Abstract
α2M* targets antigens to APCs for rapid internalization, processing, and presentation. When used as an antigen-delivery vehicle, α2M* amplifies MHC class II presentation, as demonstrated by increased antibody titers. Recent evidence, however, suggests that α2M* encapsulation may also enhance antigen-specific CTL immunity. In this study, we demonstrate that α2M*-delivered antigen (OVA) enhances the production of specific in vitro and in vivo CTL responses. Murine splenocytes expressing a transgenic TCR specific for CTL peptide OVA257–264 (SIINFEKL) demonstrated up to 25-fold greater IFN-γ and IL-2 secretion when treated in vitro with α2M*-OVA compared with soluble OVA. The frequency of IFN-γ-producing cells was increased ∼15-fold, as measured by ELISPOT. Expansion of the OVA-specific CD8+ T cell population, as assayed by tetramer binding and [3H]thymidine incorporation, and OVA-specific cell-mediated cytotoxicity, as determined by a flow cytometric assay, were also enhanced significantly by α2M*-OVA. Furthermore, significant CTL responses were observed at antigen doses tenfold lower than those required with OVA alone. Finally, we also observed enhanced humoral and CTL responses by naïve mice following intradermal immunization with α2M*-OVA. These α2M*-OVA-immunized mice demonstrated increased protection against a s.c.-implanted, OVA-expressing tumor, as demonstrated by delayed tumor growth and prolonged animal survival. The observation that α2M*-mediated antigen delivery elicits specific CTL responses suggests the cross-presentation of antigen onto MHC class I. These results support α2M* as an effective antigen-delivery system that may be particularly useful for vaccines based on weakly immunogenic subunits or requiring dose sparing.
Keywords: vaccination, cytokines, spleen
Introduction
Concerns about the safety of live attenuated and whole microorganism vaccines, as well as expanding knowledge of molecular pathobiology have stimulated increased interest in the delivery of subunit vaccines. Although generally considered safe and relatively simple to manufacture, subunit vaccines are typically poor at eliciting CD8+ T cell immunity. As a result, the use of such vaccines for the prevention and treatment of viral infections, cancers, and other diseases for which immunity is mediated by CTLs is limited. The failure of subunit vaccines to stimulate CTL immunity depends on the lack of presentation of these exogenously delivered antigens on the MHC class I molecules of professional APCs. Therefore, vaccine formulations that introduce these antigens in a manner that promotes their cross-presentation should improve CTL immunity [1]. Although numerous vaccine adjuvants and antigen-delivery vehicles have been studied, few are able to enhance cross-presentation specifically, and most are plagued by significant toxicity or manufacturing issues that often preclude human use.
Although originally characterized as a proteinase inhibitor, the serum protein α2M is also a potent antigen-delivery vehicle. This 718-kDa tetrameric glycoprotein is an abundant and highly conserved member of the thiolester superfamily, which includes complement components C3 and C4 [2]. α2M is present at micromolar concentrations in the plasma of humans, mice, and other animal species [3]. Like the complement components, α2M is cleaved proteolytically to achieve its functionally activated form, α2M*. This proteolytic cleavage of the “bait region” within each α2M subunit triggers a major conformational change that permits the entrapment and subsequent inhibition of proteinases from all four mechanistic classes [4, 5].
During this activation, the thiolesters within each α2M* subunit are subject to nucleophilic attack, allowing for incorporation of a variety of nonproteolytic peptides, proteins, and growth factors, as well as CpG oligodeoxynucleotides [3, 6,7,8]. In addition, activation of α2M exposes receptor recognition sites on each of its four subunits, which permits the binding of α2M* to the scavenger receptor LRP-1, also known as CD91. Cells known to express LRP-1/CD91 include monocytes/macrophages [9, 10], DC [11, 12], B cells [13], and fibroblasts [14, 15]. APCs and other LRP-1/CD91+ cells internalize α2M* rapidly via receptor-mediated endocytosis, resulting in the removal of entrapped proteinases from the extracellular space and the introduction into the cell of any other α2M*-bound molecules [16,17,18].
