Abstract
Throughout atherosclerotic lesion development, intimal macrophages undergo apoptosis, a form of death that usually prevents cellular necrosis. In advanced atherosclerotic lesions, however, these apoptotic macrophages become secondarily necrotic and coalesce over time into a key feature of vulnerable plaques, the necrotic core. This event is critically important, as necrotic core formation in these advanced atheromata is thought to promote plaque disruption and ultimately, acute atherothrombotic vascular disease. Increasing evidence suggests that the mechanism behind postapoptotic macrophage necrosis in advanced atherosclerosis is defective phagocytic clearance or “efferocytosis” of the apoptotic cells. Thus, understanding the cellular and molecular mechanisms of efferocytosis in atherosclerosis and why efferocytosis becomes defective in advanced lesions is an important goal. Molecular–genetic causation studies in mouse models of advanced atherosclerosis have provided evidence that several molecules known to be involved in efferocytosis, including TG2, MFG-E8, complement C1q, Mertk, lysoPC, and Fas, play important roles in the clearance of apoptotic cells in advanced plaques. These and future insights into the molecular mechanisms of defective efferocytosis in advanced atheromata may open the way for novel therapeutic strategies for atherothrombotic vascular disease, the leading cause of death in the industrialized world.
Keywords: apoptosis, phagocytosis, macrophage, necrosis, Mertk, milk fat globule-epidermal growth factor 8 (MFG-E8, lactadherin), transglutaminase 2, complement C1q, Fas, lysophosphatidylcholine
Introduction EFFEROCYTOSIS
Billions of cells in our body die each day by the process of apoptosis [1,2,3]. Coupled with each of these cell death events is a critical process, whereby professional and nonprofessional phagocytes dispose of the apoptotic cells in a rapid and efficient manner. Peter Henson and co-workers [2] have coined the term “efferocytosis”, from the Greek “to bury”, to describe this process [1, 3]. Efferocytosis, which is highly conserved throughout evolution, involves a number of molecules, including ligands on the apoptotic cells (e.g., PS); receptors on the efferocyte (e.g., Mertk2); soluble ligand–receptor bridging molecules (e.g., MFG-E8); and so-called “find-me” and “don’t-eat-me” molecules (e.g., lysosphospholipids and CD47, respectively), the expression of which by dying cells is altered to attract nearby phagocytes [1,2,3]. Efferocytosis has several critical functions. First, by clearing apoptotic cells at a relatively early stage of cell death, when the cell plasma and organelle membranes are still intact, postapoptotic, or “secondary”, necrosis is prevented [2]. Prevention of cellular necrosis, in turn, prevents the release of potentially damaging intracellular molecules into the extracellular milieu, including molecules that can stimulate inflammatory and/or autoimmune responses [4]. Second, engagement of efferocyte receptors by ligands or ligand-bridging molecule complexes often triggers an anti-inflammatory response by the efferocyte [5, 6]. For example, efferocytosis has been shown to induce the secretion of the anti-inflammatory molecules TGF-β, IL-10, and PGE2 [1,2,3], and engagement of an important efferocytosis receptor, Mertk (below), leads to suppression of NF-κB signaling [7]. Third, antigen processing by efferocytes, particularly dendritic cell efferocytes, can play a part in T cell-mediated immune recognition processes, including anti-tumor responses [8]. Efferocytosis is normally an extremely efficient and high-capacity process, and the appearance of apoptotic cells in tissues often represents defective efferocytosis rather than enhanced apoptosis. When the efficiency of efferocytosis is compromised, tissue necrosis, pathological inflammation, and/or autoimmunity often ensue.
AN OVERVIEW OF EFFEROCYTOSIS IN ATHEROSCLEROSIS
The process of efferocytosis has gained attention among researchers investigating atherosclerosis, as macrophages, the most prominent cell type in atherosclerotic lesions, undergo apoptosis throughout lesion development [9]. Therefore, the efficiency, or lack thereof, of efferocytic clearance of apoptotic macrophages in atherosclerotic lesions is likely to play an important role in the number and atherogenic properties of lesional macrophages, which are known to participate in the atherogenic process starting at a very early stage, entering into focal areas of the arterial subendothelium as blood-borne monocytes in response to the presence of subendothelially retained ApoB100 lipoproteins [10]. When the monocytes differentiate into macrophages, they internalize and metabolize the retained lipoproteins, giving rise to cells known as lipid-laden “foam cells”, which promote atherogenesis by a number of mechanisms, including secretion of inflammatory cytokines, and although they have a long half-life, lesional macrophages undergo a steady rate of apoptosis [9]. In early lesions, apoptotic macrophages are cleared rapidly by neighboring macrophages, and the net effect of this efferocytic event is to limit lesion cellularity [11]. Moreover, the anti-inflammatory signaling consequences of efferocytosis may decrease the progression of these early lesions further, including via suppression of new monocyte recruitment. For example, one of the anti-inflammatory cytokines that can result from efferocytosis, IL-10, has known antiatherogenic effects in vivo [12, 13]. Thus, experimental genetic or pharmacologic manipulations in mouse models of early atherosclerosis that promote macrophage apoptosis tend to limit lesion size and progression and vice versa for manipulations that block macrophage apoptosis [14,15,16]. These results indicate that in early atherosclerosis, as in normal physiology, efferocytosis is efficient.
