Abstract
Systemic application of the muscarinic agonist, pilocarpine, is commonly utilized to induce an acute status epilepticus that evolves into a chronic epileptic condition characterized by spontaneous seizures. Recent findings suggest that the status epilepticus induced by pilocarpine may be triggered by changes in the blood–brain barrier (BBB) permeability. We tested the role of the BBB in an acute pilocarpine model by using the in vitro model brain preparation and compared our finding with in vivo data. Arterial perfusion of the in vitro isolated guinea-pig brain with <1 mM pilocarpine did not cause epileptiform activity, but rather reduced synaptic transmission and induced steady fast (20–25 Hz) oscillatory activity in limbic cortices. These effects were reversibly blocked by co-perfusion of the muscarinic antagonist atropine sulfate (5 μM). Brain pilocarpine measurements in vivo and in vitro suggested modest BBB penetration. Pilocarpine induced epileptiform discharges only when perfused with compounds that enhance BBB permeability, such as bradykinin (n=2) or histamine (n=10). This pro-epileptic effect was abolished when the BBB-impermeable muscarinic antagonist atropine methyl bromide (5 μM) was co-perfused with histamine and pilocarpine. In the absence of BBB permeability enhancing drugs, pilocarpine induced epileptiform activity only after arterial perfusion at concentrations >10 mM. Ictal discharges correlated with a high intracerebral pilocarpine concentration measured by high pressure liquid chromatography.
We propose that acute epileptiform discharges induced by pilocarpine treatment in the in vitro isolated brain preparation are mediated by a dose-dependent, atropine-sensitive muscarinic effect promoted by an increase in BBB permeability. Pilocarpine accumulation secondary to BBB permeability changes may contribute to in vivo ictogenesis in the pilocarpine epilepsy model.
Keywords: extracellular potassium, limbic cortex, muscarinic
Pilocarpine is a non-selective muscarinic agonist (Maslanski et al., 1994) with a relatively high affinity for CNS muscarinic receptors (Hedlund and Bartfai, 1981) commonly utilized to develop an experimental model of temporal lobe epilepsy (Turski et al., 1989; Cavalheiro et al., 2006; but see Sloviter, 2005). In different animal species, i.p. injection of pilocarpine induces a convulsive status epilepticus (SE; i.e. sub-continuous generalized seizures that recur for several hours), followed within 2 weeks by a chronic epileptic condition that mimics human temporal lobe epilepsy (Cavalheiro et al., 2006). The initial SE is thought to be triggered by a cholinergic activation of excitatory neurons in specific brain regions that include limbic cortices. Such an effect is supposedly mediated by micro-molar concentrations of pilocarpine, since this drug shows a relatively poor brain penetration (Omori et al., 2004). Although studies directly assessing pilocarpine penetration across the blood–brain barrier (BBB) are lacking, a recent report demonstrated that intracerebral pilocarpine does not exceed micromolar concentration (circa 200 μM) in rats killed immediately before or after SE (Marchi et al., in press).
Interestingly, in spite of the large use of systemic pilocarpine to induce SE in vivo, very few studies utilized direct brain application of pilocarpine to evoke epileptic activity. Intra-cerebral application of very high concentrations of pilocarpine (10 mM) was found to induce seizures, enhance extracellular amino-acid levels and change neurotrophin expression (Millan et al., 1993; French et al., 1999). The dose of pilocarpine utilized in these in vivo experiments is three orders of magnitude higher than pilocarpine brain levels observed at onset or just before SE induced by i.p. injection (200±80 μM; Marchi et al., in press). In two in vitro studies on hippocampal slices, direct application of micromolar (10 μM) concentration of pilocarpine was reported to induce epileptiform activity (Nagao et al., 1996; Rutecki and Yang, 1998). However, ictal epileptiform discharges were observed only when potassium concentration was enhanced to 7.5 mM (Rutecki and Yang, 1998) or after prolonged perfusion of pilocarpine (Nagao et al., 1996). Similar conclusions were recently reported in slices by Marchi et al. (in press).
In addition to the well-described histological alterations induced by pilocarpine SE, magnetic resonance studies in the rat in vivo showed that pilocarpine treatment is associated with an enhancement of BBB permeability (Roch et al., 2002; Fabene et al., 2003). Whether such changes are the consequence of the SE or are due to a peripheral effect of pilocarpine, independent from the epileptiform activity is not known, even though data support a peripheral mechanism for BBB opening occurring prior to SE (Marchi et al., in press).
Following the preliminary observation that micromolar concentrations of pilocarpine (100–800 μM) perfused through the cerebrovascular system does not induce epileptiform discharges in the in vitro isolated guinea-pig brain preparation (Uva et al., 2006; Marchi et al., in press), we planned to study the acute pro-epileptic effects of pilocarpine in the guinea pig, which is amenable for direct comparison between in vivo and whole brain in vitro conditions.
EXPERIMENTAL PROCEDURES
In vivo experiments
Hartley guinea pigs (200–250 g, Charles River, Calco, Italy) were injected with methylscopolamine (1 mg/kg, i.p., Sigma-Aldrich, St. Louis, MO, USA) 30 min before pilocarpine to prevent peripheral cholinergic side effects. Within 14.62±5.37 min after pilocarpine injection (220 mg/kg, i. p, Sigma, St. Louis, MO, USA) 80% of the animals underwent an obvious convulsive SE, characterized by falling, simultaneous clonic jerks of the four limbs and apparent loss of consciousness. A condition defined pre-SE, characterized by coarse tremor associated to chewing and grooming, loss of hind limb posture, extension of the head, and side fall with alternating movements of the legs, was consistently observed before SE. Animals were killed by barbiturate overdose either 5 min from the onset of the pre-SE state (9 min after pilocarpine i.p. injection) or 30 min after SE onset. Intracerebral and plasma levels of pilocarpine (see below) were evaluated on brain specimens and blood samples. The experimental protocols were reviewed and approved by the Committee on Animal Care and Use and by Ethics Committee of the Fondazione Istituto Neurologico and the Mario Negri Institute, according to the international guidelines on ethical use of animals (European Communities Council Directive of 24 November 1986 (86/609/EEC)). All efforts were made to minimize the number of animals used and their suffering.
