Abstract
Yersinia pestis, the etiological agent of plague, is one of the most deadly pathogens on our planet. This organism shares important attributes with its ancestral progenitor, Yersinia pseudotuberculosis, including a 70-kb virulence plasmid, lymphotropism during growth in the mammalian host, and killing of host macrophages. Infections with both organisms are biphasic, where bacterial replication occurs initially with little inflammation, followed by phagocyte influx, inflammatory cytokine production, and tissue necrosis. During infection, plasmid-encoded attributes facilitate bacterial-induced macrophage death, which results from two distinct processes and corresponds to the inflammatory crescendo observed in vivo: Naïve cells die by apoptosis (noninflammatory), and later in infection, activated macrophages die by pyroptosis (inflammatory). The significance of this redirected cell death for the host is underscored by the importance of phagocyte activation for immunity to Yersinia and the protective role of pyroptosis during host responses to anthrax lethal toxin and infections with Francisella, Legionella, Pseudomonas, and Salmonella. The similarities of Y. pestis and Y. pseudotuberculosis, including conserved, plasmid-encoded functions inducing at least two distinct mechanisms of cell death, indicate that comparative studies are revealing about their critical pathogenic mechanism(s) and host innate immune responses during infection. Validation of this idea and evidence of similar interactions with the host immune system are provided by Y. pseudotuberculosis-priming, cross-protective immunity against Y. pestis. Despite these insights, additional studies indicate much remains to be understood concerning effective host responses against Yersinia, including chromosomally encoded attributes that also contribute to bacterial evasion and modulation of innate and adaptive immune responses.
Keywords: inflammation, pyroptosis, apoptosis, bacterial pathogenesis
Introduction
The yersiniae pathogenic for humans include Yersinia pestis, Yersinia pseudotuberculosis, and Yersinia enterocolitica. Y. pestis, the etiological agent of plague, has killed millions of humans and caused multiple pandemics [1], which provides objective legitimacy to the current concern that it could threaten public health. It was used successfully for bioterrorism in the 14th century (by catapulting corpses of plague victims over city walls); plague-infected fleas were packaged into bombs dropped on Chinese villages in the early part of the 20th century; and more sophisticated means of weaponizing aerosols were investigated by several countries later in the 20th century [2, 3]. Worldwide, the bubonic form of plague is the most common in humans [2], where bites from bacteria-infected fleas provide initial inoculation, and subsequently, bacterial replication in the draining lymph node causes tender swelling and formation of the hallmark bubo. Here, the battle with plague is won or lost [4]; left untreated, the ensuing systemic illness is often fatal. The enteropathogenic Yersinia (Y. pseudotuberculosis and Y. enterocolitica) cause a dramatically different disease but share a tropism for lymph nodes.
Y. pestis is a highly pathogenic clone that evolved from an ancestral Y. pseudotuberculosis strain 1500–20,000 years ago [5]. Two plasmids important for bacterial survival in, and transmission by, fleas [6,7,8] and just 32 additional genes have been acquired by Y. pestis since divergence from Y. pseudotuberculosis [9], indicating that most of Y. pestis virulence determinants were inherited directly from Y. pseudotuberculosis. Correspondingly, Y. pestis and Y. pseudotuberculosis share many important properties (Table 1). Both contain a 70-kb plasmid encoding a T3SS consisting of a basal structure embedded in the bacterial membrane and a needle [10] through which Yersinia deliver effector proteins into host cells. The effector proteins primarily target the host actin cytoskeleton and signaling pathways [11, 12] and include a protein tyrosine phosphatase (YopH), a GTPase activating protein (YopE), a scaffolding protein (YopM), a kinase that also contains a guanine nucleotide dissociation inhibitor domain (Yersnia protein kinase A/YopO), and a serine-threonine acetyltransferase (YopJ). Secretion of effectors is triggered by bacterial contact with host cells [13], and this is mimicked in vitro by growth in conditions resembling host tissues, i.e., 37°C in low calcium media [14,15,16]. These effectors block phagocytosis of Yersinia by macrophages in vitro, which has long been considered vital for extracellular replication in the host and for incapacitating host phagocyte function [11, 17, 18]. Importantly, this plasmid-encoded T3SS also allows Yersinia to induce macrophage cell death, which is discussed in more detail below.
TABLE 1.
