Abstract
We have previously reported that vaccination with CpG oligodeoxynucleotides delivered concomitantly with live Leishmania major (Lm/CpG) eliminates lesions associated with live vaccination in C57BL/6 mice. The absence of lesions is at least in part a result of the CpG DNA-mediated activation of dermal dendritic cells to produce cytokines such as interleukin (IL)-6. Wild-type C57BL/6 mice and IL-6−/− mice were immunized with the Lm/CpG vaccine and monitored for the development of lesions. IL-6−/− mice developed extensive, nonhealing lesions following live vaccination. The analysis of the inoculation site and draining lymph nodes of the IL-6−/− mice revealed a constitutive reduction in lymphocyte numbers, particularly CD4+ T cells. Live vaccination resulted in the specific expansion of CD4+Foxp3+ regulatory T cells in the knockout mice, and in a decrease of CD4+ IFN-γ -producing cells. These results indicate that IL-6−/− mice may have collateral immune defects that could influence the development of the natural immune response to pathogens, vaccines, or other inflammatory stimuli.
Keywords: IL-6, Leishmania, CpG DNA, leishmanization, vaccination
Introduction
Leishmania major (Lm) is the causative agent of zoonotic cutaneous leishmaniasis, the most widely distributed form of cutaneous leishmaniasis in the Old World (Desjeux 2004). The inoculation of live parasites to produce a lesion that heals (leishmanization) has been the only vaccination strategy implemented at a large scale because it provides lifelong protection against the development of lesions. This approach was discontinued because of the unacceptable frequency (10%) of lesions that were slow to heal or nonhealing (Modabber 1995).
We have shown that CpG DNA delivered at the site of intradermal vaccination with Lm moderates the pathology associated with leishmanization in C57BL/6 mice (Mendez et al. 2003). Mechanistically, we have discovered that the addition of CpG DNA to live Lm (Lm/CpG DNA) induces activation of dermal dendritic cells to produce cytokines, especially interleukin (IL)-6 (Wu et al. 2006), a pleiotropic cytokine described as a developmental factor for lymphocytes, mesangial cells (Ruef et al. 1990; Jones et al. 2005; Gabay 2006), and, most recently, CD4+ Th17 cells (Harrington et al. 2006). To investigate the role of IL-6 in our system, we immunized wild-type (WT) C57BL/6 mice and IL-6−/− mice with the Lm/CpG DNA vaccine and evaluated the development, or lack thereof, of vaccinal lesions. In this report, we present data showing the unpredicted enhanced susceptibility of the IL-6−/− mice to Lm using our intradermal, low-dose, live vaccination model. We also analyzed changes on the T cell populations to identify specific subsets that were the most affected in the knockout mouse, as well as the effect of these T cell population changes on the predicted vaccination outcome, with the purpose of identifying immune mechanisms that may be defective in the IL-6−/− mouse strain.
Materials and methods
Mice
C57BL/6 mice were purchased from the Division of Cancer Treatment, National Cancer Institute (Frederick, Maryland) or Taconic (Germantown, New York). IL-6−/− mice were purchased from Taconic. Animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animals (1996, published by National Academy Press). The use of animals was reviewed and approved by the appropriate animal care review committee at Cornell University.
Infection protocol and vaccine preparation
Lm clone V1 (MHOM/IL/80/Friedlin) promastigotes were grown at 26 °C in medium 199 supplemented with 20% fetal calf serum (Gemini, Sacramento, California), 100 U/mL penicillin, 100 µg/mL streptomycin, 2 mmol/L l-glutamine, 40 mmol/L Hepes, 0.1 mmol/L adenine (in 50 mmol/L Hepes), and 5 mg/mL hemin (in 50% triethanolamine). Infective-stage promastigotes (metacyclics) of Lm were isolated from stationary cultures (4–5 days old) by Ficoll enrichment (Spath and Beverley 2001). Mice were anesthetized and vaccinated intradermally in the ear with 1 × 104 Lm metacyclic promastigotes alone or in combination with 50 µg of CpG DNA 1826 (5′-TCCATGACGTTCCTGACGTT-3′; Coley Pharmaceutical, Ottawa, Ontario) using a 27 1/2G needle in a volume of 10 µL.
Parasite quantitation
Parasite loads in the ears were determined as previously described (Wu et al. 2006). Briefly, the ventral and dorsal sheets of the infected ears were separated and deposited in RPMI medium containing 100 U/mL penicillin, 100 µg/mL streptomycin and Liberase CI enzyme blend (0.5 mg/mL; Roche, Indianapolis, Indiana). The ears were incubated for 60 min at 37 °C. The sheets were dissociated using a handheld tissue homogenizer. The homogenates were filtered using a 70 µm cell strainer (BD Falcon, San José, California), washed in RPMI, and serially diluted (3-fold) in 96-well flat-bottom microtiter plates containing biphasic medium prepared using 50 µL of Novy–MacNeal–Nicolle (NNN) medium containing 20% of defibrinated rabbit blood overlaid with 100 µL of complete M199 medium. The number of viable parasites in each ear was estimated from the highest dilution at which promastigotes could be grown out after 7 days of incubation at 26 °C. Results were expressed as log parasite burden per ear.
Analysis of dermal leukocytes
Single cell suspensions from the ear dermis were obtained following Liberase digestion as described earlier. Cells were resuspended in supplemented RPMI, counted, and seeded in 24-well plates at a concentration of 2 × 106 cells/well. Dermal cells were then cultured at 37 °C under 5% CO2 for 6 h with Brefeldin A (10 µg/mL) and then fixed in 2% paraformaldehyde (Electron Microscopy Sciences, Hatfield, Pennsylvania).
