Abstract
Background
State AIDS Drug Assistance Programs (ADAPs) provide antiretroviral medications to patients with no access to medications. Resource constraints limit many ADAPs' ability to meet demand for services.
Objective
To determine ADAP eligibility criteria that minimize morbidity and mortality and contain costs.
Methods
We used Discrete Event Simulation to model the progression of HIV-infected patients and track utilization of an ADAP. Outcomes included five-year mortality and incidence of first opportunistic infection or death, and time to starting ART. We compared expected outcomes for two policies: 1) first-come, first-served (FCFS) eligibility for all with CD4 count ≤350/μl (current standard), and 2) CD4 count prioritized eligibility for those with CD4 counts below a defined threshold.
Results
In the base case, prioritizing patients with CD4 counts ≤250/μl led to lower five-year mortality than FCFS eligibility [2.77 vs. 3.27 deaths/1,000 person months], and to a lower incidence of first opportunistic infection or death [5.55 vs. 6.98 events/1,000 person months]. CD4-based eligibility reduced the time to starting ART for patients with CD4 counts ≤200/μl. In sensitivity analyses, CD4-based eligibility consistently led to lower morbidity and mortality than FCFS eligibility.
Conclusions
When resources are limited, programs that provide ART can improve outcomes by prioritizing patients with low CD4 counts.
Keywords: AIDS, Anti-HIV Agents*/therapeutic use, ADAP, Government Programs, Resource Allocation*, CD4 Lymphocyte Count
INTRODUCTION
State AIDS Drug Assistance Programs (ADAPs) provide antiretroviral therapy (ART) to patients with no other access to medications. ADAPs provide ART to approximately one-quarter of all patients enrolled in HIV/AIDS care in the United States (1). Approximately two-thirds of ADAP patients are people of color and three-quarters are uninsured (1). ADAPs provide ART to U.S. patients who have historically experienced decreased access to care (2–5).
ADAPs are funded by the Ryan White Program and state discretionary spending, and they utilize negotiated rebates from pharmaceutical companies (1). Because none of these resources is directly tied to the number of people seeking care, ADAPs balance program capacity with demand (1, 2). Centers for Disease Control and Prevention (CDC) recommendations for routine HIV screening, and growing demand for treatment of Hepatitis C virus co-infection, will likely increase demand for ADAP (6–8). If the current fiscal crisis forces ADAPs to limit access, how should ADAPs prioritize patients for ART?
ADAPs employ a variety of strategies for controlling costs, including restricting drug formularies and lowering income eligibility thresholds (2, 9–11). While some states utilize clinical criteria for determining program eligibility, none has used stage of disease to prioritize patients when resources are insufficient to treat all meeting current U.S. guidelines for starting ART (9). When resources are inadequate to treat all eligible patients, most programs establish a waitlist for care on a first-come, first-served (FCFS) basis (1, 2). Prior work demonstrates that because FCFS eligibility does not consider stage of disease, patients with low CD4 counts may wait for ART while those with less urgent need gain immediate access to treatment (12). The FCFS approach, therefore, may not maximize the effectiveness of limited ADAP resources. We used Discrete Event Simulation (DES), a mathematical modeling method, to estimate morbidity and mortality in a cohort of patients applying to ADAP. The analysis builds on prior findings to provide guidance to policy makers seeking to improve ADAP outcomes.
METHODS
Analytic Overview
We compared clinical outcomes and program utilization from a cohort of patients applying to an ADAP using FCFS eligibility to one prioritizing patients based on CD4 count. We used DES to track program capacity, and simulate the clinical progression of patients on and off ART. DES is a simulation method well-suited to studying competition for limited resources (13, 14). It has been used to simulate medical prioritization decisions such as the allocation of cadaveric livers for transplant (15). A DES model represents the operation of a system as a chronological sequence of events, each marking a discrete change of state in the system (13). For example, an ADAP DES model event was a new patient applying to the program. The event changed the state of the ADAP - the number of patients enrolled increased by one and the number of program slots available decreased by one. By continuously tracking enrollment, the model was able to determine when capacity existed to accept new patients.
Patients enrolled in ADAP received ART as recommended by U.S. clinical guidelines (when CD4≤ 350/μl) (16). Those ineligible for ADAP, or on the waitlist did not. Clinical outcomes included the five-year incidence of first opportunistic infection or death and all-cause mortality. Program utilization outcomes included the time to starting ART after applying to ADAP, the mean number enrolled in the program, the mean number of patient days spent on the waitlist each month, the percentage of total days that the program administered a waitlist, and the mean percentage of program capacity utilized on a daily basis.
We first simulated an ADAP with capacity as needed to treat all patients with CD4 count ≤350/μl. We then simulated a fixed capacity program facing a 10% increase in the arrival rate of new applicants and compared outcomes under two ADAP eligibility policies: 1) FCFS eligibility for all with CD4 count ≤350/μl, and 2) CD4-prioritized eligibility for all with a CD4 count below a pre-defined threshold. To identify the optimal CD4-based ADAP eligibility threshold, we repeated the simulation, systematically altering the pre-defined CD4 count and identifying the threshold value that minimized five-year mortality.
Model Overview
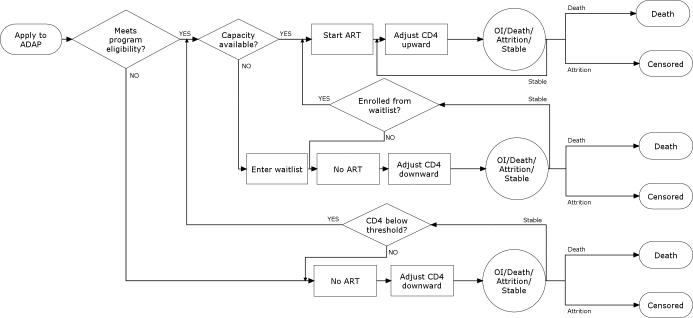
When a new patient applied to ADAP, the model checked that the patient met the ADAP eligibility criterion. If the patient was eligible for the program, the model next checked that program slots were available. If slots were available, the person enrolled in ADAP and started ART (Figure 1).
