.6. Group differences
For the survival analysis we compared the total days from baseline to failure or censor between the experimental and control groups. The groups had approximately an equal number of subjects who were censored prior to completing the study [control, n=7 (25.9%); experimental, n=9 (34.6%)] (see Table 1).
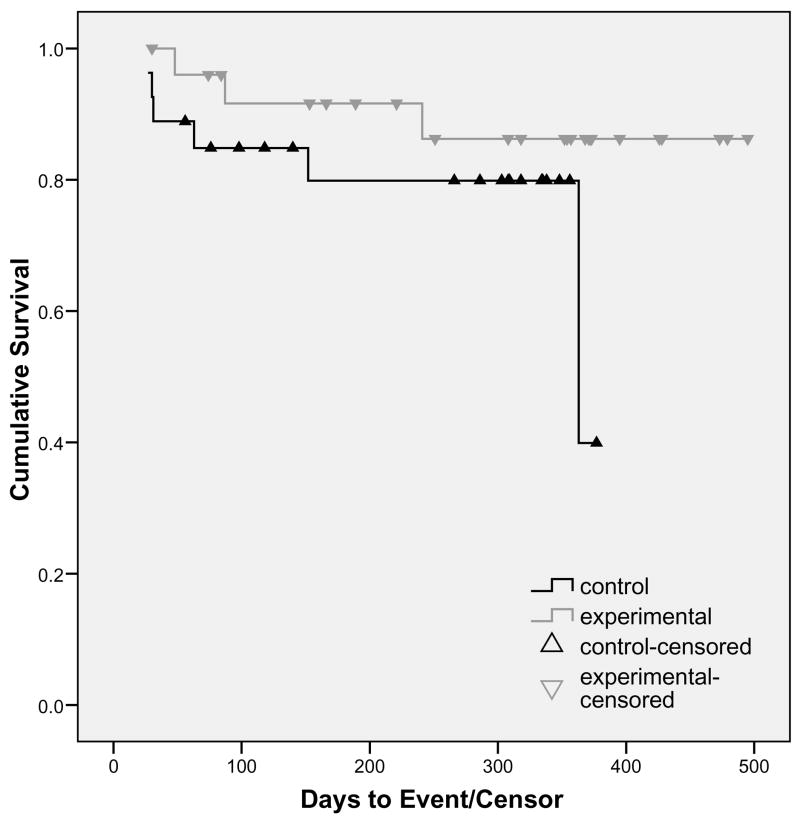
The Kaplan-Meier Survival Curve is presented in Figure 1, with the cumulative survival percent at each time point displayed in Table 1. A Mantel-Cox log rank test, used to test group differences, was not significant (Χ2 = 1.78, p = 0.18). The incidence of nighttime injuries could not be established when the study was designed; therefore, this pilot was powered on a different outcome variable (caregiver sleep), and the data collected can be used to correctly determine the power in subsequent studies.
Figure 1.
Survival Curve of Analysis by Group
Using the covariates of PWD age, gender, caregiver relation (spouse or adult child/grandchild) and the sleeping location of the caregiver (same room or not), a Cox proportional hazards regression was done to determine the influence of those covariates and the relative risk of a night event occurring. When the covariates were entered as a group, there was not a significant improvement in model fit (Χ2 = 1.72, p = 0.79). Similarly, there was not a significant improvement in fit when group was entered in the final step (Χ2 = 3.01, p = 0.08; RR = 0.26, p = 0.09). However, future studies should consider sampling until there are at least 17 events as that provides a power of 0.80 with the effect size found in this study (relative risk ~ 0.30).