Brain concentrations of pilocarpine were measured by high pressure liquid chromatography (HPLC) on frozen (−80 °C) samples (parietal neocortex and hippocampus) of guinea-pig brains obtained from in vivo experiments. The chromatographic analysis was carried out according to a method previously described by Kuks et al. (1990). Tissue samples were weighed and transferred to round bottom 15 ml tubes with 2 ml of ice-cold saline. Samples were homogenized with a sonicating probe for 10 s. A partial volume of the homogenate (1 ml) was transferred to a 20 ml extraction tube and an additional 1 ml saline added. The sample was further extracted with 7 ml methylene chloride. The organic phase was transferred to a clean tube and brought to dryness with a gentle stream of nitrogen. The residue was reconstituted with 0.1 ml of mobile phase, vortexed, transferred to the autosampler and injected on column. The chromatography system consisted of a detector (diode array (DAD)), a solvent delivery system, a reverse phase column, automatic injector. Data were measured at 220 nm and confirmed via DAD (spectra analysis). The data were normalized for the injection amount, average % recovery, and tissue amount. Routinely, approximately 10% of the samples were re-analyzed. This method shows linearity in a concentration range of 2.5–100 μg/ml pilocarpine. HPLC grade acetonitrile was purchased from Merck (Darmstadt, Germany). Milli-Q water (Milli-pore, Bedford, MA, USA) was used throughout. The other chemicals used were of analytical reagent grade. Cerebral levels of pilocarpine were measured with the same method also in brains treated with pilocarpine in vitro (see below).
In vitro experiments on isolated guinea-pig brains
Brains of 200–250 g Hartley guinea-pigs were isolated and maintained in vitro according to the standard procedure (Muhlethaler et al., 1993; de Curtis et al., 1998). Animals anesthetized with sodium thiopental (125 mg/kg i.p., Farmotal, Pharmacia, Milan, Italy) were transcardially perfused with a cold (10 °C) oxygenated (95% O2, 5% CO2) saline solution composed of 126 mM NaCl, 3 mM KCl, 1.2 mM KH2PO4, 1.3 mM MgSO4, 2.4 mM CaCl2, 26 mM NaHCO3, 15 mM glucose, 2.1 mM Hepes and 3% dextran M. W. 70,000 (pH=7.1). Following decapitation, brains were dissected out, transferred to a recording chamber and perfused via a peristaltic pump (Minipulse 3, Gilson, Villiers-le-Bel, France) through the basilar artery with the above solution (7 ml/min, pH=7.3, 15 °C). Brain temperature was slowly raised to 32 °C to perform the electrophysiological experiment.
The muscarinic receptor agonist, pilocarpine (100 μM, 800 μM, 10–13 mM; Sigma), and antagonist, atropine sulfate or atropine methyl bromide (5 μM; Sigma), bradykinin (BK; 0.6–2.5 μM; Sigma), histamine (100 μM; Sigma) and glutamate receptor agonist kainic acid (4 μM, Tocris, Ballwin, MO, USA) were dissolved in the perfusate and applied through the resident arterial system.
Extracellular recordings were simultaneously performed with NaCl-filled micropipettes (5–8 μm tip diameter, 5–10 MΩ resistance) in the piriform cortex (PC), in the medial and lateral entorhinal cortex (EC) and in the hippocampus (subfield CA1). Signals were amplified using a multichannel amplifier (Biomedical Engineering, Thornwood, NY, USA) and were digitized via an AT-MIO-64E3 National A/D Board (National Instrument, Milan, Italy). Data were acquired and analyzed with a custom-made software (ELPHO©) developed in Labview by Vadym Gnatkovsky.
The viability of isolated brains was tested by recording the responses evoked in the olfactory-limbic cortex by stimulation of the lateral olfactory tract (LOT; see Biella and de Curtis, 2000; Gnatkovsky et al., 2004). Stimuli were delivered through an isolation unit driven by a Grass S88 pulse generator (Grass Telefactor S88, West Warwick, RI, USA). Stimulating and recording electrodes were positioned under direct visual control with a stereoscopic microscope. Control brains never showed spontaneous epileptiform discharges.
For intracerebral HPLC measurements of pilocarpine, in vitro isolated brains were rapidly frozen in liquid nitrogen at different time points after the end of drug perfusion. Brains were stored at −80 °C and measurements were performed on samples of parietal neocortex and hippocampus, according to the methods described above.
In order to evaluate the brain level of extracellular potassium ([K+]o), two-barrel glass pipettes were utilized to simultaneously record ion-selective signals and field responses (tip diameter, 3–5 μm) in EC and hippocampus (Librizzi et al., 2001). The conventional electrode barrel was filled with KCl 0.2 M. The pipette barrel utilized for [K+]o measurements was filled at the tip with K+ ionophore I cocktail A (Fluka, Buchs, Switzerland) after 1 min exposure to dimethyldichlorosylane vapors (Fluka, Steinheim, Germany) and was backfilled with 0.2 M KCl following a 2-h incubation at 120 °C. K+ calibration solutions had the same composition of the solution used for arterial perfusion except for KCl concentration that was modified to obtain final K+ concentrations of 1, 2.5, 6, 12.5 and 48.2 mM. The absolute K+ values recorded during the experiment were obtained by solving the equation
where x is the [K+]o, y is the measured voltage reading induced by the changes in [K+]o and a+b is the slope coefficient derived from the calibration curve for each K+-sensitive electrode. Ion-selective and field DC signals were amplified with a high input impedance head-stage amplifier (Biomedical Engineering). Subtraction of the field potential from the K+-sensitive electrode voltage reading was performed by the amplifier circuit.
RESULTS
Intracerebral concentration of pilocarpine in vivo
We performed a first series of in vivo experiments (n=10) to ascertain whether i.p. injections of 220 mg/kg pilocarpine, applied 30 min after treatment with methylscopolamine, triggered SE in guinea pigs. Within 14.62±5.37 min 80% of the animals underwent a convulsive SE. We measured pilocarpine brain levels in guinea pigs injected i.p. with pilocarpine and killed either during the pre-SE state (n=3) or 30 min after the onset of SE (40–45 from pilocarpine injection; n=3). The intracerebral concentration of the drug was identical to what reported in rats treated with pilocarpine and methylscopolamine (Marchi et al., in press; Fig. 1). The brain levels were consistently lower than serum pilocarpine levels regardless of both the region analyzed (cortex or hippocampus) and the time of kill after i.p. injection (not shown).
Fig. 1.

Cerebral and vascular levels of pilocarpine measured in vivo in the guinea-pig brain during SE. Mean pilocarpine values and brain/ blood ratio after 220 mg/kg i.p. injection in the guinea pig revealed poor penetration of pilocarpine into the CNS.
