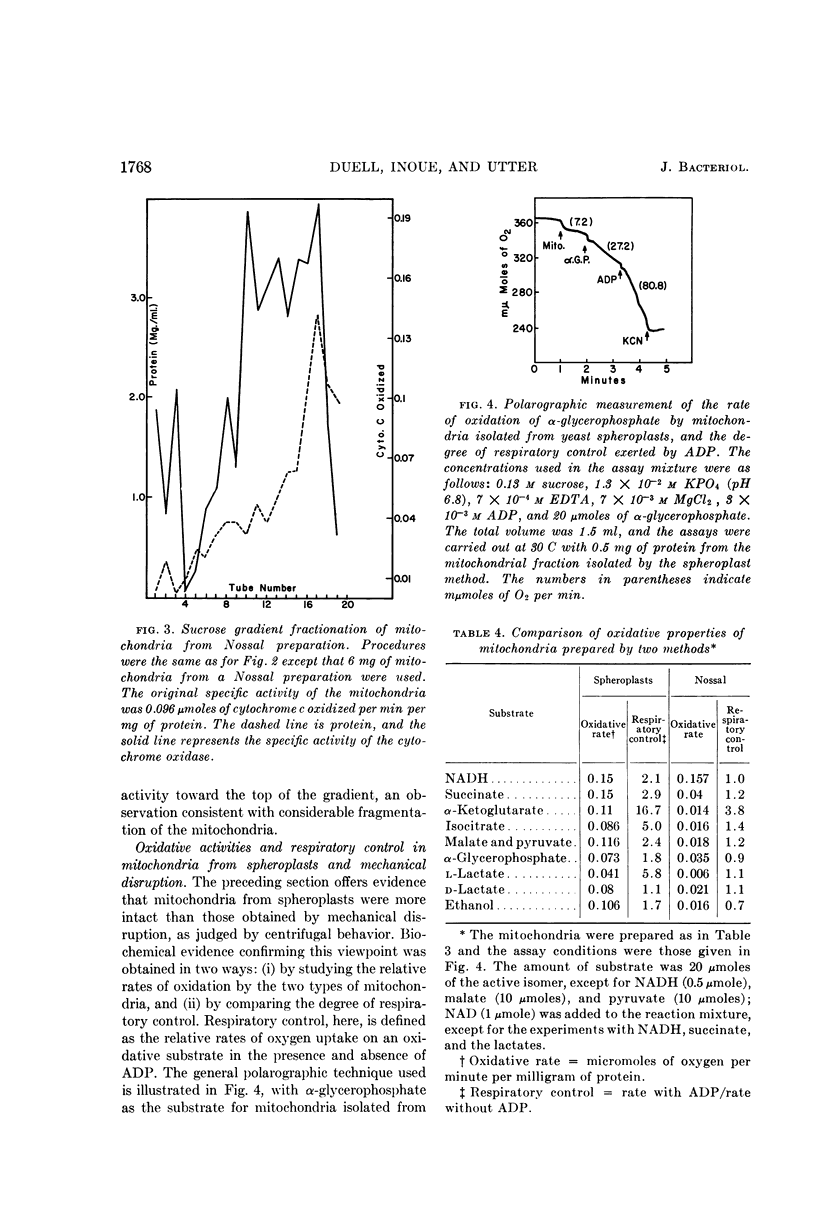
Abstract
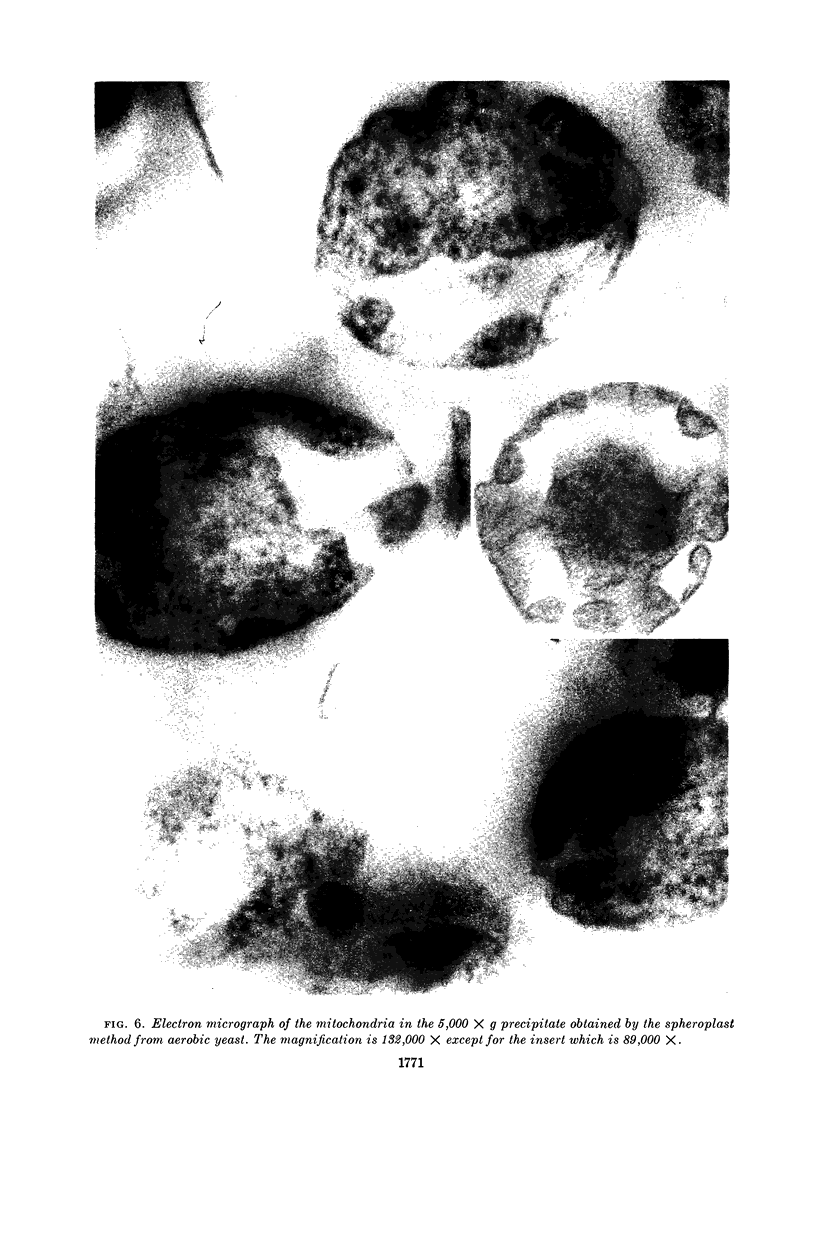
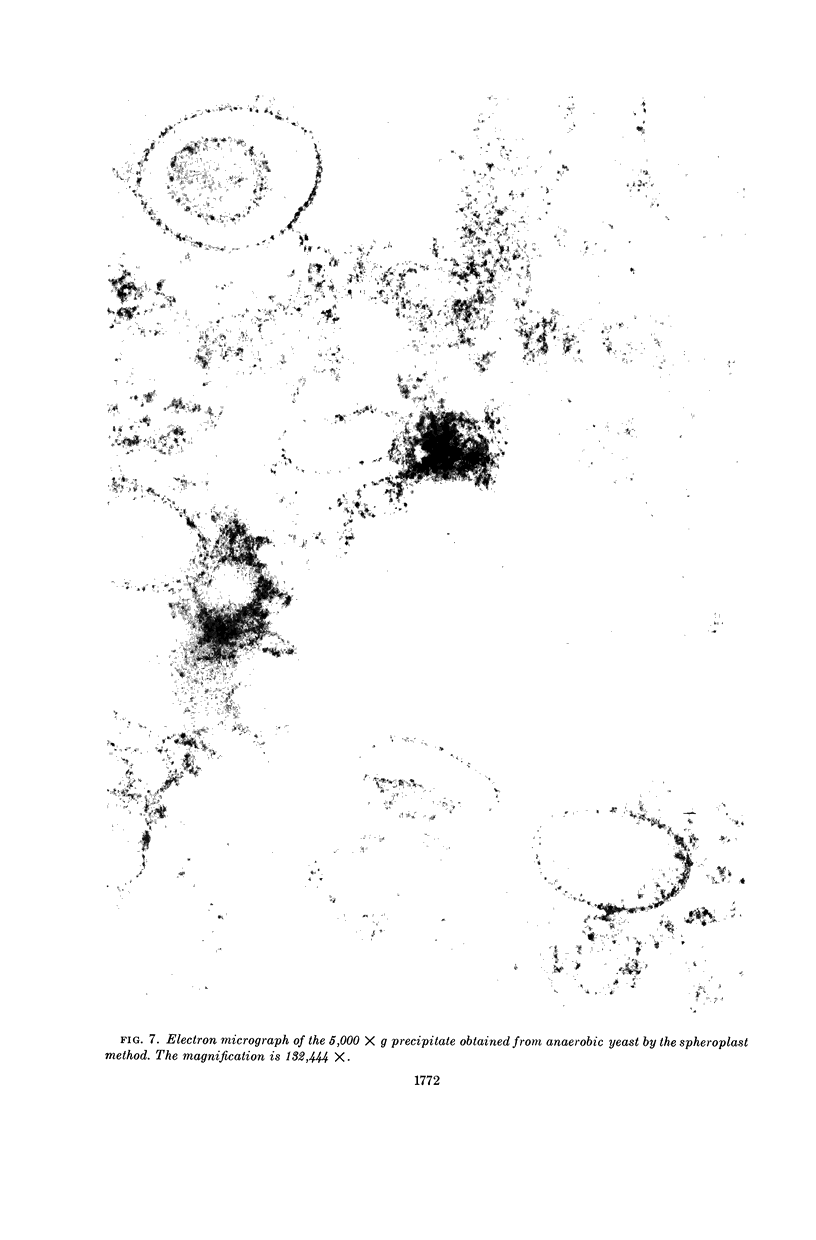
Duell, E. A. (Western Reserve University, Cleveland, Ohio), Sakae Inoue, and Merton F. Utter. Isolation and properties of intact mitochondria from spheroplasts of yeast. J. Bacteriol. 88:1762–1773. 1964.—Functionally intact mitochondria can be obtained in good yields by osmotic disruption of spheroplasts formed from yeast by treatment with an enzyme mixture from the snail digestive tract. The useful range of this method is extended greatly by pretreatment of the yeast cells with 2-mercaptoethylamine and ethylene-diaminetetraacetate. The concentration of the yeast suspension can be increased greatly, the incubation period can be shortened considerably, and the requirement for log-phase cells is obviated. Mitochondria prepared by this method have been compared with those obtained by mechanical disruption in terms of respiratory control, specific activity on a wide range of oxidizable substrates, heterogeneity during centrifugation, and structures observed by electron microscopy. In all cases, the mitochondria obtained from spheroplasts appear to be much less damaged by the preparative procedures.
