Abstract
Pulmonary fibrosis is associated with a number of disorders that affect the lung. Although there are several cellular types that are involved in the pathogenesis pulmonary fibrosis, the resident lung fibroblast has been viewed traditionally as the primary cell involved in promoting the deposition of ECM that culminates in pulmonary fibrosis. However, recent findings demonstrate that a circulating cell (i.e., the fibrocyte) can contribute to the evolution of pulmonary fibrosis. Fibrocytes are bone marrow-derived mesenchymal progenitor cells that express a variety of cell-surface markers related to leukocytes, hematopoietic progenitor cells, and fibroblasts. Fibrocytes are unique in that they are capable of differentiating into fibroblasts and myofibroblasts, as well as adipocytes. In this review, we present data supporting the critical role these cells play in the pathogenesis of pulmonary fibrosis.
Keywords: trafficking, migration
Introduction
Pulmonary fibrosis is a devastating feature of a number of disorders that affect the lung. The resident fibroblast has been portrayed historically as the critical cell that mediates the deposition of ECM associated with pulmonary fibrosis. However, recent studies have supported the notion that a circulating cell, termed fibrocyte, can promote the pathogenesis of pulmonary fibrosis significantly. Fibrocytes were first identified in 1994 as a circulating cell that extravasated into wounds and contributed to wound repair [1]. Fibrocytes are unique bone marrow-derived mesenchymal progenitor cells that are defined by their growth characteristics and surface phenotype, as they express markers compatible with leukocytes, hematopoietic progenitor cells, and fibroblasts [2]. In addition, fibrocytes express a number of other cell markers that include chemokine receptors and adhesion molecules [3, 4]. Fibrocytes participate in tissue remodeling by producing ECM proteins (i.e., collagen I and collagen III) and by secreting matrix metalloproteinases [5]. Moreover, fibrocytes are an important cellular source of inflammatory cytokines, chemokines, and growth factors that contribute to important autocrine and paracrine signals within the tissue microenvironment [4, 5]. Specific chemokine receptor/chemokine ligand biological axes are critical to the recruitment of fibrocytes to sites of tissue injury and repair, which can contribute to the propagation of the fibrotic response [5]. Fibrocytes can differentiate into other mesenchymal cells, such as myofibroblasts and adipocytes [6, 7]. Fibrocytes have been found to be important mediators of antigen-specific immunity via their ability to function as APCs [8]. Fibrocytes have been shown to deposit ECM in wound repair [1] and during fibroproliferative disorders in response to local tissue injury [9]. These unique cells have become the focus of research efforts that encompass a wide variety of focal and diffuse fibrosing disorders, including those localized to the skin, lungs, liver, kidney, pancreas, and bladder and the more diffuse involvement seen in atherosclerosis and tumors. In this review, we will concentrate on the data supporting the pivotal role of fibrocytes in the pathogenesis of pulmonary fibrosis.
THE CIRCULATING FIBROCYTE
In the context of physiologic and pathologic fibrosis, tissue fibroblasts and myofibroblasts are historically thought to be derived from resident fibroblasts that migrate, proliferate, and express constituents of the ECM in response to tissue injury [10,11,12]. However, two contemporary theories have been proposed that have added complexity to the concept that fibroblast-like cells are only derived from local, resident fibroblasts. The first theory is that tissue injury induces epithelial cells to transition to a mesenchymal phenotype (the fibroblast/myofibroblast; concept of epithelial mesenchymal transition), which subsequently contributes to the fibroproliferative process [1, 11, 13, 14]. The second theory is that circulating fibrocytes (bone marrow-derived progenitor cells) home to and extravasate into sites of tissue injury, differentiate into fibroblasts/myofibroblasts, and contribute to the generation of ECM during the fibroproliferative process in response to injury [1, 3, 9, 11, 13, 15].
The circulating fibrocyte was first described in an experimental model of wound repair [1]. Following the creation of a wound injury, fibrocytes were found in the wound within 24 h. These cells were described as spindle-shaped, coexpressed procollagen/collagen, and CD34 and represented 10% of the cells in the wound. The presence of these cells in the wound occurred faster than what would have been expected by entry of fibroblasts from the surrounding skin into the wound to begin collagen production [1, 16]. In addition, these spindle-shaped cells expressed markers of connective tissue cells, not of monocytes, macrophages, endothelial cells, or epithelial cells [1]. Thus, the word fibrocyte (a term combining fibroblast with leukocyte) was adapted for this circulating fibroblast progenitor cell that produced collagen and expressed the hematopoietic marker CD34 [1, 16]. On scanning electromagnetic, fibrocytes morphologically exhibit prominent cell-surface projections, making them distinct from the appearance of leukocytes [1, 16]. In addition to promoting fibrosis, fibrocytes have been found to function as APCs in promoting activation of T cells [8]. Fibrocytes also promote angiogenesis in vivo through the generation of a variety of proangiogenic signals [3, 5]. It has now been determined that fibrocytes comprise 0.1–1% of the nucleated cells in the peripheral blood of healthy hosts [3, 4, 9, 17] and have been found in a variety of tissues [9] under physiologic and pathologic states [3, 18].