Effects of pilocarpine on the in vitro isolated guinea-pig brain preparation: spontaneous activity
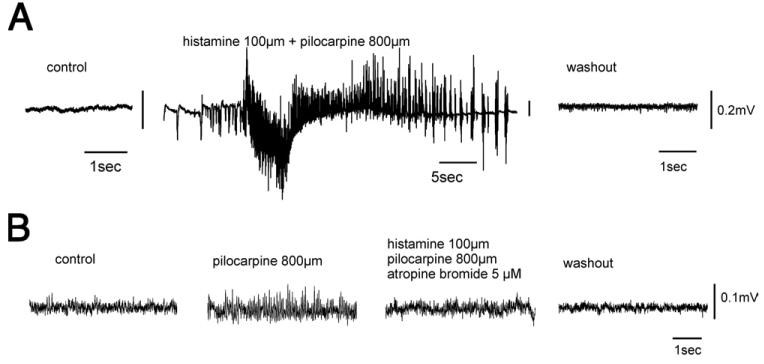
Pilocarpine at sub-millimolar concentrations of 100 μM (n=6) and 800 μM (n=22) was arterially perfused for variable periods (5–30 min) in the in vitro guinea-pig isolated brain. These values are representative and exceed the range of concentrations observed in vivo (e.g. Fig. 1). Regardless of the concentrations used, small-amplitude, fast activity at 20–25 Hz appeared in the hippocampus (n=20; Fig. 2A–D) and in the EC (n=6). Such activity was similar to the fast oscillations generated in vitro by the muscarinic cholinergic agonist, carbachol (Williams and Kauer, 1997; Fisahn et al., 1998; van der Linden et al., 1999; Dickson et al., 2000). Similar to the carbachol-induced oscillations, fast activity induced by pilocarpine was abolished by co-perfusion of 5 μM atropine sulfate (n=5; Fig. 2C). Quantitative data on the effect of atropine on pilocarpine-induced fast activity observed in four experiments are illustrated in Fig. 2D. Perfusion with atropine methyl bromide, a cholinergic antagonists that does not cross the intact BBB (Biesold et al., 1989), failed to abolish the pilocarpine induced oscillatory activity (n=2).
Fig. 2.
Submillimolar concentrations of pilocarpine induce muscarine-receptor dependent fast activity in the hippocampus of the in vitro isolated guinea-pig brain. In the upper panel, the positions of the recording electrodes in the isolated brain are illustrated: PC, EC and hippocampus (CA1 area). (A) Reversible effect of arterial perfusion of pilocarpine 100 μM in PC, EC and CA1. (B) Fast activity in CA1 region induced by different concentrations of pilocarpine (100 and 800 μM) diluted in the arterial perfusate (middle traces). Field activity before and 20 min after pilocarpine perfusion is illustrated in the upper and lower traces. (C) The effect of pilocarpine observed in CA1 is abolished by co-perfusion of the isolated brain with pilocarpine and atropine sulfate (5 μM). (D) Mean frequency power of CA1 fast activity recorded during application of 800 μM pilocarpine (n=8; continuous thick line), co-application of 800 μM pilocarpine and atropine 5 μM (n=4; thin continuous line) and after drug washout (n=8; dotted line).
Effects of pilocarpine on the in vitro isolated guinea-pig brain preparation: evoked potentials
Field potentials evoked by stimulation of the LOT in PC, EC and hippocampus were reduced in amplitude during application of micromolar concentrations of pilocarpine (Fig. 3). As previously demonstrated (Biella and de Curtis, 2000; Gnatkovsky et al., 2004), LOT-evoked potentials in PC (Fig. 3A) and EC (Fig. 3C) were characterized by a direct monosynaptic component (monosynaptic population component, mpc; arrow) followed by disynaptic associative potentials (disynaptic population component, dpc; arrowhead). LOT-evoked activity propagated to the hippocampus (asterisk in Fig. 3C). LOT pairing stimulation at 40–100 ms inter-pulse intervals showed a slight facilitation of the conditioned mpc in both PC and EC (Fig. 3A and C). The ratio between the monosynaptic components in conditioned (mpc2) and conditioning (mpc1) responses was not substantially modified during application of 100–800 μm pilocarpine (left panel in Fig. 3B; n=5). Pilocarpine induced a significant suppression of the dpc in the conditioned responses (dpc2) in PC (Fig. 3A and lower panel in 3B; two-tailed t-test, P<0.05) and in the EC (not shown). Moreover, the propagation of activity along the piriform–entorhinal–hippocampal pathway was reduced by pilocarpine, as demonstrated by the disappearance of the late potential in CA1 (asterisks) in response to the second stimulus of a pairing test (lower traces in the middle panel in Fig. 3C). The effects of pilocarpine were partially reverted upon drug washout. As reported for other muscarinic receptor agonists such as carbachol (Richter et al., 1999; Yun et al., 2000; Hamam et al., 2007), these findings confirm that pilocarpine decreases synaptic excitability and polysynaptic propagation of neuronal activity.
Fig. 3.
Pilocarpine induces depression of both paired pulse disynaptic responses and polysynaptic propagation of activity. (A) Stimulation of the LOT induces in the PC a response characterized by a monosynaptic component (arrow) and a disynaptic component (arrowhead). Paired-pulse depression of the dpc is dramatically enhanced in the conditioned response to LOT stimulation (40 ms pulse interval) after perfusion with 800 μM pilocarpine (middle trace). (B) Quantitative results obtained in five experiments demonstrate that pilocarpine does not change the ratio between the conditioned (mpc2) and the conditioning (mpc1) monosynaptic PC component evoked by LOT pairing at 40 ms inter-pulse interval (upper panel), while it reversibly reduces significantly (two-tailed t-test, P<0.05) the ratio between the conditioned (dpc2) and the conditioning (dpc1) disynaptic component (lower panel). Values expressed as mean±S.D. (C) Recordings from EC and CA1 during paired LOT stimuli (100 ms interpulse interval). A reduction of the disynaptic component is observed also in the EC (upper traces). In CA1 the polysynaptic response to the conditioned LOT stimulus (asterisk) is reversibly abolished.
Effects of pilocarpine after manipulations of BBB integrity
Since in vivo findings demonstrated that the effect of pilocarpine is associated with changes in BBB permeability (Roch et al., 2002; Fabene et al.2003; van Eijsden et al., 2004;Marchi et al., in press), we tested whether BBB damage could play a role in establishing the pro-epileptic effect of pilocarpine. We perfused pilocarpine in the in vitro isolated brain with two drugs that are known to increase BBB permeability: bradykinin 0.6–2.5 μM (n=7) or histamine 100 μM (n=14) (Greenwood, 1991; Schilling and Wahl, 1999). Under these conditions, epileptiform discharges characterized by either interictal spikes (n=10) or seizure-like activity (n=9; Fig. 4A) were observed with a mean delay of several minutes after perfusion started (on average, 12.44±4.59 min; n=2 with a pre-treatment with bradykinin and n=10 after co-perfusion with histamine). When the concentration of pilocarpine co-perfused with histamine was decreased to 100 μM (n=6), we observed epileptiform activity in only 50% of the experiments. As expected, bradykinin 2.5 μM (n=2) or histamine (100 μM; n=8) perfused for 10–15 min without pilocarpine did not induce epileptiform discharges (data not shown). In only one case out of nine epileptiform activity was recorded in hippocampus after histamine perfusion. We monitored the evoked response to paired LOT stimulation (interstimulus interval 40 ms) during histamine perfusion (n=5) and found no significant change in the ratio between the peak amplitude of mono- and disynaptic components of conditioned and conditioning responses in the PC (t-test, P<0.05). These data suggest that histamine is not modifying brain excitability in most of the experiments.
Fig. 4.