FIGURE 1. Model schematic.
A flow chart depicts the sequence of events in the Discrete Event Simulation. Diamond shapes represent decisions for program managers or policy makers such as program eligibility policy or total capacity. Rectangles represent model processes that simulate HIV disease progression. Circles represent probabilistic risk of contracting an opportunistic infection, dying, leaving the program without dying (program attrition), or remaining stable. See methods for further details.
The patient then progressed through a simulation of HIV disease while taking ART. We modeled ART efficacy as the probability of achieving an HIV RNA<400 copies/ml at 24 weeks. Each month, patients with suppressed HIV RNA faced a probability of having virologic breakthrough, triggering a change in ART to the next line of therapy (16, 17). The individual's CD4 count was adjusted upward if HIV RNA was <400 copies/ml or downward if HIV RNA was >400 copies/ml (18–20). Each patient then faced a monthly risk of developing an opportunistic infection (OI) as well as both HIV-related and non-HIV mortality. Incidence of OIs and HIV-related death were a function of the patient's CD4 count and infection type (20, 21). Non-HIV death was a function of age and sex (22). Patients surviving the month faced a probability of leaving the program due to a cause other than death such as moving, loosing ADAP eligibility, or being lost to follow-up (program attrition). When an ADAP patient died or left the program, the model enrolled the first patient on the waitlist if there were people waiting, or else increased the number of available treatment slots by one.
If the patient was eligible for ADAP, but no program slots were available, the patient entered the waitlist for care and began the simulation of HIV infection without ART. As capacity became available, the patient moved toward the top of the waitlist.
If the patient had a CD4 count above the ADAP eligibility threshold, that person entered the simulation of HIV infection without ART. When the CD4 count fell below the ADAP eligibility threshold, the patient applied to ADAP.
We simulated 5 years of follow-up with the ADAP DES model. We conducted 10 replications of each simulation and used the mean outcome to generate estimates of the incidences of death and first opportunistic infection or death.
Model Inputs
Cohort characteristics
The administrative dataset of the Massachusetts ADAP informed the arrival rate and characteristics of simulated ADAP applicants (Table 1) (23). The dataset included all patients who applied to the Massachusetts ADAP in 2004 and who had at least one CD4 count recorded within 6 months of the date of application (N=1,243). To determine the arrival date of the next applicant, the model randomly sampled from the distribution of the intervals between patient arrivals (log normal distribution mean= 0.44 days SD=0.92 days).
Table 1.
Select Input Data Values for Analysis of ADAP Eligibility
| Variable | Baseline value | Range evaluated | Source |
|---|---|---|---|
| ADAP Program characteristics | |||
| Mean number of arrivals per day (SD) | 2.34 (1.60) | 2.34–2.94 | (23) |
| Program capacity | 3,440 | 3,300–3,600 | (23) |
| Monthly probability of program attrition | (23) | ||
| CD4 > 100/μl | 0.017 | 0.0085–0.034 | |
| CD4<=100/μl | 0.000 | 0.000–0.034 | |
| ADAP applicant pool | |||
| Mean age (SD) | 41.5 (9.0) | 35–55 | (23) |
| % Male | 77.9 | 50–90 | (23) |
| Mean CD4 count/μl (SD) | 181 (106) | 100–300 | (23) |
| Distribution HIV RNA copies/ml (%) | (41) | ||
| <500 | 7.71 | 0–100 | |
| 501–3,000 | 16.33 | 0–100 | |
| 3001–10,000 | 25.21 | 0–100 | |
| 10,001–30,000 | 25.02 | 0–100 | |
| 30,001–100,000 | 25.73 | 0–100 | |
| HIV Disease Progression | |||
| Mean CD4 loss/month, no ART (SD) | (21) | ||
| HIV RNA >30,000/ml | 6.38 (5.06) | 3.19–12.76 | |
| HIV RNA 10,000–30,000/ml | 5.40 (4.33) | 2.70–10.80 | |
| HIV RNA 3,001–10,000/ml | 4.60 (3.76) | 2.30–9.20 | |
| HIV RNA 501–3,000/ml | 3.73 (3.83) | 1.87–7.46 | |
| HIV RNA <500/ml | 3.03 (2.72) | 1.52–6.06 |
| CD4 cells/ μl | PCP | MAC | TOXO | CMV | FUNG | OTHER OI | Source |
|---|---|---|---|---|---|---|---|
| Incidence of OI/1000 person months (base case values, varied as function of OI history and ART status) | (21) | ||||||
| >500 | 0.31–4.61 | 0.04–5.56 | 0.02–19.16 | 0.04–51.29 | 0.07–4.27 | 0.35–0.47 | |
| 351–500 | 0.64–4.61 | 0.04–5.56 | 0.07–19.16 | 0.10–51.29 | 0.21–4.27 | 0.65–0.87 | |
| 201–350 | 2.80–4.61 | 0.17–5.56 | 0.32–19.16 | 0.44–51.29 | 0.22–4.27 | 1.68–2.24 | |
| 101–200 | 3.46–9.65 | 0.76–5.56 | 0.50–19.16 | 1.61–51.29 | 1.01–4.27 | 5.38–7.19 | |
| 51–100 | 3.46–31.49 | 2.82–5.56 | 1.05–19.16 | 3.93–51.29 | 3.20–5.93 | 18.62–24.91 | |
| <50 | 3.46–37.70 | 4.16–12.28 | 2.03–19.16 | 14.03–51.29 | 3.20–11.29 | 30.00–40.20 | |
| Mortality rate given OI/1000 person months (base case values, varied as function of OI history and ART status) | (21) | ||||||
| >200 | 36.20–48.56 | 93.38–126.56 | 105.87–143.81 | 61.06–82.27 | 32.36–43.38 | 43.04–57.81 | |
| ≤200 | 43.60–58.56 | 113.15–153.91 | 128.46–175.23 | 73.73–99.57 | 38.95–52.28 | 51.88–69.79 | |
| Efficacy of OI prophylaxis (percent reduction in incidence of specific OI while taking prophylaxis) | |||||||
| 0.65–0.98a | 0.63–0.72b | 0.65c | (42–45) | ||||
| Efficacy of Antiretroviral Therapy | ||||||
|---|---|---|---|---|---|---|
| Regimen number/medications | % with HIV RNA<400 copies at 24 weeks | Range evaluated | Rate of virologic failure/1000 person months | Mean increase in CD4 at 12 months (cells/μl) | Range evaluated | Source |
