Abstract
Objective
To assess intermittent treatment over 12 months in patients with symptomatic gastro-oesophageal reflux disease.
Design
Randomised, multicentre, double blind, controlled study. Patients with heartburn and normal endoscopy results or mild erosive changes received omeprazole 10 mg or 20 mg daily or ranitidine 150 mg twice daily for 2 weeks. Patients remaining symptomatic had omeprazole 10 mg or ranitidine dose doubled for another 2 weeks while omeprazole 20 mg was continued for 2 weeks. Patients who were symptomatic or mildly symptomatic were followed up for 12 months. Recurrences of moderate or severe heartburn during follow up were treated with the dose which was successful for initial symptom control.
Setting
Hospitals and primary care practices between 1994 and 1996.
Subjects
677 patients with gastro-oesophageal reflux disease.
Main outcome measures
Total time off active treatment, time to failure of intermittent treatment, and outcomes ranked from best to worst.
Results
704 patients were randomised, 677 were eligible for analyses; 318 reached the end of the study with intermittent treatment without recourse to maintenance antisecretory drugs. The median number of days off active treatment during follow up was 142 for the entire study (281 for the 526 patients who reached a treatment related end point). Thus, about half the patients did not require treatment for at least 6 months, and this was similar in all three treatment groups. According to outcome, 378 (72%) patients were in the best outcome ranks (no relapse or one (or more) relapse but in remission until 12 months); 630 (93%) had three or fewer relapses in the intermittent treatment phase. Omeprazole 20 mg provided faster relief of heartburn. The results were similar in patients with erosive and non-erosive disease.
Conclusions
Intermittent treatment is effective in managing symptoms of heartburn in half of patients with uncomplicated gastro-oesophageal reflux disease. It is simple and applicable in general practice, where most patients are seen.
Key messages
Symptomatic gastro-oesophageal disease can be managed successfully in half of patients with intermittent treatment with antisecretory drugs
Omeprazole 20 mg once daily gives more rapid relief of symptoms than either omeprazole 10 mg once daily or ranitidine 150 mg twice daily. However, the choice of antisecretory drug has little effect on the overall outcome
Relapses are relatively infrequent and can be managed with short courses of repeat treatment
Starting intermittent treatment with omeprazole 20 mg once daily is more cost effective than a dose titration approach with omeprazole 10 mg once daily or ranitidine 150 mg twice daily
An intermittent treatment strategy is simple and applicable in general practice, where most of these patients are seen
Introduction
Maintenance treatment with proton pump inhibitors is the most widely recommended form of treatment for the long term management of gastro-oesophageal reflux disease. Yet in day to day clinical practice treatment is commonly given in short courses, as and when symptoms demand—that is, intermittent treatment—particularly for patients perceived to have mild or only moderately troublesome disease. Even when maintenance treatment is prescribed patients often take their drugs intermittently.1
In contrast with maintenance treatment, which has been extensively investigated in clinical trials,2–14 the strategy of intermittent treatment has not been formally assessed. We therefore assessed intermittent treatment as a strategy to manage patients with symptomatic gastro-oesophageal reflux disease over a 12 month period.
We also assessed control of symptoms after the first 2 weeks of treatment and studied the natural course of uncomplicated gastro-oesophageal reflux disease over the 12 month period.
Patients and methods
Design of trial
Our aim was to reflect common clinical practice. We therefore used both histamine H2 receptor antagonists and proton pump inhibitors. Their doses were increased if symptoms were not controlled at the lower one. Patients received maintenance treatment if intermittent treatment at the higher doses failed.
Patients
Patients were recruited into the study either after hospital consultation or directly by their general practitioners. Patients with moderate (discomfort sufficient to cause interference with usual activities) or severe (leading to inability to perform normal activities) heartburn for more than 2 days in each of the previous 2 weeks and with normal endoscopy results or with mucosal breaks in the oesophagus (Los Angeles classification, grades A to C15) were included in the study (see table 1). Heartburn was defined as epigastric or substernal burning with ororadiation related to meals, straining, or posture. Those with the most severe erosive changes (grade D) were excluded. All antisecretory treatment not to be used in the study was stopped 2 weeks before entry to the study. Helicobacter pylori infection was detected with the rapid urease test.