Pro-epileptic effect of histamine and pilocarpine mediated by enhancement of BBB permeability. (A) Arterial co-perfusion of 100 μM histamine and 800 μM pilocarpine in the isolated guinea-pig brain induces a sustained ictal epileptiform discharge in area CA1 of the hippocampus. (B) The BBB-impermeable atropine methyl bromide (5 μM) reduces fast activity induced by 800 μM pilocarpine, when co-perfused for 10 min with the BBB-opener histamine (100 μM).
We demonstrated that histamine was able to increase BBB permeability by analyzing the effects induced by simultaneous 10-min perfusion of pilocarpine (800 μM), histamine (100 μM) and atropine methyl bromide (5 μM), the BBB-impermeable derivative of atropine (Biesold et al., 1989; Librizzi et al., 2001). In all experiments (n=3) the presence of atropine methyl bromide prevented the development of pilocarpine-induced epileptiform activity and either reduced or abolished the oscillatory activity induced by a previous perfusion of pilocarpine (800 μM, 5 min; Fig. 4B). The observation that atropine bromide was able to block pilocarpine action confirmed that histamine was indeed effective in enhancing BBB permeability to this molecule.
We further confirmed that histamine induced changes in BBB permeability by comparing pilocarpine brain levels measured by HPLC on tissue samples obtained from isolated brains frozen at different time points after in vitro perfusion of 800 μM pilocarpine in the presence (open circles; n=8) or absence (filled squares; n=4) of 100 μM histamine (Fig. 5). Electrophysiological recordings were performed in all brains before freezing and the presence of seizure-like activity during histamine–pilocarpine co-application was demonstrated (n=6). Comparison between extrapolated brain values at time zero, that coincides with the end of 10 min arterial pilocarpine application, demonstrated that pilocarpine accumulates in the brain during histamine administration. Both measured and extrapolated drug values were significantly (P<0.05) higher in the presence of histamine.
Fig. 5.
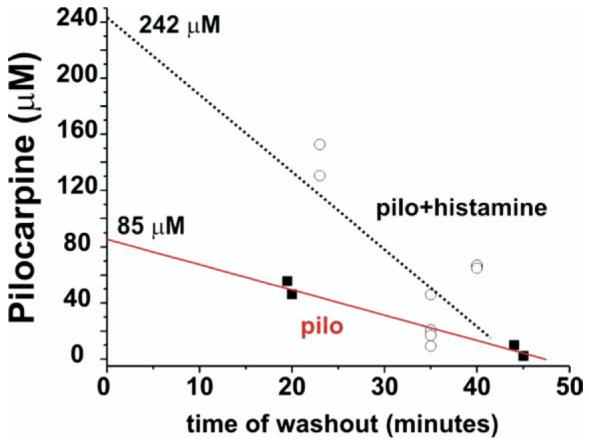
Pilocarpine levels from in vitro isolated guinea-pig brains. HPLC pilocarpine levels were measured in frozen isolated brains in which pilocarpine was perfused in vitro for 10 min at 800 μM in the presence (open circles) or in the absence (filled squares) of histamine, an agent known to increase the permeability of the BBB. The data points for pilocarpine levels are plotted against the time of washout of the drug. In these brains electrophysiological recordings were performed to verify the presence of seizure-like activity during histamine–pilocarpine co-application. The initial cerebral concentrations of the drug were obtained by extrapolating the time-dependent decay and by using y axis (mM) intercepts.
Pilocarpine effect and changes in extracellular potassium
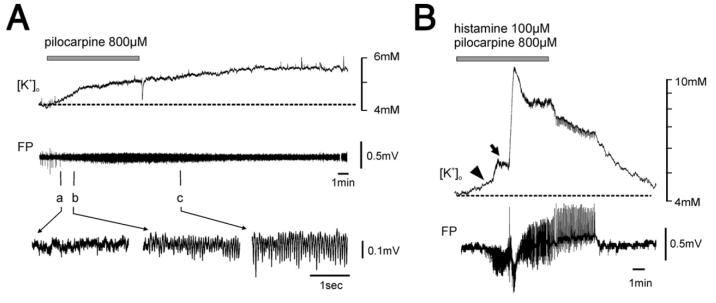
We then tested the hypothesis that increased excitability associated with enhanced BBB permeability could be due to extravasation of potassium ions from the vascular compartment to the brain. Potassium-sensitive electrodes were inserted in both hippocampus and EC. [K+]o shifts of 1.15±0.3 mM (mean±SEM.; n=7) above the baseline were observed after 10 min perfusion of 800 μM pilocarpine (Fig. 6A). These changes in [K+]o were associated with the development of fast oscillatory activity at 20–25 Hz (n=5, lower traces in Fig. 6A). Co-perfusion for 10 min of pilocarpine (800 μM) and histamine (100 μM) caused a comparable early increase of [K+]o (1.10±0.75 mM; n=3) associated with fast oscillatory activity. This was followed by a larger, delayed [K+]o increase (4.07±1.35 mM; n=5) that correlated with the onset of ictal epileptiform activity. These results demonstrate that application of pilocarpine alone and in co-perfusion with hista-mine induced similar changes in [K+]o.
Fig. 6.
Pilocarpine/histamine-induced seizure-like activity is not determined by potassium enhancement. (A) Perfusion of 800 μM pilocarpine is followed by an increase of [K+]o that correlates with the development of fast oscillatory activity in the field potential (FP) of CA1. In the bottom panel, details of the fast activity at time points a, b and c are illustrated. The duration of the pilocarpine perfusion is shown by the gray bar. (B) Comparable increase of [K+]o is observed at the onset pilocarpine 800 μM and histamine 100 μM co-perfusion (arrowhead); a subsequent large increase in [K+]o is associated to the onset of ictal discharges (arrow). The duration of the histamine–pilocarpine co-perfusion is shown by the gray bar.
Dose-dependence of pilocarpine effect in vitro
Pilocarpine-induced epileptiform activity triggered by histamine or bradykinin could be mediated by increased BBB permeability and possibly also by the drug accumulation in to the brain. To test whether the pro-epileptic effects of pilocarpine in the isolated guinea-pig brain were dose-dependent, we arterially perfused the isolated brain preparation with a much higher concentration of pilocarpine (10–13 mM; n=7) for 3–5 min. In these experiments epileptiform discharges characterized by either interictal spikes (n=4) or ictal seizure-like discharges (n=7; Fig. 7A) were observed in both EC and hippocampus within 2.92±1.55 min. Drug levels measured in the brain after perfusion at 10 mM were around 1 mM (not shown). Finally, epileptiform activity could also be induced by sustained perfusion with pilocarpine 800 μM for more then 20 min (25.6±2.51 min; n=3; not shown). The duration of seizure-like discharge induced by perfusion of pilocarpine 800 μM and histamine 100 μM was 1.80±0.73 min. The ictal events recorded after a 20-min perfusion of pilocarpine 800 μM lasted 1.91±0.66 min.
Fig. 7.

