Abstract
Previous studies by our laboratory have demonstrated that the mu opioid receptor antagonist, CTAP, blocks the rewarding effects of cocaine when it is injected directly into the nucleus accumbens or ventral tegmental area (VTA). This finding suggests that cocaine is causing the release of endogenous opioid peptides which activate mu opioid receptors within the nucleus accumbens and VTA. The purpose of the present study was to characterize the dose-response and time-course of mu receptor occupancy following systemic cocaine administration and to determine if release of endogenous opioids by cocaine is mediated by activation of D1 or D2 dopamine receptors. Quantitative in vitro receptor autoradiography was used to measure the regional displacement of 3H-DAMGO binding following cocaine administration. Adult male Sprague-Dawley rats were given intraperitoneal (i.p.) injections of cocaine and their brains were removed at various times and prepared for mu opioid receptor quantitation. To determine the role of dopamine D1 and D2 receptors in the effect of cocaine on mu receptor occupancy, rats were injected with the selective D1 or D2 receptor antagonists SCH23390 or eticlopride prior to cocaine. For all studies, 3H-DAMGO binding to mu opioid receptors was measured in the nucleus accumbens, caudate putamen, frontal cortex, olfactory tubercle and VTA. Results demonstrate that cocaine administration caused a time- and dose-dependent reduction in 3H-DAMGO binding within the nucleus accumbens core and shell. The reduction in mu receptor binding was attenuated by pretreatment with eticlopride. These results suggest that cocaine, acting via D2 dopamine receptors, can cause the release of an endogenous opioid peptide that binds to mu opioid receptors within the nucleus accumbens.
Keywords: Dopamine, nucleus accumbens, beta-endorphin, SCH23390, eticlopride, ventral tegmental area
Cocaine is a psychomotor stimulant that facilitates monoaminergic neurotransmission by binding to transporters and inhibiting the reuptake of dopamine, serotonin and norepinepherine into presynaptic neurons (Heikkila et al., 1975; Nicolaysen and Justice, 1988). Increases in extracellular dopamine within the nucleus accumbens produced by cocaine are directly related to its rewarding properties (Kiyatkin and Stein, 1995; Phillips et al., 2003). In addition to the direct effects on monoamine reuptake, cocaine can alter levels of endogenous opioid peptides (Costa et al., 1978; Sivam, 1989) and can have profound effects on the expression and function of opioid receptors (Hammer, 1989; Unterwald et al., 1994; Izenwasser et al., 1996; Schroeder et al., 2007). Data from our lab have shown that administration of the selective mu opioid receptor antagonist, CTAP (D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2), into either the nucleus accumbens core or ventral tegmental area (VTA) attenuated cocaine-induced reward and hyperactivity (Soderman and Unterwald, 2008). Further, mu opioid receptor agonists themselves are rewarding when administered in either the nucleus accumbens (Olds, 1982; van der Kooy et al., 1982) or rostral VTA (Devine and Wise, 1994). These data suggest that the opioid and dopamine systems interact to mediate the rewarding effects of cocaine.
Limbic brain regions including the nucleus accumbens, receive endorphinergic inputs from pro-opiomelanocortin (POMC)-containing neurons originating in the arcuate nucleus of the hypothalamus (Bloom et al., 1978b; Finley et al., 1981). Cocaine administration can increase beta-endorphin levels in the rat nucleus accumbens (Olive et al., 2001; Roth-Deri et al., 2003; Doron et al., 2006). The ability of cocaine to increase the levels of endorphins within the nucleus accumbens is thought to be mediated indirectly through dopamine signaling (Roth-Deri et al., 2003; Doron et al., 2006).
Cocaine has been shown to alter the levels of other opioid peptide precursors and proteins in addition to its effects on beta-endorphin. The effects of cocaine self-administration on the expression of messenger RNAs for preprodynorphin and preproenkephalin have been evaluated. Both preprodynorphin and preproenkephalin mRNA levels are elevated in the striatum following administration of cocaine (Hurd et al., 1992; Spangler et al., 1993). In separate studies, striatal peptide levels of met-enkephalin and dynorphin were measured in rats by radioimmunoassay following acute (single dose) or subchronic (one dose daily for 4 days) administration of cocaine. An acute administration of cocaine did not affect peptide levels in the striatum. Subchronic administration of cocaine increased striatal dynorphin levels without altering the levels of met-enkephalin (Sivam, 1989). Together, these results suggest that cocaine administration can alter levels of endogenous peptides to varying extents throughout the striatum.
Although previous data demonstrate that cocaine can increase levels of endogenous opioid peptides, the receptor activated by these opioid peptides in vivo has not been established. The present study determined the time– and dose-dependent occupancy of mu opioid receptors within specific brain regions following injection of cocaine. The occupancy of mu opioid receptors by endogenous ligands was assessed indirectly by displacement of 3H-DAMGO binding using in vitro autoradiography. The rationale of this technique is that opioid peptides released following cocaine administration will bind to (or occupy) mu opioid receptors and displace exogenously applied 3H-DAMGO, leading to a decrease in signal assessed by autoradiography. An additional aim of this study was to determine the contribution of dopamine D1 and D2 receptors in cocaine-induced 3H-DAMGO displacement. Results demonstrate that cocaine caused a time- and dose-dependent displacement of 3H-DAMGO binding to mu opioid receptors within the nucleus accumbens core and shell. Cocaine-induced release of endogenous peptides was mediated by dopamine D2 receptors.