FIBROCYTES CAN DIFFERENTIATE TO OTHER MESENCHYMAL LINEAGE CELLS
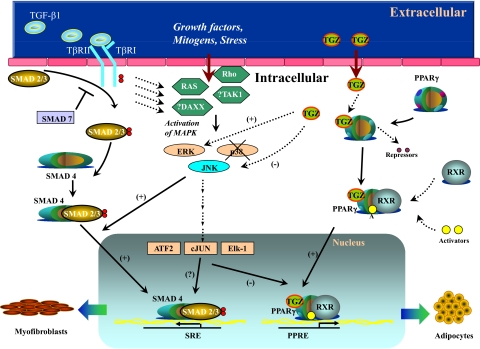
In long-term cell culture and in tissue in vivo, fibrocytes lose their expression of CD34 and CD45 [6, 13, 19,20,21,22]. Although isolated fibrocytes in cell culture (i.e., presence of serum) differentiate spontaneously into myofibroblasts [9, 21, 23], this process is augmented in the presence of TGF-β or endothelin-1 [3, 4, 7, 9, 13, 23], resulting in cells that produce fibronectin and collagen and express the myofibroblast marker αSMA. For example, using a wound-repair model, bone marrow transplantation from GFP-transgenic animals to wild-type animals showed that the cells in the wound coexpressed GFP and αSMA, suggesting that the myofibroblasts present in the wound were from the bone marrow [24]. Fibrocytes have also been found to respond to type 2 cytokines and more readily differentiate. The profibrotic cytokines IL-4 and IL-13 promote fibrocyte differentiation to αSMA-positive cells (i.e., myofibroblasts) from PBMCs without inducing proliferation, whereas the antifibrotic cytokine, IFN-γ, inhibited fibrocyte differentiation [25]. In addition to fibrocytes differentiating into αSMA-positive cells (i.e., myofibroblasts), fibrocytes have been found to differentiate into adipocytes in vitro and in vivo, which appears to be a process that is PPARγ-dependent and inhibited by TGF-β [6, 7] (Fig. 1). These findings, taken together with the previous studies, suggest that fibrocyte differentiation is influenced by a complex profile of cytokines within the local microenvironment of tissue injury.
Figure 1.
Cartoon demonstrating the different signaling pathways that participate in fibrocyte differentiation into αSMA-expressing myofibroblasts or differentiation into aP2-expressing adipocytes. Signaling pathways activated by TGF-β1, including Smad2/3 and stress-activated protein kinases/JNK MAPK, collaborate to induce αSMA transcription, whereas Troglitazone (TGZ)-mediated PPARγ activation leads to induction of aP2 expression. The cross-talk and complex balance of TGF-β with PPARγ signaling, within the context of the local microenvironmental niche, drive the selection of defined differentiation pathways. TβRII/I, TGF-β type II/I receptor; DAXX, death-associated protein 6; TAK1, TGF-β-activated kinase 1; ATF2, activating transcription factor 2; SRE, Smad response element; PPRE, peroxisome proliferator response element; RXR, retinoid X receptor.
THE ORIGIN OF FIBROCYTES APPEARS TO BE THE BONE MARROW
Fibrocytes express markers of hematopoietic cells (CD45, MHC II, and CD34) and stromal cells (collagens I and III) [1, 4, 9, 13, 15, 20, 21, 26, 27]. By microarray analysis of constitutive expression of various ECM genes, fibrocytes express high levels of collagens I, III, and IV (Table 1). The constitutive expression of collagen genes is associated with fibrocyte production of soluble collagen in their conditioned medium in vitro, as compared with lung fibroblasts and isolated peripheral blood monocytes, which can be augmented further in the presence of TGF-β (Table 2). In circulation, human fibrocytes (type I collagen positive by FACS analysis) express more of the common leukocyte antigen CD45 than CD34 (100% positive vs. only 10% positive, respectively; unpublished observation). The cells do not express T cell markers (CD3, CD4, and CD8), B cell markers (CD19), the IL-2R chain CD25, the low-affinity FcγRIII (CD16), or myeloid markers (CD14 and nonspecific esterase) [1, 3, 4, 9, 13, 23]. As the expression of CD34 by the fibrocyte has been shown to decrease over time (in culture and in vivo), depending on the inflammatory milieu [9, 16, 23], the coexpression of collagen production and the other hematologic markers (such as CD45) are used frequently to identify fibrocytes. For example, fibrocytes early in culture are associated with expression of CD34, CD45, Col1, and vimentin; however, after exposure to TGF-β or endothelin, fibrocytes differentiate into myofibroblast-type cells, resulting in expression of αSMA and loss of CD34 and CD45 expression [4, 9, 23, 28]. Thus, the classic markers for circulating fibrocytes are CD34, CD45, and Col1.
TABLE 1.
Fibrocyte Constitutive Gene Expression Determined by Microarray Analysis after Isolation from Human Peripheral Blood
| Name of gene | Relative gene expression |
|---|---|
| GAPDH | 204078 |
| Chemokine receptor (CCR2) | 91 |
| Chemokine receptor (CCR5) | 353 |
| Chemokine receptor (CCR7) | 385 |
| Chemokine receptor (CXCR4) | 7086 |
| Collagen, type I, α 1 | 223848 |
| Collagen, type I, α 2 | 30141 |
| Collagen, type III, α 1 | 122958 |
| Collagen, type IV, α 2 | 120161 |
TABLE 2.