The observation that α2M* can entrap and bind diverse macromolecules during its transient proteinase-activated state before undergoing rapid internalization by macrophages and other cells led to the hypothesis that α2M* may mediate receptor-enhanced antigen uptake by APCs. Consistent with this hypothesis, our laboratory found that HEL complexed to α2M* demonstrates enhanced macrophage uptake, processing, and presentation to T cells in vitro [19]. Furthermore, rabbits injected with α2M*-HEL demonstrate up to 500-fold greater IgG titers compared with those treated with HEL alone, a response comparable with that observed with HEL in CFA [18]. Based on these findings, it has been hypothesized that a natural role of α2M* may be to facilitate antigen delivery to APCs in areas of inflammation, the conditions of which include enriched levels of proteinases and high concentrations of native α2M [18].
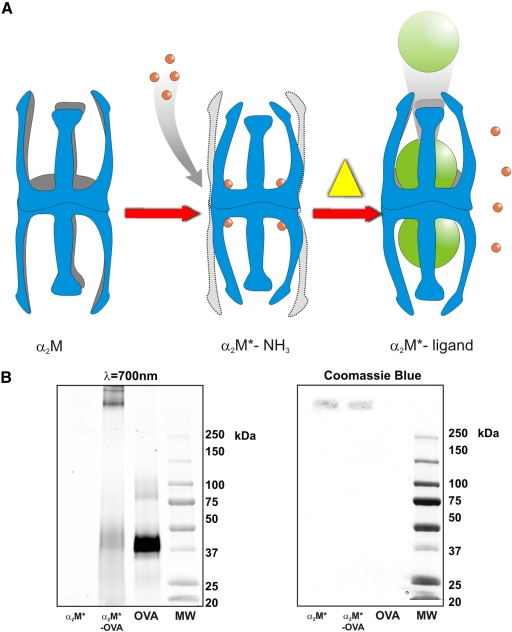
A novel, nonproteolytic method of α2M* encapsulation was developed in our laboratory to improve the efficiency of antigen incorporation [20]. When incubated with ammonia, the α2M thiolesters are cleaved to form α2M* without proteolysis. Heating α2M*-NH3 (at 50°C for 5 h or 37°C for 24 h) results in nucleophile loss and thiolester regeneration, restoring the ability of α2M to inhibit proteinases and allowing it to bind new, available proteins (Fig. 1A). This approach avoids the possibility of antigen degradation by proteinases and removes the competition between proteinase and antigen for available thiolesters, allowing for higher efficiency of antigen incorporation. Furthermore, α2M*-antigen complexes created in this manner bind to macrophages with an affinity identical to that of proteolytically activated α2M* [21]. As a result, this nonproteolytic method suggests greater potential for targeted antigen delivery via α2M*.
Figure 1.
(A) α2M activation and nonproteolytic ligand incorporation. Native α2M is converted to its receptor-recognized conformation, α2M*, by reaction with a proteinase or small nucleophiles, such as NH3 or primary amines. When heated in the presence of excess ligand, α2M* produced with NH3 undergoes a transient conversion to α2M, the ligand is covalently incorporated into the α2M cage-like structures, and α2M reverts to receptor-recognized α2M*. This can be viewed chemically as a mechanism of nucleophilic exchange. (B) Isolation of α2M*-encapsulated OVA. As shown here, 4–15% SDS-PAGE, under nonreducing conditions, demonstrates isolated α2M*-OVA complexes after column purification and concentration, OVA, α2M*, and MW markers. OVA is present at twice the concentration as that in α2M*-OVA, and α2M* concentrations in both lanes are equivalent. α2M* (MW 718 kDa) dissociates into disulfide-linked dimers (MW 360 kDa) under nonreducing conditions and is observed migrating primarily as a single band. In the infrared fluorescence scan, free OVA (MW 45 kDa) can be identified as a major band in Lane 3. In Lane 2, however, OVA is shown to migrate in association with α2M*, and α2M* but not OVA is visible on Coomassie staining as a result of the small mass of OVA loaded on the gel. Infrared fluorescence scan at λ = 700 nm (left) and Coomassie stain (right) are shown.