Epileptiform activity can be induced by supra-millimolar concentration of pilocarpine. (A) Arterial perfusion of the isolated guinea-pig brain preparation with supra-millimolar concentration of pilocarpine (13 mM) induces seizure-like activity in the CA1–EC region. (B) Epileptiform discharges induced by arterial application of a micromolar solution of kainic acid (4 μM).
Such ictal events were comparable in time and duration to those elicited by pilocarpine administration during BBB opener. Finally, seizure-like discharges were precipitated by 5-min arterial application of concentrations of kainic acid (4 μM; Fig. 7B) commonly utilized to induce epileptiform discharges on in vitro slices and in vivo.
DISCUSSION
We demonstrated that, as for other animal species (Turski et al., 1984, 1989), in vivo i.p. injection of pilocarpine in the guinea pig is able to induce an SE. We further observed that arterial perfusion of pilocarpine at serum concentrations measured in vivo prior to or during SE does not induce epileptiform activity in the in vitro isolated guinea-pig brain preparation. This is consistent with the observations reported in several studies, where direct intracerebral application of pilocarpine exerted pro-epileptic effect only at extremely high (10 mM) concentrations (Millan et al., 1993; French et al., 1999). Unlike pilocarpine, other proconvulsive compounds, such as kainic acid (see Fig. 7B), bicuculline (Uva et al., 2005) or pentylenetetrazole (Uva, Carriero and de Curtis, unpublished observations) were effective in the isolated guinea-pig brain at a concentration range similar to that utilized on in vitro slices.
Our experiments confirmed that pilocarpine penetration in the brain is significantly lower than expected based on its oil:water partition coefficient and molecular weight (Omori et al., 2004; Marchi et al., in press). The muscarinic-dependent fast activity observed in the isolated in vitro brain preparation demonstrates that pilocarpine reaches brain concentrations sufficient to activate neurons. However, at the brain levels measured in vivo and in vitro after peripheral administration of pilocarpine sufficient to cause SE in vivo, epileptiform activity was not detected, suggesting that the mechanisms underlying the genesis of SE may differ from those responsible for the generation of fast activity and may require the additional inputs derived from the periphery that are absent in the isolated brain preparation.
A number of human and animal studies suggested that breaching the BBB either promotes epileptiform events (Pavlovsky et al., 2005; Marchi et al., 2007) or may lead to progression of the epileptogenic process (Seiffert et al., 2004; van Vliet et al., 2007; Tomkins et al., 2007). The presence of epileptiform activity when pilocarpine was coperfused with agents that mediate a transient enhancement of BBB permeability may suggest that opening of the BBB is the main event responsible for the induction of epileptiform discharges. This however seems unlikely, since perfusion with either histamine or bradykinin alone failed to affect neuronal firing. Thus, accumulation of intra-cerebral pilocarpine facilitated by concomitant opening of the BBB may be required to promote the pro-epileptic effect in our experiments. The demonstration that histamine was, indeed, able to open BBB is provided by the experiments with atropine bromide, a BBB-impermeable compound (Biesold et al., 1989). If the BBB is functionally intact, atropine bromide does not block muscarinic-dependent activity (Librizzi et al., 2001). When BBB-opener histamine was co-perfused in the isolated brain, atropine bromide either hindered epileptiform discharges or substantially reduced fast oscillatory activity induced by pilocarpine.
Since elevated potassium is a potent convulsant (Traynelis and Dingledine, 1988), intracerebral potassium rise could contribute to facilitate pilocarpine-induced epileptiform discharges. Rutecki and Yang (1998) showed that ictal epileptiform discharges in hippocampal slices occurred exclusively when potassium in the perfusate was increased to 7.5 mM. Treatment of EC slices with the muscarinic agonist, carbachol, was also capable of inducing epileptiform discharges only when extracellular potassium concentration was raised above 5 mM (Dickson and Alonso, 1997). The pro-epileptic effect of carbachol was not observed in the isolated guinea-pig preparation (van der Linden and de Curtis, 1998; Dickson et al., 2000) and in hippocampal slices bathed with a 4.2 mM potassium solution (Fisahn et al., 1998; Gloveli et al., 1999). When BBB permeability is enhanced, potassium may leak into brain from the blood compartment, where its concentration is higher (Lux and Neher, 1973; Somjen, 1979). This mechanism is not likely to occur in our experiments, since measurements with ion-selective electrodes did not show significant differences in [K+]o rise during histamine coperfusion, before seizure onset. The [K+]o shifts with or without histamine were, indeed, below the average values observed during epileptiform discharges. Interestingly, small [K+]o increases were observed during arterial perfusion with pilocarpine alone. This effect was not correlated to seizure-like events and was most likely associated to neuronal activation during fast oscillatory activity.
Histamine has an anticonvulsant action in the amygdaloid-kindled rats (Kamei et al., 1998), in a genetic model of temporal lobe epilepsy (Yawata et al., 2004). On the other hand, Yanovsky and Haas (1998) showed that, in vitro histamine application in the presence of 5 mM extracellular potassium has an excitatory effect mediated by H2 receptors. B1 bradykinin receptors mediate excita-tory effects in the peripheral nervous system and have little constitutional expression in the CNS (Walker et al., 1995). Bradykinin receptors are upregulated during epileptogenesis (Bregola et al., 1999). Whether this phenomenon has a protective role or represents an epileptogenic event is still debated. A direct role of the BBB openers, bradykinin and histamine, in precipitating epileptiform discharges in our experimental conditions can be excluded, since brain perfusion with either histamine or bradykinin without pilocarpine did not induce epileptiform activity. In addition, the demonstration that atropine bromide prevents epileptiform activity when co-perfused with histamine and pilocarpine further confirms that the pro-epileptic effect of pilocarpine during BBB opening is mediated by an agonistic action on atropine-sensitive muscarinic receptors.
Since in the isolated guinea-pig brain pilocarpine does not induce epileptiform activity in the absence of BBB opening, the possibility that also in the in vivo condition pilocarpine may require BBB permeability changes to induce SE should be considered. In vivo studies show that pilocarpine injections induce the release of histamine from cat submandibular gland and rat peritoneal mast cells (Erjavec and Ferjan, 1994). Therefore, pilocarpine could affect BBB permeability either by a direct action on intravascular muscarinic receptors, or by modulating histamine production. Increased BBB permeability was observed after SE in the lithium–pilocarpine model (Roch et al., 2002) or after pilocarpine alone (Marchi et al., in press). In the former model the concentration of pilocarpine necessary to induce a SE is 20 times lower than the dose required to induce SE with pilocarpine alone. Interestingly, molar concentration of lithium chloride has been used as a hyperos-molar compound to open BBB (Spatz et al., 1976). Therefore, the enhancing effect of LiCl could be attributed to the breakdown of BBB that subsequently increases the permeability to either pilocarpine or other ions/molecules that are usually excluded from brain parenchyma (Marchi and Janigro, unpublished observations). Finally, a recent in vivo report (Marchi et al., in press) demonstrated that serum levels of IL-1β increase and white blood cell are activated during pilocarpine-induced SE, therefore decreasing the CD4/CD8 T-lymphocyte ratio (Arzt et al., 1989; Prync et al., 1992). These pro-inflammatory events may synergistically potentate direct CNS action of pilocarpine leading to seizures (Simard and Rivest, 2005).