Fibrocytes Produce Significant Collagen In Vitro
| Soluble collagen (Sircol; ug/ml in conditioned medium)
|
|||
|---|---|---|---|
| Condition | Week 1 | Week 2 | Week 3 |
| Fibrocytes unstimulated | 80.9 | 86.7 | 95.8 |
| Fibroblasts unstimulated | 72.9 | 86.7 | 102.6 |
| Fibrocytes stimulated with 10 ng/ml TGF-β | 144.9 | 182.8 | 206.5 |
| Fibroblasts stimulated with 10 ng/ml TGF-β | 138.7 | 196.8 | 225.1 |
| Monocytes | ND | ND | ND |
| Monocytes stimulated with 10 ng/ml TGF-β | ND | ND | ND |
ND, None detected.
Some studies suggest that fibrocytes may differentiate from CD14+ peripheral blood monocytes that express the receptors for the Fc portion of IgG, CD64, and CD32 [20,21,22, 27]. Circulating fibrocytes may be present in a subset of CD14+ CD16– monocytes that carry the chemokine receptor CCR2 on their surface [29, 30]. At the time of tissue injury, this monocyte subset is released from the bone marrow into the peripheral blood and migrates to inflamed sites via a CCR2-mediated pathway [29, 30]. Other studies suggest that human fibrocytes may represent an intermediate stage of differentiation of a monocyte subset into mature fibroblasts and myofibroblasts in tissue [31]. This hypothesis is supported by the fact that fibrocytes express the MHC class I and class II and CD80 and CD86 [1, 8, 20, 21, 32], exhibit antigen-presenting activity [8], and activate CD4+ and CD8+ lymphocytes [8, 32] but do not express markers of monocyte-derived dendritic cells such as CD1a, CD10, and CD83. However, quantitative FACS analysis of freshly isolated human fibrocytes from peripheral blood shows that the majority of these fibrocytes are CD14– and CXCR4+, not CD14+ and CCR2+ (unpublished observation). Although it is increasingly clear that fibrocytes are most likely myeloid lineage in character, it remains to be fully elucidated whether they are truly derived from a CD14+ progenitor cell.
Bone marrow-derived fibrocytes appear to traffic to the lung during the pathogenesis of pulmonary fibrosis
A recent study has demonstrated that CD45+Col1+CXCR4+ fibrocytes contribute to bleomycin-induced pulmonary fibrosis and found that similar cells were expanded in the bone marrow [9]. In addition, another study has shown using a chimeric model of GFP+ bone marrow transplanted into wild-type mice that bone marrow-derived GFP+Col1+ cells were found in the lungs of mice exposed to bleomycin [33]. Based on these two studies, one could speculate that CD45+Col1+CXCR4+ cells and the GFP+Col1+ cells were the same cells (i.e., fibrocytes); however, the two studies did not fully confirm that fibrocytes were bone marrow-derived or that bone marrow-derived fibrocytes actually expand, mobilize, home, and extravasate into the lungs of bleomycin-exposed mice in response to the CXCL12/CXCR4 biological axis. On this basis, bone marrow from GFP+C57BL/6 transgenic mice, which express GFP under the direction of the ubiqutin C promoter, was transplanted into lethally irradiated wild-type mice. After durable bone marrow transplantation, mice were exposed to intratracheal bleomycin. At Day 8, the mice were killed, and single-cell suspensions of their lungs were performed to assess for the presence of fibrocytes in their lungs by quantitative FACS analysis. As shown in Table 3, similar levels of fibrocytes (CD45+Col1+) were found in the lungs as seen previously [9]. More than 60% were CD45+GFP+Col1+ fibrocytes, suggesting that the origin of these cells was from the bone marrow. However, ∼30% were noted to be CD45+GFP–Col1+ fibrocytes. These findings could suggest that CD45+GFP–Col1+ fibrocytes were not from the bone marrow; however, when the bone marrow from these mice was examined, two populations of CD45+Col1+ fibrocytes were found: a larger population that was CD45+GFP+Col1+ and a smaller population of fibrocytes that was CD45+GFP–Col1+ in character (Table 4). These findings suggest that although the bone marrow transplantation is effective in repopulating the bone marrow with CD45+GFP+ cells, it also supports the notion that residual recipient CD45+GFP– cells can be found in the bone marrow of these mice even after lethal total body irradiation.
TABLE 3.
Bone Marrow-Derived GFP+ Fibrocytes in the Lung during Bleomycin-Induced Pulmonary Fibrosis
| Fibrocytes | Saline [cells/lung (×106)] | Bleomycin [cells/lung (×106)] |
|---|---|---|
| CD45+Col1+ | 1.5 ± 0.09 | 3.3 ± 0.12 |
| CD45+Col1+GFP+ | 0.97 ± 0.2 | 2.26 ± 0.15 |
| CD45+Col1+GFP– | 0.48 ± 0.1 | 1.1 ± 0.48 |
A larger population (CD45+Col1+GFP+) and a smaller population (CD45+Col1+GFP–) are found in the lungs of chimeric mice at Day 8 after exposure to bleomycin.
TABLE 4.
Bone Marrow-Derived GFP+ Fibrocytes in the Bone Marrow during Bleomycin-Induced Pulmonary Fibrosis
| Fibrocytes | Saline [cells/BM (×105)] | Bleomycin [cells/BM (×105)] |
|---|---|---|
| CD45+Col1+ | 0.95 ± 0.08 | 3.7 ± 0.12 |
| CD45+Col1+GFP+ | 0.92 ± 0.09 | 3.2 ± 0.2 |
| CD45+Col1+GFP– | 0.03 ± 0.001 | 0.5 ± 0.015 |
A larger population (CD45+Col1+GFP+) and a smaller population (CD45+Col1+GFP–) are found in the bone marrow (BM) of chimeric mice at Day 8 after exposure to bleomycin.