Results
Cocaine Time-Course Study
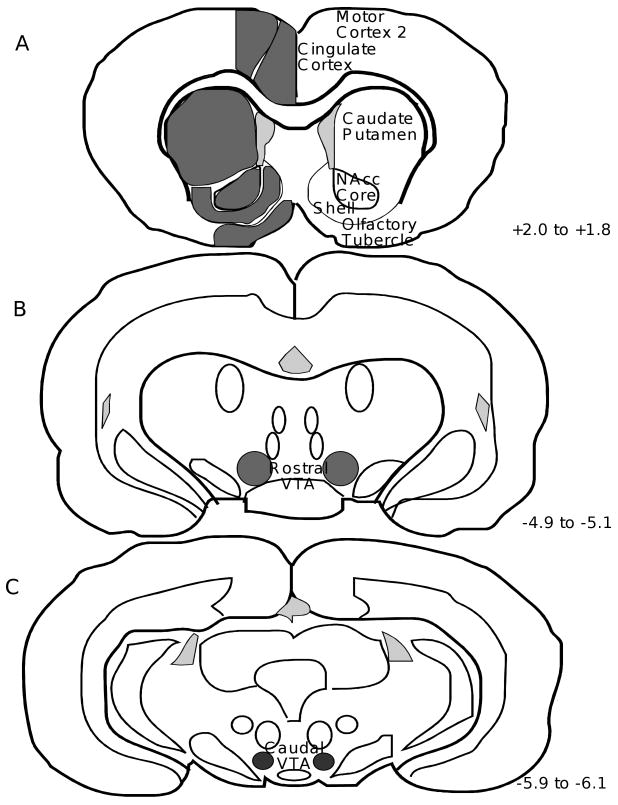
3H-DAMGO binding was measured by quantitative in vitro receptor autoradiography to indirectly determine the time-course of occupancy of mu opioid receptors by endogenous ligand(s) following cocaine administration. Figure 1 shows the brain regions in which mu receptor binding was measured. A representative total binding section for each time point is shown in Figure 2 (A-E), along with a representative non-specific binding section for the 0 min time point (F).
Figure 1. Coronal Rat Brain Sections Illustrating the Regions of Quantitation of Mu Opioid Receptor Binding.
Receptor densities from both the left and right hemisphere for each region of interest were obtained. The distance of each section from bregma is given in millimeters (Paxinos and Watson, 1986). NAcc=nucleus accumbens; VTA=ventral tegmental area.
Figure 2. Total and Non-Specific 3H-DAMGO Binding to Representative Brain Sections.
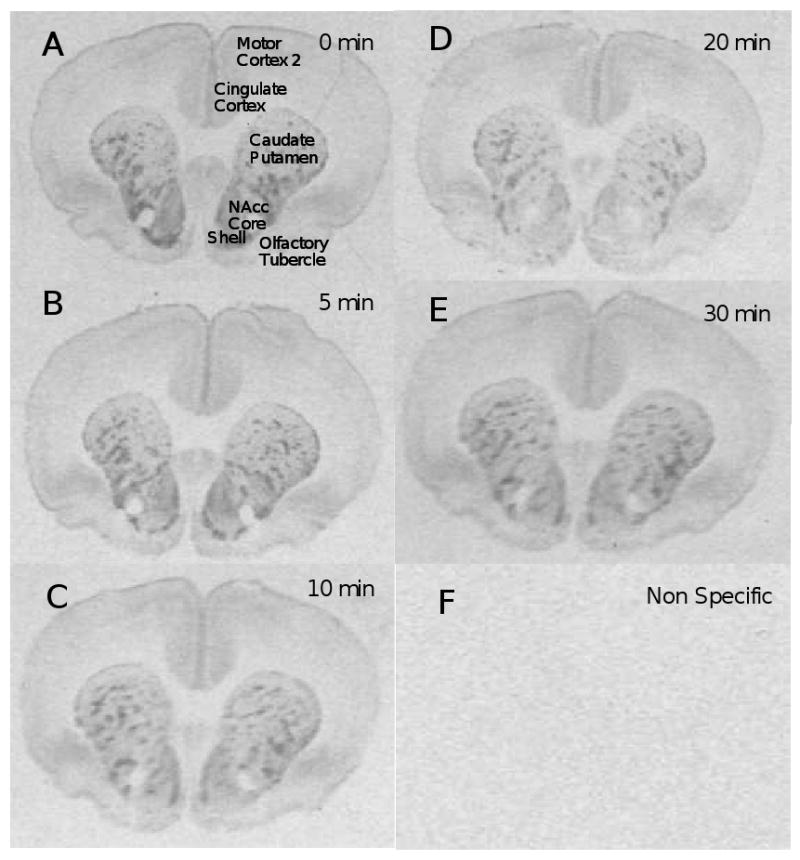
Brains were obtained at various times following injection of 10 mg/kg cocaine: time=0 min (2A), time=5 min (2B), time=10 min (2C), time=20 min (2D), time=30 min (2E), and non-specific binding for T=0 min (2F).
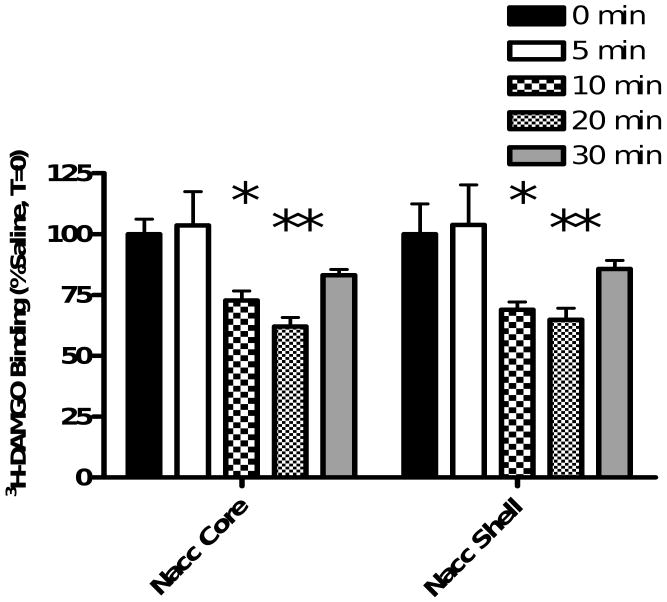
A significant effect of cocaine on 3H-DAMGO binding within the nucleus accumbens core was found (F(4,10)=8.908, p=0.0007) (Figure 3). Bonferroni post-hoc analyses revealed that cocaine administration caused a time-dependent decrease in 3H-DAMGO binding in the nucleus accumbens core (0 min vs 5 min = p>0.05; 0 min vs 10 min = p<0.05; 0 min vs 20 min = p<0.01; 0 min vs 30 min = p>0.05). Similarly, analysis of data obtained from the shell of the nucleus accumbens revealed a significant decrease in 3H-DAMGO binding (F(4,10)=8.445, p=0.0009) (Figure 3). Bonferroni post-hoc analyses indicated a significant reduction in 3H-DAMGO binding at 10 and 20 minutes following cocaine administration (0 min vs 5 min = p>0.05; 0 min vs 10 min = p<0.05; 0 min vs 20 min = p<0.01; 0 min vs 30 min = p>0.05). Data obtained for the caudate putamen, olfactory tubercle, cingulate cortex, motor cortex 2, rostral VTA and caudal VTA are shown in Table 1. There were no significant differences between saline and cocaine groups at any time point in any of these brain regions (all ANOVAs p>0.05). Results show a time-dependent decrease in 3H-DAMGO binding following cocaine administration within the core and shell of the nucleus accumbens.