Table 1.
Demography of patients with gastro-oesophageal reflux according to randomised group of treatment (all treatments twice daily). Figures are numbers (percentages) of patients unless stated otherwise
| Detail | Ranitidine 150 mg (n=229) | Omeprazole 10 mg (n=227) | Omeprazole 20 mg (n=221) | All (n=677)* |
|---|---|---|---|---|
| Men | 124 (54) | 127 (56) | 128 (58) | 379 (56) |
| No who smoked | 57 (25) | 66 (29) | 57 (26) | 183 (27) |
| Median (range) age (years): | ||||
| Men | 45 (21-75) | 46 (18-74) | 44 (21-76) | 45 (18-76) |
| Women | 52 (19-75) | 50 (19-74) | 51 (21-75) | 51 (19-75) |
| Mean body mass index (kg/m2) | 26 | 27 | 27 | 27 |
| Duration of reflux (months): | ||||
| 3-6 | 34 (15) | 36 (16) | 24 (11) | 95 (14) |
| 7-12 | 18 (8) | 27 (12) | 20 (9) | 68 (10) |
| > 12 | 176 (77) | 163 (72) | 177 (80) | 515 (76) |
| Endoscopic grade of mucosal breaks†: | ||||
| Normal | 80 (35) | 79 (35) | 64 (29) | 223 (33) |
| Grade A | 57 (25) | 59 (26) | 73 (33) | 190 (28) |
| Grade B | 71 (31) | 75 (33) | 69 (31) | 217 (32) |
| Grade C | 23 (10) | 14 (6) | 18 (8) | 54 (8) |
| Positive for H pylori‡ | 80/206 (39) | 84/205 (41) | 79/202 (39) | 245/613 (40) |
Only 6/677 were non-white.
Endoscopy grade (Los Angeles classification15): grade A: 1 or more mucosal breaks not more than 3 mm maximum length; grade B: 1 or more mucosal breaks >3 mm maximum length but not continuous between two mucosal folds; grade C: mucosal breaks continuous between tops of two or more mucosal folds but not circumferential; grade D (excluded): circumferential mucosal breaks. Grades A and B are broadly similar to Savary-Miller grades 1 and 2.
On basis of numbers of patients who had rapid urease test.
Initial treatment
The patients were allocated to treatment according to a computer generated randomisation list. At each centre patients were allocated to the next available treatment number and received 2 weeks of double blind, double dummy treatment with ranitidine 150 mg twice daily, omeprazole 10 mg daily, or omeprazole 20 mg daily. After 2 weeks patients who had had no symptoms over the previous 7 days entered a follow up period (see below) for up to 12 months from randomisation. Those with symptoms had the omeprazole 10 mg or ranitidine dose doubled for a further 2 weeks, whereas those on omeprazole 20 mg continued on this dose. After 4 weeks patients who had no or only mild symptoms also entered the follow up period. A compound antacid preparation (Maalox) was provided for the control of reflux symptoms during the study.
Follow up period: intermittent treatment strategy
Patients received no further treatment until moderate or severe symptoms of heartburn recurred for at least 2 days in each of the previous 2 weeks or if more than three antacid tablets were needed per day to control symptoms, whereupon patients returned to the clinic for assessment of symptoms. Confirmation that the patient was experiencing moderate or severe symptoms triggered repeat treatment for 2 or 4 weeks with the dose initially found to control symptoms, and the cycle was repeated for subsequent relapses. Patients were reviewed routinely at 3, 6, 9, and 12 months or whenever the need for repeat treatment arose.