Common Features of Y. pestis and Y. pseudotuberculosis
| • 70 kb Virulence plasmid encodes injectisome and common effector proteins |
| • Temperature-dependent phenotypes |
| • Ability of kill macrophages: apoptosis and pyroptosis |
| • Lymphotropism during infection: buboes and mesenteric lymphadenitis |
| • Bi-phasic infection: |
| • initial bacterial replication without inflammation |
| • host response from phagocytes with cytokine production, inflammation, and tissue necrosis |
ENIGMATIC BI-PHASIC CLINICAL DISEASES
Y. pestis is most commonly transmitted by flea bites and Y. pseudotuberculosis by fecal-oral contamination, and the clinical diseases arising from infection are also different. Y. pestis, replicating in a draining lymph node, eventually causes acutely lethal systemic infection, and Y. pseudotuberculosis causes chronic gastrointestinal symptoms, localized mesenteric lymphadenitis, and occasionally systemic infections. However, both organisms display a proclivity for replication in lymph nodes [4, 19,20,21]. These infections are notably bi-phasic: Bacteria initially replicate without a measurable host response for periods up to 36–48 h, but eventually, phagocyte influx into infected tissues and lymph nodes results in inflammation, cytokine production, and tissue necrosis [4, 19, 21,22,23,24,25]. The lack of inflammation during the initial stages of bacterial growth is consistent with the plasmid-encoded T3SS and its truly impressive arsenal of effector molecules limiting phagocyte function; however, clinical disease also encompasses profoundly inflammatory consequences not included by such a model. A more complete explanation would necessarily account for a transition from the remarkable lack of inflammation to a markedly inflammatory host response and suggests the possibility of threshold-gated host responses governing this key transition during Yersinia infections.
CELL DEATH: QUIET COOPERATION YIELDS TO INFLAMMATORY OUTRAGE
Yersinia cause apoptosis in naïve macrophages (Table 2), which requires the translocated effector YopJ. Maximal induction depends on signaling through TLR4 [26, 27], which occurs upon contact of the bacterial LPS with the host macrophage. LPS activates proapoptotic signaling via the adaptor protein Toll/IL-R domain-containing adaptor-inducing IFN-β [28, 29]. LPS also up-regulates cell survival genes and inflammatory cytokine production controlled by MAPK and NF-κB [30,31,32], but YopJ inhibits their activation [33, 34], and therefore, apoptotic signaling predominates [29, 35]: This leads to cleavage and activation of the initiator caspase-8, a cysteine protease, triggers mitochondrial release of cytochrome c and activation of the executioner caspase-3, -7, and -9 [36]. Apoptotic cells conserve ATP to maintain plasma membrane integrity and retain intracellular contents and display surface molecules that direct their uptake by phagocytes [36]. This encounter triggers production of anti-inflammatory cytokines TGF-β and IL-10 [36,37,38,39], making the process noninflammatory. This is not to say that the functions of YopJ, important for inactivating or avoiding host phagocytes, are limited to causing apoptosis in naïve macrophages. The acetyltransferase activity of YopJ may facilitate other forms of cell death [40], and it is important to note that distinct host responses result from delivery of the appropriate effector “cocktail”: Y. enterocolitica YopJ(P) translocates into host cells more efficiently and is more cytotoxic than YopJ alleles commonly expressed by Y. pestis and Y. pseudotuberculosis [41]. Replacing the Y. pseudotuberculosis YopJ with the more cytotoxic Y. enterocolitica YopJ(P) attenuates Y. pseudotuberculosis infection [42]. Thus, appropriate modulation of host cell responses during infection is sensitive to the stoichiometry of bacterial effectors received. Further, YopJ has been shown to be dispensable for pathogenesis [43,44,45], although this remains controversial, and others observe attenuation [42, 46]. These observations underscore the significance of chromosomal genes playing critical, albeit yet undefined, roles in Yersinia pathogenesis (see below), in concert with a highly potent, plasmid-encoded T3SS.
TABLE 2.
Contrast in Cell Death: Apoptosis and Pyroptosis
| Apoptosis | Pyroptosis | |
|---|---|---|
| caspases activated | caspase-3,-8,-9 | caspase-1 |
| membrane damage and cell lysis | phagocytic uptake before lysis | early |
| outcome | noninflammatory | inflammatory |
In contrast, Yersinia cause cell death in activated macrophages by inflammatory pyroptosis (Table 2). This requires the plasmid-encoded T3SS but not YopJ or any of the other known effector molecules [20]. Pyroptosis results from the activation of caspase-1, which is functionally distinct from the structurally related apoptotic caspases [36]. Caspase-1-dependent DNA damage occurs during pyroptosis but by a mechanism distinct from DNA cleavage in cells undergoing apoptosis [20, 36, 47]. Caspase-1 also stimulates maturation and secretion of the inflammatory cytokines IL-1 and IL-18 [48]. It also causes pore formation in the plasma membrane, pathological ion fluxes that lead to cellular swelling, and eventually, lysis and release of inflammatory intracellular contents, which serve to amplify the physiological impact of inflammatory cytokines [49].