Figure 3. Cocaine Time-Course Study.
Animals received a single injection of saline (1 ml/kg i.p.) or cocaine (10 mg/kg i.p.) and were euthanized either 0, 5, 10, 20 or 30 minutes later. 3H-DAMGO binding was quantified in the nucleus accumbens and plotted as a % of binding in the saline-injected rat at T=0. One-way ANOVA with Bonferroni post-hoc analyses revealed a significant reduction in 3H-DAMGO binding in the nucleus accumbens (NAcc) core and shell at 10 and 20 minutes following cocaine injection (*=P<0.05, **=P<0.01 vs 0 min). Data are presented as mean (±SD). (N=4 animals/group)
Table 1. Cocaine Time-Course Study.
Data are expressed as mean (±SD) of 3H-DAMGO binding. Animals received a single injection of saline (1 ml/kg i.p.) or cocaine (10 mg/kg i.p.) and were euthanized at either 0, 5, 10, 20 or 30 minutes later. 3H-DAMGO binding was quantified and expressed as a % of binding measured in saline-injected controls. One-way ANOVA show no significant differences in 3H-DAMGO binding in any region listed. (N=3/group)
| 0 min | 5 min | 10 min | 20 min | 30 min | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| CP | 100 | 15.7 | 100.4 | 16 | 86.9 | 24.1 | 87.3 | 13 | 95 | 15.8 |
| OT | 100 | 10.1 | 97.5 | 9.2 | 82.3 | 10.5 | 101.9 | 13.6 | 102.5 | 15.1 |
| Cingulate Cortex | 100 | 1.9 | 101.9 | 6.3 | 85.8 | 21.4 | 95.7 | 3.3 | 97.5 | 6.6 |
| Motor Cortex 2 | 100 | 3.5 | 110.9 | 7.7 | 96.9 | 18 | 98.8 | 2.9 | 99.5 | 4.2 |
| Rostral VTA | 100 | 4.6 | 101.4 | 4.3 | 89.4 | 2.1 | 96.4 | 8.4 | 100.8 | 15.6 |
| Caudal VTA | 100 | 6.2 | 92.6 | 3.1 | 100.4 | 2.9 | 99.9 | 9.4 | 94.7 | 5.1 |
Cocaine Dose-Response Study
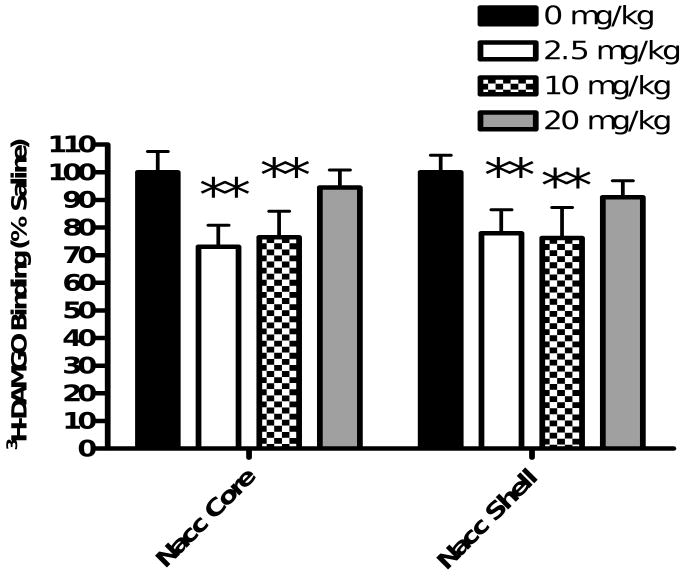
In vitro receptor autoradiography was employed to quantitate 3H-DAMGO binding to mu opioid receptors 10 minutes following administration of three doses of cocaine. The means (± SD) of 3H-DAMGO binding in the nucleus accumbens core and shell as a percentage of mu receptor binding to the saline control group are shown in Figure 4.
Figure 4. Cocaine Dose-Response Study.
Animals received a single injection of saline (1 ml/kg i.p.) or cocaine (2.5, 10 or 20 mg/kg i.p.) and were euthanized ten minutes later. 3H-DAMGO binding was quantified in the nucleus accumbens and plotted as a % of binding in saline controls. One-way ANOVA with Bonferroni post-hoc analyses revealed a significant reduction in 3H-DAMGO binding in the nucleus accumbens core and shell for animals injected with 2.5 and 10 mg/kg cocaine (**=P<0.01 vs 0 mg/kg). Data are presented as mean (±SD). (N=4 animals/group)
Results show a decrease in 3H-DAMGO binding following cocaine administration within the nucleus accumbens core and shell. One-way ANOVA revealed a significant effect of cocaine on 3H-DAMGO binding within the core (F(3,12)=11.56, p=0.0007). Bonferroni post-hoc analyses revealed that 2.5 mg/kg and 10 mg/kg cocaine significantly decreased 3H-DAMGO binding (saline vs 2.5 mg/kg cocaine = p<0.01; saline vs 10 mg/kg cocaine = p<0.01). One-way ANOVA of data obtained from the nucleus accumbens shell also showed significant differences between treatment groups (F(3,12)=7.634, p=0.0041). Bonferroni post-hoc analyses revealed that both 2.5 and 10 mg/kg cocaine produced a significant decrease in 3H-DAMGO binding (saline vs 2.5 mg/kg cocaine = p<0.01; saline vs 10 mg/kg cocaine = p<0.01). 3H-DAMGO binding in the caudate putamen, olfactory tubercle, cingulate cortex, motor cortex 2, rostral VTA and caudal VTA for all cocaine doses were not significant by one-way ANOVA (Table 2). These results demonstrate that 2.5 or 10 mg/kg cocaine reduces 3H-DAMGO binding within the nucleus accumbens core and shell.