Full text
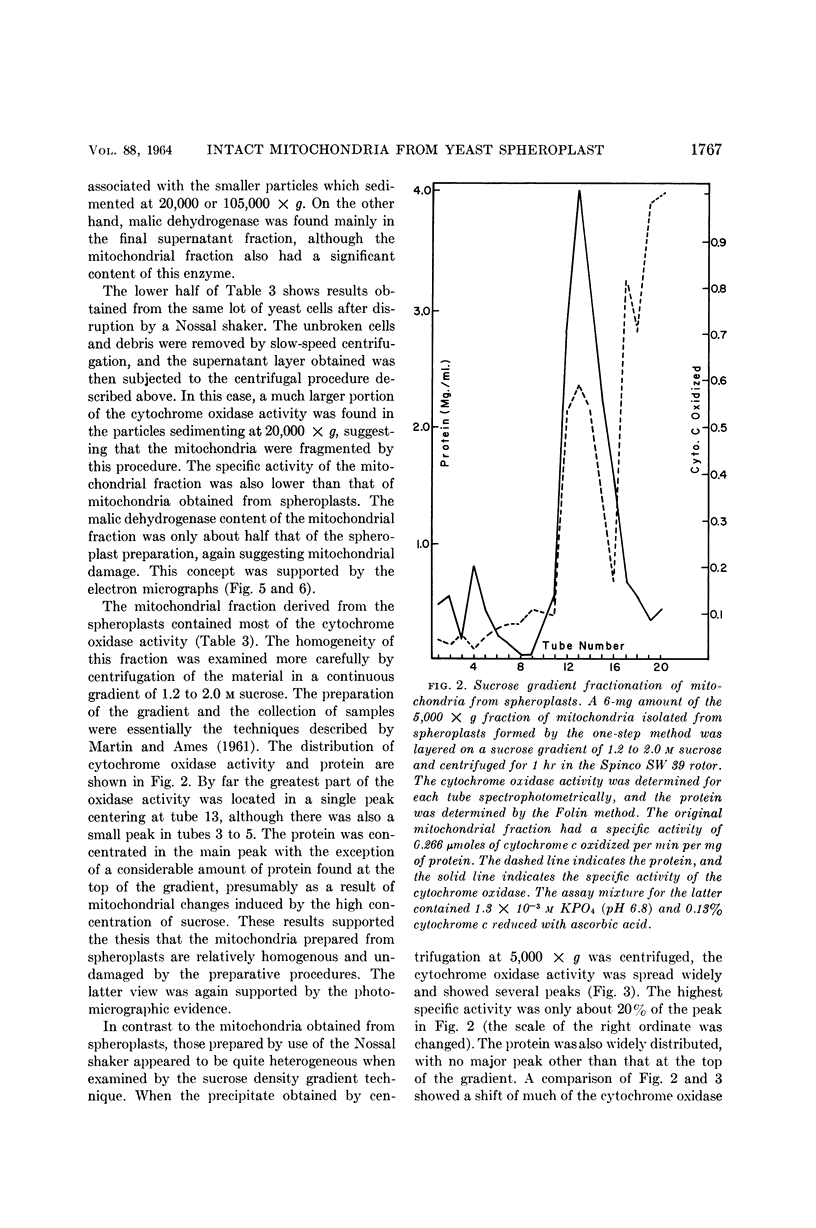
PDF




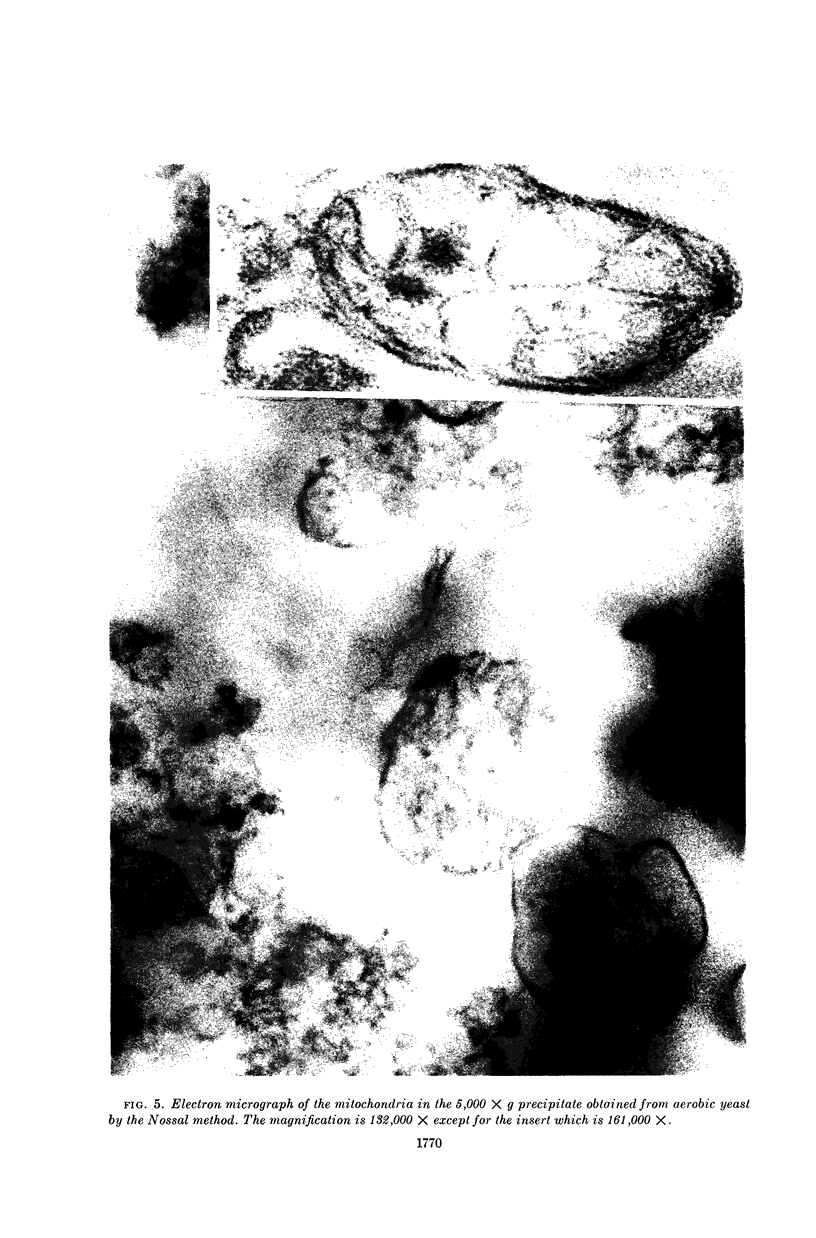

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGAR H. D., DOUGLAS H. C. Studies on the cytological structure of yeast: electron microscopy of thin sections. J Bacteriol. 1957 Mar;73(3):365–375. doi: 10.1128/jb.73.3.365-375.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGER M., BACON E. E., BACON J. S. Some observations on the form and location of invertase in the yeast cell. Biochem J. 1961 Mar;78:504–511. doi: 10.1042/bj0780504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEBB C. R., MONTGOMERY J. D., SLEBODNIK J. Particles exhibiting oxidative enzyme activity in yeast. Exp Cell Res. 1958 Jun;14(3):495–509. doi: 10.1016/0014-4827(58)90157-5. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J. Simple methods for "staining with lead" at high pH in electron microscopy. J Biophys Biochem Cytol. 1961 Dec;11:729–732. doi: 10.1083/jcb.11.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Polakis E. S., Bartley W., Meek G. A. Changes in the structure and enzyme activity of Saccharomyces cerevisiae in response to changes in the environment. Biochem J. 1964 Feb;90(2):369–374. doi: 10.1042/bj0900369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVIHLA G., SCHLENK F., DAINKO J. L. Spheroplasts of the yeast Candida utilis. J Bacteriol. 1961 Dec;82:808–814. doi: 10.1128/jb.82.6.808-814.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UTTER M. F., KEECH D. B., NOSSAL P. M. Oxidative phosphorylation by subcellular particles from yeast. Biochem J. 1958 Mar;68(3):431–440. doi: 10.1042/bj0680431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VITOLS E., LINNANE A. W. Studies on the oxidative metabolism of Saccharomyces cerevisiae. II. Morphology and oxidative phosphorylation capacity of mitochondria and derived particles from baker's yeast. J Biophys Biochem Cytol. 1961 Mar;9:701–710. doi: 10.1083/jcb.9.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VITOLS E., NORTH R. J., LINNANE A. W. Studies on the oxidative metabolism of Saccharomyces cerevisiae. I. Observations on the fine structure of the yeast cell. J Biophys Biochem Cytol. 1961 Mar;9:689–699. doi: 10.1083/jcb.9.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]