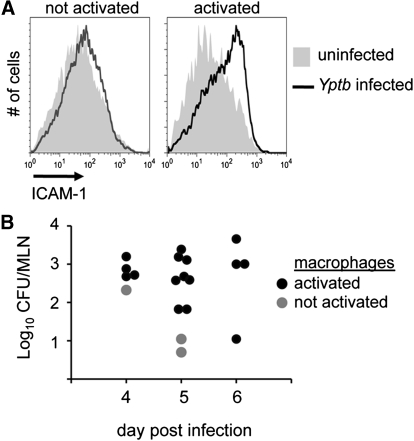
Although some stimuli trigger pyroptosis in naïve cells, such as anthrax lethal toxin and Salmonella infection [50, 51], Yersinia trigger pyroptosis in activated macrophages [20]. Activation of macrophages with TLR ligands, including LPS and TLR2 (palmitoyl-3-cysteine-serine-lysine-4) and TLR3 (polyinosinic:polycytidylic acid) ligands, inhibits caspase-3 activation and renders macrophages susceptible to Yersinia-mediated pyroptosis [20]. Exposure of macrophages to these stimuli up-regulates surface expression of ICAM-1, and this can be used to assess macrophage activation during infection in vivo (Fig. 1A) [20]. In the early stages of infection, significant levels of Yersinia are required for macrophage activation, but this threshold declines as infection progresses (Fig. 1B), suggesting that host functions are amplified or the macrophage populations are exposed to greater concentrations of host- or bacteria-derived activating molecules. Pyroptosis generates inflammation, and these observations suggest the initial apoptotic macrophage response may benefit Yersinia, but redirecting cell death to pyroptosis benefits the host by accelerating inflammation to combat bacterial growth (Fig. 2).
Figure 1.
Threshold-dependent macrophage activation during Yersinia infection. Macrophages from Y. pseudotuberculosis (Yptb)-infected mice were assessed for activation by ICAM-1 expression and compared with macrophages from uninfected mice (A). Shown are representative histograms from infected mice, with (right panel) and without (left panel) activated macrophages. Bacterial colonization of infected mice was quantified by plating and plotted as cfu at Days 4–6 postinfection. The presence of activated macrophages is indicated in the accompanying legend (B). Representative results from one of several experiments; data from ref. [20]. MLN, mesenteric lymph nodes.
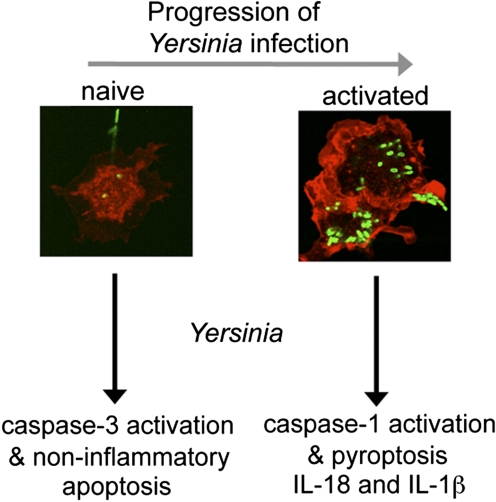
Figure 2.
Redirected cell death during host response to Yersinia infection. Initial interaction of Yersinia with naïve macrophages results in the activation of caspase-3 and induction of noninflammatory apoptosis (left). With additional bacterial replication, macrophage activation occurs as a result of TLR stimulation (or other yet-to-be-defined pathways), and interaction with Yersinia results in caspase-1 activation, maturation, and release of IL-1β and IL-18 and cell lysis with the release of inflammatory intracellular contents (right).
Redirected cell death provides new insight into several observations about host responses to Yersinia infection. First, the ability of Yersinia to trigger noninflammatory death (apoptosis [52,53,54]) of naïve macrophages is consistent with initial bacterial growth in the relative absence of inflammation. Induction of inflammatory death (pyroptosis [20, 55, 56]) in activated macrophages corresponds to later stages of infection, where indicators of protective host responses and inflammation are evident from clinical signs as well as tissue histology and cytokine production (Fig. 2). Activation of macrophages and caspase-1 activation has been demonstrated in vivo during infection [20], and macrophage activation appears to be a threshold-dependent process influenced by level (cfu) and duration (time postinoculation) of infection (Fig. 1). These observations provide an initial mechanistic understanding of the bi-phasic nature of Yersinia infections. Second, during bacterial infection, multiple TLR ligands capable of activating macrophages [57] are inevitably present in vivo. Thus, betrayal of Yersinia by ligands recognized by TLRs facilitates macrophage activation. With the activation of pyroptosis by unknown ligand(s) delivered by the T3SS [20], these events together cause inflammatory cytokine production and the release of inflammatory intracellular contents, which is consistent with an orchestrated and generalized host response to pathological stimuli [50]. Third, macrophage activation is key to controlling Yersinia infection. Macrophage-activating cytokines IFN-γ and TNF-α are critical to host defense against Yersinia [58,59,60,61], and pretreatment of mice with these cytokines confers protection to lethal challenge [62]. Yersinia normally modifies LPS to a less-stimulatory form during growth at 37°C [63], and disrupting this ability reduces bacterial virulence dramatically [64]. Differences in the extent of LPS structural modification(s) [63] may enhance the ability of Y. pestis to replicate systemically and in lymphoid tissues [21], which could contribute significantly to the distinct clinical diseases caused by Y. pestis and Y. pseudotuberculosis. Further, antecedent viral infection that leads to macrophage activation and IFN-γ production protects mice from subsequent challenge with Yersinia [65]. Thus, avoiding phagocyte detection is important for initial bacterial replication and establishing Yersinia infection, and in contrast, phagocyte activation underlies host mechanisms directed toward resisting continued bacterial growth and the clinical signs associated with disease.