Table 2. Cocaine Dose-Response Study.
Data are expressed as mean (±SD) of 3H-DAMGO binding. Animals received a single injection of saline (1 ml/kg i.p.) or cocaine (2.5, 10 or 20 mg/kg i.p.) and were euthanized ten minutes later. 3H-DAMGO binding was determined and expressed as a % of that in saline controls. One-way ANOVA showed no significant differences in 3H-DAMGO binding in any region listed. (N=4/group)
| Saline | 2.5 mg/kg Cocaine | 10 mg/kg Cocaine | 20 mg/kg Cocaine | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| CP | 99.9 | 18.8 | 96.2 | 16.5 | 95.6 | 21.2 | 115.1 | 19.7 |
| OT | 100.1 | 16.9 | 98.6 | 33.9 | 85 | 10.9 | 91.2 | 10.9 |
| Cingulate Cortex | 100 | 9.1 | 97.6 | 8.8 | 90.3 | 9.9 | 97.3 | 4.8 |
| Motor Cortex 2 | 99.9 | 9.9 | 97.3 | 7.9 | 84.9 | 13.4 | 92.2 | 7.4 |
| Rostral VTA | 100 | 9.1 | 102.9 | 14.6 | 89.2 | 14.6 | 103.8 | 23.8 |
| Caudal VTA | 100 | 6.2 | 105.7 | 17.1 | 102.7 | 11.9 | 107.2 | 6.7 |
Involvement of Dopamine D1 and D2 Receptors
In order to determine if the effects of cocaine on mu opioid receptor binding were mediated by dopamine D1 or D2 receptors, the effects of selective blockade of dopamine D1 and D2 receptors prior to cocaine administration on the reduction of 3H-DAMGO binding were investigated.
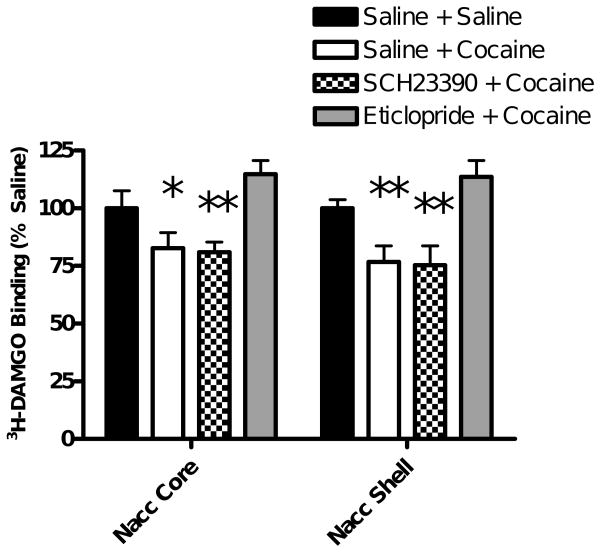
One-way ANOVA revealed a significant effect of cocaine on 3H-DAMGO binding within the nucleus accumbens core (F(3,12)=25.35, p<0.0001). Bonferroni post-hoc analysis show a significant decrease in 3H-DAMGO binding in the nucleus accumbens core 10 minutes following 10 mg/kg cocaine (saline + saline vs saline + cocaine = p< 0.05; Figure 5) in agreement with the data shown in Figures 3 and 4. Pretreatment with eticlopride, but not SCH23390, significantly attenuated the reduction in 3H-DAMGO binding seen following cocaine administration (saline + cocaine vs eticlopride + cocaine = p<0.001; saline + cocaine vs SCH23390 + cocaine= p>0.05). Mu receptor binding in the nucleus accumbens core of rats administered eticlopride plus cocaine was not significantly different from that in saline controls (saline + saline vs eticlopride + cocaine= p>0.05).
Figure 5. Dopamine D1 and D2 Receptor Antagonist Study.
Animals were injected with saline (1 ml/kg i.p.), the D1 selective antagonist SCH23390 (0.5 mg/kg i.p.) or the D2 selective antagonist eticlopride (1 mg/kg i.p.). Thirty minutes later, animals in one of the two saline-injected groups received saline (1 ml/kg i.p.) and all other animals received cocaine (10 mg/kg i.p.). Ten minutes later, brains were obtained. 3H-DAMGO binding was quantified in the nucleus accumbens and plotted as a % of that in saline controls. One-way ANOVA with Bonferroni post-hoc analyses revealed a significant reduction in 3H-DAMGO binding in the NAcc core and shell in animals pretreated with saline prior to 10 mg/kg cocaine when compared to saline-saline animals. This reduction was attenuated in animals pretreated with eticlopride, but not SCH23390. Data are presented as mean (±SD). (N=4 animals/group) (*=P<0.05, **=P<0.01 vs saline + saline).
For the nucleus accumbens shell, one-way ANOVA showed a significant difference between treatment groups (F(3,12)=30.14, p<0.0001). Bonferroni post-hoc analyses revealed that pretreatment with eticlopride significantly attenuated the decrease in 3H-DAMGO binding following cocaine (saline + cocaine vs eticlopride + cocaine = p<0.001) and was not significantly different from the saline controls (saline + saline vs eticlopride + cocaine= p>0.05). Pretreatment with SCH23390 did not alter the cocaine-induced reduction in 3H-DAMGO binding (saline + cocaine vs SCH23390 + cocaine= p>0.05). 3H-DAMGO binding in the caudate putamen, olfactory tubercle, cingulate cortex, motor cortex 2, rostral VTA and caudal VTA were not significantly different between treatment groups (Table 3). These data demonstrate that the cocaine-induced decreases in 3H-DAMGO binding within the nucleus accumbens core and shell was blocked by pretreatment with a dopamine D2 receptor antagonist.
Table 3. Dopamine D1 and D2 Receptor Antagonist Study.