Flow cytometry staining
Prior to staining, cells were incubated with an anti-Fcγ III/II receptor and 10% normal mouse serum in phosphate-buffered saline containing 0.1% BSA, and 0.01% NaN3. Cells were permeabilized with saponin and stained for the surface markers CD3 (clone 145-2C11), CD4 (clone RM4-5), and CD25 (clone PC61.5). Then, the cells were permeabilized with saponin and stained with antibodies against the transcription factor Foxp3 (clone FJK-16s) and for the cytokines interferon-gamma (IFN-γ; clone XMG1.2) and IL-10 (clone JES5-16E3). Incubations were carried out for 30 min on ice. All antibodies were purchased from BD Biosciences (San José, California) or eBioscience (San Diego, California). For each sample, at least 50 000 cells were analyzed. Data were collected and analyzed using a FACScalibur flow cytometer and CELLQuest software (Becton Dickinson, San José, California).
Cytokine measurements
To determine the levels of secretion of the cytokines IL-10, IL-6, IL-12 p70, tumor necrosis factor-alpha (TNF-α), IFN-γ, and monocyte chemoattractant protein-1 (MCP-1), dermal cells were obtained from 3 mice (6 ears) as described earlier, resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum and antibiotics, seeded into 24-well plates at a concentration of 2 × 106 cells/well, and incubated at 37 °C under 5% CO2 to allow for the spontaneous release of cytokines. Twenty-four hours later, supernatants were collected. Cytokine levels in these supernatants were measured using the BD CBA Mouse Inflammation kit following the manufacturer’s instructions (BD Biosciences). The theoretical limit of detection of the kit is 20 pg/mL.
In vivo anti-IL-6R treatment
C57BL/6 mice were injected with 5 µg of anti-IL-6R (Catalogue No. AF1830; R&D Systems, Minneapolis, Minnesota) by intraperitoneal injection on days −1, 1, and 3 relative to vaccination with 1 × 104 Lm alone or in combination with 50 µg of CpG DNA.
Statistical analysis
All comparisons of non-normally distributed continuous data were analyzed with the Mann–Whitney U test using GraphPad Prism (San Diego, California).
Results
IL-6−/− mice develop nonhealing lesions following vaccination with live Lm alone or in combination with CpG DNA
In previous work, we have shown that vaccination with a combination of live Lm parasites and CpG DNA (Lm/CpG) protected animals against the development of vaccinal lesions associated with the inoculation of infective parasites (Mendez et al. 2003; Wu et al. 2006). We have also reported that one of the most striking effects of vaccination with Lm/CpG was the enhanced secretion of IL-6 by dermal dendritic cells (Wu et al. 2006). Hence, we decided to investigate whether the elevated IL-6 production observed as a consequence of Lm/CpG vaccination was associated with disease prevention. We demonstrated that neutralization of the cytokine by anti-IL-6 antibodies abrogated the protective effect of the Lm/CpG vaccine (Wu et al. 2006), thus confirming that IL-6 is linked to vaccine protection. To further demonstrate the effect of IL-6, WT C57BL/6 mice and IL-6−/− mice were immunized with Lm alone (to mimic classical leishmanization) or Lm/CpG.
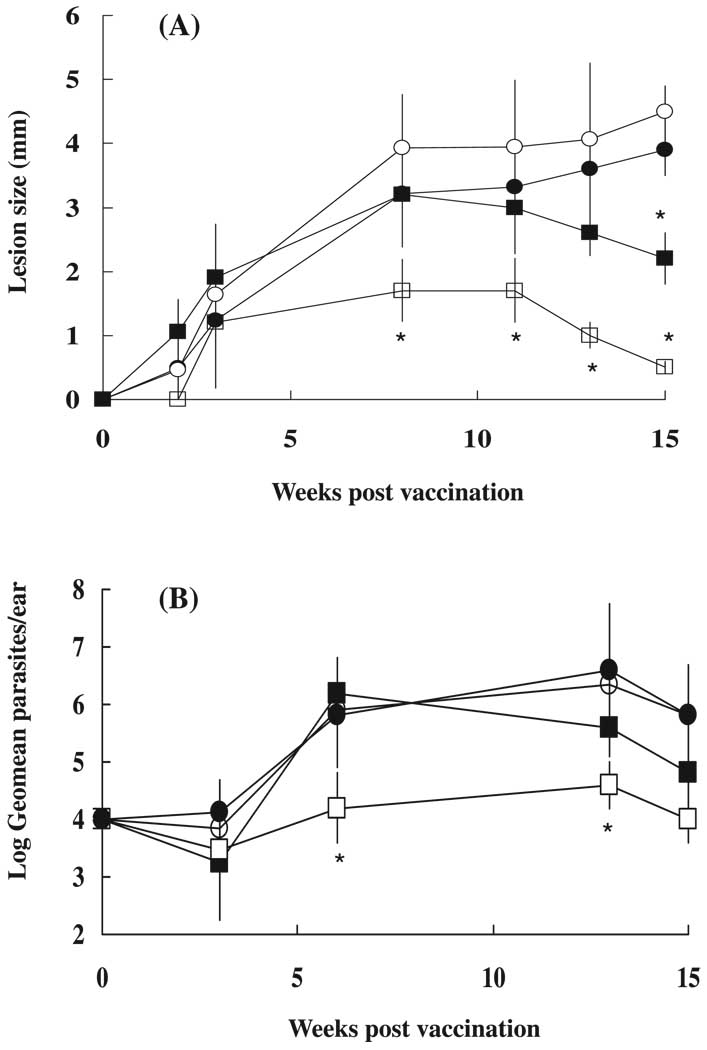
Lesion size was measured for 15 weeks and parasite numbers in the ears were determined in the experimental groups at weeks 3, 6, 13, and 15 post vaccination. WT mice vaccinated with Lm/CpG showed a striking reduction in dermal lesions compared with mice infected with Lm alone (Fig. 1A; p < 0.001). Because WT mice exhibit a self-healing phenotype, lesion size was also significantly reduced (p < 0.001) in these animals at week 15 post vaccination when compared with the IL-6−/− mice, whose lesions did not decrease in size and developed necrosis.