Open maintenance treatment
When intermittent treatment failed maintenance treatment with omeprazole 20 mg daily was given until 12 months after randomisation.
The end points for intermittent treatment were changing to maintenance treatment because of unsuccessful intermittent treatment (symptoms remaining after 4 consecutive weeks of treatment or symptoms controlled on treatment but intermittent treatment unacceptable to the patient); exhaustion of drug supply, sufficient for up to 12 courses of treatment each of 2 weeks’ duration over 12 months; and completion of the study with patient still on intermittent treatment.
Statistical analysis
There were two approaches to the analyses: firstly, an intention to treat approach, which included those patients correctly randomised and treated and for whom there were follow up data on efficacy (n=677) and, secondly, an analysis of those 526 patients for whom final outcome was known—that is, they reached an end point while still on intermittent treatment. Formal statistical comparison between the groups was possible only when the patients were still being treated as randomised—that is, for the proportion of patients without symptoms after the initial 2 week treatment period and the proportions of patients reaching an end point on intermittent treatment who had no adjustment of dose during the initial treatment period. Elsewhere descriptive statistics have been used.
The assessment of outcomes in intermittent treatment was evaluated in three different but complementary ways.
Total time off treatment
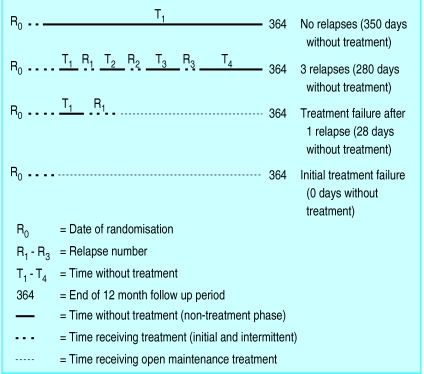
was defined as the sum of days during which the patient did not receive active treatment (fig 1 shows examples of calculations).
Figure 1.
Calculation of total time off treatment
Time to failure of intermittent treatment
was analysed by life table methods with censored follow up times for patients discontinuing treatment or withdrawn for other reasons. Cox proportional hazards regression was used to assess the influence of the following prognostic factors: randomised treatment, week of response to initial treatment, erosive or non-erosive gastro-oesophageal reflux disease, sex, smoking habit, duration of gastro-oesophageal reflux disease, age, body mass index, and H pylori status at baseline.
Outcomes ranked from best to worst
—Outcomes were ranked as follows: rank 1: no relapse (best outcome); rank 2: one (or more) relapses but thereafter in remission until 12 months; rank 3: exhaustion of the drug supply; rank 4: treatment failure after relapse on intermittent treatment; and rank 5: failure of the initial treatment (worst outcome).
Other details
The presentation of results meets the criteria of the CONSORT statement.16 This study was approved by the ethics committees of the participating centres and each patient gave written informed consent.
Results
The study involved 56 centres in the United Kingdom, Republic of Ireland, Germany, France, Spain, and Italy. The first patient entered the study in March 1994, and recruitment lasted for 1 year. Patients were followed up for 1 year, and the last patient completed the study in March 1996. A total of 704 patients were enrolled; about 10% were taking H2 receptor antagonists or proton pump inhibitors before entry to the study. Twenty seven patients were excluded (six did not return for reassessment; 21 did not fulfil the inclusion criteria). The 677 remaining patients had data valid for the intention to treat analysis. Over half (54%) of the patients had been recruited after hospital consultation and the remainder directly by their general practitioners. The patients in the three treatment groups were well matched for all baseline characteristics (table 1).
Broad outcome of intermittent treatment
Three hundred and eighteen (47%) patients completed this study while they were still receiving intermittent treatment. One hundred and sixty one (24%) continued in the study on maintenance treatment, and 197 (29%) discontinued the study at some stage mainly because of unwillingness to continue (21), adverse events (51), and loss to follow up (58). Other patients discontinued for various reasons that were unrelated to treatment or symptoms of heartburn. No patient completed the study prematurely because of exhaustion of their drug supply. There were no differences with respect to these outcomes between the three initially randomised groups.