Data are expressed as mean (±SD) of 3H-DAMGO binding as a percent of saline controls. Animals were injected with saline (1 ml/kg i.p.), the D1 receptor antagonist SCH23390 (0.5 mg/kg i.p.) or the selective D2 receptorantagonist eticlopride (1 mg/kg i.p.) Thirty minutes later, animals in the first saline group received saline (1 ml/kg i.p.) and all other groups received cocaine (10 mg/kg i.p.). Ten minutes later, brains were obtained and 3H-DAMGO binding was quantified and expressed as a % of saline controls. One-way ANOVA show no significant differences in 3H-DAMGO binding in any region listed. (N=4/group)
| Saline + Saline | Saline + Cocaine | SCH23390 + Cocaine | Eticloprode + Cocaine | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| CP | 100 | 10.4 | 89.3 | 8.7 | 87.4 | 3.2 | 100.6 | 2.0 |
| OT | 100 | 25.5 | 83.2 | 10.0 | 75.8 | 7.6 | 88.4 | 7.2 |
| Cingulate Cortex | 100 | 5.2 | 91.6 | 6.5 | 92.6 | 10.6 | 91.9 | 12.5 |
| Motor Cortex 2 | 100 | 6.6 | 91.5 | 4.6 | 89.3 | 7.0 | 92.9 | 13.3 |
| Rostral VTA | 100 | 12.0 | 93.1 | 3.9 | 91.5 | 9.2 | 115.6 | 9.0 |
| Caudal VTA | 100 | 15.0 | 98.3 | 7.6 | 96.5 | 14.9 | 100.7 | 10.8 |
Discussion
The findings of this study demonstrate that cocaine, acting via D2 dopamine receptors, can cause a time-dependent decrease in 3H-DAMGO binding within the nucleus accumbens core and shell, suggesting that cocaine is causing the release of endogenous opioids that binds to mu opioid receptors in this brain region. This finding is consistent with previously published studies showing that cocaine can elevate beta-endorphin levels (Olive et al., 2001; Roth-Deri et al., 2003) and preprodynorphin and preproenkephalin mRNA expression (Hurd et al., 1992) within the nucleus accumbens, and extends these findings to show that elevations in opioid peptides in the nucleus accumbens are coupled with increases in binding of these peptides to mu opioid receptors. 3H-DAMGO binding was measured by in vitro autoradiography under conditions that minimized endogenous ligand dissociation. This method allows for the visualization of changes in opioid receptor occupancy with high anatomical resolution. The hypothesis tested was that cocaine causes opioid peptide release and these peptides bind to mu opioid receptors. The opioid peptides, having occupied the mu opioid receptors, would competitively displace subsequent exogenously applied 3H-DAMGO, leading to a decrease in 3H-DAMGO binding assessed by autoradiography.
Previous results from our lab have shown that mu opioid receptors within the nucleus accumbens, VTA and caudate putamen are involved in cocaine-induced behaviors (Soderman and Unterwald, 2008) and as a result these regions were chosen for study. The present results show that cocaine decreased 3H-DAMGO binding in the nucleus accumbens core and shell suggesting an increase in mu opioid receptor occupancy by endogenous opioid peptides. A time-dependent reduction in 3H-DAMGO binding was observed in both the core and shell of the nucleus accumbens, with maximal reduction occurring 20 minutes following cocaine administration. These results are consistent with a previous study showing a significant increase in beta-endorphin in nucleus accumbens 20 minutes following i.v. administration of cocaine which returned to basal levels within 40 minutes (Roth-Deri et al., 2003). The results of the present study show that mu opioid receptor occupancy in the nucleus accumbens returned to basal levels 30 minutes following cocaine administration We hypothesize that the opioid peptide occupying mu opioid receptors following cocaine within the nucleus accumbens is beta-endorphin as previous studies have demonstrated increases in beta-endorphin in the accumbens following acute administration of cocaine (Roth-Deri et al., 2003; Doron et al., 2006) within the time period of this study (≤30 min). Although other opioid peptides including the enkephalins and dynorphin have been shown to be released following cocaine administration, previous studies failed to see increases in dynorphin or enkephalin peptide or mRNA levels in this region following acute administration of cocaine within the time period of this study (<30 min) (Sivam, 1989; Andrea et al., 1992; Hurd et al., 1992).
The results of this study showed that 2.5 and 10 mg/kg of cocaine produced a decrease in 3H-DAMGO binding in the nucleus accumbens measured 10 minutes following cocaine administration. The current study used doses of cocaine shown to be reinforcing in the rat by cocaine conditioned place preference models (Nomikos and Spyraki, 1988; Soderman and Unterwald, 2008). The lack of effect seen with the 20 mg/kg dose of cocaine is hypothesized to be a result of the effects of this dose on other neurotransmitter systems. Previous studies have shown higher doses of cocaine (including 20 mg/kg) to be less rewarding in rats than lower doses (Durazzo et al., 1994; Russo et al., 2003). It is possible that at higher doses of cocaine, increased inhibition of norepinephrine reuptake inhibits the release of beta-endorphin. Previous studies have shown that norepinephrine can have an inhibitory effect on a subset of arcuate nucleus neurons (Lin et al., 1993; Kang et al., 2000) where beta-endorphin-containing neurons arise.
The results of the present study indicate that cocaine-induced endogenous opioid binding to mu opioid receptors within the nucleus accumbens is mediated by the dopamine D2 receptor. The reduction in 3H-DAMGO binding following cocaine administration was attenuated by pretreatment with the selective D2 dopamine receptor antagonist eticlopride but not the selective D1 dopamine receptor antagonist SCH23390. A previous study has shown that blockade of dopamine D1 receptors attenuates cocaine-induced beta-endorphin release within the nucleus accumbens, although D2 receptors were not investigated in that study (Roth-Deri et al., 2003). The difference in the results reported herein and the previous study by Roth-Deri et al. (2003) may be due to the dose of either cocaine (2.0 mg/kg i.v. vs 10 mg/kg i.p.) or SCH23390 (0.25 vs 0.5 mg/kg). A complete dose-response curve for SCH23390 is needed to fully characterize the involvement of dopamine D1 receptors in the effect found herein. Previous studies have shown the involvement of dopamine D2 receptors in cocaine-induced beta-endorphin release. Doron et al. (2006) demonstrated that blockade of dopamine D2 receptors in the arcuate nucleus with eticlopride significantly attenuates cocaine-induced increases of beta-endorphin in the nucleus accumbens, suggesting that dopaminergic neurons in the arcuate nucleus regulate beta-endorphin release in the nucleus accumbens. These results are consistent with our present findings that dopamine D2 receptor blockade inhibits mu opioid receptor occupancy within the nucleus accumbens.