A prominent feature of advanced atherosclerotic lesions is the necrotic core, or lipid core, which is a collection of dead and necrotic macrophages surrounded by inflammatory cells [17, 18] (Fig. 1). Necrotic cores are thought to be a major feature responsible for plaque “vulnerability”—i.e., plaques capable of undergoing disruption and triggering acute lumenal thrombosis [19]. Plaque disruption and acute thrombosis are the events that trigger acute coronary syndromes, including myocardial infarction, unstable angina, sudden cardiac death, and stroke [19]. Necrotic cores activate harmful inflammatory processes in lesions; are a source of tissue-disrupting proteases and procoagulant/thrombotic molecules; and contribute to physical stress on the overlying fibrous cap, a structure of nonvulnerable plaques that prevent exposure of blood platelets to thrombotic material in the underlying lesion [20, 21]. In view of the principles of efferocytosis described above, investigators have hypothesized that necrotic cores arise from a combination of macrophage apoptosis coupled with defective efferocytosis in advanced lesions [11, 22]. A corollary of this hypothesis is that macrophage apoptosis in earlier lesions, where efferocytosis is presumably not defective, actually leads to less cellular lesions and decreased atherosclerosis progression [11, 22]. Experimental proof has come from a number of mouse models using genetics to force macrophage apoptosis in early versus advanced lesions. In these models, induction of early lesional apoptosis leads to smaller lesions, and advanced lesional apoptosis promotes plaque necrosis [14, 16, 23]. Similarly, forced macrophage survival in early lesions promotes lesion expansion [15]. Moreover, Schrijvers et al. [24] compared human advanced plaques and as a control, human tonsillar tissue for the number of apoptotic cells that were closely associated with phagocytes or free. As expected, most apoptotic cells were associated with phagocytes in tonsils, but the percentage of free apoptotic cells was increased markedly in the atherosclerotic lesions. These data are consistent with defective efferocytosis in advanced human atherosclerosis, but they do not address the critical issues of causation.
Figure 1.
Cross-section of an advanced human coronary plaque showing a large necrotic core, plaque rupture, and lumenal thrombosis. Mφs, Macrophages.
CAUSATION STUDIES LINKING DEFECTIVE EFFEROCYTOSIS TO PLAQUE NECROSIS IN ADVANCED ATHEROSCLEROTIC LESIONS
Although a number of efferocyte molecules have thus far emerged as playing a role in the clearance of apoptotic macrophages in atherosclerosis, only few of these have been subjected to genetic causation studies related specifically to plaque necrosis in advanced murine atheromata. One such molecule is TG2 [25], which with its “coreceptor” αvβ3 integrin, bind to extracellular molecule MFG-E8 (also known as lactadherin), a macrophage-secreted protein that acts as a bridge between PS on apoptotic cells and the TG2–αvß3 complex on efferocytes [26, 27]. Boisvert et al. [28] used lethally irradiated atherosclerosis-prone Ldlr−/− mice transplanted with Tg2−/− bone marrow to create chimeric mice. After 16 weeks on a high-fat diet, the mice transplanted with Tg2−/− bone marrow had larger aortic root lesions and most importantly, increased plaque necrosis compared with mice transplanted with Tg2+/+ bone marrow. A second in vivo study by the Mallat group [29] focused on the aforementioned efferocytosis-bridging molecule MFG-E8, which is known to be expressed in atheromata. Using Mfge8−/− mice, the investigators first demonstrated that the molecule was needed for the uptake of apoptotic cells in vitro and in a peritoneal cavity assay in vivo. Atherosclerosis studies were conducted using Ldlr−/− mice reconstituted with Mfge8−/− bone marrow. When these and Mfge8+/+-reconstituted control mice were fed a high-fat diet to induce atherosclerosis, the advanced aortic root atheromata of the MFG-E8-deficient mice had larger numbers of apoptotic cells, increased plaque necrosis, and increased indices of inflammation, particularly increased IFN-γ, compared with the control lesions. Another bridging molecule involved in the recognition of apoptotic cells by phagocytes is the complement factor C1q [30], and C1qa−/− mice on the fat-fed Ldlr−/− background had larger and more complex aortic root lesions and an increase in the number of apoptotic cells in these lesions compared with C1qa+/+;Ldlr−/− mice [31].
A fourth efferocyte molecule investigated in advanced atheromata is Mertk, which is a cell-surface receptor tyrosine kinase that plays roles in efferocytosis and in anti-inflammatory responses [7, 32]. Mertk participates in efferocytosis by binding one of two other PS-binding bridging molecules, Gas6 and protein S [33]. Mice deficient in Mertk by virtue of a mutation in the tyrosine kinase domain—MertkKD mice—show evidence of defective efferocytosis and susceptibility to a lupus-like autoimmune syndrome [32]. Early in vitro studies by our laboratory showed that Mertk played a major role in the uptake of macrophages rendered apoptotic by inducers thought to be important in advanced atherosclerosis, notably those inducers that trigger endoplasmic reticulum stress-induced apoptosis [34,35,36]. Most importantly, advanced aortic root lesions of fat-fed MertkKD;Apoe−/− mice had large areas of plaque necrosis compared with those of control Apoe−/− mice [37]. This finding was associated with an increase in the number of apoptotic cells in the MertkKD lesions and importantly, an increase in the percentage of apoptotic macrophages that were not associated with phagocytes, which is a measure of defective efferocytosis [37]. Mallat and colleagues [38] published a similar study showing increased plaque necrosis in the mutant mice, and these authors also found evidence of increased inflammation in the spleens of the MertkKD mice, consistent with the anti-inflammatory effect of efferocytosis and Mertk signaling.