Intermittent treatment: outcome measures
Total time off treatment
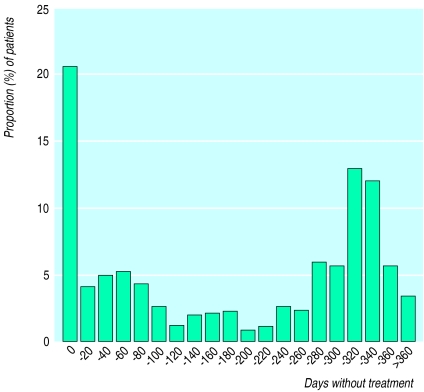
—The median number of days off treatment for all 677 patients was 142 days and for the 526 patients who reached a treatment related end point was 281 days. Thus half the patients did not require treatment for at least 6 months in total over the 1 year period. Eighty six patients failed to respond to initial treatment and another 55 were lost to follow up in this phase, representing all patients who had no days off treatment. Five more patients relapsed within 7 days after completing initial treatment but did not respond to 4 weeks’ further treatment; these are included in the 0 days off treatment column in figure 2. In contrast most patients had no relapse (217; 32%) or only one (163; 24%) or two (81; 12%) relapses. These patients generally had more than 280 days off treatment (fig 2). A similar pattern was seen for the 526 patients who reached a treatment related end point.
Figure 2.
Distribution of total number of days off treatment for 677 patients (all patients treated analysis)
Time to failure of intermittent treatment
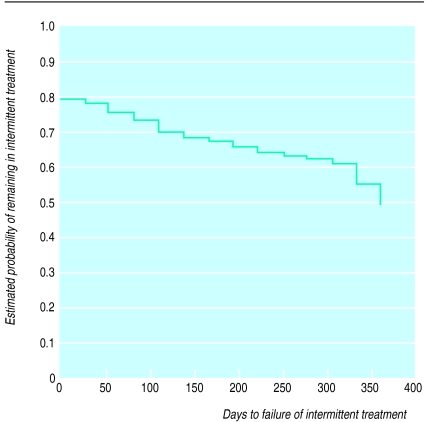
—Life table analysis on all 677 patients showed that 318 (47%) patients reached the end of the study using an intermittent treatment strategy without recourse to maintenance antisecretory treatment for at least 1 year (fig 3).
Figure 3.
Time to failure of intermittent treatment in 677 patients (all patients treated analysis)
Outcomes ranked from best to worst
—This analysis was based on the 526 patients for whom assessment of final outcome was available and could be ranked. Most patients (378; 72%) were in the first (172; 33%) or second (206; 39%) ranks, indicating they had a satisfactory outcome. Conversely, about a quarter (148; 28%) had more severe disease and were ranked 4 (62; 12%) and 5 (86; 16%).
Relapses
—Most patients relapsed infrequently on intermittent treatment: 271 (40%) had none, 203 (30%) had one, 102 (15%) had two, and 54 (8%) had three relapses. Analysis of the 156 patients who relapsed twice or more showed an association between the first and second remission periods when the cut off (for time to relapse) was arbitrarily set at 14, 28, or 56 days (table 2). There was a tendency for patients with a longer time to first relapse to have a longer time to the next relapse; the converse was also true. Despite this association, which was significant for the large group, the predictive value for an individual patient was limited.
Table 2.
Relation between time to second relapse and time to first relapse for 156 patients who had two or more relapses
| Time to first relapse (days) | Time to second relapse (days)
|
|||||
|---|---|---|---|---|---|---|
| 0-14 | >14 | 0-28 | >28 | 0-56 | >56 | |
| 0-14 | 15 | 25 | ||||
| >14 | 22 | 94* | ||||
| P value* | <0.05 | |||||
| 0-28 | 45 | 28 | ||||
| >28 | 21 | 62 | ||||
| P value* | <0.01 | |||||
| 0-56 | 75 | 25 | ||||
| >56 | 23 | 33 | ||||
| P value* | <0.01 | |||||
In relation to tests of association in each of 2×2 tables (χ2 with one degree of freedom).