3.7. Secondary analyses
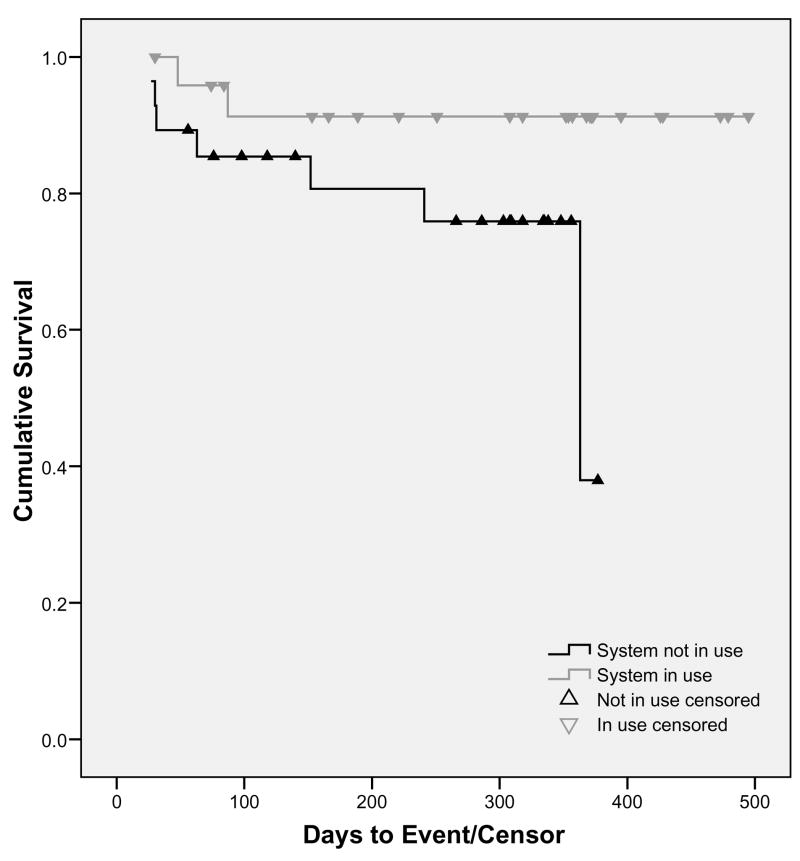
In a secondary analysis of the data, subjects were grouped by whether or not the intervention was active at the time of an event (e.g., the NMS was in use at the time of the injury/exit). Using this strategy, there were seven injuries/exits sustained when no NMS was used (6 control subjects and 1 experimental subject), and two injuries/exits while the system was in use. The Kaplan-Meier survival curve is presented in Figure 2. A Mantel-Cox log rank test demonstrated that when the intervention was active, subjects were less likely to sustain injuries/exits than when no monitoring system was being used (Χ2 = 3.58, p = 0.058).
Figure 2.
Survival Curve of Analysis by Actual Use of Intervention
Using the same procedure as above, the Cox proportional hazards regression was run. Again, covariates had no significant effect, but stepping in the group variable significantly improved model fit (Χ2 = 5.85, p = 0.02). The relative risk was reduced by 86% when an NMS was used (RR = 0.24, p = .03; 95%CI = 0.02–0.82).
In order to determine whether night events were more likely to be associated with nursing home placement, a 3 × 4 Chi-square analysis was done comparing event (none, day event, night event) to final disposition (remained home, nursing home placement resulting from event, nursing home placement/death not a result of an event). There were 13 subjects who had only day events (injuries, exits) with none of these events resulting in nursing home placement. There was a trend of nighttime injuries to be more likely associated with nursing home placement as compared to no event or daytime events (Χ2 = 7.96, p = 0.09). Of subjects who had nighttime injuries, 30% resulted in nursing home placement. Subjects who had no injuries or only daytime ones had a much lower possibility of nursing home placement (7%, 0%, respectively).
Discussion
The NMS was a highly reliable system for monitoring nighttime activity in PWDs living in their own homes with family caregivers. Caregivers reported satisfaction with the NMS’s ease of use and its features, and expressed confidence that the system prevented nighttime injuries and home exits. Even after the study was over, caregivers continued to use the NMS. When used correctly, the NMS was effective in reducing events during times caregivers were asleep.
The NMS addresses a particularly difficult behavior in PWDs, that of nighttime activity. Other researchers have identified that caregiver fatigue resulting from trying to manage the PWD during the night is a primary reason for nursing home placement. In this study, it was evident that night exits and injuries can also precipitate nursing home placement, generally a solution of last resort for caregivers. The NMS has the potential to ameliorate the devastating triad of consequences that results from nighttime activity and possibly delay nursing home placement.