Fig. 1.
Wild-type (WT) C57BL/6 mice and IL-6−/− mice were vaccinated in the ear dermis with 1 × 104 Leishmania major (Lm) alone (■ and ●, respectively) or in combination with 50 µg of CpG DNA (□ and ○, respectively). (A) Lesion size, expressed as mean induration ± SD, 12–20 ears/group. *, Indicates statistically significant difference (p < 0.001). (B) Parasite numbers per ear, expressed as log geometric mean ± SD, 6 ears/group. *, Indicates statistically significant difference (p < 0.05).
Ear parasite burdens were also determined. At 3 weeks post vaccination, no differences were detected in parasite loads among the different experimental groups. However, at 6 and 13 weeks post vaccination, WT mice inoculated with Lm/CpG showed a statistically significant reduction (p < 0.05) in ear parasite loads when compared with animals vaccinated with Lm alone (Fig. 1B). IL-6 −/− mice vaccinated with either Lm or Lm/CpG not only were not protected against the development of cutaneous lesions, but also showed elevated parasite numbers from weeks 6 to 15. The slight reduction in parasite numbers in IL-6 −/− mice at week 15 could be attributable to the concurrent necrosis and loss of ear tissue. Our results indicate that IL-6−/− mice appear to be more susceptible to cutaneous leishmaniasis (Lm) than their WT counterparts and are incapable of self-healing. As a consequence of this, the relationship between IL-6 and vaccine protection cannot be properly established using this mouse model.
Cytokine responses to Lm/CpG DNA vaccination are decreased in IL-6−/− mice
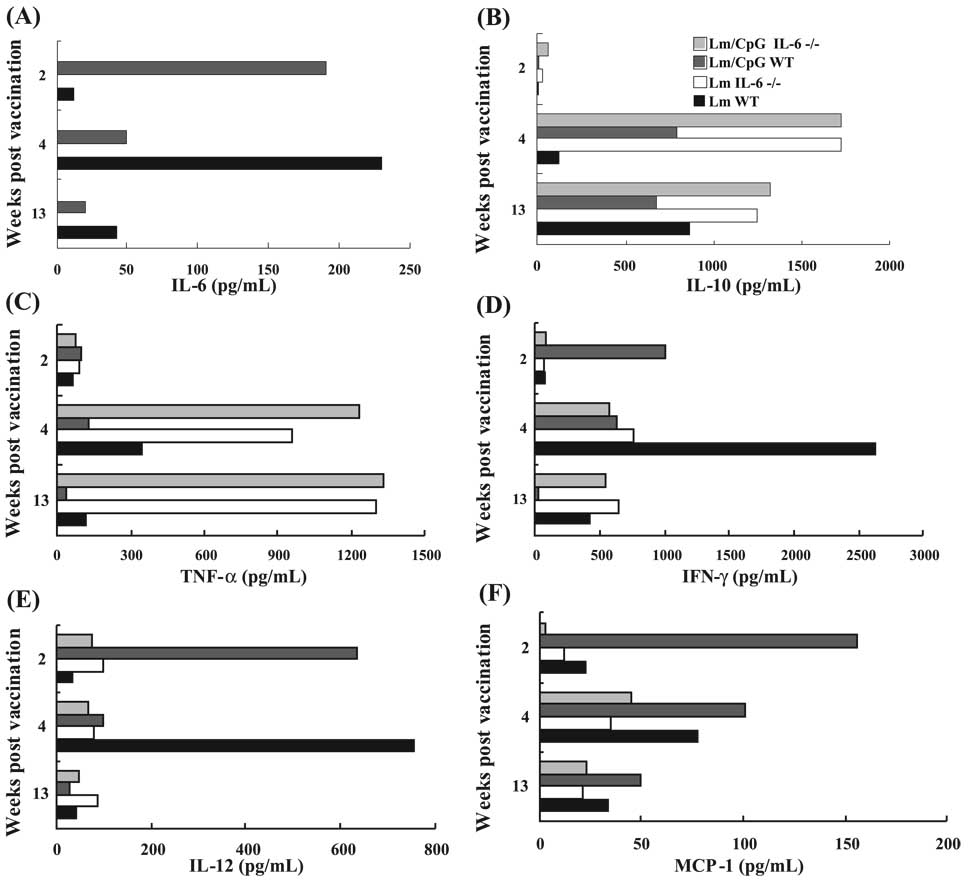
We determined the cytokine profile of dermal cells extracted from WT and IL-6−/− mice vaccinated with Lm or Lm/CpG at different time points. As expected, no IL-6 was detected in the supernatants collected from IL-6−/− mice. As shown by us, Lm/CpG vaccination induced early production of IL-6 in the WT mice (Fig. 2A) that decreased over time; the production of this cytokine was the greatest at week 4 in WT mice vaccinated with Lm alone, when the lesions developed. Following Lm/CpG vaccination, IL-10 was not detectable at week 2 (concomitant to the peak of IL-6) in WT mice, but the levels of this cytokine increased at weeks 4 and 13 (Fig. 2B). Interestingly, IL-10 values were always higher in IL-6−/− mice when compared with WT mice at weeks 4 and 13. We found no differences in TNF-α production between WT and IL-6−/− mice at week 2, although its secretion was elevated at weeks 4 and 13 in the IL-6−/− mice, probably because of the development of necrotic lesions (Fig. 2C). IFN-γ production was increased in Lm/CpG-vaccinated WT mice at week 2 post vaccination (Fig. 2D), and, as expected, in the Lm-vaccinated WT mice at week 4 post vaccination (owing to the onset of the immune response and lesion development in this group). Distinctly in the IL-6−/− mice, the level of IFN-γ remained low at weeks 4 and 13. Also as expected, IL-12 secretion was elevated in the Lm/CpG-vaccinated WT mice at the early time point (Fig. 2E), and at week 4 in the Lm-vaccinated WT mice. Conversely, IL-12 levels were never prominent in the IL-6−/− mice. MCP-1 displayed similar kinetics (Fig. 2F), with the WT mice releasing higher amounts than the IL-6−/− mice, especially at the earliest time point.