Prognostic factors
—Symptoms controlled at 2 weeks was a powerful prognostic factor (P<0.0001). A higher proportion of such patients required no further treatment (rank 1—that is, no relapse; 33%). Smokers had a shorter time to final treatment failure (P=0.03). The other factors had no significant effect: endoscopic grade of oesophagitis at entry (P=0.59), sex (P=0.48), duration of reflux symptoms (P=0.39), age (P=0.54), body mass index (P=0.26), and presence of H pylori (P=0.63).
Treatment comparison
Omeprazole was significantly superior to ranitidine in outcome at week 2; the proportions without symptoms were 55% on omeprazole 20 mg, 40% on omeprazole 10 mg, and 26% on ranitidine (P<0.001; χ2 test). The outcome was similar in patients with and without oesophageal mucosal breaks at baseline.
As more patients responded to initial treatment with omeprazole compared with ranitidine a greater proportion were in remission in the early part of follow up. The long term outcome, however, was not affected by initial treatment at randomisation (table 3), similar proportions (22-27%) ultimately needing maintenance treatment or completing intermittent treatment over 12 months (46-48%).
Table 3.
Outcome in short and long term in patients with gastro-oesophageal reflux randomised to three different treatments. Figures are numbers (percentages) of patients unless stated otherwise
| Outcome | Ranitidine 150 mg (n=229) | Omeprazole 10 mg (n=227) | Omeprazole 20 mg (n=221) |
|---|---|---|---|
| Asymptomatic at 2 weeks | 60 (26) | 91 (40) | 122 (55) |
| Completed intermittent treatment | 108 (47) | 104 (46) | 106 (48) |
| Transferred to maintenance treatment | 62 (27) | 50 (22) | 49 (22) |
Discussion
Intermittent treatment: its rationale and outcome
The naturally relapsing nature of symptoms of gastro-oesophageal reflux is not altered by current management regimens. Patients with severe erosive or frequently relapsing disease require long term daily treatment.17 Most patients, however, have less severe disease and so may not require maintenance treatment. All of the patients in our study had a history of symptoms of reflux and, at entry, one third had a normal mucosa and 60% had mild erosive disease (grades A or B). Thus, they seemed suitable candidates for intermittent treatment. In fact, our study showed that intermittent treatment was effective for about half of such patients and drug treatment was unnecessary for much of the year. Relapses were, in general, infrequent and control of symptoms was achieved rapidly with a short course of repeat treatment. Omeprazole 20 mg was the most effective treatment for initial control of symptoms and might be expected to control symptoms in subsequent relapses.
Factors which influence outcome
In the attempt to target intermittent treatment, knowledge of factors which may indicate a good outcome is important. Others have shown that in patients who receive placebo after healing and control of symptoms only higher grades of oesophagitis and regurgitation before treatment clearly increase the risk of relapse. Older patients and smokers have a slightly increased risk of relapse.18
The most important factor in this study was response to initial treatment; patients who had no symptoms after 2 weeks of treatment had a better outcome on intermittent treatment than those who required treatment for 4 weeks. This corresponds with the observations of others who showed that patients who remain symptomatic at the time of mucosal healing relapse more rapidly.19 There was a marginally poorer outcome in patients who smoked. Other factors which may have been expected to have an effect, such as age and obesity, however, had no bearing on the outcome nor did infection with H pylori.