Findings of this study indicate that informal caregivers, even older caregivers, can reliably and correctly use technological solutions to assist them in care of their relatives. It is critical to bring this technology to market and continue to develop other technologies that can assist informal caregivers. Future research should be conducted including a randomized clinical trial of the NMS. Measures of nighttime injury and exit should be improved to provide more detail about the events; this information can be used to make adjustments to the system that may improve its utility in this setting. Data from this study on caregiver outcomes will be published separately.
In conclusion, the NMS represents a technology that can significantly improve the ability of informal caregivers to provide a safe environment throughout the night in homes of PWDs.
Acknowledgments
The authors wish to acknowledge the study funding agency: National Institute for Nursing Research, Night Alert Prompting System, 1R41N004952-01A1 2R42NR004952-02A2. The authors thank the following individuals for their work on the project and/or manuscript: Amy Lawson, Suzanne Aparicio, Kevin Strauss, and Hyo-Chol Ahn.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Alzheimer’s disease facts and figures. Alzheimer & Demen. 2008;4:1–41. doi: 10.1016/j.jalz.2008.02.005. (Appendix) [DOI] [PubMed] [Google Scholar]
- 2.Statistics and prevalence of Alzheimer’s disease. Chicago: Alzheimer’s Association; 1998. Jul, [Google Scholar]
- 3.Schulz R, Belle SH, Czaja SJ, McGinnis KA, Stevens A, Zhang S. Long-term care placement of dementia patients and caregiver health and well-being. JAMA. 2004;292:961–7. doi: 10.1001/jama.292.8.961. [DOI] [PubMed] [Google Scholar]
- 4.Michel JP, Zekry D, Mulligan R, Giacobini E, Gold G. Economic considerations of Alzheimer’s disease and related disorders. Aging (Milano) 2001;13:255–60. doi: 10.1007/BF03351484. [DOI] [PubMed] [Google Scholar]
- 5.Karlawish JH, Klocinski JL, Merz J, Clark CM, Asch DA. Caregivers’ preferences for the treatment of patients with Alzheimer’s disease. Neurology. 2000;55:1008–14. doi: 10.1212/wnl.55.7.1008. [DOI] [PubMed] [Google Scholar]
- 6.Hope T, Keene J, Gedling K, Fairburn C, Jacoby R. Predictors of institutionalization for people with dementia living at home with a carer. Int J of Geriatr Psychiatry. 1998;13:682–90. doi: 10.1002/(sici)1099-1166(1998100)13:10<682::aid-gps847>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 7.Pollak CP, Perlick D, Alexopoulos G, Gonzales A. Disruptive nighttime behaviors in elder caregiver pairs. Sleep Res. 1994;23:305. [Google Scholar]
- 8.Pollak CP, Perlick D. Sleep problems and institutionalization of elderly. Journal of Geriatr Psychiatry and Neurology. 1991;4:204–10. doi: 10.1177/089198879100400405. [DOI] [PubMed] [Google Scholar]
- 9.Ancoli-Israel S, Klauber MR, Gillin JC, Campbell SS, Hofstetter CR. Sleep in non-institutionalized Alzheimer’s disease patients. Aging Clin Exp Res. 1994;6:1–8. doi: 10.1007/BF03324277. [DOI] [PubMed] [Google Scholar]
- 10.Klein DA, Steinberg M, Galik E, Steele C, Sheppard JM, Warren A, et al. Wandering behaviour in community-residing persons with dementia. Int J Geriatr Psychiatry. 1999;14:272–9. doi: 10.1002/(sici)1099-1166(199904)14:4<272::aid-gps896>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 11.McCurry SM, Gibbons LE, Logsdon RG, Vitiello MV, Teri L. Nighttime insomnia treatment and education for Alzheimer’s disease: a randomized, controlled trial. J Am Geriatr Soc. 2005;53:793–802. doi: 10.1111/j.1532-5415.2005.53252.x. [DOI] [PubMed] [Google Scholar]