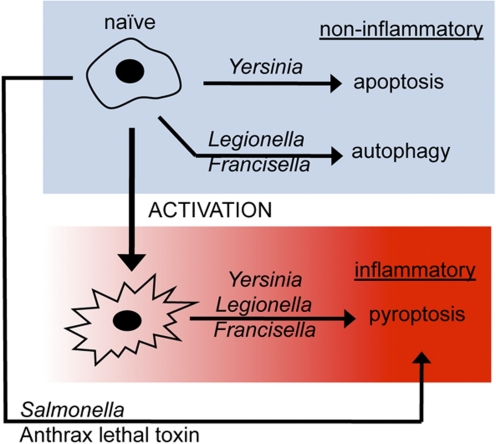
These observations clearly indicate the mechanism of cell death, and the attendant physiological outcome(s) directed by such processes are an important component of host response to infection. The recent literature supports this idea, as a variety of pathogens have evolved the means to battle the host for control of phagocyte function and the physiological outcome(s) provided by biochemically discrete cell death pathways (Fig. 3). This research also suggests the possibility of modifying susceptibility to cell death or changing the pathway(s) used by host phagocytes as alternatives to traditional chemotherapeutic antimicrobials for combating infection.
Figure 3.
Pyroptosis is a conserved effector pathway important in combating infection. The interaction of naïve macrophages with anthrax lethal toxin or Salmonella results in pyroptosis [51]; with Yersinia, noninflammatory apoptosis occurs but can be redirected to pyroptosis (see text for more details [20]); with Legionella [66] or Francisella [67] infection, noninflammatory autophagy occurs and transitions to pyroptosis. Physiological outcome is dictated by cell death mechanism; control is subject to influence by pathogen (ligands and virulence determinants translocated into host cell) and host (macrophage activation and redirected use of cell death pathway) [50].
CHROMOSOMAL ATTRIBUTES PROVIDE COMPLEX, MULTIPROLONGED DECEPTION OF PHAGOCYTES
The 70-kb virulence plasmid plays an important role during Yersinia interactions with phagocytes and remains a primary target of intensive research; however, chromosomally encoded traits also significantly influence bacterial interactions with the host immune system. A commonly held belief is that the primary role of the virulence plasmid is to maintain Yersinia in an extracellular niche. Extracellular bacterial replication in vivo has been well documented [4, 19, 21, 25, 68], but in early stages of infection, the organisms have been found inside macrophages [61, 69,70,71]. Intracellular growth is also observed in vitro and does not require the 70-kb virulence plasmid [72, 73], suggesting the possibility that early in infection, Yersinia replicates inside macrophages prior to maximal expression of plasmid-encoded traits [74]. Chromosomally encoded genes, [75] including the global regulator PhoP [76], are important for growth in macrophages [77, 78]. PhoP also regulates covalent modifications of LPS that reduce its stimulatory capacity [63]. Thus, the ability to avoid, minimize, or delay macrophage activation is an important theme in Yersinia virulence and is not limited to the virulence plasmid.
The virulence plasmid is also dispensable for substantial persistence and/or localized growth in vivo [19, 20, 25, 79, 80]. This is especially true of Y. pseudotuberculosis in lymph nodes, where strains without the virulence plasmid replicate at wild-type levels [19, 20], and remarkably, the bacteria remain extracellular [19]. Indeed, clinical illness has been caused by strains lacking the 70-kb virulence plasmid [81]. Yersinia lacking the virulence plasmid are attenuated in vivo, but their ability to grow extracellularly in lymphoid organs clearly indicates these bacteria have additional capabilities to be uncovered. For example, Y. pseudotuberculosis, without the 70-kb virulence plasmid [82, 83] or with mutations in the T3SS [84], primes protective immunity against Y. pestis, indicating that responses to plasmid-encoded determinants are apparently not necessary for immunity. These data also suggest conservation of chromosomally encoded antigens and critical bacterial interactions with the host immune system and that the initial courses of these two infections must therefore be similarly susceptible to inactivation by specific acquired host immune functions.
CONCLUDING REMARKS
Phagocyte death results from biochemically distinct pathways with discrete and distinguishable outcomes. Cell death may be triggered by pathogens for their benefit or result from activation of biochemical fail-safe systems that sacrifice an infected cell to help defend the surrounding tissue [50]. The mechanism of cell death dictates subsequent physiological consequences, which range from quiescent cell removal to inflammation. Inflammatory cytokine maturation and secretion, together with the release of inflammatory intracellular contents during pyroptosis, recruit additional host defense functions. Pyroptosis can therefore provide the critical milieu for generating adaptive immunity in the case of infection, and in response to other pathological conditions, it stimulates tissue repair. But just beyond this threshold, further inflammation leads to tissue damage and organ dysfunction exemplified by heart attack and stroke, and persistent inflammation is often the harbinger of malignancy. Pyroptosis is a fundamental cellular process significantly contributing to optimal functioning of host immune responses but also, the pathology underlying the major causes of human morbidity and mortality in the developed world. Viewed from the perspective that microbes and the mammalian immune system have been co-evolving for millions of years, bacterial pathogens provide useful tools for probing host cell death. Redirected cell death, as demonstrated during Yersinia infection, suggests the possibility of therapeutic interventions to control cell death responses in favor of quality outcomes for hosts suffering from a variety of clinically important pathological insults. Recent observations support this idea: Using pathogens as bioprobes (Fig. 3) reveals an extensively interconnected system of innate immune responses, where host cell death pathways are redirected to pyroptosis upon phagocyte activation.