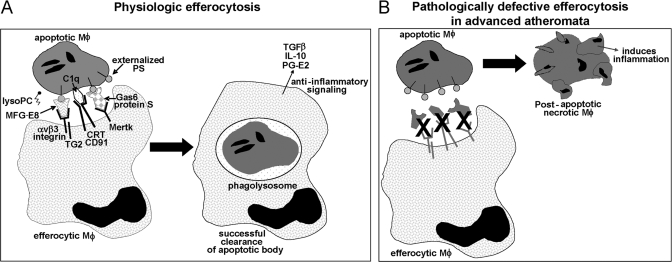
Evidence for a defect in lesional efferocytosis has also been found in the aortic root lesions of fat-fed Apoe−/− mice, particularly on the Fas-deficiency (gld) background [39, 40], and Ldlr−/− mice transplanted with bone marrow from gld mice developed lesions with increased inflammation and apoptotic debris [41]. In this study, infusion of lysoPC into chow-fed gld;Apoe+/+ mice caused a defect in efferocytosis in lymph nodes, which the authors suggested might mediate the defect in efferocytosis in fat-fed Apoe−/− mice, which have elevated levels of lysoPC in their plasma [39, 42]. However, lysoPC can function as a so-called find-me signal in efferocytosis [42], and so, how elevated lysoPC would impair efferocytosis is unclear. The authors speculate that excess lysoPC may impair the normal gradient between apoptotic cells and efferocytes that is necessary for this find-me signal to function properly [42]. In terms of the possible role of Fas in efferocytosis, Fas engagement was found to increase the amount of oxidized PS on the apoptotic cell surface, which is a potent recognition ligand for phagocytes [43]. In addition, Fas engagement increased the expression of two efferocytosis-enhancing molecules, annexin I and IL-10 [44]. The combined studies in this section provide evidence for two major points: A number of molecules involved directly in efferocytosis or in its regulation, including TG2, MFG-E8, C1q, Mertk, lysoPC, and Fas, have important roles in clearance of apoptotic cells in advanced atherosclerotic lesions, and manipulations that block efferocytosis promote advanced plaque progression, particularly plaque necrosis and inflammation (Fig. 2).
Figure 2.
Efferocytosis of apoptotic macrophages and how it may go awry in advanced atheroma. (A) Physiologic efferocytosis can involve many molecules, including a diverse array of receptors, ligands, and bridging molecules. An increase in the ratio of so-called find-me:don’t-eat-me signals also enables efferocytosis. Depicted here are several molecules that have been shown to a play a role in murine models of atherosclerosis. Successful recognition and engulfment of apoptotic bodies lead to avoidance of cellular necrosis and anti-inflammatory signaling. CRT, Calreticulin. (B) In advanced atheromata, there is evidence that efferocytosis becomes less effective, leading to secondary macrophage necrosis and inflammation, two processes that likely promote plaque vulnerability. There are several hypotheses as to why efferocytosis loses efficiency in advanced plaques, including dysfunction of the molecules depicted in A as a result of cleavage, decreased expression, or competitive inhibition of binding by other plaque molecules. See text for details.
WHY IS EFFEROCYTOSIS “DEFECTIVE” IN ADVANCED ATHEROMATA?
As mentioned previously, there is evidence that efferocytosis is defective in advanced human lesions [24]. In theory, what we are calling “defective efferocytosis” could represent overwhelming apoptosis. Although this possibility needs to be considered, efferocytosis, when functioning properly, has a high capacity of efferocytosis [2], which is consistent with the finding that when apoptosis is increased artificially by genetic manipulations in early lesions, where efferocytosis is functioning properly, there is not an accumulation of apoptotic cells [15, 16]. Defective efferocytosis could also result from alterations in the apoptotic cells themselves that render them poor substrates for efferocytic recognition and engulfment. Studies in our laboratory, however, have revealed that macrophages rendered apoptotic by many different atherosclerosis-relevant mechanisms are equally good substrates for healthy efferocytes (unpublished data). Thus, we favor the concept that efferocytosis per se loses efficiency in advanced lesions (Fig. 2). One possibility is that the quintessential macrophage alteration in atheromata, namely, foam cell formation, compromises the ability of the cells, acting as efferocytes, to recognize and/or engulf apoptotic cells. However, we have found that cholesteryl-ester-laden macrophage foam cells are excellent efferocytes in vitro [34]. Moreover, to the extent that advanced lesional macrophages accumulate excess unesterified cholesterol [45], we found that this perturbation also does not compromise efferocytosis, unless the loading is prolonged enough to cause cell death [34]. It should be noted, however, that in vitro assays of this nature would not necessarily pick up disturbances of other efferocytic processes, such as migration of efferocytes to the sites of apoptosis. Another possibility is that advanced lesional macrophage death per se limits efferocytosis by decreasing the pool of efferocytes. Although the fact that advanced lesions have a large population of living macrophages might make this mechanism seem unlikely [46], it is possible that the number of living macrophages is rate-limiting near those areas in plaques where apoptotic cells accumulate. Macrophages in advanced plaques are known to accumulate ROS, and there is some in vitro evidence that ROS may perturb efferocytosis, perhaps through activation of 12/15-lipoxygenase [47, 48]. Other studies have shown that extracellular-oxidized molecules known to be present in atheromata, such as OxLDL, can compete with apoptotic cells for recognition by efferocytes in vitro, for example, through the OxLDLR, lectin-like OxLDLR1 [49, 50]. Moreover, fully OxLDL as well as LDL that is only minimally oxidized, referred to as “minimally modified” LDL, have been shown to inhibit the engulfment of apoptotic cells by cultured macrophages by altering actin signaling through a pathway involving CD14 and TLR4 [51].