- 12.Craig D, Mirakhur A, Hart DJ, McIlroy SP, Passmore AP. A cross-sectional study of neuropsychiatric symptoms in 435 patients with Alzheimer’s disease. Am J Geriatr Psychiatry. 2005;13:460–8. doi: 10.1176/appi.ajgp.13.6.460. [DOI] [PubMed] [Google Scholar]
- 13.McCurry SM, Logsdon RG, Teri L, Vitiello MV. Sleep disturbances in caregivers of persons with dementia: contributing factors and treatment implications. Sleep Med Rev. 2007;11:143–53. doi: 10.1016/j.smrv.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurley AC, Gauthier MA, Horvath KJ, Harvey R, Smith SJ, Trudeau S, et al. Promoting safer home environments for persons with Alzheimer’s disease. The Home Safety/Injury Model. J Gerontol Nurs. 2004;30:43–51. doi: 10.3928/0098-9134-20040601-09. [DOI] [PubMed] [Google Scholar]
- 15.Rowe MA, Fehrenbach N. Injuries sustained by community-dwelling individuals with dementia. Clin Nurs Res. 2004;13:98–110. doi: 10.1177/1054773803262520. discussion 1–6. [DOI] [PubMed] [Google Scholar]
- 16.Rowe MA, Glover JC. Antecedents, descriptions and consequences of wandering in cognitively-impaired adults and the Safe Return (SR) program. Am J Alzheimers Dis Other Demen. 2001;16:344–52. doi: 10.1177/153331750101600610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowe MA, Bennett V. A look at deaths occurring in persons with dementia lost in the community. Am J Alzheimers Dis Other Demen. 2003;18:343–8. doi: 10.1177/153331750301800612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richards K. Effect of activity on sleep in cognitively-impaired veterans: A pilot study. Sleep Res. 1997;26:587. [Google Scholar]
- 19.Gaugler JE, Edwards AB, Femia EE, Zarit SH, Stephens MA, Townsend A, et al. Predictors of institutionalization of cognitively impaired elders: family help and the timing of placement. J Gerontol B Psychol Sci Soc Sci. 2000;55:P247–55. doi: 10.1093/geronb/55.4.p247. [DOI] [PubMed] [Google Scholar]
- 20.Rowe M, Lane S, Phipps C. CareWatch: A home monitoring system for use in homes of persons with cognitive impairment. Top in Geriatr Rehab. 2007;23:3–8. doi: 10.1097/00013614-200701000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braekhus A, Oksengard AR, Engedal K, Laake K. Social and depressive stress suffered by spouses of patients with mild dementia. Scand J Prim Health Care. 1998;16:242–6. doi: 10.1080/028134398750003034. [DOI] [PubMed] [Google Scholar]
- 22.Demers L, Weiss-Lambrou R, Ska B. Item analysis of the Quebec User Evaluation of Satisfaction with Assistive Technology (QUEST) Assist Technol. 2000;12:96–105. doi: 10.1080/10400435.2000.10132015. [DOI] [PubMed] [Google Scholar]
- 23.American National Standard method of recording basic facts relating to the nature and occurrence of work injuries. New York: American National Standards Institute; 1969. Report No.: ANAI Z16.2–1962 (R1969) [Google Scholar]
- 24.Oleske DM, Wilson RS, Bernard BA, Evans DA, Terman EW. Epidemiology of injury in people with Alzheimer’s disease. J Am Geriatr Soc. 1995;43:741–6. doi: 10.1111/j.1532-5415.1995.tb07042.x. [DOI] [PubMed] [Google Scholar]
- 25.Rowe M, Fehrenbach N. Injuries sustained by individuals with dementia living in the community living in the community: Classification and disposition. Appl Nurs Res. submitted. [Google Scholar]
- 26.O’Rourke N, Tuokko HA. Psychometric properties of an abridged version of The Zarit Burden Interview within a representative Canadian caregiver sample. Gerontologist. 2003;43:121–7. doi: 10.1093/geront/43.1.121. [DOI] [PubMed] [Google Scholar]
- 27.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–9. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]