ACKNOWLEDGMENTS
We thank National Institutes of Health for grants P50 HG02360 and U54 AI57141 supporting our work about inflammation and innate immune responses and Robert Brubaker and B. T. C. lab members for useful discussions. We apologize to those whose work we could not reference as a result of size limitations.
Footnotes
Abbreviations: T3SS=type III secretion system, Yop=Yersinia outer protein
References
- Stenseth N C, Atshabar B B, Begon M, Belmain S R, Bertherat E, Carniel E, Gage K L, Leirs H, Rahalison L. Plague: past, present, and future. PLoS Med. 2008;5:e3. doi: 10.1371/journal.pmed.0050003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglesby T V, Dennis D T, Henderson D A, Bartlett J G, Ascher M S, Eitzen E, Fine A D, Friedlander A M, Hauer J, Koerner J F, Layton M, McDade J, Osterholm M T, O'Toole T, Parker G, Perl T M, Russell P K, Schoch-Spana M, Tonat K. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 2000;283:2281–2290. doi: 10.1001/jama.283.17.2281. [DOI] [PubMed] [Google Scholar]
- Koirala J. Plague: disease, management, and recognition of act of terrorism. Infect Dis Clin North Am. 2006;20:273–287. doi: 10.1016/j.idc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Sebbane F, Gardner D, Long D, Gowen B B, Hinnebusch B J. Kinetics of disease progression and host response in a rat model of bubonic plague. Am J Pathol. 2005;166:1427–1439. doi: 10.1016/S0002-9440(10)62360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M, Zurth K, Morelli G, Torrea G, Guiyoule A, Carniel E. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc Natl Acad Sci USA. 1999;96:14043–14048. doi: 10.1073/pnas.96.24.14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch B J, Rudolph A E, Cherepanov P, Dixon J E, Schwan T G, Forsberg A. Role of Yersinia murine toxin in survival of Yersinia pestis in the midgut of the flea vector. Science. 2002;296:733–735. doi: 10.1126/science.1069972. [DOI] [PubMed] [Google Scholar]
- Sebbane F, Jarrett C O, Gardner D, Long D, Hinnebusch B J. Role of the Yersinia pestis plasminogen activator in the incidence of distinct septicemic and bubonic forms of flea-borne plague. Proc Natl Acad Sci USA. 2006;103:5526–5530. doi: 10.1073/pnas.0509544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodeinde O A, Subrahmanyam Y V, Stark K, Quan T, Bao Y, Goguen J D. A surface protease and the invasive character of plague. Science. 1992;258:1004–1007. doi: 10.1126/science.1439793. [DOI] [PubMed] [Google Scholar]
- Chain P S, Carniel E, Larimer F W, Lamerdin J, Stoutland P O, Regala W M, Georgescu A M, Vergez L M, Land M L, Motin V L, Brubaker R R, Fowler J, Hinnebusch J, Marceau M, Medigue C, Simonet M, Chenal-Francisque V, Souza B, Dacheux D, Elliott J M, Derbise A, Hauser L J, Garcia E. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc Natl Acad Sci USA. 2004;101:13826–13831. doi: 10.1073/pnas.0404012101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G R. The type III secretion injectisome. Nat Rev Microbiol. 2006;4:811–825. doi: 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]
- Cornelis G R. Yersinia type III secretion: send in the effectors. J Cell Biol. 2002;158:401–408. doi: 10.1083/jcb.200205077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trosky J E, Liverman A D, Orth K. Yersinia outer proteins: Yops. Cell Microbiol. 2008;10:557–565. doi: 10.1111/j.1462-5822.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- Pettersson J, Nordfelth R, Dubinina E, Bergman T, Gustafsson M, Magnusson K E, Wolf-Watz H. Modulation of virulence factor expression by pathogen target cell contact. Science. 1996;273:1231–1233. doi: 10.1126/science.273.5279.1231. [DOI] [PubMed] [Google Scholar]
- Motin V L, Georgescu A M, Fitch J P, Gu P P, Nelson D O, Mabery S L, Garnham J B, Sokhansanj B A, Ott L L, Coleman M A, Elliott J M, Kegelmeyer L M, Wyrobek A J, Slezak T R, Brubaker R R, Garcia E. Temporal global changes in gene expression during temperature transition in Yersinia pestis. J Bacteriol. 2004;186:6298–6305. doi: 10.1128/JB.186.18.6298-6305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S C, Perry R D. Environmental modulation of gene expression and pathogenesis in Yersinia. Trends Microbiol. 1995;3:310–317. doi: 10.1016/s0966-842x(00)88960-x. [DOI] [PubMed] [Google Scholar]
- Straley S C, Plano G V, Skrzypek E, Haddix P L, Fields K A. Regulation by Ca2+ in the Yersinia low-Ca2+ response. Mol Microbiol. 1993;8:1005–1010. doi: 10.1111/j.1365-2958.1993.tb01644.x. [DOI] [PubMed] [Google Scholar]
- Cornelis G R, Boland A, Boyd A P, Geuijen C, Iriarte M, Neyt C, Sory M P, Stainier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viboud G I, Bliska J B. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu Rev Microbiol. 2005;59:69–89. doi: 10.1146/annurev.micro.59.030804.121320. [DOI] [PubMed] [Google Scholar]