Finally, a possible mechanism related directly to the mouse studies described above is that atherosclerosis-relevant efferocytosis molecules may be suppressed or defective in advanced lesions (Fig. 2B). The possibility that Mertk function is defective is intriguing in view of the fact that this molecule gets cleaved by one or more plasma membrane sheddases under inflammatory conditions [52]. The cleavage of Mertk suppresses efferocytosis by destroying the receptor and by creating soluble Mer, which competes for the efferocytosis bridging molecules Gas6 and protein S [52]. Whether Mertk is cleaved in advanced atheromata and whether this accounts for the defective efferocytosis in these lesions remain to be tested experimentally. In a similar vein, MFG-E8 has been shown to be down-regulated in splenic macrophages in a mouse model of sepsis [53]. Concomitant with this down-regulation, which was TLR4-dependent, was a decrease in efferocytosis and an increase in apoptotic bodies in the spleen. As advanced atheromata are at a heightened state of inflammation and have functional TLR4 signaling [54], a similar process might contribute to phagocytic inefficiency in advanced lesions.
FUTURE DIRECTIONS
In industrialized societies, almost all subjects have early atherosclerotic lesions by the time they reach their early 20s [55]. The vast majority of these lesions will not cause clinical disease [56]. However, the few lesions that progress to the vulnerable plaque stage can trigger an acute disease process that accounts for the leading cause of death in this population. Given the role of plaque necrosis in vulnerable plaque formation and our developing concepts about how defective efferocytosis can contribute to plaque necrosis, the area of research covered in this review may reveal specific, mechanism-based targets for antivulnerable plaque therapy to prevent acute coronary syndromes in subjects with established atherosclerotic lesions. The number of molecules that participate in the efferocytosis process is large, and so, it will be important to determine whether other efferocytosis-related molecules play roles in advanced lesions. This goal can be accomplished through a candidate molecule approach such as those summarized in this review. In addition, advanced proteomic techniques may enable a more global, unbiased analysis of a larger set of efferocytosis molecules throughout atherogenesis, particularly in mouse models of large necrotic cores that are highly relevant to human CAD, such as insulin resistance and aging [57,58,59,60]. In each case, causation can be tested in mouse models of plaque necrosis, as illustrated by the studies described above. However, it should be noted that although fat-fed Ldlr−/− and Apoe−/− mice are excellent models of plaque necrosis, they do not develop plaque disruption or acute lumenal thrombosis. Perhaps the extreme plaque necrosis that might develop in atherosclerosis-prone mice lacking more than one atheromata-relevant efferocytosis molecule, whose functions are nonoverlapping, such as the combination of MGF-E8 and Mertk deficiency, would be enough to trigger plaque disruption.
The ultimate goal in this area of research is to identify strategies to enhance efferocytosis in advanced human atheromata. Enhancing efferocytosis will likely decrease necrosis, inflammation, and macrophage content in advanced lesions. We favor this approach over blocking macrophage apoptosis, as living macrophages also contribute to vulnerable plaque formation [46], and blocking apoptosis may promote early lesion development (above). A theoretic disadvantage of efferocytosis enhancement might be promotion of cholesterol-induced death in the efferocyte through uptake of cholesterol-laden macrophages. However, efferocytosis is associated with cell-survival signaling [61], and we have shown that when macrophage efferocytes engage cholesterol-loaded apoptotic macrophages, they are protected from cytotoxicity through enhanced cholesterol esterification and efflux and through activation of cell-survival pathways involving Akt and NF-κB [62].