| 1.TDF/FTC + EFV | 86 | 43–96 | 11.8 | 190 | 95–380 | (27, 32) |
| 2. ATV/r + TDF/FTC | 77 | 39–88 | 9.6 | 110 | 55–220 | (29, 30) |
| 3. LPV/r + TDF/FTC | 63 | 32–76 | 15.2 | 121 | 61–242 | (26, 29, 30) |
| 4. RAL + OBR | 71 | 36–82 | 13.1 | 102 | 51–204 | (28) |
| 5. 50% ENF + OBR; | 38 | 19–56 | 22.1 | 117 | 59–234 | (31) |
| 50% MVC + OBR +/− ENF | ||||||
SD = standard deviation; PCP = Pneumocystis carinii pneumonia; MAC = Mycobacterium avium complex; TOXO = Toxoplasmosis; CMV = Cytomegalovirus; FUNG = disseminated fungal disease; OTHER OI = other opportunistic infections, including conditions such as bacterial infection, lymphoma, and Kaposi's sarcoma; OI = opportunistic infection; TDF = tenofovir; FTC = emtricitabine; EFV = efavirenz; ATV/r = ritonavir-boosted atazanavir; LPV/r = ritonavir-boosted lopinavir; RAL = raltegravir; OBR = optimized background regimen; ENF = enfuvirtide; MVC = maraviroc
efficacies of Bactrim, Dapsone, and Pentamidine
efficacies of Azithromycin and Clarithromycin
Efficacies of Bactrim and Atovaquone
To simulate the demographic and clinical characteristics of applicants, we limited the dataset to patients who had a current or nadir CD4 count <350/μl (N=577). Because the Massachusetts ADAP provides an open formulary including non-ART medications, patients with high CD4 counts apply. To increase the generalizability of results, we used the demographic characteristics of only those applicants who would be eligible for every state's ADAP (those with CD4<350). The model randomly sampled from the list of first-time applicants with CD4≤350/μl to simulate a cohort of patients applying to ADAP.
Disease progression parameters
Monthly probabilities of clinical events were from the Cost Effectiveness of Preventing AIDS Complications (CEPAC) model and based on data from the Multicenter AIDS cohort study (MACS) (21, 24, 25). Incidences of opportunistic infections were a function of the patient's CD4 count and prior history of having had an opportunistic infection (20, 21). The rate of decline of CD4 count with unsuppressed HIV RNA was stratified by the patient's HIV RNA level (20, 21).
ART efficacy parameters
The probabilities of achieving an HIV RNA<400 copies/ml for each regimen and of subsequent virologic failure, as well as regimen specific rates of CD4 rise were derived from clinical trials and observational cohorts (Table 1) (26–32). We utilized data from intention to treat analyses of clinical trials for each simulated regimen to capture underlying rates of adherence and viral resistance in the study populations.
Analyses
To estimate the morbidity and mortality associated with various ADAP eligibility policies, we conducted simulations under a variety of scenarios.
Base case scenario - 10% increase in the arrival rate of new patients
We first used the model to simulate outcomes for a cohort applying to an ADAP with capacity as needed to treat all patients whose CD4 count met current U.S. guidelines for starting ART (CD4 count ≤350/μl)(16). Next, we simulated a fixed capacity program that faced a 10% increase in new applicants and used a FCFS ADAP eligibility policy for all patients meeting clinical guidelines for starting ART (CD4 count ≤350/μl). The model began with no patients enrolled in ADAP. Enrollment began and rose rapidly until it reached a steady state plateau. At steady state, we capped program capacity and increased the new patient arrival rate by 10%. All patients with CD4 counts ≤350/μl were eligible for ADAP. When capacity was insufficient to treat all eligible patients, the ADAP started a wait-list on a FCFS basis. The model simulated 5 years of program outcomes under these conditions.
We next simulated outcomes for an identical cohort applying to a program that utilized CD4-prioritized eligibility. We began with a CD4 eligibility threshold of 300/μl and repeated the analysis, systematically altering the ADAP CD4 eligibility threshold to ≤250/μl, ≤200/μl, ≤150/μl, and ≤100/μl. We defined the optimal CD4-based ADAP eligibility threshold as the value that minimized the five-year incidence of mortality. We then compared clinical outcomes and program utilization for the FCFS approach and the CD4-based approach using the optimal CD4 eligibility threshold.
Sensitivity analyses on the rate of patient arrival and stage of disease
To investigate the impact of increased demand on outcomes, we repeated the analysis described in the base case scenario, but increased the rate of arrival of new applicants by 25%. To simulate a population with more advanced HIV infection, we reduced the mean baseline CD4 count from 181/μl to 150/μl. To simulate a population with less advanced HIV infection, we increased the mean CD4 count to 250/μl.
Additional Sensitivity Analyses
We performed a series of additional sensitivity analyses on parameters that might be expected to affect outcomes including: mean age, percent male, baseline HIV RNA, program attrition rate, the number of available lines of ART, the availability of salvage regimens that included darunavir and etravirine, ART efficacy, the rate of CD4 rise with suppressed HIV RNA (Table 1), and the incidence of opportunistic infections. We systematically varied the value of each parameter and observed outcomes associated with the minimum and maximum plausible values. In addition, to simulate disease progression rates in a cohort with different demographics than the MACS, we repeated the base case scenario using data from using the Women's Interagency HIV Study (WIHS) as the source informing HIV natural history parameters (33).