Fig. 2.
Comparative analysis of cytokine production by cells extracted from the dermis of either wild-type (WT) C57BL/6 or IL-6−/− mice at weeks 2, 4, and 13, following vaccination with 1 × 104 Leishmania major (Lm) alone or in combination with 50 µg of CpG DNA. Cells extracted from the dermis of 3 mice (6 ears) were pooled. After 24 h, supernatants were collected and analyzed by flow cytometry using the BD CBA Mouse Inflammation kit. Data show the average of 2 independent experiments.
Cytokine responses were also determined in the lymph nodes, with comparable results (Fig. S1; Supplementary data).2 Together, the decrease in proinflammatory cytokines and the increase in IL-10 (that in turn could favor uncontrolled parasite expansion) may explain the exacerbated pathology and lack of healing in the IL-6−/− mice after vaccination.
Lymphocyte numbers are reduced in ears and lymph nodes of uninfected IL-6−/− mice
Since IL-6 is considered a competence factor for the development of T cells (Uyttenhove et al. 1988), we aimed to determine whether IL-6−/− mice had a constitutive deficiency in lymphocyte numbers. Table 1 shows the mean number of CD3+ T cells per ear and in the draining submandibular lymph node collected from uninfected WT mice and IL-6−/− mice in the absence of infection. Upon comparison, it was evident that IL-6−/− mice had significantly reduced numbers of lymphocytes in the ears and lymph nodes. When we analyzed specific populations, we found that the frequency of CD4+ T cells was dramatically decreased in IL-6−/− mice (17.6-fold reduction), whereas the reduction in CD8+ T cells was not as striking (1.5-fold). We also determined the numbers of CD4+Foxp3+ Treg cells. We found that Treg cells were 17.4-fold lower in the IL-6−/− mice when compared with the WT mice. These results demonstrate that IL-6−/− mice are constitutively lymphopenic, and that some T cell populations appear to be more affected than others.
Table 1.
Cell numbers in the ears and submandibular lymph nodes of uninfected wild-type (WT) C57BL/6 mice and IL-6−/− mice (n = 3 mice, 6 samples).
| No. of cells | |||
|---|---|---|---|
| Cell type | WT mice | IL-6−/− mice | p |
| Lymph node CD3+ T cells | 1.4×106±2×105 | 7.0×105±9×104 | 0.0001 |
| Dermal CD3+ T cells | 8.6×103±2×103 | 1.1×103±5×102 | 0.0001 |
| Dermal CD4+ T cells | 5290±756 | 300±221 | 0.0001 |
| Dermal CD8+ T cells | 922±121 | 634±234 | 0.02 |
| Dermal CD4+ Foxp3+ Treg cells | 1322±344 | 84±12 | 0.0001 |
Note: Data are expressed as means ± SD, representative of 2 independent experiments.
Effector CD4+ T cells are decreased and Treg cells are increased following Lm/CpG DNA vaccination in IL-6−/− mice
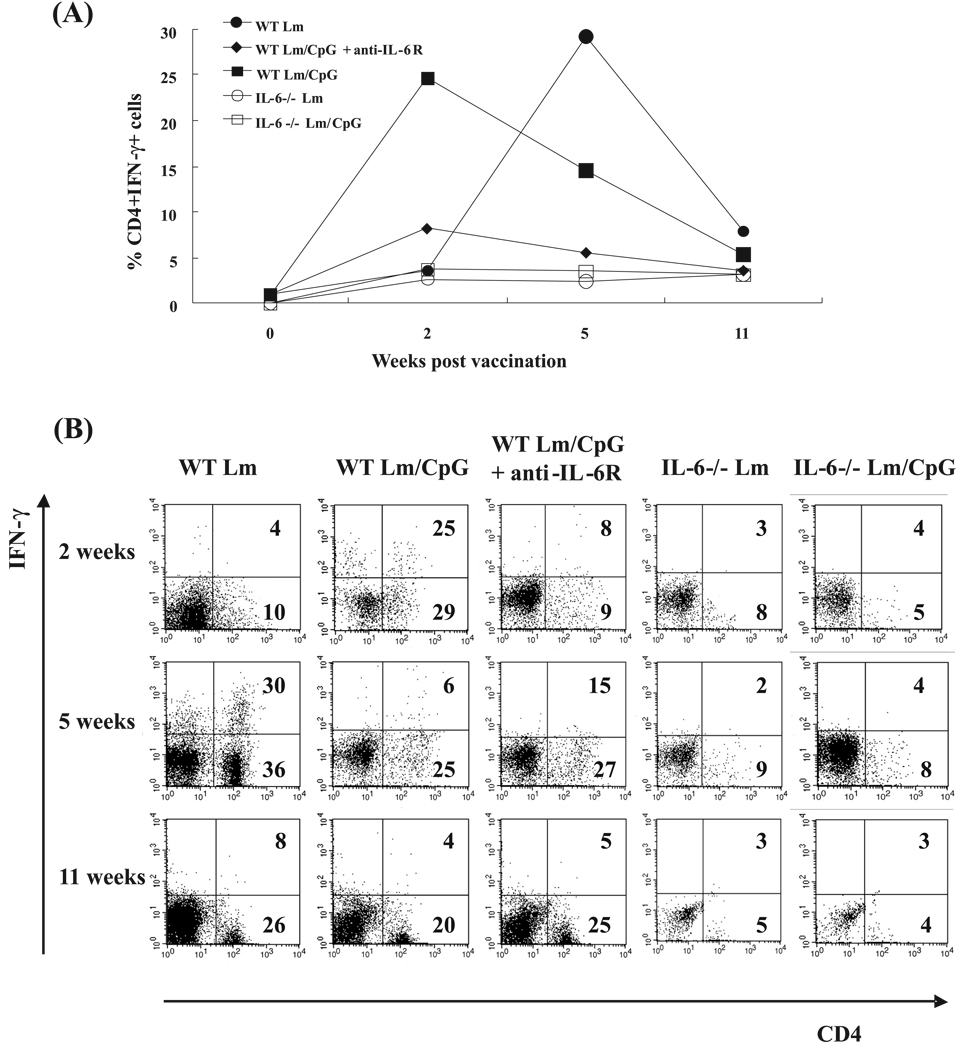