The goal of enhancing efferocytosis may be approached by more general proefferocytosis strategies or by targeting specific processes that are defective in advanced atherosclerosis. An example of the former would be to consider the emerging concept that some macrophage subpopulations are more efficient at efferocytosis than others. For example, some studies have shown that alternatively activated macrophages are better efferocytes than classically activated macrophages [63, 64], which is consistent with the former’s role in resolution of inflammation. Thus, molecules involved in alternative macrophage development, such as PPARγ or PPARδ [65, 66], or those that alter the behavior of fully differentiated macrophages into a more anti-inflammatory, proresolution state, such as lipoxins, can be investigated as potential drug targets or drugs. Of interest in this regard, lipoxins have been explored as efferocytosis enhancers in lupus, where defective efferocytosis of neutrophils in joints is thought to promote disease progression [67]. Interestingly, the hydroxymethylglutaryl-CoA reductase inhibitor lovastatin increases efferocytosis of apoptotic T cells by human monocyte-derived macrophages in vitro and by alveolar macrophages in vivo [68]. The mechanism appears to be through inhibition of the geranylgeranyl/farnesyl-RhoA pathway of actin signaling, which is known to suppress the engulfment of apoptotic cells [69]. Whether statins in doses administered to humans enhance efferocytosis in atherosclerotic lesions and if so, whether this effect adds to the protective effect of cholesterol-lowering by statins in decreasing atherothrombotic vascular disease remain to be investigated.
A more specific approach would be to define molecular mechanisms of defective efferocytosis in human lesions—perhaps involving some of the molecules discussed in this review—and then to base therapeutic strategy on this information. For example, the fact that Mertk is rendered inactive through sheddase-mediated cleavage (above) may provide a therapeutic opportunity. Thus, if excess Mertk cleavage were a culprit in human advanced plaques, inhibition of cleavage by drugs might suppress plaque necrosis. In general, showing correlations between alterations of efferocytosis molecules in advanced human lesions and plaque vulnerability and cardiovascular disease may be helpful in identifying potentially promising drug targets. However, the strongest evidence is likely to emerge from human genetic studies if functionally important polymorphisms in specific efferocytosis genes can be shown to be associated with plaque necrosis and acute coronary syndromes.
In summary, an appreciation of the importance of atherosclerotic plaque necrosis in cardiovascular disease and the knowledge that plaque necrosis results from the linked processes of macrophage apoptosis and defective efferocytic clearance of these apoptotic cells have revealed a specific set of molecular events that may play a critical role in plaque progression and human heart disease. This concept opens up novel opportunities for mechanism-based therapy designed to combat the leading cause of death in the industrialized world. To pursue this goal, investigators will need to understand the in-depth molecular and cellular biology of efferocytosis, define mechanisms of defective efferocytosis in advanced atheromata, and assign causation between defective efferocytosis and atherothrombotic vascular disease through animal and human genetic studies.
Acknowledgments
This work was supported by an American Heart Association postdoctoral training grant (to E. T.) and National Institutes of Health grants HL54591 and HL75662 (to I. T.). The authors acknowledge the outstanding contributions of Drs. Dongying Cui, Yankun Li, and Dorien M. Schrijvers.
Footnotes
Abbreviations: Apo=apolipoprotein, C1q=component 1 q subcomponent, C1qa=component 1 q subcomponent a chain, CAD=coronary artery disease, Gas6=growth arrest-specific 6, Ldlr−/−=low-density lipoprotein receptor-deficient, lysoPC=lysophosphatidylcholine, Mertk=Mer tyrosine kinase, MertkKD=Mertk kinase-defective, MFG-E8=milk fat globule-epidermal growth factor 8, OxLDL=oxidized LDL, PPAR=peroxisome proliferator-activated receptor, PS=phosphatidylserine, ROS=reactive oxygen species, TG2=transglutaminase 2
References
- Savill J. Recognition and phagocytosis of cells undergoing apoptosis. Br Med Bull. 1997;53:491–508. doi: 10.1093/oxfordjournals.bmb.a011626. [DOI] [PubMed] [Google Scholar]
- Henson P M, Bratton D L, Fadok V A. Apoptotic cell removal. Curr Biol. 2001;11:R795–R805. doi: 10.1016/s0960-9822(01)00474-2. [DOI] [PubMed] [Google Scholar]