RESULTS
Base case scenario
Clinical Outcomes
An ADAP with capacity as needed to treat all patients with CD4 counts ≤350/μl led to a five-year mortality rate of 2.50 deaths/1,000 person months and an incidence of first OI or death of 4.56/1,000 person months (Table 2). With fixed capacity and a 10% increased arrival rate, an eligibility policy that prioritized patients with current or nadir CD4 count ≤250/μl had substantially lower mortality than a program that served patients on a FCFS basis [2.77 deaths/1,000 person months vs. 3.27/1,000 person months. In addition, the CD4-based approach led to a lower five-year incidence of first OI or death [5.55/1,000 person months vs. 6.98/1,000 person months].
Table 2.
Base Case Results and Selected Sensitivity Analyses
| Deaths/1000 person months | First OI or Death/1000 person months | Median months wait to ART (IQR) CD4 at presentation (cells/μl) | Average Number enrolled | Mean patient days on waitlist/month | % capacity held in reserve | |||
|---|---|---|---|---|---|---|---|---|
| <100 | 101–200 | 201–350 | ||||||
| Base case scenario: Patient arrival increased 10% | ||||||||
| Unlimited capacity | 2.50 | 4.56 | 0 (0–0) | 0 (0–0) | 0 (0–0) | 3,571 | - | - |
| Capacity cappeda | ||||||||
| FCFS CD4 ≤350/μl* | 3.27 | 6.98 | 3 (1–3) | 3 (2–3) | 3 (2–3) | 3,373 | 4,749 | 1.9 |
| Prioritize CD4≤250/μl | 2.77 | 5.55 | 0 (0–1) | 0 (0–1) | 4 (0–12) | 3,349 | 735 | 2.6 |
| Scenario 2: Sensitivity analysis on the rate of patient arrival - patient arrival increased 25% | ||||||||
| Unlimited capacity. | 2.46 | 4.60 | 0 (0–0) | 0 (0–0) | 0 (0–0) | 3,883 | - | - |
| Capacity capped | ||||||||
| FCFS CD4≤350/μl | 4.12 | 9.43 | 6 (3–7) | 6 (3–7) | 6 (3–7) | 3,376 | 11,039 | 1.9 |
| Prioritize CD4≤200/μl | 3.04 | 6.46 | 0 (0–1) | 0 (0–1) | 15 (9–23) | 3,326 | 1,314 | 3.3 |
| Scenario 3: Sensitivity analysis on CD4 count - CD4 count = 150/μl; Arrival increased 10% | ||||||||
| Unlimited capacity | 2.80 | 5.17 | 0 (0–0) | 0 (0–0) | 0 (0–0) | 3,550 | - | - |
| Capacity capped | ||||||||
| FCFS CD4≤350/μl | 3.70 | 8.01 | 3 (2–4) | 3 (2–4) | 3 (2–4) | 3,321 | 4,697 | 2.0 |
| Prioritize CD4≤200/μl | 3.13 | 6.42 | 0 (0–1) | 0 (0–1) | 9 (5–15) | 3,293 | 854 | 2.8 |
| Scenario 4: Sensitivity analysis on CD4 count - CD4 count = 250/μl; Arrival increased 10% | ||||||||
| Unlimited capacity | 2.14 | 4.02 | 0 (0–0) | 0 (0–0) | 0 (0–0) | 3,367 | - | - |
| Capacity capped | ||||||||
| FCFS CD4≤350/μl | 2.60 | 5.51 | 2 (1–3) | 2 (1–3) | 2 (1–3) | 3,188 | 3,766 | 2.0 |
| Prioritize CD4≤300/μl | 2.42 | 4.82 | 0 (0–0) | 0 (0–0) | 0 (0–6) | 3,142 | 528 | 3.4 |
Capacity was fixed at the level it reached at the end of the simulation warm-up period. During the warm-up, patients arrived to and exited from ADAP at rates observed in the Massachusetts ADAP dataset. Program enrollment increased steeply from 0 patients until it reached a steady state plateau (base case = 3440 patients). When the program reached a steady state of enrollment, capacity was capped and the arrival rate of new patients increased by a defined percentage (10%, 20%, etc.). FCFS = first-come first-served
all CD4 counts are cells/μl.
OI: Opportunistic infection; IQR: Inter-quartile range
Impact of eligibility policies on time to starting ART
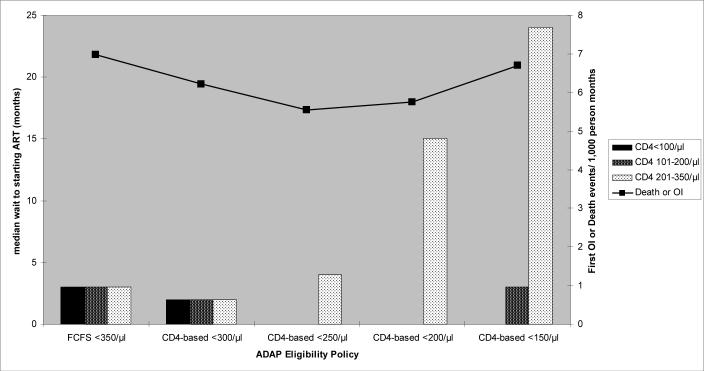
The CD4-based eligibility policy (≤250/μl) minimized the number of months that patients with low CD4 counts waited for ART, but increased the wait time for patients who applied to the program when their CD4 count was 201–350/μl. The FCFS approach resulted in an equal distribution of wait-times [median wait-time 3 months (IQR 1–3 months)] for patients with CD4 counts <100/μl, 101–200/μl and 201–350/μl (Figure 2). The CD4-based approach resulted in 0 months of waiting for patients with CD4 counts ≤100/μl and 101–200/μl but a 4 month wait (IQR 0–12) for patients with CD4 counts 201–350/μl.