Cocaine administration had no effect on mu opioid receptor binding in any other area studied besides the nucleus accumbens. Cocaine did not decrease 3H-DAMGO binding in the caudate putamen, olfactory tubercle, cingulate cortex, rostral motor cortex, rostral or caudal VTA. The effect of cocaine in the nucleus accumbens can be explained by the neuronal pathways involved in beta-endorphin neurotransmission. Neurons in the brain that synthesize beta-endorphin are predominantly present in the hypothalamic arcuate nucleus (Bloom et al., 1978a). Beta-endorphin neurons originating in the arcuate nucleus innervate the nucleus accumbens (Bloom et al., 1978b; Finley et al., 1981). The nucleus accumbens is also a terminal projection site of the mesolimbic dopamine system. Previous studies have shown that systemic cocaine administration results in beta-endorphin outflow into the nucleus accumbens (Olive et al., 2001; Roth-Deri et al., 2003; Doron et al., 2006). It is not surprising, therefore, that decreased mu opioid receptor binding by 3H-DAMGO was detected in the nucleus accumbens following cocaine administration. The lack of a significant decrease in 3H-DAMGO binding within the VTA was unexpected considering previous results by our lab showing a role for mu opioid receptors within the VTA in cocaine-induced reward (Soderman and Unterwald 2008). The most likely explanation is that the density of mu opioid receptors in the VTA coupled with the sensitivity of the assay was not adequate to see a significant difference between groups. The lack of significant findings in the caudate putamen was also unexpected because our previous results showed a role for mu opioid receptors within the caudate putamen in cocaine-induced hyperactivity (Soderman and Unterwald 2008). Although a trend for reduced 3H-DAMGO binding within this region was seen at the 10 and 20 minute time points, the data were not significantly different from the 0 minute control group. It is possible that small increases in mu receptor activation in the caudate putamen are sufficient to induce the behavioral effects seen previously.
The decreases in 3H-DAMGO binding that occurred following administration of cocaine are thought to be due to increases in endogenous opioids binding to mu opioid receptors. However, there are other possibilities for the reduced 3H-DAMGO binding seen in these experiments. For example, mu receptor internalization might be increased following cocaine administration, although this internalization would be expected to result from increased opioid ligand binding. Another possibility includes cocaine-induced heterologous desensitization or down-regulation of the mu opioid receptor. Mu opioid and D2 receptors are co-expressed by individual neurons in the rat striatum (Ambrose et al., 2004). Increased dopamine D2 receptor activation following cocaine administration could lead to heterologous desensitization of the mu receptor, similar to what has been shown previously for other GPCRs (Unterwald et al., 2000; Rogers et al., 2000). Additional studies are needed to determine if this plays a role in the observed effects.
In summary, previous studies have shown that acute cocaine administration can increase beta-endorphin levels in the nucleus accumbens (Olive et al., 2001; Roth-Deri et al., 2003; Doron et al., 2006) and that beta-endorphin binds mu opioid receptors (Hazum et al., 1979). The present study adds to previous data by showing that cocaine causes a time-dependent decrease in 3H-DAMGO binding within the nucleus accumbens core and shell, suggesting that cocaine causes the release of opioid peptides which bind to mu opioid receptors in this region. In addition, this study shows that the reduction in 3H-DAMGO binding is mediated by dopamine D2 receptors.
Materials and Methods
Drugs
Cocaine HCl was generously provided by the National Institute on Drug Abuse, dissolved in sterile saline, and injected intraperitoneally (i.p.) in doses ranging from 2.5 to 20 mg/kg. The dose is based on the weight of the salt. Eticlopride and SCH23390 were obtained from Sigma-Aldrich and diluted in sterile saline to a concentration of 1 mg/ml and 0.5 mg/ml respectively. All drugs were administered i.p. at a volume of 1 ml/kg of body weight.
Animals
All animal procedures were approved by Temple University's Institutional Animal Care and Use Committee and followed guidelines set forth in the NIH's Guide for the Care and Use of Laboratory Animals. Male Sprague Dawley rats (approximately 60 days old) were obtained from Charles River Laboratory (Raleigh, NC), maintained on a 12-hour light/dark cycle (lights on 6 AM), and had ad libitum access to standard food and water. Animals were housed 3/cage for the duration of the experiments. Animals were acclimatized to the animal facility for 1 week prior to initiation of experiments.
Cocaine Time-Course Study
Animals were weighed and randomly assigned to one of five experimental groups (N=3/group). Animals received a single i.p. injection of saline (1 ml/kg i.p.) or cocaine (10 mg/kg i.p.). Animals receiving saline were immediately exposed to CO2 for 15 seconds and decapitated in an unconscious state. Brains from animals receiving cocaine were obtained at 5, 10, 20 and 30 minutes following injection. Brains were rapidly removed and frozen by immersion in 2-methylbutane cooled to -30°C.
Cocaine Dose-Response Study
Animals were weighed and randomly assigned to one of three experimental groups (N=4/group). Animals received a single injection of saline (1 ml/kg i.p.) or cocaine (2.5, 10 or 20 mg/kg i.p.). Ten minutes following injection, animals were exposed to CO2 for 15 seconds and decapitated in an unconscious state. Brains were rapidly removed and frozen by immersion in 2-methylbutane at -30°C.
Involvement of Dopamine D1 and D2 Receptors in Cocaine-Induced Endogenous Opioid Peptide Binding
Animals were weighed and randomly assigned to one of four experimental groups (N=4/group). Animals in groups 1 and 2 were injected with saline (1 ml/kg i.p.), group 3 received the dopamine D1 receptor selective antagonist SCH23390 (0.5 mg/kg i.p.) and group 4 received the dopamine D2 receptor selective antagonist eticlopride (1 mg/kg i.p.). Thirty minutes later, animals in group 1 received saline (1 ml/kg i.p.) and animals in group 2, 3 and 4 received cocaine (10 mg/kg i.p.). Ten minutes later, brains were rapidly removed and frozen by immersion in 2-methylbutane at -30°C.