Differences in outcome between the treatment groups
Over 1 year we could find no difference between the groups, as randomised, in terms of overall outcome of intermittent treatment—for example, in time to treatment failure or number of relapses. This is to be expected for two reasons. Firstly, an effective dose was determined for each patient by titration. As this dose was then used in all subsequent relapses a broadly similar response was to be expected across the treatment groups. Secondly, treatment with antisecretory drugs will only temporarily interrupt the natural history of gastro-oesophageal reflux disease.20
Patients who do not respond to intermittent treatment
Two groups of patients who did not respond to intermittent treatment require special mention: those with initial treatment failure and those who failed subsequently. Despite initial dose titration, 13% (86/677) of patients remained symptomatic. As mentioned earlier, it was assumed that patients would respond in a similar manner to repeat treatment with the optimal dose, but 23% (121/526) ultimately failed to do so, suggesting that with time symptoms become less responsive in a proportion of patients. This has not been shown before to our knowledge, perhaps because the design of earlier studies precluded sequential observations on repeated relapses. It may also reflect the changing natural history of the disease in this subgroup of patients.
Since we completed our investigation a study has been reported concerning on demand treatment of patients with reflux symptoms and with normal results at endoscopy.21 Only half of the patients required treatment over 6 months’ follow up and omeprazole 20 mg proved superior to omeprazole 10 mg, which, in turn, was superior to placebo. These findings are broadly similar to our observations.
Cost effectiveness
A comment on cost effectiveness of treatment is appropriate. A recent report compared the costs of intermittent versus maintenance treatments. When the outcome was acceptable to the patient intermittent treatment was likely to be more cost effective than maintenance treatment.22 Our study included a parallel cost effectiveness analysis within intermittent treatment. The preliminary results indicate that starting intermittent treatment with omeprazole 20 mg is more cost effective than a dose titration approach with either omeprazole 10 mg or ranitidine 150 mg twice daily.23
Practical application
Our findings encourage us to recommend intermittent treatment for the long term management of patients with heartburn and with normal results on endoscopy or with mild erosive disease. Starting treatment with omeprazole 20 mg minimises the need for subsequent adjustments of dose. The strategy of intermittent treatment is suitable for about half the patients. Those who respond quickly to initial treatment are more likely to have a better outcome. Conversely, those who take longer to respond to treatment or who relapse quickly when treatment stops are likely to require maintenance treatment. Such a strategy allows better targeting of intermittent and maintenance treatments and is applicable to general practice, where most patients are seen.
Acknowledgments
The members of the European Study Group were as follows. France: Dr Abensur, Pont a Mousson; Dr Begue, Hayange; Professor Bigard, Dr Hudziak, Dr P Dieterling, Dr Pitoy, Dr Simon, Dr Protte, Nancy; Dr Courrier, Metz; Dr G Dalstein, Thionville; Dr R Fiorucci, Longwy; Dr Geoffroy, Epernay; Dr Goldfain, Dreux; Dr J Granguillaume, Belfort; Dr Jouin, Saint Avold; Dr J I Kolopp, Metz; Dr Laugros, Dr Seng, Freyming Merlebach; Dr Lirzin, Dr Salas, Troyes; Dr Pasqual, Dr Dahlab, Troyes; Dr Rotenberg, Dreux; Dr D Schmitz, Forbach; Dr Zahm, Hagondange; Professor Zeitoun, Reims. Germany: Dr G Buttner, Dr Ch von Wolff, Dr W Dubel, Dr E Gozdowsky, Dr H Hartmann, Dr U Kernchen, Dr J Machens, Dr B Marowski, Dr V Olivier, Dr H U Rehs, Dr A Ryschka, Dr K Scholze, Dr P Semier, Dr Med K Uhlig, Dr R Wach, Berlin. Republic of Ireland: Dr J P Crowe, Dr P W N Keeling, Professor D G Weir, Dublin. Italy: Dr A Andriulli, Rotondo (FG); Professor P Bianchi, Professor G Bianchi Porro, Milan; Dr E Colombo, Garbagnate (M1); Professor M Curzio, Varese; Professor G Mazzacca, Napoli; Dr G Minoli, Como. Spain: Dr D Abascal, Sevilla; Dr L Martin, Cadiz; Dr G Mino, Cordoba; Dr Julio Ponce, Valencia; Dr L Rodrigo, Oviedo; Professor Dr Diaz Rubio, Madrid. United Kingdom: Dr K D Bardhan, Rotherham; Dr R Cockel, Birmingham; Dr A Cowie, Bath; Dr T K Daneshmend, Exeter; Dr N Gough, Bradford-on-Avon; Dr K Gruffydd-Jones, Wiltshire; Dr J Hampton, Dr J Playfair, Bath; Dr J Hosie, Dr M G B Scott, Glasgow; Dr A P S Hungin, Stockton on Tees; Dr S Rowlands, Trowbridge; Dr S Wilkinson, Plymouth.