Although Hashimoto and colleagues [33] suggested that if they isolated GFP+Col1+ cells from the lungs of bleomycin-induced murine pulmonary fibrosis, they were unable to detect that these same cells expressed αSMA compatible with differentiation to a myofibroblast-like cell. However, their analysis was performed in vitro after isolation of these cells from the lungs [33]. In contrast, several studies have demonstrated that fibrocytes can differentiate into αSMA+ cells in vivo and in vitro [3, 4, 7, 9, 13, 23, 24, 34] and can be found in the lungs of patients with IPF [18]. To further clarify this potential disparity in the bleomycin murine model of pulmonary fibrosis, studies were performed to analyze the presence of αSMA+ cells from single-cell suspension of lungs of the same GFP+ chimeric mice that were exposed to bleomycin to assess for the presence of αSMA+ fibrocytes in their lungs by quantitative FACS analysis. As shown in Table 5, a significant number of CD45+Col1+αSMA+ cells were found in the lungs of these mice at Day 8 after bleomycin exposure, the majority of which was CD45+GFP+Col1+αSMA+; however, a second, smaller population of CD45+GFP–Col1+αSMA+ cells in the lungs was also detected at the same time-point (Table 5). To confirm that these CD45+GFP–Col1+αSMA+ cells were still bone marrow-derived, the bone marrow of these mice was analyzed and found to demonstrate two populations of CD45+ Col1+αSMA+ cells: a larger population that was CD45+GFP+Col1+αSMA+ and a smaller population that was CD45+GFP–Col1+αSMA+ (Table 6). These findings again suggest that although the bone marrow transplantation is effective in repopulating the recipient bone marrow with cells that can differentiate to GFP+αSMA+ cells, it also supports the notion that residual recipient cells in the bone marrow can differentiate to GFP–αSMA+ cells in these mice, even after lethal total body irradiation. Moreover, although the numbers of these αSMA+ fibrocytes were lower than αSMA– fibrocytes in the lungs and bone marrow of bleomycin-exposed mice, these findings support the notion that fibrocytes can undergo differentiation to αSMA+ cells in vivo. Furthermore, these studies support the notion that fibrocytes found in the lung are bone marrow-derived.
TABLE 5.
Bone Marrow-Derived GFP+ Fibrocytes Undergoing Differentiation to αSMA+ Cells in the Lung during Bleomycin-Induced Pulmonary Fibrosis
| Fibrocytes (αSMA+) | Saline [cells/lung (×105)] | Bleomycin [cells/lung (×105)] |
|---|---|---|
| CD45+Col1+αSMA+ | 1.0 ± 0.2 | 2.4 ± 0.1 |
| CD45+Col1+αSMA+GFP+ | 0.68 ± 0.1 | 1.6 ± 0.1 |
| CD45+Col1+αSMA+GFP– | 0.34 ± 0.12 | 0.77 ± 0.005 |
CD45+Col1+αSMA+ cells in the lungs of chimeric mice at Day 8 after bleomycin exposure. Note: The majority of cells was CD45+Col1+αSMA+GFP+, as compared with CD45+Col1+αSMA+GFP– fibrocytes.
TABLE 6.
Bone Marrow-Derived GFP+ Fibrocytes Undergoing Differentiation to αSMA+ Cells in the Bone Marrow during Bleomycin-Induced Pulmonary Fibrosis
| Fibrocytes (αSMA+) | Saline [cells/BM (×105)] | Bleomycin [cells/BM (×105)] |
|---|---|---|
| CD45+Col1+αSMA+ | 0.46 ± 0.05 | 1.8 ± 0.082 |
| CD45+Col1+αSMA+GFP+ | 0.42 ± 0.05 | 1.4 ± 0.08 |
| CD45+Col1+αSMA+GFP– | 0.04 ± 0.05 | 0.4 ± 0.04 |
CD45+Col1+αSMA+ cells in the bone marrow of chimeric mice at Day 8 after bleomycin exposure. Note: The majority of cells was CD45+Col1+αSMA+GFP+, as compared with CD45+Col1+αSMA+GFP–fibrocytes.
THE CHEMOKINE RECEPTOR/CHEMOKINE (CXCR4/CXCL12) BIOLOGICAL AXIS PLAYS A CRITICAL ROLE IN TRAFFICKING OF CIRCULATING FIBROCYTES INTO THE LUNGS DURING THE PATHOGENESIS OF PULMONARY FIBROSIS
Several lines of evidence support the role of circulating fibrocytes in the development of lung fibrosis [35]. The interest in studying the potential role of fibrocytes in the pathophysiology of lung fibrosis stems from the well-known characteristics of fibrocytes themselves, including: fibrocytes can differentiate into fibroblasts and myofibroblasts [3, 4, 9, 13, 23]; fibrocytes can produce cytokines that induce collagen deposition [3,4,5, 9, 13, 23]; fibrocytes can produce proangiogenic mediators and promote angiogenesis [36]; and fibrocytes are potent APCs that can recruit and activate T cells [8]. Human fibrocytes express several chemokine receptors, including CCR2, CCR5, CCR7, and CXCR4; in contrast, mouse fibrocytes express predominately CCR2, CCR7, and CXCR4 [4, 9, 13, 37]. Fibrocyte migration into wound sites has been quantified previously by ex vivo labeling of cells with a fluorescent dye, followed by intravascular injection and monitoring of their trafficking to intradermal injection sites of CCL21 (chemokine ligand to CCR7) and CXCL12 (chemokine ligand to CXCR4) [13]. The trafficking of CXCR4+ fibrocytes in response to CXCL12 is especially apropos to the importance of chemokine signaling during pulmonary fibrosis and will be discussed in more detail.