Quantitative In Vitro Mu Opioid Receptor Autoradiography
Brains were sectioned into 15 μm coronal sections throughout the striatum and VTA for all 3 studies described and thaw mounted on Superfrost-Plus Microslides (VWR, Pennsylvania). Slides were air dried on ice for 20 minutes before being stored at -30°C for 2 days in a vacuum desiccator. Eight to ten sections for total binding and eight to ten sections for non-specific binding from each of the striatum and VTA from each animal were used for 3H-DAMGO autoradiography. Sections for the striatum were chosen between A/P +2.0 and +1.8 from bregma, sections for the rostral VTA were chosen between A/P -4.9 to -5.1 from bregma and the caudal VTA between -5.9 and -6.1 from bregma according to Paxinos and Watson (1986). Sections were thawed at room temperature for 2 minutes and then incubated in 5 nM 3H-DAMGO (Amersham Biosciences, New Jersey) in 50 mM Tris HCl pH 7.4 at 4°C to measure total binding or 5 nM 3H-DAMGO plus 5 μM naloxone in 50 mM Tris HCl pH 7.4 to measure nonspecific binding. Sections were washed 6 × 20 seconds in ice cold 50 mM Tris pH 7.4 and dried under a cold stream of air. Labeled sections were exposed to tritium-sensitive film (Biomax MR, Kodak, New York) along with tritium standards (Amersham, NJ) for 14 weeks. Optical densities for brain regions of interest were determined using a computer imaging device (AIS 6.0, MCID Imaging, Cambridge, UK) and converted to fmol of radioligand bound per mg of tissue by reference to the tritium standards. The optical density from the left and right aspects of each brain region were obtained and averaged for each section. Eight to ten sections per brain region per animal were analyzed. Specific radioligand binding was calculated by subtracting nonspecific binding from total binding. This mean from each brain region was independently calculated for the saline treated animals and was set to 100%. These values were used to calculate % increase or decrease in 3H-DAMGO binding for each individual animal. Data from all animals in the treatment group were averaged to obtain a mean specific binding value relative to the saline treated group.
Data Analysis
Data were analyzed by one-way ANOVA. Bonferroni's post-hoc analysis was performed after significance was determined by ANOVA (Graphpad Prism V.4). The null hypothesis was rejected when p<0.05.
Acknowledgments
This work was supported by NIH/NIDA R01 DA09580 (EMU).
Abbreviations
- cVTA
Caudal Ventral Tegmental Area
- CPu
Caudate Putamen
- CTAP
D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2
- DAMGO
[D-Ala2, N-Me-Phe4, Gly-ol5]enkephalin
- DAT
Dopamine Transporter
- mPFC
Medial Prefrontal Cortex
- NAcc
Nucleus Accumbens
- RMoCo
Rostral Motor Cortex
- rVTA
Rostral Ventral Tegmental Area
Footnotes
Section: Cognitive and Behavioral Neuroscience
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams DH, Hanson GR, Keefe KA. Cocaine and methamphetamine differentially affect opioid peptide mRNA expression in the striatum. J Neurochem. 2001;75(5):2061–70. doi: 10.1046/j.1471-4159.2000.0752061.x. [DOI] [PubMed] [Google Scholar]
- Ambrose LM, Unterwald EM, Van Bockstaele EJ. Ultrastructural evidence for co-localization of dopamine D2 and micro-opioid receptors in the rat dorsolateral striatum. Anat Rec A Discov Mol Cell Evol Biol. 2004;279:583–591. doi: 10.1002/ar.a.20054. [DOI] [PubMed] [Google Scholar]
- Bloom F, Battenberg E, Rossier J, Ling N, Guillemin R. Neurons containing beta-endorphin rat brain exist separately from those containing enkephalin: immunocytochemical studies. Proc Natl Acad Sci USA. 1978a;75(3):1591–5. doi: 10.1073/pnas.75.3.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom FE, Rossier J, Battenberg EL, Bayon A, French E, Henriksen SJ, Siggins GR, Segal D, Browne R, Ling N, Guillemin R. Beta-endorphin: cellular localization, electrophysiological and behavioral effects. Adv Biochem Psychopharmacol. 1978b;18:89–109. [PubMed] [Google Scholar]
- Branch AD, Unterwald EM, Lee SE, Kreek MJ. Quantitation of preproenkephalin mRNA levels in brain regions from male Fisher rats following chronic cocaine treatment using a recently developed solution hybridization assay. Brain Res Mol Brain Res. 1992;14(3):231–238. doi: 10.1016/0169-328x(92)90178-e. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Devine DP, Wise RA. Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacology (Berl) 1995;122(2):194–7. doi: 10.1007/BF02246095. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. Microinjections of phencyclidine (PCP) and related drugs into nucleus accumbens shell potentiate medial forebrain bundle brain stimulation reward. Psychopharmacology (Berl) 1996;128(4):413–20. doi: 10.1007/s002130050151. [DOI] [PubMed] [Google Scholar]
- Costa E, Fratta W, Hong JS, Moroni F, Yang HY. Interactions between enkephalinergic and other neuronal systems. Adv Biochem Psychopharmacol. 1978;18:217–26. [PubMed] [Google Scholar]
- Devine DP, Leone P, Pocock D, Wise RA. Differential involvement of ventral tegmental mu, delta, and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies. J Pharmacol Exp Ther. 1993;266(3):1236–46. [PubMed] [Google Scholar]
- Devine DP, Wise RA. Self-administration of morphine, DAMGO, and DPDPE into the ventral tegmental area of rats. J Neurosci. 1994;14:1978–84. doi: 10.1523/JNEUROSCI.14-04-01978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Gauvin DV, Goulden KL, Briscoe RJ, Halloway FA. Cocaine-induced conditioned place approach in rats: the role of dose and route of administration. Pharmacol Biochem Behav. 1994;49(4):1001–1005. doi: 10.1016/0091-3057(94)90255-0. [DOI] [PubMed] [Google Scholar]
- Doron R, Fridman L, Yadid G. Dopamine-2 receptors in the arcuate nucleus modulate cocaine-seeking behavior. Neuroreport. 2006;17(15):1633–6. doi: 10.1097/01.wnr.0000234755.88560.c7. [DOI] [PubMed] [Google Scholar]
- Finley JC, Lindstrom P, Petrusz P. Immunocytochemical localization of beta-endorphin-containing neurons in the rat brain. Neuroendocrinology. 1981;33(1):28–42. doi: 10.1159/000123197. [DOI] [PubMed] [Google Scholar]