Several studies suggest a crucial role for central cholinergic activation in seizure initiation. Interestingly, in vivo experiments demonstrated that treatment with atropine before pilocarpine application is able to block epileptiform activity (Maslanski et al., 1994). Acetylcholine itself (Domer et al., 1983) and acetylcholine-esterase inhibitors, such as sarin (Turski et al., 1989; Abdel-Rahman et al., 2002) and soman (Grauer et al., 2001) are able to induce an increase in rats BBB permeability in vivo. Grauer et al. (2001) showed that the opening of the BBB after soman administration was observed even with doses that do not induce convulsions, suggesting that the disruption of the BBB is not only a consequence, but can precede seizures. It should be mentioned that, unlike sarin, soman and similar agents that cross the BBB, acetylcholine-esterase inhibitors impermeable to the BBB (such as pyridostigmine) do not cause seizures, unless applied at very high doses or in association with the insect repellent N-N-diethyl-m-toluamide (Chaney et al., 1997). The pro-epileptic effect of muscarine receptor activation is in partial contradiction with the evidence that muscarinic activation depresses synaptic activity in cortical structures and specifically in the EC (Richter et al., 1999; Yun et al., 2000; Kunitake et al., 2004; Hamam et al., 2007). Muscarinic synaptic dampening possibly occurs via a modulation of presynaptic potassium conductances that inhibits presynaptic voltage-dependent calcium channels (Egorov et al., 1996) therefore reducing neurotransmitter release. Low concentration of pilocarpine is indeed depressing synaptic propagation in our experiments. The reason why higher pilocarpine brain concentration promotes epileptiform discharge is still to be determined and is probably due to the multiple effects on membrane excitability mediated by muscarinic modulation of potassium conductances. Muscarine receptor agonists induce direct membrane potential depolarization (see Dickson et al., 2003) that might enhance intrinsic neuronal excitability and sustain the generation of hypersynchronous discharges.
CONCLUSION
In conclusion, we demonstrate that pilocarpine acutely induces epileptiform activity in the isolated guinea-pig brain only when BBB permeability is enhanced or when exorbitant brain drug levels are attained. Since in vivo administration of pilocarpine can promote BBB changes via a still undetermined direct (central) or indirect (peripheral) mechanism (Marchi et al., 2007) we propose that enhanced brain penetration of pilocarpine, together with other pro-epileptic blood-borne elements, contributes to the acute SE in the pilocarpine model of temporal lobe epilepsy.
Acknowledgments
The study was supported by the Italian Health Ministry (Ricerca Corrente N. 703901-L1) and by the Mariani Foundation grant No. R 06-50. This work was also supported in part by the National Institutes of Health (NIH-NS43284, NIH-HL51614, NIH-NS46513, NIH-NS049514 and NIH-NS38195) to D.J.
Abbreviations
- BBB
blood–brain barrier
- DAD
diode array
- dpc
di-synaptic population component
- EC
entorhinal cortex
- HPLC
high pressure liquid chromatography
- LOT
lateral olfactory tract
- mpc
monosynaptic population component
- PC
piriform cortex
- SE
status epilepticus
REFERENCES
- Abdel-Rahman A, Shetty AK, Abou-Donia MB. Acute exposure to sarin increases blood brain barrier permeability and induces neuropathological changes in the rat brain: dose-response relationships. Neuroscience. 2002;113:721–741. doi: 10.1016/s0306-4522(02)00176-8. [DOI] [PubMed] [Google Scholar]
- Arzt ES, Fernandez-Castelo S, Diaz A, Finkielman S, Nahmod VE. The muscarinic agonist pilocarpine inhibits DNA and inter-feron-gamma synthesis in peripheral blood mononuclear cells. Int J Immunopharmacol. 1989;11:275–281. doi: 10.1016/0192-0561(89)90165-3. [DOI] [PubMed] [Google Scholar]
- Biella G, de Curtis M. Olfactory inputs activate the medial entorhinal cortex via the hippocampus. J Neurophysiol. 2000;83:1924–1931. doi: 10.1152/jn.2000.83.4.1924. [DOI] [PubMed] [Google Scholar]
- Biesold D, Inanami O, Sato A, Sato Y. Stimulation of the nucleus basalis of Meynert increases cerebral cortical blood flow in rats. Neurosci Lett. 1989;98:39–44. doi: 10.1016/0304-3940(89)90370-4. [DOI] [PubMed] [Google Scholar]
- Bregola G, Varani K, Gessi S, Beani L, Bianchi C, Borea PA, Regoli D, Simonato M. Changes in hippocampal and cortical B1 bradykinin receptor biological activity in two experimental models of epilepsy. Neuroscience. 1999;92:1043–1049. doi: 10.1016/s0306-4522(99)00075-5. [DOI] [PubMed] [Google Scholar]
- Cavalheiro EA, Naffah-Mazzacoratti MG, Mello LE, Leite JP. The pilocarpine model of seizures. In: Pitkanen A, Schwartzkroin PA, Moshé SL, editors. Models of seizures and epilepsy. Elsevier Academic Press; Burlington, MA: 2006. pp. 433–446. [Google Scholar]
- Chaney LA, Rockhold RW, Mozingo JR, Hume AS, Moss JI. Potentiation of pyridostigmine bromide toxicity in mice by selected adrenergic agents and caffeine. Vet Hum Toxicol. 1997;39:214–219. [PubMed] [Google Scholar]
- de Curtis M, Biella G, Buccellati C, Folco G. Simultaneous investigation of the neuronal and vascular compartments in the guinea pig brain isolated in vitro. Brain Res Brain Res Protoc. 1998;3:221–228. doi: 10.1016/s1385-299x(98)00044-0. [DOI] [PubMed] [Google Scholar]
- Dickson CT, Alonso A. Muscarinic induction of synchronous population activity in the entorhinal cortex. J Neurosci. 1997;17:6729–6744. doi: 10.1523/JNEUROSCI.17-17-06729.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson CT, Biella G, de Curtis M. Evidence for spatial modules mediated by temporal synchronisation of carbachol induced gamma rhythm in medial entorhinal cortex. J Neurosci. 2000;20:7846–7854. doi: 10.1523/JNEUROSCI.20-20-07846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson CT, Biella G, de Curtis M. Slow periodic events and their transition to gamma oscillations in the entorhinal cortex of the isolated guinea pig brain. J Neurophysiol. 2003;90:39–46. doi: 10.1152/jn.01063.2002. [DOI] [PubMed] [Google Scholar]