FIGURE 2. Median time to starting ART stratified by CD4 count.
A bar and line graph illustrates the median wait-time to starting ART for each CD4 count stratum, as well as the associated morbidity and mortality for a cohort of ADAP applicants with mean CD4=181/μl (base case scenario). The horizontal axis is denominated in terms of the specific ADAP eligibility policy simulated. The left vertical axis is denominated in terms of the median wait-time to starting ART. Bars are graphed on the left vertical axis. Each bar represents a specific CD4 count stratum. The line is graphed against the right vertical axis, which is denominated in terms of the incidence of opportunistic events or death/1,000 person months observed for each ADAP eligibility strategy.
CD4-eligibility thresholds above the optimal value (250/μl) inadequately controlled the number of patients meeting the ADAP eligibility criterion, increasing the time to starting ART for patients with low CD4 counts (Figure 2). CD4 thresholds below the optimal cutoff reduced the number of patients eligible for ADAP too much, such that the program maintained too many treatment slots in reserve. As a result, patients with CD4 counts of 201–350/μl experienced increased wait-times, with no associated decrease in wait-times for patients with lower CD4 counts.
Impact of eligibility policies on wait-lists
An ADAP utilizing CD4-based eligibility maintained a wait-list on 34% of program days with a mean of 735 patient days spent on the waitlist each month. An ADAP using FCFS eligibility maintained a waitlist on 96% of program days with a mean of 4,749 patient days spent on the waitlist each month (Table 2). To minimize the need for a waitlist and preserve adequate capacity to treat patients with lower CD4 counts, the CD4-based approach required that ADAP enroll fewer than the maximum possible number of patients (3,349 vs. 3,373), maintaining an average of 2.6% of treatment slots in reserve at any time.
Scenario 2 - Sensitivity analysis on rate of new patient arrival
When the patient arrival rate increased by 25%, CD4 prioritized eligibility again led to lower mortality compared to FCFS eligibility [3.04 deaths/1,000 person months vs. 4.12 deaths/1,000 person months], and to a lower incidence of first OI or death [6.46/1,000 person months vs. 9.43/1,000 person months]. With this increased demand, the optimal CD4 eligibility threshold decreased to ≤200/μl. With CD4-based eligibility, the median time to starting ART for patients with CD4 counts ≤200/μl was 0 months (IQR 0–1), compared to 6 months (IQR 3–7) with FCFS eligibility. CD4-based eligibility led to ADAP maintaining a waitlist for care on 37% of program days, with a mean of 1,314 patient days spent on the waitlist each month. The FCFS approach led to ADAP maintaining a waitlist on 99% of program days with a mean of 11,039 patient days spent on the waitlist each month. With this higher level of demand, CD4-based eligibility required that ADAP maintain 3.3% of total slots in reserve for applicants who may arrive with lower CD4 counts (Table 2).
Scenario 3 - Sensitivity analysis on CD4 count – more advanced disease
When the mean CD4 count of the applicant pool was reduced to 150/μl, CD4-prioritized eligibility again led to lower mortality than FCFS eligibility [3.13 deaths/1,000 person months vs. 3.70 deaths/1,000 person months] and to a lower incidence of first OI or death [6.42/1,000 person months vs. 8.01/1,000 person months]. With this cohort with more advanced disease, the optimal CD4 eligibility threshold decreased to ≤200/μl. The median time to starting ART for patients with CD4 count ≤200/μl remained 0 months (IQR 0–1), compared to 3 months (IQR 2–4) for FCFS eligibility. CD4-based eligibility resulted in ADAP maintaining a waitlist for care on 38% of program days with a mean of 854 patient days spent on the waitlist each month. FCFS eligibility resulted in ADAP maintaining a waitlist on 93% of program days with a mean of 4,697 patient days spent on the waitlist each month. CD4-based eligibility, with these cohort characteristics, required that ADAP maintain 2.8% of program slots in reserve (Table 2).
Scenario 4 - Sensitivity analysis on CD4 count – less advanced disease
When the mean CD4 count of the applicant pool was increased to 250/μl, CD4-prioritized eligibility again resulted in lower mortality [2.42 deaths/1,000 patient months vs. 2.60 deaths/1,000 patient months], and a lower incidence of first opportunistic infection or death (4.82/1,000 person months vs. 5.51/1,000 person months] compared with FCFS eligibility. The optimal CD4 threshold value increased, however, to ≤300/μl. The median time to starting ART for patients with CD4 count ≤200/μl was 0 months (IQR 0–0), compared to 2 months (IQR 1–3) for FCFS eligibility. CD4-based eligibility resulted in ADAP maintaining a waitlist for care on 23% of program days with a mean of 528 patient days spent on the waitlist each month. FCFS eligibility resulted in the program maintaining a waitlist on 90% of program days with a mean of 3,766 patient days spent on the waitlist each month. CD4-based eligibility required that ADAP maintain 3.4% of program capacity in reserve (Table 2).
Additional sensitivity analyses
In sensitivity analyses, though the CD4 eligibility threshold varied, CD4-based ADAP eligibility consistently resulted in fewer deaths and OIs than FCFS eligibility. CD4-based eligibility also consistently shortened the time to starting ART for patients with CD4 counts below the ADAP eligibility threshold, but increased wait-time for patients with CD4 counts above the threshold. Factors such as the age and sex of applicants, the number of lines of ART available, the availability of darunavir and etravirine, the effectiveness of ART, and the attrition rate of the ADAP had little impact on the marginal benefit of CD4-based over FCFS eligibility. In the base case scenario, only when the incidence of OIs for patients with CD4 counts >350/μl increased ten fold did FCFS eligibility become the preferred strategy.