- Balada-Llasat J M, Mecsas J. Yersinia has a tropism for B and T cell zones of lymph nodes that is independent of the type III secretion system. PLoS Pathog. 2006;2:e86. doi: 10.1371/journal.ppat.0020086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsbaken T, Cookson B T. Macrophage activation redirects Yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog. 2007;3:e161. doi: 10.1371/journal.ppat.0030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinet F, Ave P, Jones L, Huerre M, Carniel E. Defective innate cell response and lymph node infiltration specify Yersinia pestis infection. PLoS ONE. 2008;3:e1688. doi: 10.1371/journal.pone.0001688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autenrieth I B, Hantschmann P, Heymer B, Heesemann J. Immunohistological characterization of the cellular immune response against Yersinia enterocolitica in mice: evidence for the involvement of T lymphocytes. Immunobiology. 1993;187:1–16. doi: 10.1016/S0171-2985(11)80241-X. [DOI] [PubMed] [Google Scholar]
- Dube P H, Revell P A, Chaplin D D, Lorenz R G, Miller V L. A role for IL-1 α in inducing pathologic inflammation during bacterial infection. Proc Natl Acad Sci USA. 2001;98:10880–10885. doi: 10.1073/pnas.191214498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley S A, Dube P H, Revell P A, Miller V L. Characterization of oral Yersinia enterocolitica infection in three different strains of inbred mice. Infect Immun. 2004;72:1645–1656. doi: 10.1128/IAI.72.3.1645-1656.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathem W W, Crosby S D, Miller V L, Goldman W E. Progression of primary pneumonic plague: a mouse model of infection, pathology, and bacterial transcriptional activity. Proc Natl Acad Sci USA. 2005;102:17786–17791. doi: 10.1073/pnas.0506840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase R, Kirschning C J, Sing A, Schrottner P, Fukase K, Kusumoto S, Wagner H, Heesemann J, Ruckdeschel K. A dominant role of Toll-like receptor 4 in the signaling of apoptosis in bacteria-faced macrophages. J Immunol. 2003;171:4294–4303. doi: 10.4049/jimmunol.171.8.4294. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bliska J B. Role of Toll-like receptor signaling in the apoptotic response of macrophages to Yersinia infection. Infect Immun. 2003;71:1513–1519. doi: 10.1128/IAI.71.3.1513-1519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim S O, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B. Identification of Lps2 as a key transducer of MyD88-independent TIR signaling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- Ruckdeschel K, Pfaffinger G, Haase R, Sing A, Weighardt H, Hacker G, Holzmann B, Heesemann J. Signaling of apoptosis through TLRs critically involves Toll/IL-1 receptor domain-containing adapter inducing IFN-β, but not MyD88, in bacteria-infected murine macrophages. J Immunol. 2004;173:3320–3328. doi: 10.4049/jimmunol.173.5.3320. [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A. NF-κB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- Park J M, Greten F R, Wong A, Westrick R J, Arthur J S, Otsu K, Hoffmann A, Montminy M, Karin M. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis—CREB and NF-κB as key regulators. Immunity. 2005;23:319–329. doi: 10.1016/j.immuni.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ting A T, Marcu K B, Bliska J B. Inhibition of MAPK and NF-κ B pathways is necessary for rapid apoptosis in macrophages infected with Yersinia. J Immunol. 2005;174:7939–7949. doi: 10.4049/jimmunol.174.12.7939. [DOI] [PubMed] [Google Scholar]
- Palmer L E, Hobbie S, Galan J E, Bliska J B. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-α production and downregulation of the MAP kinases p38 and JNK. Mol Microbiol. 1998;27:953–965. doi: 10.1046/j.1365-2958.1998.00740.x. [DOI] [PubMed] [Google Scholar]
- Schesser K, Spiik A K, Dukuzumuremyi J M, Neurath M F, Pettersson S, Wolf-Watz H. The yopJ locus is required for Yersinia-mediated inhibition of NF-κB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol Microbiol. 1998;28:1067–1079. doi: 10.1046/j.1365-2958.1998.00851.x. [DOI] [PubMed] [Google Scholar]
- Denecker G, Declercq W, Geuijen C A, Boland A, Benabdillah R, van Gurp M, Sory M P, Vandenabeele P, Cornelis G R. Yersinia enterocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of bid. J Biol Chem. 2001;276:19706–19714. doi: 10.1074/jbc.M101573200. [DOI] [PubMed] [Google Scholar]