We investigated whether the constitutive lymphopenia of the IL-6−/− mice (apparently affecting some lymphocyte populations more than others) had an effect on the expected response to Lm/CpG vaccination. In this experiment, WT mice and IL-6−/− mice were vaccinated with Lm or Lm/CpG. A group of WT mice vaccinated with Lm/CpG and treated with anti-IL-6R antibody was included. In previous work, we showed that this treatment neutralized IL-6 production and abrogated protection against vaccinal lesions in Lm/CpG-vaccinated mice (Wu et al. 2006). Single cell suspensions were generated from the ears of vaccinated mice at weeks 0, 2, 5, and 11 post vaccination and analyzed by flow cytometry. To obtain enough cells to perform intracellular staining, cells from 3 mice (6 ears) had to be pooled. First, the frequency of CD4+ T cells expressing IFN-γ was determined (Fig. 3A). Representative dot plots including frequencies of CD4+ T cells and CD4+IFN-γ+ T cells are also shown (Fig. 3B). As expected, mice vaccinated with Lm/CpG had increased frequencies of CD4+ T cells (29%) and IFN-γ-expressing CD4+ T cells (25%) early (2 weeks) after vaccination. Also as expected, the peak in the effector response did not take place in the Lm-vaccinated animals until week 5 (36% CD4+ T cells, 30% of them expressing IFN-γ). IL-6−/− mice immunized with Lm alone or in combination with CpG DNA showed decreased frequencies of IFN-γ-expressing CD4+ T cells throughout the duration of the experiment (<5%); the overall frequencies of CD4+ T cells were diminished when compared with WT mice (<10%). Treatment of Lm/CpG-vaccinated WT mice with anti-IL-6R antibodies (that increases the frequency of regulatory Treg cells) decreased the ability of CD4+ T cells to express IFN-γ, although it did not affect the overall frequency of CD4+ T cells in the dermis.
Fig. 3.
(A) Frequencies of CD4+IFN-γ+ T cells in the inoculation site of wild-type C57BL/6 (WT) mice and IL-6−/− mice at weeks 0, 2, 5, and 11 following vaccination with 1 × 104 Leishmania major (Lm) alone (● and ○, respectively) or in combination with 50 µg of CpG DNA (■ and □, respectively). A group of WT mice vaccinated with Lm plus CpG DNA treated with anti-IL-6R antibodies prior to and after immunization was also included in the study (◆). (B) Flow cytometry dot plots representative of the experiment. The frequencies of CD4+ T cells and CD4+IFN-γ+ T cells are included in the figure at the bottom right and upper right corners, respectively. Antibody staining was performed on dermal cells pooled from 3 mice per time point (6 ears) following in vitro restimulation with Lm or Lm/CpG DNA. The experiment was repeated twice and the average frequencies are shown.