Changing the source of disease progression parameters to the WIHS dataset led to higher expected rates of morbidity and mortality, but did not alter the conclusion that CD4-based eligibility leads to fewer deaths and OIs. Replicating the base case scenario of 10% excess demand, but using the WIHS dataset input parameters, the optimal CD4-based eligibility threshold remained 250/μl.
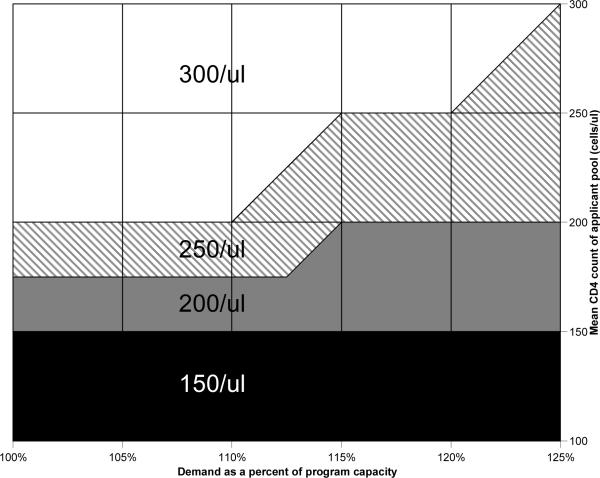
The mean CD4 count of the applicant pool was the largest driver of the optimal CD4 count threshold. For every combination of arrival rate and mean CD4 count, there was a CD4 count threshold that maintained the program within its fixed capacity, and resulted in fewer deaths and OIs than FCFS eligibility (Figure 3).
FIGURE 3. Nomogram providing the optimal CD4 count eligibility threshold as a function of program demand and mean applicant CD4 count.
The nomogram provides the CD4 count eligibility threshold that minimizes morbidity and mortality when resources are inadequate to provide ART to all patients with CD4 count ≤350/μl. The horizontal axis provides program demand at any point denominated in terms of percentage of available capacity. The right vertical axis provides the mean CD4 count of the applicant pool. To use the nomogram, extend a vertical line upward from the appropriate level of program demand and a horizontal line leftward from the appropriate mean CD4 count. The color at the point of intersection of the two lines provides the optimal CD4 count threshold for eligibility.
DISCUSSION
State ADAPs continue to struggle with limited budgets and over-enrollment. Recent economic trends will likely exacerbate the situation (34–36). This study provides evidence that ADAPs facing budget constraints can use limited resources most efficiently by changing eligibility criteria to prioritize patients with low CD4 counts, rather than serving all patients clinically eligible for treatment on a FCFS basis. Prior studies show that CD4-based prioritization would lead to ADAP serving patients with lower CD4 counts, likely reducing morbidity and mortality (12). This analysis quantified the survival benefit of such CD4-based ADAP eligibility, demonstrated the consistent marginal benefit of CD4-based eligibility under a variety of scenarios, and provided program directors a tool for determining the best CD4 count eligibility threshold for their program.
Under nearly every tested scenario, CD4-based eligibility led to lower morbidity and mortality than FCFS eligibility. The improved outcomes, however, come with costs. First, the approach requires the real-time collection of CD4 counts and their application to enrollment criteria. The administrative resources needed to collect, confirm, and analyze CD4 counts could reduce ADAP capacity, potentially increasing the length of waitlists. Second, though CD4-based eligibility is associated with improved morbidity and mortality, it leads to longer wait-times for patients with higher CD4 counts. Third, because CD4-based programs maintain a percentage of capacity in reserve for low CD4-count applicants who may arrive, they will face moments when they must defer treatment for patients who meet clinical criteria for starting ART, despite having immediately available capacity. Confronting these administrative challenges, however, will allow ADAP to contain costs while shortening wait times for the sickest patients and improving clinical outcomes.
It is important to note that this analysis does not suggest that ADAPs with adequate resources should defer ART for patients with CD4 200–350/μl. The best outcome, without exception, was the scenario in which ADAP had the capacity necessary to provide ART to all patients with a CD4 count ≤350/μl. Given data suggesting the ART provides a survival benefit when started at CD4≤ 500/ul (37), ADAPs might see the best outcomes if they expanded ART availability to those with CD4 350–500/μl. When resources are limited, prioritizing patients with advanced disease will lead to the best short-term outcomes. In the long-term, however, the only way to minimize HIV-related morbidity and mortality is to adequately fund ADAP.
There are several limitations to this analysis. First, as a model-based analysis, the findings are not based on empiric observation. It would be impossible, however, to conduct a randomized trial of ADAP eligibility criteria. An ecologic analysis comparing outcomes in states employing different ADAP eligibility criteria would also be difficult, as most states do not track ADAP outcomes. This model used inputs from the Massachusetts ADAP administrative dataset, and we conducted a variety of sensitivity analyses on key input parameters. The core finding, that CD4-based eligibility outperforms FCFS eligibility, was consistent in every analysis.
Second, we used clinical trial data to derive estimates of ART efficacy. ADAP patients may be less likely to adhere to ART regimens than are clinical trial patients, resulting in decreased ART efficacy, and limiting the benefit of ADAP enrollment. Sensitivity analyses that limited ART efficacy to 50% of that observed in clinical trials, however, still found that CD4-based ADAP enrollment leads to fewer opportunistic infections and deaths.
Third, we used only one state's ADAP data to generate cohort and ADAP program characteristics. We observed outcomes, however, with a wide variety of possible cohort and program characteristics. CD4-based eligibility was consistently the best performing strategy.
Fourth, the model did not include mortality and costs related to chronic ART toxicities such as dyslipidemia. ADAPs deciding whether to provide medications to treat hypercholesterolemia and/or cardiovascular disease may balance the cost of these medications with those of providing ART to a larger group of applicants. While this model cannot provide estimates of the costs and benefits of such trade-offs, ADAPs nationally used 91% of budgeted funds to provide ART (1). Limiting the availability of non-ART medications, therefore, will likely not generate the extent of cost savings that many programs need in order to remain solvent.