The CXCR4/CXCL12 biological axis plays an important role in the homing of bone marrow-derived progenitor cells [38]. CXCR4 is an important chemokine receptor in stem-cell trafficking, and the differential expression of CXCL12 in tissues creates the chemotactic gradient required for trafficking of CXCR4+ cells. In a mouse model of bleomycin-induced pulmonary fibrosis, fibrocytes have been shown to home to the lungs and contribute to fibrosis [9]. In initial studies, isolated human fibrocytes were found to have the increased capacity to home to the lungs of bleomycin-exposed SCID mice during the pathogenesis of pulmonary fibrosis [9]. Similarly, in immunocompetent bleomycin-treated mice, the magnitude of lung procollagen I and III expression correlated with the number of CD45+Col1+CXCR4+ fibrocytes in the bone marrow, blood, and lungs [9]. In addition, CXCL12 was found to be increased significantly in the lungs of mice that were treated with bleomycin, supporting the notion that a CXCL12 gradient between the lungs and the plasma promoted the recruitment of the CD45+Col1+CXCR4+ fibrocytes to the fibrotic lungs. In subsequent experiments, the administration of specific neutralizing anti-CXCL12 antibodies to bleomycin-treated mice resulted in markedly reduced fibrocyte extravasation into the lungs, reduced pulmonary collagen deposition, and reduced morphometric expression of collagen deposition and αSMA. However, the effect of blocking CXCL12 did not alter the magnitude of other leukocyte populations in the lungs under these conditions [9].
Fibrocytes appear to traffic to the lung during the pathogenesis of human pulmonary fibrosis
The finding of the importance of CXCR4/CXCL12 in the mouse model of pulmonary fibrosis has also been found to be important in human pulmonary fibrosis. Recently, a study of patients with idiopathic fibrotic interstitial lung disease demonstrated that the numbers of circulating CD45+, Col1+, CXCR4+ fibrocytes were an order of magnitude higher than in healthy control subjects and accounted for 6–10% of their circulating nucleated cell population. In addition, this study demonstrated that the expression of CXCL12 was markedly elevated in the lungs and plasma of the patients with pulmonary fibrosis [17]. The predominate cell type in the lungs that appeared to express CXCL12 was the hyperplastic type II pneumocyte [17]. This study has been confirmed further in a recent study of patients with IPF [18]. In IPF lung tissue, morphometric analysis by immunofluorescence and confocal microscopy demonstrated the presence of CD34+ or CD45+ CXCR4+ fibrocytes, which coexpressed markers of collagen production and αSMA [18]. In addition, CXCL12 was found to be increased significantly in the plasma of patients with IPF, as compared with healthy controls, and CXCL12 was detectable in the BAL fluid of 40% of the IPF patients but not in control subjects [18]. Similar to the above study, this study demonstrated that CXCL12 was strongly expressed by hyperplastic type II pneumocytes within the lungs [18]. To extend the above studies further, Moeller and colleagues [39] found that the magnitude of circulating fibrocytes in IPF patients correlates directly with their disease exacerbations, and these elevated levels were shown to return to their typical baseline levels with resolution of the exacerbations. Furthermore, this study demonstrated that IPF patients with >5% circulating levels of fibrocytes had a worse prognosis than IPF patients with circulating levels of fibrocytes <5% of the circulating, nucleated cell population. The results of these studies underscore the importance of chemokine-mediated fibrocyte influx in pulmonary fibrosis and indicate that circulating fibrocytes, likely recruited through the CXCR4/CXCL12 axis, may contribute to the expansion of the fibroblast/myofibroblast population in IPF.