Footnotes
Funding: Astra Clinical Research Unit, Edinburgh.
Competing interests: MAB has acted as consultant to Astra (manufacturers of Losec (omeprazole)) and Glaxo-Wellcome Laboratories (manufacturers of Zantac (ranitidine)). RAP is employed by Astra as a biostatistician and holds shares in the company.
References
- 1.Hungin APS, Rubin GP, O’Flanagan H. How regularly do patients on long term PPIs take their therapy? Gastroenterology. 1997;112:A19. [Google Scholar]
- 2.Staerk Laursen L, Havelund T, Bondesen S, Hansen J, Sanchez G, Sebelin E, et al. Omeprazole in the long term treatment of gastro-oesophageal reflux disease. A double-blind randomised dose finding study. Scand J Gastroenterol. 1995;30:839–846. doi: 10.3109/00365529509101589. [DOI] [PubMed] [Google Scholar]
- 3.Zeitoun P, Isal JP, Barbier P. Comparison of two dosage regimens of omeprazole—10mg once daily and 20mg weekends—as prophylaxis against recurrence of reflux oesophagitis. Hepatogastroenterol. 1989;36:279–280. [Google Scholar]
- 4.Dent J, Yeomans ND, Mackinnon M, Reed W, Narielvala FM, Hetzel DJ, et al. Omeprazole v ranitidine for prevention of relapse in reflux oesophagitis. A controlled double-blind trial of their efficacy and safety. Gut. 1995;35:590–598. doi: 10.1136/gut.35.5.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vigneri S, Termini R, Leandro G, Badalamenti S, Panatalena M, Savarino V, et al. A comparison of five maintenance therapies for reflux oesophagitis. N Engl J Med. 1995;333:1106–1110. doi: 10.1056/NEJM199510263331703. [DOI] [PubMed] [Google Scholar]
- 6.Bate CM, Booth SM, Crowe JP, Mountford RA, Keeling PWN, Hepworth-Jones B. Omeprazole 10mg or 20mg once daily in the prevention of recurrence of reflux oesophagitis. Gut. 1995;36:492–498. doi: 10.1136/gut.36.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallerbäck B, Unge P, Carling L, Edwin B, Glise H, Havu N, et al. Omeprazole or ranitidine in longterm treatment of reflux oesophagitis. Gastroenterology. 1994;107:1305–1311. doi: 10.1016/0016-5085(94)90531-2. [DOI] [PubMed] [Google Scholar]
- 8.Bardhan KD, Cherian P, Vaishnavi A, Jones RB, Thompson M, Morris P, et al. Erosive oesophagitis: outcome of repeated long term treatment with low dose omeprazole 10mg or placebo. Gut. 1998;43:458–464. doi: 10.1136/gut.43.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson MJ, Lanza F, Avner F, Haber M. Effective maintenance treatment of reflux oesophagitis with low dose lansoprazole. Ann Intern Med. 1996;124:859–867. doi: 10.7326/0003-4819-124-10-199605150-00001. [DOI] [PubMed] [Google Scholar]
- 10.Gough AL, Long RG, Cooper BT, Fosters CF, Garrett AD, Langworthy CH. Lansoprazole vs ranitidine in the maintenance treatment of reflux oesophagitis. Aliment Pharmacol Ther. 1996;10:529–539. doi: 10.1046/j.1365-2036.1996.14156000.x. [DOI] [PubMed] [Google Scholar]
- 11.Hatlebakk JG, Berstad A. Lansoprazole 15 and 30mg daily in longterm treatment of erosive reflux oesophagitis. Gastroenterology. 1995;108:A111. [Google Scholar]