Faced with limited resources, ADAPs can minimize morbidity and mortality by basing eligibility on CD4 count rather than using a FCFS approach. Program directors seeking to employ CD4-based ADAP eligibility can use the nomogram from this analysis (Figure 3) to identify the optimal CD4 threshold for their state's program as a function of demand and mean applicant CD4 count.
Currently, few states maintain an ADAP waitlist, but many have in the recent past and concern remains for the future. Twenty-two states currently report budget shortfalls totaling $11.2 billion (35, 40). Many economists feel that this number signals the onset of a historic fiscal crisis for state governments. In the setting of ongoing economic stress, ADAPs will need to consider means of controlling costs. Ideally, programs should serve all patients who meet U.S. treatment guidelines (16). If they cannot, however, CD4-based eligibility can contain costs, minimize morbidity and mortality, and reduce the need for waitlists.
ACKNOWLEDGEMENTS
We thank A. David Paltiel, PhD. for his thoughtful review of the manuscript, and Brandon Morris for his technical assistance.
Supported by grants from the National Institute of Allergy and Infectious Diseases (K01AI073193, K24AI062476, R37AI42006, P30AI060354), the National Institute of Mental Health (R01MH073445), and by the Massachusetts Department of Public Health HIV/AIDS Bureau.
REFERENCES
- 1.Carbaugh A, Kates J, Crutsinger-Perry B, Penner M, Ginsburg B. National ADAP Monitoring Project Annual Report. Henry J. Kaiser Family Foundation; Menlo Park, CA: Apr, 2008. July 8, 2008. [Google Scholar]
- 2.Health Resources and Service Administration HIV/AIDS Bureau . Maximizing Access to Medications Through Efficient Use of Care Act Resources. Washington, DC: May, 2005. July 8, 2008. [Google Scholar]
- 3.Gebo KA, Fleishman JA, Conviser R, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38:96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 4.Reif S, Whetten K, Thielman N. Association of race and gender with use of antiretroviral therapy among HIV-infected individuals in the Southeastern United States. South Med J. 2007;100:775–81. doi: 10.1097/SMJ.0b013e3180f626b4. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham WE, Markson LE, Andersen RM, et al. Prevalence and predictors of highly active antiretroviral therapy use in patients with HIV infection in the united states. HCSUS Consortium. HIV Cost and Services Utilization. J Acquir Immune Defic Syndr. 2000;25:115–23. doi: 10.1097/00042560-200010010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Grant WC, Jhaveri RR, McHutchison JG, Schulman KA, Kauf TL. Trends in health care resource use for hepatitis C virus infection in the United States. Hepatology. 2005;42:1406–13. doi: 10.1002/hep.20941. [DOI] [PubMed] [Google Scholar]
- 7.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. quiz CE1-4. [PubMed] [Google Scholar]
- 8.Bassett IV, Farel C, Szmuilowicz ED, Walensky RP. HIV/AIDS: AIDS Drug Assistance Programs in the era of routine HIV testing. Clin Infect Dis. 2008;47:695–701. doi: 10.1086/590936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Association of State and Territorial AIDS Directors . AIDS Drug Assistance Programs and Cost Containment Strategies: Eligibility Criteria Options. National Association of State and Territorial AIDS Directors; Washington, DC: 2007. [Google Scholar]
- 10.National Association of State and Territorial AIDS Directors . AIDS Drug Assisstance Programs and Cost Containment Strategies: Waiting List Management. National Association of State and Territorial AIDS Directors; Washington, DC: 2007. [Google Scholar]
- 11.National Association of State and Territorial AIDS Directors . AIDS Drug Assisstance Programs and Cost Containment Strategies: Managing Prescription Utilization. National Association of State and Territorial AIDS Directors; Washington, DC: 2007. [Google Scholar]
- 12.Linas BP, Zheng H, Losina E, et al. Optimizing resource allocation in United States AIDS drug assistance programs. Clin Infect Dis. 2006;43:1357–64. doi: 10.1086/508657. [DOI] [PubMed] [Google Scholar]
- 13.Fishman GS. Discrete-Event Simulation: Modeling, Programming, and Analysis. Springer-Verlag; Berlin: 2001. [Google Scholar]
- 14.Robinson S. Simulation : The Practice of Model Development and Use. John Wiley & Sons; 2004. [Google Scholar]
- 15.Shechter SM, Bryce CL, Alagoz O, et al. A clinically based discrete-event simulation of end-stage liver disease and the organ allocation process. Med Decis Making. 2005;25:199–209. doi: 10.1177/0272989X04268956. [DOI] [PubMed] [Google Scholar]
- 16.Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Jan 29, 2008. pp. 1–128. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed (September 25, 2008) [Google Scholar]
- 17.Lee KJ, Dunn D, Porter K, et al. Treatment switches after viral rebound in HIV-infected adults starting antiretroviral therapy: multicentre cohort study. AIDS. 2008;22:1943–50. doi: 10.1097/QAD.0b013e32830e4cf3. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann GR, Perrin L, Pantaleo G, et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med. 2003;163:2187–95. doi: 10.1001/archinte.163.18.2187. [DOI] [PubMed] [Google Scholar]
- 19.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44:441–6. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 20.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 21.Multicenter AIDS Cohort Study (MACS) Public Dataset: Release PO4. National Technical Information Service; Springfield, VA: 1995. [Google Scholar]