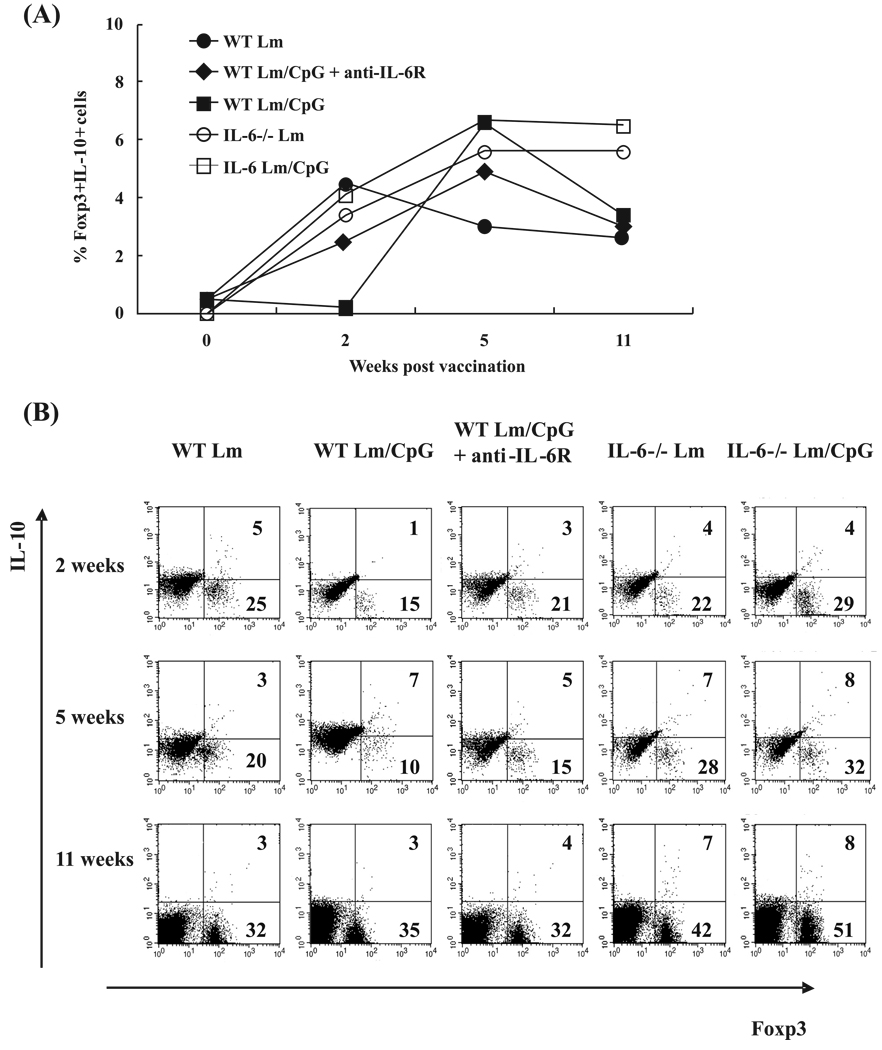
A different profile was found when the frequency of Foxp3+IL-10+ T cells was analyzed. At week 2, IL-10-producing Treg cell numbers were the highest in mice vaccinated with Lm alone as well as in IL-6−/− mice (4%–5%, Fig. 4A). Mice vaccinated with Lm/CpG showed the lowest frequency of IL-10-producing Treg cells (1%); the frequency increased in these mice when treated with anti-IL-6R (3%). Figure 4B shows representative flow cytometry dot plots and demonstrates that, in our system, IL-10 is mostly expressed in Foxp3+ cells (because Foxp3− cells do not express IL-10). At week 2, the percentage of CD4+Foxp3+ (shown in Fig. 4B at the bottom right corner) was slightly >20%, with the exception of that in the Lm/CpG-vaccinated animals, whose frequencies were decreased at this time point (15% coinciding with the onset of the effector immune response). At week 5, the frequency of double-positive cells increased in all experimental groups, with the exception of Lm-vaccinated and Lm/CpG-vaccinated mice treated with anti-IL-6R antibodies (again, concomitant with the onset of the effector immune response in those experimental groups). At week 11, although the percentage of IL-10-producing Treg cells remained constant in the WT mice, the frequency of Foxp3+ cells was much higher, indicating the establishment of a regulatory response. The frequencies of double-positive cells remained elevated in the IL-6−/− mice, supporting the hypothesis that the IL-6−/− mouse is biased towards anti-inflammatory responses and that IL-10-producing cells expand to a larger extent in these mice following live vaccination. This expansion appears to be associated with IL-6 production, since its neutralization increased the frequency of Foxp3+ Treg cells expressing IL-10.
Fig. 4.
(A) Frequencies of Foxp3+IL-10+ cells in the inoculation site of wild-type C57BL/6 (WT) mice and IL-6−/− mice at weeks 0, 2, 5, and 11 following vaccination with 1 × 104 Leishmania major (Lm) alone (● and ○, respectively) or in combination with 50 µg of CpG DNA (■ and □, respectively). A group of WT mice vaccinated with Lm plus CpG DNA treated with anti-IL-6R antibodies prior to and after immunization was also included in the study (◆). (B) Flow cytometry dot plots representative of the experiment. The frequencies of Foxp3+ cells and Foxp3+IL-10+ cells are included in the figure at the bottom right and upper right corners, respectively. Antibody staining was performed on dermal cells pooled from 3 mice per time point (6 ears) following in vitro restimulation with Lm or Lm/CpG DNA. The experiment was repeated twice and the average frequencies are shown.
Discussion
The use of knockout mice is instrumental to many studies. Thus, it is important to document phenotypical and functional changes that immune-deficient mice could present to properly interpret experimental results. We discovered that IL-6−/− mice are not protected by a live vaccine against cutaneous Lm containing CpG DNA. We have shown that lymphocyte numbers are constitutively reduced in IL-6−/− mice. Moreover, in our system, IL-6−/− mice appear to develop higher Treg cell frequencies for producing IL-10 and decreased γ responses, leading to a disequilibrium in which anti-inflammatory responses prevail to enhance the development of vaccinal lesions.
IL-6 is a multifunctional cytokine with regulatory effects on B-lymphocytes, hematopoiesis, and inflammation (Kopf et al. 1995; Mangan et al. 2004). Following Lm infection, IL-6−/− mice presented progressive disease that resulted in the necrotic destruction of the inoculation site. These results confirm the enhanced susceptibility of the IL-6−/− mice to microbial infections such as Listeria monocytogenes (Kopf et al. 1994; Ryffel 1996).