- 12.Poynard T, Staub JL, Lemerez M, Deltenre M, Rekacevica C, Sallerin V. Efficacy and safety of lansoprazole 15mg OAD or 30mg OAD as one year maintenance treatment for erosive reflux oesophagitis, a randomised trial. Gastroenterology. 1995;108:A195. [Google Scholar]
- 13.Baldi F, Bardhan KD, Borman BC, Brullet E, Dent J, Galmiche JP, et al. Lansoprazole maintains healing in patients with reflux oesophagitis. Gastroenterology. 1996;110:A55. [Google Scholar]
- 14.Sontag S. Rolling review; gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 1993;7:293–312. doi: 10.1111/j.1365-2036.1993.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong D, Bennett JR, Blum AL, Dent J, de Dombal FT, Galmiche J-P, et al. The endoscopic assessment of oesophagitis: a progress report on observer agreement. Gastroenterology. 1996;111:85–92. doi: 10.1053/gast.1996.v111.pm8698230. [DOI] [PubMed] [Google Scholar]
- 16.Altman DG. Better reporting of randomised controlled trials: the CONSORT statement. BMJ. 1996;313:570–571. doi: 10.1136/bmj.313.7057.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambert R. Review article. Current practice and future perspectives in the management of gastroesophageal reflux disease. Aliment Pharmacol Ther. 1997;11:651–662. doi: 10.1046/j.1365-2036.1997.00181.x. [DOI] [PubMed] [Google Scholar]
- 18.Carlsson R, Galmiche J-P, Dent J, Lundell L, Frison L. Prognostic factors influencing relapse of oesophagitis during maintenance therapy with antisecretory drugs: a meta-analysis of long-term omeprazole trials. Aliment Pharmacol Ther. 1997;11:473–482. doi: 10.1046/j.1365-2036.1997.00167.x. [DOI] [PubMed] [Google Scholar]
- 19.Tytgat GNJ, Blum AL, Verlinden M. Prognostic factors for relapse and maintenance treatment with cisapride in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 1995;9:271–280. doi: 10.1111/j.1365-2036.1995.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 20.Sandmark S, Carlsson R, Fausa O, Lundell L. Omeprazole or ranitidine in the treatment of reflux oesophagitis: results of a double-blind, randomised, Scandinavian multicenter study. Scand J Gastroenterol. 1988;23:625–632. doi: 10.3109/00365528809093923. [DOI] [PubMed] [Google Scholar]
- 21.Lind T, Havelund T, Carlsson R, Eriksson G, Glise H, Junghard O, et al. The effect of omeprazole (OME) 20mg and 10mg daily on heartburn in patients with endoscopy negative reflux disease (ENRD) treated on an on-demand basis. Gastroenterology. 1996;100:A178. [Google Scholar]
- 22.Harris RA, Kupperman M, Richter JE. Prevention of recurrences of erosive reflux oesophagitis: a cost-effectiveness analysis of maintenance proton pump inhibition. Am J Med. 1997;102:78–88. doi: 10.1016/s0002-9343(96)00301-4. [DOI] [PubMed] [Google Scholar]
- 23.Bardhan KD, Müller-Lissner S, Bigard MA, Bianchi-Porro G, Ponce J, Hosie J, et al. Cost-effectiveness (C-E) of omeprazole (OM) and ranitidine (RAN) in intermittent treatment (IT) of symptomatic gastroesophageal reflux disease (GERD) Gut. 1997;41(suppl 3):A203. [Google Scholar]