- Grimsley C, Ravichandran K S. Cues for apoptotic cell engulfment: eat-me, don’t eat-me and come-get-me signals. Trends Cell Biol. 2003;13:648–656. doi: 10.1016/j.tcb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Fink S L, Cookson B T. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok V A, Bratton D L, Konowal A, Freed P W, Westcott J Y, Henson P M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E Y, Liu J, Homma Y, Zhang Y, Brendolan A, Saggese M, Han J, Silverstein R, Selleri L, Ma X. Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity. 2007;27:952–964. doi: 10.1016/j.immuni.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch T D, Koller B H, Earp H S, Matsushima G K. A novel receptor tyrosine kinase, Mer, inhibits TNF-α production and lipopolysaccharide-induced endotoxic shock. J Immunol. 1999;162:3498–3503. [PubMed] [Google Scholar]
- Albert M L, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Kockx M M. Apoptosis in the atherosclerotic plaque: quantitative and qualitative aspects. Arterioscler Thromb Vasc Biol. 1998;18:1519–1522. doi: 10.1161/01.atv.18.10.1519. [DOI] [PubMed] [Google Scholar]
- Tabas I, Williams K J, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- Caligiuri G, Rudling M, Ollivier V, Jacob M P, Michel J B, Hansson G K, Nicoletti A. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med. 2003;9:10–17. [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li D, Chen J, Xie J, Bandyopadhyay S, Zhang D, Nemarkommula A R, Liu H, Mehta J L, Hermonat P L. Inhibition of atherogenesis in LDLR knockout mice by systemic delivery of adeno-associated virus type 2-hIL-10. Atherosclerosis. 2006;188:19–27. doi: 10.1016/j.atherosclerosis.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Arai S, Shelton J M, Chen M, Bradley M N, Castrillo A, Bookout A L, Mak P A, Edwards P A, Mangelsdorf D J, Tontonoz P, Mivazaki T. A role for the apoptosis inhibitory factor AIM/Spα/Api6 in atherosclerosis development. Cell Metab. 2005;1:201–213. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Liu J, Thewke D P, Su Y R, Linton M F, Fazio S, Sinensky M S. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler Thromb Vasc Biol. 2005;25:174–179. doi: 10.1161/01.ATV.0000148548.47755.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier E L, Huby T, Witztum J L, Ouzilleau B, Miller E R, Saint-Charles F, Aucouturier P, Chapman M J, Lesnik P. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation. 2009;119:1795–1804. doi: 10.1161/CIRCULATIONAHA.108.806158. [DOI] [PubMed] [Google Scholar]
- Ball R Y, Stowers E C, Burton J H, Cary N R, Skepper J N, Mitchinson M J. Evidence that the death of macrophage foam cells contributes to the lipid core of atheroma. Atherosclerosis. 1995;114:45–54. doi: 10.1016/0021-9150(94)05463-s. [DOI] [PubMed] [Google Scholar]
- Kolodgie F D, Virmani R, Burke A P, Farb A, Weber D K, Kutys R, Finn A V, Gold H K. Pathologic assessment of the vulnerable human coronary plaque. Heart. 2004;90:1385–1391. doi: 10.1136/hrt.2004.041798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virmani R, Burke A P, Kolodgie F D, Farb A. Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol. 2002;15:439–446. doi: 10.1111/j.1540-8183.2002.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Shah P K. Molecular mechanisms of plaque instability. Curr Opin Lipidol. 2007;18:492–499. doi: 10.1097/MOL.0b013e3282efa326. [DOI] [PubMed] [Google Scholar]
- Ohayon J, Finet G, Gharib A M, Herzka D A, Tracqui P, Heroux J, Rioufol G, Kotys M S, Elagha A, Pettigrew R I. Necrotic core thickness and positive arterial remodeling index: emergent biomechanical factors for evaluating the risk of plaque rupture. Am J Physiol Heart Circ Physiol. 2008;295:H717–H727. doi: 10.1152/ajpheart.00005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers D M, De Meyer G R, Herman A G, Martinet W. Phagocytosis in atherosclerosis: molecular mechanisms and implications for plaque progression and stability. Cardiovasc Res. 2007;73:470–480. doi: 10.1016/j.cardiores.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Thorp E, Li Y, Bao L, Yao P M, Kuriakose G, Rong J, Fisher E A, Tabas I. Brief report: increased apoptosis in advanced atherosclerotic lesions of Apoe−/− mice lacking macrophage Bcl-2. Arterioscler Thromb Vasc Biol. 2009;29:169–172. doi: 10.1161/ATVBAHA.108.176495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers D M, De Meyer G R, Kockx M M, Herman A G, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- Szondy Z, Sarang Z, Molnar P, Nemeth T, Piacentini M, Mastroberardino P G, Falasca L, Aeschlimann D, Kovacs J, Kiss I, Szegezdi E, Lakos G, Rajnavolgyi E, Birckbichler P J, Melino G, Fesus L. Transglutaminase 2−/− mice reveal a phagocytosis-associated crosstalk between macrophages and apoptotic cells. Proc Natl Acad Sci USA. 2003;100:7812–7817. doi: 10.1073/pnas.0832466100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth B, Garabuczi E, Sarang Z, Vereb G, Vamosi G, Aeschlimann D, Blasko B, Becsi B, Erdodi F, Lacy-Hulbert A, Zhang A, Falasca L, Birge R B, Balajthy Z, Melino G, Fesus L, Szondy Z. Transglutaminase 2 is needed for the formation of an efficient phagocyte portal in macrophages engulfing apoptotic cells. J Immunol. 2009;182:2084–2092. doi: 10.4049/jimmunol.0803444. [DOI] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- Boisvert W A, Rose D M, Boullier A, Quehenberger O, Sydlaske A, Johnson K A, Curtiss L K, Terkeltaub R. Leukocyte transglutaminase 2 expression limits atherosclerotic lesion size. Arterioscler Thromb Vasc Biol. 2006;26:563–569. doi: 10.1161/01.ATV.0000203503.82693.c1. [DOI] [PubMed] [Google Scholar]