- Hammer RP., Jr Cocaine alters opiate receptor binding in critical brain reward regions. Synapse. 1989;3:55–60. doi: 10.1002/syn.890030108. [DOI] [PubMed] [Google Scholar]
- Hazum E, Chang KJ, Cuatrecasas P. Interaction of iodinated human [D-Ala2]beta-endorphin with opiate receptors. J Biol Chem. 1979;254(6):1765–7. [PubMed] [Google Scholar]
- Heikkila RE, Orlansky H, Cohen G. Studies on the distinction between uptake inhibition and release of [3H]dopamine in rat brain tissue slices. Biochem Pharmacol. 1975;24:847–852. doi: 10.1016/0006-2952(75)90152-5. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. Influence of a single injection of cocaine, amphetamine or GBR 12909 on mRNA expression of striatal neuropeptides. Brain Res Mol Brain Res. 1992;16(12):97–104. doi: 10.1016/0169-328x(92)90198-k. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci. 1997b;17(21):8580–7. doi: 10.1523/JNEUROSCI.17-21-08580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izenwasser S, Heller B, Cox BM. Continuous cocaine administration enhances mu- but not delta-opioid receptor-mediated inhibition of adenylyl cyclase activity in nucleus accumbens. Eur J Pharmacol. 1996;297:187–91. doi: 10.1016/0014-2999(95)00828-4. [DOI] [PubMed] [Google Scholar]
- Kang YM, Ouyang W, Chen JY, Qiao JT, Dafny N. Norepinephrine modulates single hypothalamic arcuate neurons via alpha(1) and beta adrenergic receptors. Brain Res. 2000;869(12):146–57. doi: 10.1016/s0006-8993(00)02380-5. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Stein EA. Fluctuations in nucleus accumbens dopamine during cocaine self-administration behavior: an in vivo electrochemical study. Neuroscience. 1995;64(3):599–617. doi: 10.1016/0306-4522(94)00436-9. [DOI] [PubMed] [Google Scholar]
- Lin JY, Li CS, Pan JT. Effects of various neuroactive substances on single unit activities of hypothalamic arcuate neurons in brain slices. Brain Res Bull. 1993;31(5):587–94. doi: 10.1016/0361-9230(93)90127-w. [DOI] [PubMed] [Google Scholar]
- Nicolaysen LC, Justice JB., Jr Effects of cocaine on release and uptake of dopamine in vivo: differentiation by mathematical modeling. Pharmacol Biochem Behav. 1988;31:327–35. doi: 10.1016/0091-3057(88)90354-1. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Spyraki C. Cocaine-induced place conditioning: importance of route of administration and other procedural variables. Psychopharmacology. 1988;94(1):119–25. doi: 10.1007/BF00735892. [DOI] [PubMed] [Google Scholar]
- Olds ME. Reinforcing effects of morphine in the nucleus accumbens. Brain Res. 1982;237:429–40. doi: 10.1016/0006-8993(82)90454-1. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Mannini MA, Hodge CW. Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci. 2001;21(23):RC184. doi: 10.1523/JNEUROSCI.21-23-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press Inc.; 1986. [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422(6932):614–18. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Rogers TJ, Steele AD, Howard OM, Oppenheim JJ. Bidirectional heterologous desensitization of opioid and chemokine receptors. Ann N Y Acad Sci. 2000;917:19–28. doi: 10.1111/j.1749-6632.2000.tb05369.x. [DOI] [PubMed] [Google Scholar]
- Ross JW, Laska FJ, Fennessy MR. Brain biogenic amines and intravenous self-administration of cocaine in rats. Clin Exp Pharmacol Physiol. 1978;5(4):351–9. doi: 10.1111/j.1440-1681.1978.tb00684.x. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Zangen A, Aleli M, Goelman RG, Pelled G, Nakash R, Gispan-Herman I, Green T, Shaham Y, Yadid G. Effect of experimenter-delivered and self-administered cocaine on extracellular beta-endorphin levels in the nucleus accumbens. J Neurochem. 2003;84:930–8. doi: 10.1046/j.1471-4159.2003.01584.x. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003;970(12):214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Hummel M, Simpson AD, Sheikh R, Soderman AR, Unterwald EM. A role for mu opioid receptors in cocaine-induced activity, sensitization, and reward in the rat. Psychopharmacology (Berl) 2007;195(2):265–72. doi: 10.1007/s00213-007-0883-z. [DOI] [PubMed] [Google Scholar]
- Sivam SP. Cocaine selectively increases striatonigral dynorphin levels by a dopaminergic mechanism. J Pharmacol Exp Ther. 1989;250(3):818–24. [PubMed] [Google Scholar]
- Soderman AR, Unterwald EM. Cocaine reward and hyperactivity in the rat: sites of mu opioid receptor modulation. Neuroscience. 2008;154(4):1506–16. doi: 10.1016/j.neuroscience.2008.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem. 1990a;55(5):1734–40. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Identification of the opioid receptor types modulating beta-endorphin-induced alterations in dopamine release in the nucleus accumbens. Eur J Pharmacol. 1990b;190(12):177–84. doi: 10.1016/0014-2999(90)94124-g. [DOI] [PubMed] [Google Scholar]
- Spangler R, Unterwald EM, Kreek MJ. ‘Binge’ cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudate-putamen. Brain Res Mol Brain Res. 1993;19(4):323–7. doi: 10.1016/0169-328x(93)90133-a. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Rubenfeld JM, Kreek MJ. Repeated cocaine administration upregulates kappa and mu, but not delta, opioid receptors. NeuroReport. 1994;5(13):1613–16. doi: 10.1097/00001756-199408150-00018. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Anton B, To T, Lam H, Evans CJ. Quantitative immunolocalization of mu opioid receptors: regulation by naltrexone. Neuroscience. 1998;85(3):897–905. doi: 10.1016/s0306-4522(97)00659-3. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Mucha RF, O'Shaughnessy M, Bucenieks P. Reinforcing effects of brain microinjections of morphine revealed by conditioned place preference. Brain Res. 1982;243:107–17. doi: 10.1016/0006-8993(82)91124-6. [DOI] [PubMed] [Google Scholar]
- Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51(12):13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]