- Domer FR, Boertje SB, Bing EG, Reddix I. Histamine- and acetylcholine-induced changes in the permeability of the blood-brain barrier of normotensive and spontaneously hypertensive rats. Neuropharmacology. 1983;22:615–619. doi: 10.1016/0028-3908(83)90153-3. [DOI] [PubMed] [Google Scholar]
- Egorov AV, Heinemann U, Muller W. Muscarinic activation reduces changes in [Ca2+]o evoked by stimulation of Schaffer collaterals during blocked synaptic transmission in rat hippocampal slices. Neurosci Lett. 1996;214:187–190. doi: 10.1016/0304-3940(96)12933-5. [DOI] [PubMed] [Google Scholar]
- Erjavec F, Ferjan I. The mechanism of histamine release induced by pilocarpine from different tissues: studies on rat perito-neal mast cells. Agents Actions. 1994;41(Spec No):C32–C33. doi: 10.1007/BF02007754. [DOI] [PubMed] [Google Scholar]
- Fabene PF, Marzola P, Sbarbati A, Bentivoglio M. Magnetic resonance imaging of changes elicited by status epilepticus in the rat brain: diffusion-weighted and T2-weighted images, regional blood volume maps, and direct correlation with tissue and cell damage. Neuroimage. 2003;18:375–389. doi: 10.1016/s1053-8119(02)00025-3. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- French SJ, Humby T, Horner CH, Sofroniew MV, Rattray M. Hippocampal neurotrophin and trk receptor mRNA levels are altered by local administration of nicotine, carbachol and pilocarpine. Brain Res Mol Brain Res. 1999;67:124–136. doi: 10.1016/s0169-328x(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Gloveli T, Egorov AV, Schmitz D, Heinemann U, Muller W. Carbachol-induced changes in excitability and [Ca2+]i signalling in projection cells of medial entorhinal cortex layers II and III. Eur J Neurosci. 1999;11:3626–3636. doi: 10.1046/j.1460-9568.1999.00785.x. [DOI] [PubMed] [Google Scholar]
- Gnatkovsky V, Uva L, de Curtis M. Topographic distribution of direct and hippocampus-mediated entorhinal cortex activity evoked by olfactory tract stimulation. Eur J Neurosci. 2004;20:1897–1905. doi: 10.1111/j.1460-9568.2004.03627.x. [DOI] [PubMed] [Google Scholar]
- Grauer E, Ben Nathan D, Lustig S, Kobiler D, Kapon J, Danenberg HD. Viral neuroinvasion as a marker for BBB integrity following exposure to cholinesterase inhibitors. Life Sci. 2001;68:985–990. doi: 10.1016/s0024-3205(00)01000-6. [DOI] [PubMed] [Google Scholar]
- Greenwood J. Mechanisms of blood-brain barrier breakdown. Neuroradiology. 1991;33:95–100. doi: 10.1007/BF00588242. [DOI] [PubMed] [Google Scholar]
- Hamam BN, Sinai M, Poirier G, Chapman CA. Cholinergic suppression of excitatory synaptic responses in layer II of the medial entorhinal cortex. Hippocampus. 2007;17:103–113. doi: 10.1002/hipo.20249. [DOI] [PubMed] [Google Scholar]
- Hedlund B, Bartfai T. Binding of [3H]-pilocarpine to membranes from rat cerebral cortex. Naunyn Schmiedebergs Arch Pharmacol. 1981;317:126–130. doi: 10.1007/BF00500067. [DOI] [PubMed] [Google Scholar]
- Kamei C, Ishizawa K, Kakinoki H, Fukunaga M. Histaminergic mechanisms in amygdaloid-kindled seizures in rats. Epilepsy Res. 1998;30:187–194. doi: 10.1016/s0920-1211(98)00005-9. [DOI] [PubMed] [Google Scholar]
- Kuks PF, Weekers LE, Goldhoorn PB. Decomposition of pilocarpine eye drops assessed by a highly efficient high pressure liquid chromatographic method. Pharm Weekbl Sci. 1990;12:196–199. doi: 10.1007/BF01980046. [DOI] [PubMed] [Google Scholar]
- Kunitake A, Kunitake T, Stewart M. Differential modulation by carbachol of four separate excitatory afferent systems to the rat subiculum in vitro. Hippocampus. 2004;14:986–999. doi: 10.1002/hipo.20016. [DOI] [PubMed] [Google Scholar]
- Librizzi L, Janigro D, De Biasi S, de Curtis M. Blood-brain barrier preservation in the in vitro isolated guinea pig brain preparation. J Neurosci Res. 2001;66:289–297. doi: 10.1002/jnr.1223. [DOI] [PubMed] [Google Scholar]
- Lux HD, Neher E. The equilibration time course of (K +) 0 in cat cortex. Exp Brain Res. 1973;17:190–205. doi: 10.1007/BF00235028. [DOI] [PubMed] [Google Scholar]
- Marchi N, Angelov L, Masaryk T, Fazio V, Granata T, Hernandez N, Hallene K, Diglaw T, Franic L, Najm I, Janigro D. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia. 2007;48:732–742. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Oby E, Fernandez N, Uva L, de Curtis M, Batra A, Santaguida S, Barnes V, Van Boxel A, Najim I, Janigro D. In vivo and in vitro effects of pilocarpine: relevance to epileptogenesis. Epilepsia. doi: 10.1111/j.1528-1167.2007.01185.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslanski JA, Powelt R, Deirmengiant C, Patelt J. Assessment of the muscarinic receptor subtypes involved in pilocarpine-induced seizures in mice. Neurosci Lett. 1994;168:225–228. doi: 10.1016/0304-3940(94)90456-1. [DOI] [PubMed] [Google Scholar]
- Millan MH, Chapman AG, Meldrum BS. Extracellular amino acid levels in hippocampus during pilocarpine-induced seizures. Epilepsy Res. 1993;14:139–148. doi: 10.1016/0920-1211(93)90018-3. [DOI] [PubMed] [Google Scholar]
- Muhlethaler M, de Curtis M, Walton K, Llinas R. The isolated and perfused brain of the guinea-pig in vitro. Eur J Neurosci. 1993;5:915–926. doi: 10.1111/j.1460-9568.1993.tb00942.x. [DOI] [PubMed] [Google Scholar]
- Nagao T, Alonso A, Avoli M. Epileptiform activity induced by pilocarpine in the rat hippocampal-entorhinal slice preparation. Neuroscience. 1996;72:399–408. doi: 10.1016/0306-4522(95)00534-x. [DOI] [PubMed] [Google Scholar]
- Omori Y, Endo T, Hara Y, Nishiyama M, Midgley I, Smart CI, John AJ, Chasseaud LF, McBurney A, John BA. Absorption, distribution and excretion of 14C-pilocarpine following oral administration to rats. Arzneimittelforschung. 2004;54:171–178. doi: 10.1055/s-0031-1296955. [DOI] [PubMed] [Google Scholar]
- Pavlovsky L, Seiffert E, Heinemann U, Korn A, Golan H, Friedman A. Persistent BBB disruption may underlie alpha interferon-induced seizures. J Neurol. 2005;252:42–46. doi: 10.1007/s00415-005-0596-3. [DOI] [PubMed] [Google Scholar]