- Ait-Oufella H, Kinugawa K, Zoll J, Simon T, Boddaert J, Heeneman S, Blanc-Brude O, Barateau V, Potteaux S, Merval R, Esposito B, Teissier E, Daemen M J, Leseche G, Boulanger C, Tedgui A, Mallat Z. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation. 2007;115:2168–2177. doi: 10.1161/CIRCULATIONAHA.106.662080. [DOI] [PubMed] [Google Scholar]
- Ogden C A, deCathelineau A, Hoffmann P R, Bratton D, Ghebrehiwet B, Fadok V A, Henson P M. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–795. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia V K, Yun S, Leung V, Grimsditch D C, Benson G M, Botto M B, Boyle J J, Haskard D O. Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. Am J Pathol. 2007;170:416–426. doi: 10.2353/ajpath.2007.060406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R S, McMahon E J, Pop S M, Reap E A, Caricchio R, Cohen P L, Earp H S, Matsushima G K. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- Hall M O, Obin M S, Heeb M J, Burgess B L, Abrams T A. Both protein S and Gas6 stimulate outer segment phagocytosis by cultured rat retinal pigment epithelial cells. Exp Eye Res. 2005;81:581–591. doi: 10.1016/j.exer.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Li Y, Gerbod-Giannone M C, Seitz H, Cui D, Thorp E, Tall A R, Matsushima G K, Tabas I. Cholesterol-induced apoptotic macrophages elicit an inflammatory response in phagocytes, which is partially attenuated by the Mer receptor. J Biol Chem. 2006;281:6707–6717. doi: 10.1074/jbc.M510579200. [DOI] [PubMed] [Google Scholar]
- Feng B, Yao P M, Li Y, Devlin C M, Zhang D, Harding H P, Sweeney M, Rong J X, Kuriakose G, Fisher E A, Marks A R, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- Thorp E, Li G, Seimon T A, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 2009;9:474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp E, Cui D, Schrijvers D M, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of Apoe−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:1421–1428. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Oufella H, Pouresmail V, Simon T, Blanc-Brude O, Kinugawa K, Merval R, Offenstadt G, Leseche G, Cohen P L, Tedgui A, Mallat Z. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1429–1431. doi: 10.1161/ATVBAHA.108.169078. [DOI] [PubMed] [Google Scholar]
- Aprahamian T, Rifkin I, Bonegio R, Hugel B, Freyssinet J M, Sato K, Castellot J J, Jr, Walsh K. Impaired clearance of apoptotic cells promotes synergy between atherogenesis and autoimmune disease. J Exp Med. 2004;199:1121–1131. doi: 10.1084/jem.20031557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger D J, Reckless J, McKilligin E. Apolipoprotein E modulates clearance of apoptotic bodies in vitro and in vivo, resulting in a systemic proinflammatory state in apolipoprotein E-deficient mice. J Immunol. 2004;173:6366–6375. doi: 10.4049/jimmunol.173.10.6366. [DOI] [PubMed] [Google Scholar]
- Sturley S L, Patterson M C, Balch W, Liscum L. The pathophysiology and mechanisms of NP-C disease. Biochim Biophys Acta. 2004;1685:83–87. doi: 10.1016/j.bbalip.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Peter C, Waibel M, Radu C G, Yang L V, Witte O N, Schulze-Osthoff K, Wesselborg S, Lauber K. Migration to apoptotic “find-me” signals is mediated via the phagocyte receptor G2A. J Biol Chem. 2008;283:5296–5305. doi: 10.1074/jbc.M706586200. [DOI] [PubMed] [Google Scholar]
- Kagan V E, Gleiss B, Tyurina Y Y, Tyurin V A, Elenstrom-Magnusson C, Liu S X, Serinkan F B, Arroyo A, Chandra J, Orrenius S, Fadeel B. A role for oxidative stress in apoptosis: oxidation and externalization of phosphatidylserine is required for macrophage clearance of cells undergoing Fas-mediated apoptosis. J Immunol. 2002;169:487–499. doi: 10.4049/jimmunol.169.1.487. [DOI] [PubMed] [Google Scholar]
- Zhang S, Witasp E, Lauwen M, Fadeel B. Brief cross-linking of Fas/APO-1 (CD95) triggers engulfment of pre-apoptotic target cells. FEBS Lett. 2008;582:3501–3508. doi: 10.1016/j.febslet.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Tabas I. Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J Clin Invest. 2002;110:905–911. doi: 10.1172/JCI16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Geng Y J, Aikawa M, Schoenbeck U, Mach F, Clinton S K, Sukhova G K, Lee R T. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol. 1996;7:330–335. doi: 10.1097/00041433-199610000-00012. [DOI] [PubMed] [Google Scholar]
- Swarnakar S, Temel R E, Connelly M A, Azhar S, Williams D L. Scavenger receptor class B, type I, mediates selective uptake of low density lipoprotein cholesteryl ester. J Biol Chem. 1999;274:29733–29739. doi: 10.1074/jbc.274.42.29733. [DOI] [PubMed] [Google Scholar]