- Fink S L, Cookson B T. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok V A, Bratton D L, Konowal A, Freed P W, Westcott J Y, Henson P M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- Voll R E, Herrmann M, Roth E A, Stach C, Kalden J R, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- Lilo S, Zheng Y, Bliska J B. Caspase-1 activation in macrophages infected with Yersinia pestis KIM requires the type III secretion system effector YopJ. Infect Immun. 2008;76:3911–3923. doi: 10.1128/IAI.01695-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauberman A, Cohen S, Mamroud E, Flashner Y, Tidhar A, Ber R, Elhanany E, Shafferman A, Velan B. Interaction of Yersinia pestis with macrophages: limitations in YopJ-dependent apoptosis. Infect Immun. 2006;74:3239–3250. doi: 10.1128/IAI.00097-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky I E, Medzhitov R. Reduced secretion of YopJ by Yersinia limits in vivo cell death but enhances bacterial virulence. PLoS Pathog. 2008;4:e1000067. doi: 10.1371/journal.ppat.1000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galyov E E, Hakansson S, Wolf-Watz H. Characterization of the operon encoding the YpkA Ser/Thr protein kinase and the YopJ protein of Yersinia pseudotuberculosis. J Bacteriol. 1994;176:4543–4548. doi: 10.1128/jb.176.15.4543-4548.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre N, Sebbane F, Long D, Hinnebusch B J. Yersinia pestis YopJ suppresses tumor necrosis factor α induction and contributes to apoptosis of immune cells in the lymph node but is not required for virulence in a rat model of bubonic plague. Infect Immun. 2006;74:5126–5131. doi: 10.1128/IAI.00219-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S C, Bowmer W S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986;51:445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack D M, Mecsas J, Bouley D, Falkow S. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J Exp Med. 1998;188:2127–2137. doi: 10.1084/jem.188.11.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M A, Cookson B T. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G, Dinarello C A. Interleukin-18 and interleukin-1 β: two cytokine substrates for ICE (caspase-1) J Clin Immunol. 1999;19:1–11. doi: 10.1023/a:1020506300324. [DOI] [PubMed] [Google Scholar]
- Fink S L, Cookson B T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- Bergsbaken T, Fink S L, Cookson B T. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink S L, Bergsbaken T, Cookson B T. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc Natl Acad Sci USA. 2008;105:4312–4317. doi: 10.1073/pnas.0707370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S D, Boland A, Sory M P, van der Smissen P, Kerbourch C, Finlay B B, Cornelis G R. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack D M, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckdeschel K, Roggenkamp A, Lafont V, Mangeat P, Heesemann J, Rouot B. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect Immun. 1997;65:4813–4821. doi: 10.1128/iai.65.11.4813-4821.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotte P, Denecker G, Van Den Broeke A, Vandenabeele P, Cornelis G R, Beyaert R. Targeting Rac1 by the Yersinia effector protein YopE inhibits caspase-1-mediated maturation and release of interleukin-1β. J Biol Chem. 2004;279:25134–25142. doi: 10.1074/jbc.M401245200. [DOI] [PubMed] [Google Scholar]
- Shin H, Cornelis G R. Type III secretion translocation pores of Yersinia enterocolitica trigger maturation and release of pro-inflammatory IL-1β. Cell Microbiol. 2007;9:2893–2902. doi: 10.1111/j.1462-5822.2007.01004.x. [DOI] [PubMed] [Google Scholar]
- Martinez F O, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Autenrieth I B, Heesemann J. In vivo neutralization of tumor necrosis factor-α and interferon-γ abrogates resistance to Yersinia enterocolitica infection in mice. Med Microbiol Immunol. 1992;181:333–338. doi: 10.1007/BF00191545. [DOI] [PubMed] [Google Scholar]
- Parent M A, Wilhelm L B, Kummer L W, Szaba F M, Mullarky I K, Smiley S T. γ Interferon, tumor necrosis factor α, and nitric oxide synthase 2, key elements of cellular immunity, perform critical protective functions during humoral defense against lethal pulmonary Yersinia pestis infection. Infect Immun. 2006;74:3381–3386. doi: 10.1128/IAI.00185-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bliska J B. Role of macrophage apoptosis in the pathogenesis of Yersinia. Curr Top Microbiol Immunol. 2005;289:151–173. doi: 10.1007/3-540-27320-4_7. [DOI] [PubMed] [Google Scholar]
- Lukaszewski R A, Kenny D J, Taylor R, Rees D G, Hartley M G, Oyston P C. Pathogenesis of Yersinia pestis infection in BALB/c mice: effects on host macrophages and neutrophils. Infect Immun. 2005;73:7142–7150. doi: 10.1128/IAI.73.11.7142-7150.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima R, Brubaker R R. Association between virulence of Yersinia pestis and suppression of γ interferon and tumor necrosis factor α. Infect Immun. 1993;61:23–31. doi: 10.1128/iai.61.1.23-31.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeil R, Ernst R K, Gowen B B, Miller S I, Hinnebusch B J. Variation in lipid A structure in the pathogenic yersiniae. Mol Microbiol. 2004;52:1363–1373. doi: 10.1111/j.1365-2958.2004.04059.x. [DOI] [PubMed] [Google Scholar]