Our results prominently contrast with those obtained by other investigators. Moskowitz et al. (1997) reported that IL-6−/− mice controlled Lm infection and mounted strong Th1 responses. Similarly, Titus et al. (2001) could not find differences in susceptibility between WT BALB/c and IL-6−/− mice. More recently, Murray (2008) found that IL-6−/− mice show an enhanced control of Leishmania donovani infection. This inconsistency is hard to interpret, but could be explained by the disparity of the experimental systems. We employed IL-6−/− (C57BL/6 background) mice and inoculated L. major (Friedlin strain) intradermally at a dose of 1 × 104 parasites/ear. Moskowitz et al. (1997) used IL-6−/− (C57BL/6 background) mice and infected them with the L. major strain 173, subcutaneously in the footpad, at a dose of 5 × 105 parasites. Titus et al. (2001) employed IL-6−/− mice generated on the susceptible, Th2-prone, BALB/c mouse background to subcutaneously (footpad) inoculate the L. major strain LV39 (2 × 106 parasites). Finally, Murray et al. (2008) employed a different Leishmania species (the visceralizing L. donovani), a different inoculation route (intravenous), a high inoculum (1 × 107 parasites), and, most importantly, a different life-cycle phase (amastigotes instead of promastigotes). It has been well documented that the genetic background of T lymphocytes influences development of the T helper phenotype, resulting in either resistance (i.e., C57BL/6, Th1) or susceptibility (i.e., BALB/c, Th2) of different mouse strains to L. major (Sacks and Noben-Trauth 2002). Thus, immunological outcomes can be very different depending upon the genetic background of the mice. Moreover, the immune features associated with Leishmania infection vary widely depending on the parasite species (cutaneous versus visceral). Moreover, differences in the parasite strain, inoculation dose, and, especially, inoculation site (ear dermis versus subcutaneous footpad) may have had an impact on the intensity of T cell priming and on the initiation of adaptive immune responses (Uzonna et al. 2004; Tabbara et al. 2005). In fact, we have already reported the profound divergence on the kinetics and quantity of T cell responses between dermal and subcutaneous inoculation sites (Tabbara et al. 2005). Thus, it is possible that any of those factors could change the expected outcome of infection in the IL-6−/− mice; thus, the results obtained with each experimental system may not be extrapolated and must be examined on a case-by-case basis.
There is an emerging interest in studying the role of IL-6 in T cell development, since IL-6 is now considered to be responsible for Th17 cells generation (Harrington et al. 2006). Our data showed that IL-6−/− mice have decreased lymphocyte numbers, and that the CD4+ T cell population was the most affected. Vaccination with Lm/CpG induced an abnormal expansion of Treg cells in these mice and produced disequilibrium between effector CD4+ T lymphocytes and Treg cells, causing nonhealing lesions. The lymphopenic environment of the IL-6 mouse must be taken into consideration when employing this mouse in experimental models, since it could have important repercussions on phenotypic outcome and results interpretation.
IL-10 production has been linked to susceptibility to Leishmania (Murray et al. 2002). Thus, the increased dermal IL-10 production in the deficient mice could explain their susceptibility to Lm. We found no differences in IL-10 production by dendritic cells between IL-6−/− mice and WT animals. In our intradermal challenge/vaccination model, Treg cells are the most important source of IL-10 (Wu et al. 2006). We have found that Treg cells expand in IL-6−/− mice vaccinated with Lm/CpG. It remains to be elucidated whether this is a vaccine-associated effect. We cannot discard, however, that in the presence of insufficiently activated DCs, other T cells, such as Th2, which are also associated with progressive cutaneous Lm, are generated (Aseffa et al. 2002). The study of Th2 cytokines in IL-6−/− mice vaccinated with Lm/CpG will be considered in future studies.
Taken together, our data suggest that the immune response of the IL-6−/− mice has been affected by the genetic manipulation. These defects must be reported to make scientists aware of functional defects in immune-deficient mice strains. It also confirms that experiments performed in gene knockout animals must be validated in intact mice using other methods, such as cytokine or cell population antibody neutralizations.
Supplementary Material
Acknowledgements
We thank M. Hinchman and Dr. J.M. Alunda Rodriguez for the editorial review of the manuscript. This work was supported by the National Institute of Allergy and Infectious Diseases, NIH Research Grant No. R21AI61379.
Footnotes
Supplementary data for this article are available on the journal Web site (http://cjm.nrc.ca) or may be purchased from the Depository of Unpublished Data, Document Delivery, CISTI, National Research Council Canada, Building M-55, 1200 Montreal Road, Ottawa, ON K1A 0R6, Canada. DUD 3961. For more information on obtaining material refer to http://cisti-icist.nrc-cnrc.gc.ca/eng/ibp/cisti/collection/unpublished-data.html.