- Miller Y I, Worrall D S, Funk C D, Feramisco J R, Witztum J L. Actin polymerization in macrophages in response to oxidized LDL and apoptotic cells: role of 12/15-lipoxygenase and phosphoinositide 3-kinase. Mol Biol Cell. 2003;14:4196–4206. doi: 10.1091/mbc.E03-02-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird D A, Gillotte K L, Horkko S, Friedman P, Dennis E A, Witztum J L, Steinberg D. Receptors for oxidized low-density lipoprotein on elicited mouse peritoneal macrophages can recognize both the modified lipid moieties and the modified protein moieties: implications with respect to macrophage recognition of apoptotic cells. Proc Natl Acad Sci USA. 1999;96:6347–6352. doi: 10.1073/pnas.96.11.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka K, Sawamura T, Kikuta K, Itokawa S, Kume N, Kita T, Masaki T. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc Natl Acad Sci USA. 1998;95:9535–9540. doi: 10.1073/pnas.95.16.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Y I, Viriyakosol S, Binder C J, Feramisco J R, Kirkland T N, Witztum J L. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem. 2003;278:1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- Sather S, Kenyon K D, Lefkowitz J B, Liang X, Varnum B C, Henson P M, Graham D K. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2007;109:1026–1033. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komura H, Miksa M, Wu R, Goyert S M, Wang P. Milk fat globule epidermal growth factor-factor VIII is down-regulated in sepsis via the lipopolysaccharide-CD14 pathway. J Immunol. 2009;182:581–587. doi: 10.4049/jimmunol.182.1.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss L K, Tobias P S. Emerging role of Toll-like receptors in atherosclerosis. J Lipid Res. 2009:S340–S345. doi: 10.1194/jlr.R800056-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill H C, Jr, Herderick E E, McMahan C A, Zieske A W, Malcolm G T, Tracy R E, Strong J P. Atherosclerosis in youth. Minerva Pediatr. 2002;54:437–447. [PubMed] [Google Scholar]
- Cheruvu P K, Finn A V, Gardner C, Caplan J, Goldstein J, Stone G W, Virmani R, Muller J E. Frequency and distribution of thin-cap fibroatheroma and ruptured plaques in human coronary arteries: a pathologic study. J Am Coll Cardiol. 2007;50:940–949. doi: 10.1016/j.jacc.2007.04.086. [DOI] [PubMed] [Google Scholar]
- Burke A P, Kolodgie F D, Zieske A, Fowler D R, Weber D K, Varghese P J, Farb A, Virmani R. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- Hazzard W R, Ettinger W H., Jr Aging and atherosclerosis: changing considerations in cardiovascular disease prevention as the barrier to immortality is approached in old age. Am J Geriatr Cardiol. 1995;4:16–36. [PubMed] [Google Scholar]
- Han S, Liang C P, DeVries-Seimon T, Ranalletta M, Welch C L, Collins-Fletcher K, Accili D, Tabas I, Tall A R. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Collins A R, Lyon C J, Xia X, Liu J Z, Tangirala R K, Yin F, Boyadjian R, Bikineyeva A, Pratico D, Harrison D G, Hsueh W A. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104:e42–e54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
- Weigert A, Johann A M, von Knethen A, Schmidt H, Geisslinger G, Brune B. Apoptotic cells promote macrophage survival by releasing the anti-apoptotic mediator sphingosine-1-phosphate. Blood. 2006;108:1635–1642. doi: 10.1182/blood-2006-04-014852. [DOI] [PubMed] [Google Scholar]
- Cui D, Thorp E, Li Y, Wang N, Yvan-Charvet L, Tall A R, Tabas I. Pivotal advance: macrophages become resistant to cholesterol-induced death after phagocytosis of apoptotic cells. J Leukoc Biol. 2007;82:1040–1050. doi: 10.1189/jlb.0307192. [DOI] [PubMed] [Google Scholar]
- Loke P, Gallagher I, Nair M G, Zang X, Brombacher F, Mohrs M, Allison J P, Allen J E. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol. 2007;179:3926–3936. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- Peng Y, Latchman Y, Elkon K B. Ly6C(low) monocytes differentiate into dendritic cells and cross-tolerize T cells through PDL-1. J Immunol. 2009;182:2777–2785. doi: 10.4049/jimmunol.0803172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard J I, Ricardo-Gonzalez R R, Goforth M H, Morel C R, Subramanian V, Mukundan L, Eagle A R, Vats D, Brombacher F, Ferrante A W, Chawla A. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard J I, Ricardo-Gonzalez R R, Red E A, Vats D, Morel C R, Goforth M H, Subramanian V, Mukundan L, Ferrante A W, Chawla A. Alternative M2 activation of Kupffer cells by PPARδ ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S, Thomas G, Harvey K, Cottell D, Reville K, Berlasconi G, Petasis N A, Erwig L, Rees A J, Savill J, Brady H R, Godson C. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol. 2002;13:2497–2507. doi: 10.1097/01.asn.0000032417.73640.72. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Janssen W J, Fessler M B, McPhillips K A, Borges V M, Bowler R P, Xiao Y Q, Kench J A, Henson P M, Vandivier R W. Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J Immunol. 2006;176:7657–7665. doi: 10.4049/jimmunol.176.12.7657. [DOI] [PubMed] [Google Scholar]
- Leverrier Y, Ridley A J. Requirement for Rho GTPases and PI 3-kinases during apoptotic cell phagocytosis by macrophages. Curr Biol. 2001;11:195–199. doi: 10.1016/s0960-9822(01)00047-1. [DOI] [PubMed] [Google Scholar]