REGULATION OF CXCR4 EXPRESSION ON FIBROCYTES: THE ROLE OF HYPOXIA AND ACTIVATION OF THE PI3K/AKT/mTOR PATHWAY
The chemokine receptor CXCR4 appears to be the predominant chemokine receptor expressed on human and mouse fibrocytes [40] and is important in trafficking of fibrocytes from the bone marrow to the lungs during the pathogenesis of pulmonary fibrosis in mice [9]. In human pulmonary fibrosis, there is a direct correlation between lung and plasma levels of CXCL12 and the circulating and lung fibrocyte numbers [17, 18]. These findings support the notion that the CXCL12-CXCR4 axis is a target for therapeutic intervention in pulmonary fibrosis. Further understanding of the mechanisms that regulate the expression of CXCR4 on fibrocytes should facilitate the design of future strategies to target this receptor therapeutically. Previous studies have determined that HIF-1α is the major transcriptional factor for induction of CXCR4 [41,42,43]. Activation of the PI3K/phosphatase and tensin homolog/AKT/mTOR (i.e., normoxia) pathway in the setting of hypoxia results in an additive effect for up-regulating the expression and function of CXCR4 on lung cancer cells in a HIF-1α-dependent manner [41]. This pathway of activation may also be relevant to regulation of CXCR4 on fibrocytes. Moreover, the bone marrow microenvironment is known to be hypoxic relative to arterial blood [44,45,46,47], and examination of hypoxic regulation of CXCR4 expression in fibrocytes is likely relevant to their function in vivo, especially in IPF patients with hypoxemia. Human fibrocytes cultured in hypoxic as compared with normoxic conditions demonstrated marked induction of CXCR4 mRNA in fibrocytes cultured in the hypoxic conditions [40]. The hypoxic bone marrow microenvironment under these conditions would favor the expression of genes that are regulated by HIF-1α. Fibrocytes exposed to hypoxia demonstrated markedly increased cytosolic and intranuclear levels of HIF-1α [40]. In conjunction with increased fibrocyte intranuclear levels of HIF-1α, increased expression of CXCR4 mRNA was seen under hypoxic conditions. When the fibrocytes were exposed to normoxia or hypoxia and then assessed for cell-surface expression of CXCR4, fibrocytes exposed to hypoxia demonstrated a marked increase in their expression of CXCR4. To assess whether hypoxia-induced CXCR4 was functional in promoting fibrocyte migration, fibrocytes were exposed to normoxia or hypoxia and then evaluated for chemotaxis to CXCL12. Fibrocytes preconditioned by hypoxia exhibited enhanced migration to CXCL12. Chromatin immunoprecipitation assay demonstrated the in situ binding of HIF-1α to its cognate DNA-binding motif (hypoxic response element) on the CXCR4 promoter of fibrocytes, which was confirmed by increased transactivation of the CXCR4 promoter under hypoxic conditions. These findings support the notion that bone marrow hypoxia relevant to hypoxemia in patients with IPF is an important factor for promoting the expression of CXCR4 on fibrocytes.
A substantial body of literature supports a role for growth factors in the pathogenesis of pulmonary fibrosis [48,49,50,51], and previous studies have shown that growth factors that activate TKRs increase CXCR4 expression in cancer cells [41]. A number of profibrotic cytokines, such as PDGF, EGF, and IGF-1, have been found to be present in BAL, tissue, and in the circulation of patients with fibroproliferative pulmonary disorders [48,49,50,51]. Although these growth factors have been shown to alter proliferation, matrix production, and intracellular signaling in fibroblasts, little is known about the effect of these cytokines on fibrocytes and whether they would be influential in augmenting migration of these cells in response to conventional chemokine ligands. Gene microarray analysis of fibrocytes demonstrated that these cells express TKR for PDGF, EGF, and IGF (ref. [6], and data not shown). To determine whether TKR activation leads to a change in CXCR4 expression in fibrocytes, cells were treated with PDGF, IGF-1, or EGF under normoxic conditions. PDGF, EGF, and IGF-1 were found to increase the expression of CXCR4. To assess signaling downstream of TKR activation of fibrocytes, fibrocytes were exposed to PDGF under normoxic or hypoxic conditions in the presence or absence of a PI3K inhibitor, with and without the mTOR inhibitor (rapamycin). PDGF markedly increased the expression of CXCR4 mRNA in fibrocytes under normoxic conditions, and this effect was attenuated significantly in the presence of a PI3K or rapamycin. In addition, PDGF stimulation in the presence of hypoxia was additive for induction of CXCR4 expression. However, even under hypoxic conditions, inhibition of PI3K or mTOR in the presence of PDGF resulted in reduced expression of CXCR4 in fibrocytes.
Based on the above in vitro data indicating that inhibition of the PI3K/mTOR pathways abrogates PDGF and hypoxia-induced CXCR4, we sought to determine whether pharmacological inhibition of this pathway in vivo could be used to impair homing and extravasation of fibrocytes in the lungs during bleomycin-induced pulmonary fibrosis. Mice were challenged with intratracheal bleomycin or saline and were treated with daily administration of rapamycin, as compared with vehicle control. Rapamycin treatment inhibited the number of circulating fibrocytes that expressed CXCR4 and markedly attenuated bleomycin-induced CXCR4+ fibrocyte infiltration into the lungs that was accompanied by a concomitant reduction in lung collagen deposition. In addition, the inhibition of fibrocyte homing to the lungs in response to rapamycin treatment was more marked than our previous observation with depletion of CXCL12 [9]. Previous studies have demonstrated that transcriptional regulation of the CXCL12 gene is also under the influence of HIF-1α [52]. Treatment with rapamycin resulted in a 50% decrease in lung tissue levels of CXCL12. These findings support the notion that interruption of the PI3K/AKT/mTOR pathway in vivo inhibits the expression of lung-derived CXCL12 and fibrocyte-derived CXCR4. This therapeutic strategy may have a profound effect on attenuation of the CXCL12-CXCR4 biological axis relevant to fibrocyte trafficking and extravasation in the lungs (Fig. 2).
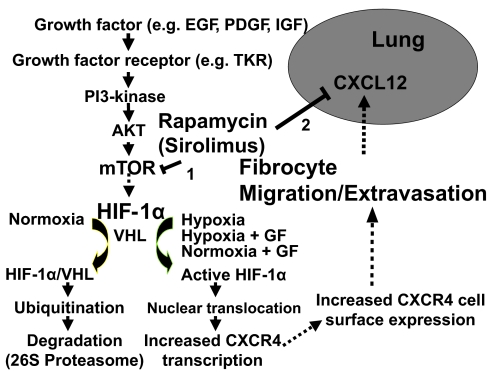
Figure 2.