References
- Aseffa A, Gumy A, Launois P, MacDonald HR, Louis JA, Tacchini-Cottier F. The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+ T cells. J. Immunol. 2002;169(6):3232–3241. doi: 10.4049/jimmunol.169.6.3232. PMID:12218142. [DOI] [PubMed] [Google Scholar]
- Desjeux P. Leishmaniasis: current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 2004;27(5):305–318. doi: 10.1016/j.cimid.2004.03.004. doi:10.1016/j.cimid.2004.03.004. PMID:15225981. [DOI] [PubMed] [Google Scholar]
- Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006;8 Suppl 2:S3. doi: 10.1186/ar1917. doi:10.1186/ar1917. PMID:16899107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr. Opin. Immunol. 2006;18(3):349–356. doi: 10.1016/j.coi.2006.03.017. doi:10.1016/j.coi.2006.03.017. PMID:16616472. [DOI] [PubMed] [Google Scholar]
- Jones SA, Richards PJ, Scheller J, Rose-John S. IL-6 transsignaling: the in vivo consequences. J. Interferon Cytokine Res. 2005;25(5):241–253. doi: 10.1089/jir.2005.25.241. doi:10.1089/jir.2005.25.241. PMID:15871661. [DOI] [PubMed] [Google Scholar]
- Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368(6469):339–342. doi: 10.1038/368339a0. doi:10.1038/368339a0. PMID:8127368. [DOI] [PubMed] [Google Scholar]
- Kopf M, Ramsay A, Brombacher F, Baumann H, Freer G, Galanos C, et al. Pleiotropic defects of IL-6-deficient mice including early hematopoiesis, T and B cell function, and acute phase responses. Ann. N. Y. Acad. Sci. 1995;762:308–318. doi: 10.1111/j.1749-6632.1995.tb32335.x. PMID:7545368. [DOI] [PubMed] [Google Scholar]
- Mangan JK, Rane SG, Kang AD, Amanullah A, Wong BC, Reddy EP. Mechanisms associated with IL-6-induced up-regulation of Jak3 and its role in monocytic differentiation. Blood. 2004;103(11):4093–4101. doi: 10.1182/blood-2003-06-2165. doi:10.1182/blood-2003-06-2165. PMID:14976041. [DOI] [PubMed] [Google Scholar]
- Mendez S, Tabbara K, Belkaid Y, Bertholet S, Verthelyi D, Klinman D, et al. Coinjection with CpG-containing immunostimulatory oligodeoxynucleotides reduces the pathogenicity of a live vaccine against cutaneous leishmaniasis but maintains its potency and durability. Infect. Immun. 2003;71(9):5121–5129. doi: 10.1128/IAI.71.9.5121-5129.2003. doi:10.1128/IAI.71.9.5121-5129.2003. PMID:12933855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modabber F. Vaccines against leishmaniasis. Ann. Trop. Med. Parasitol. 1995;89 Suppl 1:83–88. doi: 10.1080/00034983.1995.11813017. PMID:8745930. [DOI] [PubMed] [Google Scholar]
- Moskowitz NH, Brown DR, Reiner SL. Efficient immunity against Leishmania major in the absence of interleukin-6. Infect. Immun. 1997;65(6):2448–2450. doi: 10.1128/iai.65.6.2448-2450.1997. PMID:9169788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray HW. Accelerated control of visceral Leishmania donovani infection in interleukin-6-deficient mice. Infect. Immun. 2008;76(9):4088–4091. doi: 10.1128/IAI.00490-08. doi:10.1128/IAI.00490-08. PMID:18573898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray HW, Lu CM, Mauze S, Freeman S, Moreira AL, Kaplan G, Coffman RL. Interleukin-10 (IL-10) in experimental visceral leishmaniasis and IL-10 receptor blockade as immunotherapy. Infect. Immun. 2002;70(11):6284–6293. doi: 10.1128/IAI.70.11.6284-6293.2002. doi:10.1128/IAI.70.11.6284-6293.2002. PMID:12379707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033–1036. doi: 10.1126/science.1078231. doi:10.1126/science.1078231. PMID:12532024. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Regulation of the T cell response. Clin. Exp. Allergy. 2006;36(11):1357–1366. doi: 10.1111/j.1365-2222.2006.02606.x. doi:10.1111/j.1365-2222.2006. 02606.x. PMID:17083345. [DOI] [PubMed] [Google Scholar]
- Ruef C, Budde K, Lacy J, Northemann W, Baumann M, Sterzel RB, Coleman DL. Interleukin 6 is an autocrine growth factor for mesangial cells. Kidney Int. 1990;38(2):249–257. doi: 10.1038/ki.1990.193. doi:10.1038/ki.1990.193. PMID:2402117. [DOI] [PubMed] [Google Scholar]
- Ryffel B. Gene knockout mice as investigative tools in pathophysiology. Int. J. Exp. Pathol. 1996;77(4):125–141. doi: 10.1046/j.1365-2613.1996.d01-215.x. doi:10.1046/j.1365-2613.1996.d01-215.x. PMID:8943731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2002;2(11):845–858. doi: 10.1038/nri933. doi:10.1038/nri933. PMID:12415308. [DOI] [PubMed] [Google Scholar]
- Spath GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp. Parasitol. 2001;99(2):97–103. doi: 10.1006/expr.2001.4656. doi:10.1006/expr.2001.4656. PMID:11748963. [DOI] [PubMed] [Google Scholar]
- Tabbara KS, Peters NC, Afrin F, Mendez S, Bertholet S, Belkaid Y, Sacks DL. Conditions influencing the efficacy of vaccination with live organisms against Leishmania major infection. Infect. Immun. 2005;73(8):4714–4722. doi: 10.1128/IAI.73.8.4714-4722.2005. doi:10.1128/IAI.73.8.4714-4722.2005. PMID:16040984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus RG, DeKrey GK, Morris RV, Soares MB. Interleukin-6 deficiency influences cytokine expression in susceptible BALB mice infected with Leishmania major but does not alter the outcome of disease. Infect. Immun. 2001;69(8):5189–5192. doi: 10.1128/IAI.69.8.5189-5192.2001. doi:10.1128/IAI.69.8.5189-5192.2001. PMID:11447205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttenhove C, Coulie PG, Van Snick J. T cell growth and differentiation induced by interleukin-HP1/IL-6, the murine hybridoma/plasmacytoma growth factor. J. Exp. Med. 1988;167(4):1417–1427. doi: 10.1084/jem.167.4.1417. doi:10.1084/jem.167.4.1417. PMID:2965738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzonna JE, Spath GF, Beverley SM, Scott P. Vaccination with phosphoglycan-deficient Leishmania major protects highly susceptible mice from virulent challenge without inducing a strong Th1 response. J. Immunol. 2004;172(6):3793–3797. doi: 10.4049/jimmunol.172.6.3793. PMID:15004184. [DOI] [PubMed] [Google Scholar]
- Wu W, Weigand L, Belkaid Y, Mendez S. Immunomodulatory effects associated with a live vaccine against Leishmania major containing CpG oligodeoxynucleotides. Eur. J. Immunol. 2006;36(12):3238–3247. doi: 10.1002/eji.200636472. doi:10.1002/eji.200636472. PMID:17109471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.