- Prync AE, Arzt E, Fernandez Castelo S, Finkielman S, Nahmod V. The inhibitory effect of the muscarinic agonist pilocarpine on lymphocyte activation involves the IL-2 pathway and the increase in suppressor cell function. Int J Neurosci. 1992;62:277–285. doi: 10.3109/00207459108999780. [DOI] [PubMed] [Google Scholar]
- Richter M, Schilling T, Müller W. Muscarinic control of intracortical connections to layer II in rat entorhinal cortex slice. Neurosci Lett. 1999;273:200–202. doi: 10.1016/s0304-3940(99)00643-6. [DOI] [PubMed] [Google Scholar]
- Roch C, Leroy C, Nehlig A, Namer IJ. Magnetic resonance imaging in the study of the lithium-pilocarpine model of temporal lobe epilepsy in adult rats. Epilepsia. 2002;43:325–335. doi: 10.1046/j.1528-1157.2002.11301.x. [DOI] [PubMed] [Google Scholar]
- Rutecki PA, Yang Y. Ictal epileptiform activity in the CA3 region of hippocampal slices produced by pilocarpine. J Neurophysiol. 1998;79:3019–3029. doi: 10.1152/jn.1998.79.6.3019. [DOI] [PubMed] [Google Scholar]
- Schilling L, Wahl M. Mediators of cerebral edema. Adv Exp Med Biol. 1999;474:123–141. doi: 10.1007/978-1-4615-4711-2_11. [DOI] [PubMed] [Google Scholar]
- Seiffert E, Dreier JP, Ivens S, Bechmann I, Tomkins O, Heinemann U, Friedman A. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004;24:7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard AR, Rivest S. Do pathogen exposure and innate immunity cause brain diseases? Neurol Res. 2005;27:717–725. doi: 10.1179/016164105X49526. [DOI] [PubMed] [Google Scholar]
- Sloviter R. The neurobiology of temporal lobe epilepsy: too much information, not enough knowledge. C R Biol. 2005;328(2):143–153. doi: 10.1016/j.crvi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Extracellular potassium in the mammalian central nervous system. Annu Rev Physiol. 1979;41:159–177. doi: 10.1146/annurev.ph.41.030179.001111. [DOI] [PubMed] [Google Scholar]
- Spatz M, Rap AM, Rapoport SI, Klatzo I. Effect of hypertonic solution and of HgCl2 on the uptake of 14C-glucose analogue by rabbit brain. In: Rapoport S, editor. Blood brain barrier in physiology and medicine. Raven Press; New York: 1976. pp. 185–186. [Google Scholar]
- Tomkins O, Friedman O, Ivens S, Reiffurth C, Major S, Dreier JP, Heinemann U, Friedman A. Blood-brain barrier disruption results in delayed functional and structural alterations in the rat neocortex. Neurobiol Dis. 2007;25:367–377. doi: 10.1016/j.nbd.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Dingledine R. Potassium-induced spontaneous electrographic seizures in the rat hippocampal slice. J Neurophysiol. 1988;59:259–276. doi: 10.1152/jn.1988.59.1.259. [DOI] [PubMed] [Google Scholar]
- Turski L, Ikonomidou C, Turski WA, Bortolotto ZA, Cavalheiro EA. Review: cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: a novel experimental model of intractable epilepsy. Synapse. 1989;3:154–171. doi: 10.1002/syn.890030207. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Bortolotto ZA, Mello LM, Schwarz M, Turski L. Seizures produced by pilocarpine in mice: a behavioral, electroencephalographic and morphological analysis. Brain Res. 1984;321:237–253. doi: 10.1016/0006-8993(84)90177-x. [DOI] [PubMed] [Google Scholar]
- Uva L, Librizzi L, Wendling F, de Curtis M. Propagation dynamics of epileptiform activity acutely induced by bicuculline in the hippocampal-parahippocampal region of the isolated guinea pig brain. Epilepsia. 2005;46(12):1914–1925. doi: 10.1111/j.1528-1167.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- Uva L, Janigro D, de Curtis M. Submillimolar and supramillimolar concentrations of pilocarpine induce, respectively, gamma oscillations and epileptiform activity in the in vitro isolated guinea pig brain. Society for Neuroscience, 36th annual meeting. 2006 abstract 796.3/D35. [Google Scholar]
- van der Linden S, de Curtis M. Carbachol-induced fast oscillatory activity is generated in medial and not in lateral entorhinal cortex of the isolated guinea pig brain preparation. Society for Neuroscience, 28th Annual Meeting. 1998 [Google Scholar]
- van der Linden S, de Curtis M, Panzica F. Carbachol induces fast oscillations in the medial but not in the later entorhinal cortex of the isolated guinea pig brain. J Neurophysiol. 1999;82:2441–2450. doi: 10.1152/jn.1999.82.5.2441. [DOI] [PubMed] [Google Scholar]
- van Eijsden P, Notenboom RG, Wu O, de Graan PN, van Nieuwenhuizen O, Nicolay K, Braun KP. In vivo 1H magnetic resonance spectroscopy, T2-weighted and diffusion-weighted MRI during lithium-pilocarpine-induced status epilepticus in the rat. Brain Res. 2004;1030:11–18. doi: 10.1016/j.brainres.2004.09.025. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, da Costa Araujo S, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- Walker K, Perkins M, Dray A. Kinins and kinin receptors in the nervous system. Neurochem Int. 1995;26:1–16. doi: 10.1016/0197-0186(94)00114-a. [DOI] [PubMed] [Google Scholar]
- Williams JH, Kauer JA. Properties of carbachol-induced oscillatory activity in the rat hippocampus. J Neurophysiol. 1997;78:2631–2640. doi: 10.1152/jn.1997.78.5.2631. [DOI] [PubMed] [Google Scholar]
- Yanovsky Y, Haas HL. Histamine increases the bursting activity of pyramidal cells in the CA3 region of mouse hippocampus. Neurosci Lett. 1998;240:110–112. doi: 10.1016/s0304-3940(97)00925-7. [DOI] [PubMed] [Google Scholar]
- Yawata I, Tanaka K, Nakagawa Y, Watanabe Y, Murashima YL, Nakano K. Role of histaminergic neurons in development of epileptic seizures in EL mice. Brain Res Mol Brain Res. 2004;132:13–17. doi: 10.1016/j.molbrainres.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Yun SH, Cheong MY, Mook-Jung I, Huh K, Lee C, Jung MW. Cholinergic modulation of synaptic transmission and plasticity in entorhinal cortex and hippocampus of the rat. Neuroscience. 2000;97:671–676. doi: 10.1016/s0306-4522(00)00108-1. [DOI] [PubMed] [Google Scholar]