Cartoon demonstrating the signaling pathways for the up-regulation of CXCR4 expression on fibrocytes related to the role of hypoxia and activation of the PI3K/AKT/mTOR pathway that culminates in promoting trafficking of CXCR4+ fibrocytes to the lung in response to CXCL12. VHL, Von Hippel-Lindau; GF, growth factor.
CONCLUSION
The fibrocyte is a recently described and unique mesenchymal progenitor cell. Increasing evidence points to a pivotal role for these cells as an important source of fibroblasts and myofibroblasts during physiologic and pathologic tissue remodeling and repair processes. Fibrocytes have been detected in the lungs during the pathogenesis of pulmonary fibrosis, and attenuation of their trafficking in mouse models correlates directly with a reduction in pulmonary fibrosis. Furthermore, their circulating levels may represent a biomarker that indicates which IPF patients may be at greater risk for worse prognosis. Additional data are needed to ultimately translate these experimental and clinical findings to the development of novel, therapeutic tools to manipulate these cells selectively and reduce pulmonary fibrosis.
ACKNOWLEDGMENT
Funding for this study was from National Institutes of Health CA87879 and HL66027.
Footnotes
Abbreviations: αSMA=α-smooth muscle actin, aP2=adipocyte protein 2, BAL=bronchoalveolar lavage, Col1=type 1 collagen, ECM=extracellular matrix, EGF=epidermal growth factor, HIF-1α=hypoxia-inducible factor-1α protein, IGF=insulin growth factor, IPF=idiopathic pulmonary fibrosis, mTOR=mammalian target of rapamycin, PDGF=platelet-derived growth factor, PPARγ=peroxisome proliferator-activated receptor-γ, TKR=tyrosine kinase receptor
References
- Bucala R, Spiegel L A, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- Ebihara Y, Masuya M, Larue A C, Fleming P A, Visconti R P, Minamiguchi H, Drake C J, Ogawa M. Hematopoietic origins of fibroblasts: II. In vitro studies of fibroblasts, CFU-F, and fibrocytes. Exp Hematol. 2006;34:219–229. doi: 10.1016/j.exphem.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Metz C N. Fibrocytes: a unique cell population implicated in wound healing. Cell Mol Life Sci. 2003;60:1342–1350. doi: 10.1007/s00018-003-2328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T E, Cowper S, Wu S P, Bockenstedt L K, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Chesney J, Metz C, Stavitsky A B, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160:419–425. [PubMed] [Google Scholar]
- Hong K M, Burdick M D, Phillips R J, Heber D, Strieter R M. Characterization of human fibrocytes as circulating adipocyte progenitors and the formation of human adipose tissue in SCID mice. FASEB J. 2005;19:2029–2031. doi: 10.1096/fj.05-4295fje. [DOI] [PubMed] [Google Scholar]
- Hong K M, Belperio J A, Keane M P, Burdick M D, Strieter R M. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-β and peroxisome proliferator-activated receptor γ. J Biol Chem. 2007;282:22910–22920. doi: 10.1074/jbc.M703597200. [DOI] [PubMed] [Google Scholar]
- Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci USA. 1997;94:6307–6312. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R J, Burdick M D, Hong K, Lutz M A, Murray L A, Xue Y Y, Belperio J A, Keane M P, Strieter R M. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y, Ishizaki M, Masuda Y, Kimura G, Kawanami O, Masugi Y. The role of intraalveolar fibrosis in the process of pulmonary structural remodeling in patients with diffuse alveolar damage. Am J Pathol. 1987;126:171–182. [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Neilson E G. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R, Bellingan G, Laurent G. The acute respiratory distress syndrome: fibrosis in the fast lane. Thorax. 1998;53:815–817. doi: 10.1136/thx.53.10.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe R, Donnelly S C, Peng T, Bucala R, Metz C N. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff T M, Xue C, Okada H, Neilson E G. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter R M, Gomperts B N, Keane M P. The role of CXC chemokines in pulmonary fibrosis. J Clin Invest. 2007;117:549–556. doi: 10.1172/JCI30562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucala R. Circulating fibrocytes: cellular basis for NSF. J Am Coll Radiol. 2008;5:36–39. doi: 10.1016/j.jacr.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Mehrad B, Burdick M D, Zisman D A, Keane M P, Belperio J A, Strieter R M. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–108. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- Andersson-Sjoland A, de Alba C G, Nihlberg K, Becerril C, Ramirez R, Pardo A, Westergren-Thorsson G, Selman M. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–2140. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Kuwana M, Okazaki Y, Kodama H, Izumi K, Yasuoka H, Ogawa Y, Kawakami Y, Ikeda Y. Human circulating CD14+ monocytes as a source of progenitors that exhibit mesenchymal cell differentiation. J Leukoc Biol. 2003;74:833–845. doi: 10.1189/jlb.0403170. [DOI] [PubMed] [Google Scholar]