- 22.Arias E. United States Life Tables, 2003. National Vital Statistics Reports. 2007;54:1–40. [PubMed] [Google Scholar]
- 23.Massachusetts HIV Drug Assistance Program (HDAP) administrative dataset FY. Provided by Massachusetts Department of Public Health AIDS Bureau; Boston, MA: 19972004. [Google Scholar]
- 24.Freedberg KA, Losina E, Weinstein MC, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344:824–31. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 25.Freedberg KA, Scharfstein JA, Seage GR, 3rd, et al. The cost-effectiveness of preventing AIDS-related opportunistic infections. JAMA. 1998;279:130–6. doi: 10.1001/jama.279.2.130. [DOI] [PubMed] [Google Scholar]
- 26.Cohen C, Nieto-Cisneros L, Zala C, et al. Comparison of atazanavir with lopinavir/ritonavir in patients with prior protease inhibitor failure: a randomized multinational trial. Curr Med Res Opin. 2005;21:1683–92. doi: 10.1185/030079905x65439. [DOI] [PubMed] [Google Scholar]
- 27.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 28.Grinsztejn B, Nguyen BY, Katlama C, et al. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet. 2007;369:1261–9. doi: 10.1016/S0140-6736(07)60597-2. [DOI] [PubMed] [Google Scholar]
- 29.Johnson M, Grinsztejn B, Rodriguez C, et al. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005;19:685–94. doi: 10.1097/01.aids.0000166091.39317.99. [DOI] [PubMed] [Google Scholar]
- 30.Johnson M, Grinsztejn B, Rodriguez C, et al. 96-week comparison of once-daily atazanavir/ritonavir and twice-daily lopinavir/ritonavir in patients with multiple virologic failures. AIDS. 2006;20:711–8. doi: 10.1097/01.aids.0000216371.76689.63. [DOI] [PubMed] [Google Scholar]
- 31.Nelson M, Arasteh K, Clotet B, et al. Durable efficacy of enfuvirtide over 48 weeks in heavily treatment-experienced HIV-1-infected patients in the T-20 versus optimized background regimen only 1 and 2 clinical trials. J Acquir Immune Defic Syndr. 2005;40:404–12. doi: 10.1097/01.qai.0000185314.56556.c3. [DOI] [PubMed] [Google Scholar]
- 32.Pozniak AL, Gallant JE, DeJesus E, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz versus fixed-dose zidovudine/lamivudine and efavirenz in antiretroviral-naive patients: virologic, immunologic, and morphologic changes--a 96-week analysis. J Acquir Immune Defic Syndr. 2006;43:535–40. doi: 10.1097/01.qai.0000245886.51262.67. [DOI] [PubMed] [Google Scholar]
- 33.Women's Interangency HIV Study (WIHS) Dossier. Oct, 2008. Available at http://statepiaps.jhsph.edu/wihs/Invest-info/dossier.pdf. Accessed January 9, 2008.
- 34.Smith VK. State Budget Cuts: How Will Health Care Fare in FY 2009. Presented at the National Health Policy Forum; Washington, DC. July 11, 2008; Available at http://www.nhpf.org/handouts/Smith.slides_07-11-08.pdf. Accessed January 9, 2009. [Google Scholar]
- 35.Viser Matt. Budget gap estimated as high as $1.5 billion; service cuts, layoffs expected. Boston Globe. 2008 October 14; [Google Scholar]
- 36.Associated Press State's Expected Shortfall Shoots Up, May Force Deep Cuts. Tuscon Citizen. 2008 May 30; [Google Scholar]
- 37.Kitahata MM, Gange SJ, Moore RD, The North American AIDS Cohort Collaboration On ReSearch And Design . Program and abstracts of the 48th ICAAC/46th IDSA. Washington, DC: Oct 25–28, 2008. Initiating rather than deferring HAART at a CD4+ count between 351-500 cells/mm3 is associated with improved survival. Abstract H-896b. [Google Scholar]
- 38.World Health Organization . 2006 Rev. Geneva, Switzerland: 2006. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a public health approach. [PubMed] [Google Scholar]
- 39.May M, Sterne JA, Sabin C, et al. Prognosis of HIV-1-infected patients up to 5 years after initiation of HAART: collaborative analysis of prospective studies. AIDS. 2007;21:1185–97. doi: 10.1097/QAD.0b013e328133f285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richard Fausset, Nicholas Riccardi. States face new budget shortfalls. Los Angeles Times. 2008 October 19; [Google Scholar]
- 41.Samet JH, Freedberg KA, Savetsky JB, Sullivan LM, Stein MD. Understanding delay to medical care for HIV infection: the long-term non-presenter. AIDS. 2001;15:77–85. doi: 10.1097/00002030-200101050-00012. [DOI] [PubMed] [Google Scholar]
- 42.El-Sadr WM, Murphy RL, Yurik TM, et al. Atovaquone compared with dapsone for the prevention of Pneumocystis carinii pneumonia in patients with HIV infection who cannot tolerate trimethoprim, sulfonamides, or both. Community Program for Clinical Research on AIDS and the AIDS Clinical Trials Group. N Engl J Med. 1998;339:1889–95. doi: 10.1056/NEJM199812243392604. [DOI] [PubMed] [Google Scholar]
- 43.Havlir DV, Dube MP, Sattler FR, et al. Prophylaxis against disseminated Mycobacterium avium complex with weekly azithromycin, daily rifabutin, or both. California Collaborative Treatment Group. N Engl J Med. 1996;335:392–8. doi: 10.1056/NEJM199608083350604. [DOI] [PubMed] [Google Scholar]
- 44.Benson CA, Williams PL, Cohn DL, et al. Clarithromycin or rifabutin alone or in combination for primary prophylaxis of Mycobacterium avium complex disease in patients with AIDS: A randomized, double-blind, placebo-controlled trial. The AIDS Clinical Trials Group 196/Terry Beirn Community Programs for Clinical Research on AIDS 009 Protocol Team. J Infect Dis. 2000;181:1289–97. doi: 10.1086/315380. [DOI] [PubMed] [Google Scholar]
- 45.Ioannidis JP, Cappelleri JC, Skolnik PR, Lau J, Sacks HS. A meta-analysis of the relative efficacy and toxicity of Pneumocystis carinii prophylactic regimens. Arch Intern Med. 1996;156:177–88. [PubMed] [Google Scholar]