- Montminy S W, Khan N, McGrath S, Walkowicz M J, Sharp F, Conlon J E, Fukase K, Kusumoto S, Sweet C, Miyake K, Akira S, Cotter R J, Goguen J D, Lien E. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol. 2006;7:1066–1073. doi: 10.1038/ni1386. [DOI] [PubMed] [Google Scholar]
- Barton E S, White D W, Cathelyn J S, Brett-McClellan K A, Engle M, Diamond M S, Miller V L, Virgin H W., IV Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:326–329. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- Swanson M S, Molofsky A B. Autophagy and inflammatory cell death, partners of innate immunity. Autophagy. 2005;1:174–176. doi: 10.4161/auto.1.3.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry T, Brotcke A, Weiss D S, Thompson L J, Monack D M. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med. 2007;204:987–994. doi: 10.1084/jem.20062665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet M, Richard S, Berche P. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect Immun. 1990;58:841–845. doi: 10.1128/iai.58.3.841-845.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosio C M, Goodyear A W, Dow S W. Early interaction of Yersinia pestis with APCs in the lung. J Immunol. 2005;175:6750–6756. doi: 10.4049/jimmunol.175.10.6750. [DOI] [PubMed] [Google Scholar]
- Finegold M J. Pneumonic plague in monkeys. An electron microscopic study. Am J Pathol. 1969;54:167–185. [PMC free article] [PubMed] [Google Scholar]
- Meyer K F. Immunity in plague; a critical consideration of some recent studies. J Immunol. 1950;64:139–163. [PubMed] [Google Scholar]
- Pujol C, Bliska J B. The ability to replicate in macrophages is conserved between Yersinia pestis and Yersinia pseudotuberculosis. Infect Immun. 2003;71:5892–5899. doi: 10.1128/IAI.71.10.5892-5899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S C, Harmon P A. Growth in mouse peritoneal macrophages of Yersinia pestis lacking established virulence determinants. Infect Immun. 1984;45:649–654. doi: 10.1128/iai.45.3.649-654.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska J B, Casadevall A. Intracellular pathogenic bacteria and fungi—a case of convergent evolution? Nat Rev Microbiol. 2009;7:165–171. doi: 10.1038/nrmicro2049. [DOI] [PubMed] [Google Scholar]
- Pujol C, Grabenstein J P, Perry R D, Bliska J B. Replication of Yersinia pestis in interferon γ-activated macrophages requires ripA, a gene encoded in the pigmentation locus. Proc Natl Acad Sci USA. 2005;102:12909–12914. doi: 10.1073/pnas.0502849102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman E A. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol. 2001;183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenstein J P, Marceau M, Pujol C, Simonet M, Bliska J B. The response regulator PhoP of Yersinia pseudotuberculosis is important for replication in macrophages and for virulence. Infect Immun. 2004;72:4973–4984. doi: 10.1128/IAI.72.9.4973-4984.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyston P C, Dorrell N, Williams K, Li S R, Green M, Titball R W, Wren B W. The response regulator PhoP is important for survival under conditions of macrophage-induced stress and virulence in Yersinia pestis. Infect Immun. 2000;68:3419–3425. doi: 10.1128/iai.68.6.3419-3425.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet M, Mazigh D, Berche P. Growth of Yersinia pseudotuberculosis in mouse spleen despite loss of a virulence plasmid of mol. wt 47 × 10(6) J Med Microbiol. 1984;18:371–375. doi: 10.1099/00222615-18-3-371. [DOI] [PubMed] [Google Scholar]
- Une T, Brubaker R R. In vivo comparison of avirulent Vwa- and Pgm- or Pstr phenotypes of yersiniae. Infect Immun. 1984;43:895–900. doi: 10.1128/iai.43.3.895-900.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima H, Sato T, Nagasako R, Takeda I. Acute mesenteric lymphadenitis due to Yersinia pseudotuberculosis lacking a virulence plasmid. J Clin Microbiol. 1991;29:1271–1275. doi: 10.1128/jcm.29.6.1271-1275.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet M, Berche P, Mazigh D, Veron M. Protection against Yersinia infection induced by non-virulence-plasmid-encoded antigens. J Med Microbiol. 1985;20:225–231. doi: 10.1099/00222615-20-2-225. [DOI] [PubMed] [Google Scholar]
- Taylor V L, Titball R W, Oyston P C. Oral immunization with a dam mutant of Yersinia pseudotuberculosis protects against plague. Microbiology. 2005;151:1919–1926. doi: 10.1099/mic.0.27959-0. [DOI] [PubMed] [Google Scholar]
- Balada-Llasat J M, Panilaitis B, Kaplan D, Mecsas J. Oral inoculation with type III secretion mutants of Yersinia pseudotuberculosis provides protection from oral, intraperitoneal, or intranasal challenge with virulent Yersinia. Vaccine. 2007;25:1526–1533. doi: 10.1016/j.vaccine.2006.10.016. [DOI] [PubMed] [Google Scholar]