- Pilling D, Buckley C D, Salmon M, Gomer R H. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;171:5537–5546. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling D, Tucker N M, Gomer R H. Aggregated IgG inhibits the differentiation of human fibrocytes. J Leukoc Biol. 2006;79:1242–1251. doi: 10.1189/jlb.0805456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varcoe R L, Mikhail M, Guiffre A K, Pennings G, Vicaretti M, Hawthorne W J, Fletcher J P, Medbury H J. The role of the fibrocyte in intimal hyperplasia. J Thromb Haemost. 2006;4:1125–1133. doi: 10.1111/j.1538-7836.2006.01924.x. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Sun G, Stacey M A, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- Mori L, Bellini A, Stacey M A, Schmidt M, Mattoli S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res. 2005;304:81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Shao D D, Suresh R, Vakil V, Gomer R H, Pilling D. Pivotal advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83:1323–1333. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba S, Tagami H. Inverse correlation between CD34 expression and proline-4-hydroxylase immunoreactivity on spindle cells noted in hypertrophic scars and keloids. J Cutan Pathol. 1997;24:65–69. doi: 10.1111/j.1600-0560.1997.tb01098.x. [DOI] [PubMed] [Google Scholar]
- Yang L, Scott P G, Giuffre J, Shankowsky H A, Ghahary A, Tredget E E. Peripheral blood fibrocytes from burn patients: identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Invest. 2002;82:1183–1192. doi: 10.1097/01.lab.0000027841.50269.61. [DOI] [PubMed] [Google Scholar]
- Chauhan H, Abraham A, Phillips J R, Pringle J H, Walker R A, Jones J L. There is more than one kind of myofibroblast: analysis of CD34 expression in benign, in situ, and invasive breast lesions. J Clin Pathol. 2003;56:271–276. doi: 10.1136/jcp.56.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Taylor P R. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Tacke F, Randolph G J. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- Balmelli C, Ruggli N, McCullough K, Summerfield A. Fibrocytes are potent stimulators of anti-virus cytotoxic T cells. J Leukoc Biol. 2005;77:923–933. doi: 10.1189/jlb.1204701. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Jin H, Liu T, Chensue S W, Phan S H. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperly M W, Guo H, Gretton J E, Greenberger J S. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:213–224. doi: 10.1165/rcmb.2002-0069OC. [DOI] [PubMed] [Google Scholar]
- Gomperts B N, Strieter R M. Fibrocytes in lung disease. J Leukoc Biol. 2007;82:449–456. doi: 10.1189/jlb.0906587. [DOI] [PubMed] [Google Scholar]
- Hartlapp I, Abe R, Saeed R W, Peng T, Voelter W, Bucala R, Metz C N. Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J. 2001;15:2215–2224. doi: 10.1096/fj.01-0049com. [DOI] [PubMed] [Google Scholar]
- Moore B B, Kolodsick J E, Thannickal V J, Cooke K, Moore T A, Hogaboam C, Wilke C A, Toews G B. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch C. CXCR4: chemokine receptor extraordinaire. Immunol Rev. 2000;177:175–184. doi: 10.1034/j.1600-065x.2000.17715.x. [DOI] [PubMed] [Google Scholar]
- Moeller A, Gilpin S E, Ask K, Cox G, Cook D, Gauldie J, Margetts P J, Farkas L, Dobranowski J, Boylan C, O'Byrne P M, Strieter R M, Kolb M. Circulating fibrocytes are an indicator for poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- Mehrad B, Burdick M D, Strieter R M. Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis. Int J Biochem Cell Biol. 2009;41:1708–1718. doi: 10.1016/j.biocel.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R J, Mestas J, Gharaee-Kermani M, Burdick M D, Sica A, Belperio J A, Keane M P, Strieter R M. Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1{α} J Biol Chem. 2005;280:22473–22481. doi: 10.1074/jbc.M500963200. [DOI] [PubMed] [Google Scholar]
- Schioppa T, Uranchimeg B, Saccani A, Biswas S K, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, Mantovani A, Melillo G, Sica A. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley E J, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumor suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- Harrison J S, Rameshwar P, Chang V, Bandari P. Oxygen saturation in the bone marrow of healthy volunteers. Blood. 2002;99:394. doi: 10.1182/blood.v99.1.394. [DOI] [PubMed] [Google Scholar]
- Parmar K, Mauch P, Vergilio J A, Sackstein R, Down J D. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz B M, Stats D. Oxygen saturation of sternal marrow blood in polycythemia vera. J Clin Invest. 1949;28:736–740. doi: 10.1172/JCI102125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skouby A R. Hematologic adaptation in patients with chronic bronchitis and pulmonary insufficiency. Acta Med Scand. 1976;199:185–190. doi: 10.1111/j.0954-6820.1976.tb06714.x. [DOI] [PubMed] [Google Scholar]
- Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, Lee L B, McMahon G, Grone H J, Lipson K E, Huber P E. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med. 2005;201:925–935. doi: 10.1084/jem.20041393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades H N, Bravo M A, Avila R E, Galanopoulos T, Neville-Golden J, Maxwell M, Selman M. Platelet-derived growth factor in idiopathic pulmonary fibrosis. J Clin Invest. 1990;86:1055–1064. doi: 10.1172/JCI114808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston C, Jagirdar J, Lee T C, Hur T, Hintz R L, Rom W N. Enhanced insulin-like growth factor molecules in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1995;151:1597–1603. doi: 10.1164/ajrccm.151.5.7537587. [DOI] [PubMed] [Google Scholar]
- Yasuoka H, Jukic D M, Zhou Z, Choi A M, Feghali-Bostwick C A. Insulin-like growth factor binding protein 5 induces skin fibrosis: a novel murine model for dermal fibrosis. Arthritis Rheum. 2006;54:3001–3010. doi: 10.1002/art.22084. [DOI] [PubMed] [Google Scholar]
- Ceradini D J, Kulkarni A R, Callaghan M J, Tepper O M, Bastidas N, Kleinman M E, Capla J M, Galiano R D, Levine J P, Gurtner G C. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]