The ability of α2M*-mediated antigen delivery to enhance specific humoral immune responses has been demonstrated in several studies. In addition to the findings with α2M*-HEL discussed above, α2M* encapsulation produces 100- to 1000-fold enhancement of antibody titers to established but poorly immunogenic vaccine antigens, such as HBsAg [22], and to peptide-based vaccine candidates, such as the HIV envelope gp120 C4-V3 peptide [23]. The observation that α2M* encapsulation causes increased humoral immune responses to antigen is expected based on its rapid and efficient delivery of antigens to endosomal compartments, where they can be processed and presented on MHC class II. Although it has been reported that gp96 [24] and heat shock fusion proteins [13] can enhance cross-presentation in a CD91-dependent process, until recently, little has been known about the impact of α2M* encapsulation on CTL responses.
In this study, we examine the effect of receptor-mediated antigen (OVA) delivery by α2M* on in vitro and in vivo antigen-specific CTL responses. We find that delivery of OVA by α2M* increases the secretion of Tc1 cytokines, expands OVA-specific CD8+ T cells, and enhances antigen-specific, cell-mediated cytotoxicity. Mice immunized with α2M*-OVA demonstrate enhanced protection against an OVA-expressing s.c. tumor. These results suggest that α2M* is an effective antigen-delivery system, capable of enhancing antigen-specific CTL responses, thus supporting α2M* as a promising candidate to improve the immunogenicity of subunit vaccines.
MATERIALS AND METHODS
Mice
Female C57BL/6 mice were obtained from Charles River Laboratories (Raleigh, NC, USA). Female MHC class I-restricted, OVA-specific TCR transgenic mice [C57BL/6-Tg(TcraTcrb)1100Mjb/J, commonly called OT-1 mice] were purchased from Jackson Laboratories (Bar Harbor, ME, USA). All mice were housed in the Duke University Animal Facility (Durham, NC, USA), an Association for Assessment and Accreditation of Laboratory Animal Care International-approved facility. All experiments were conducted under an Institutional Animal Care and Use Committee-approved protocol.
Purification and activation of murine α2M
Purification of α2M was performed using endotoxin-free plasma, columns, and buffers and followed a protocol modified from that described previously [20, 21, 25]. Mouse plasma was purchased from Harlan (Indianapolis, IN, USA). α2M was separated from mouse plasma by consecutive precipitation at 4% and 16% polyethylene glycol (Fluka, Switzerland), followed by DEAE Sephacel fractionation (Sigma-Aldrich, St. Louis, MO, USA) and purified further over a Sephacryl S-300 sizing column (Amersham Biosciences, Uppsala, Sweden). Native α2M was converted to the “activated” form (α2M*) by incubation in 200 mM ammonium bicarbonate at 37°C overnight. Activated protein was then dialyzed for 4 h into PBS and stored at 4°C. Purified protein contained <10 pg endotoxin/mg protein, as determined by a commercial assay kit (Limulus amoebocyte lysate kinetic-quantitative chromogenic by Cambrex, Walkersville, MD, USA).
Encapsulation of OVA into α2M*
Amine-activated α2M* was incubated with a 40-fold molar excess of Alexa Fluor® 647-conjugated OVA (Invitrogen-Molecular Probes, Eugene, OR, USA) for 18 h at 37°C. The resultant α2M*-encapsulated OVA was purified by Sephacryl S-300 size exclusion chromatography (Amersham Biosciences) to remove unbound OVA. Purified α2M*-OVA contained <23 pg endotoxin/mg protein, as determined by the above-mentioned commercial assay kit. Successful incorporation and purification were confirmed by native and SDS-PAGE of the α2M*-OVA complex. Molar ratio of incorporation was determined by fluorescence quantification.
IFN-γ and IL-2 ELISAs
Spleens were harvested from OT-1 mice, and single cell suspensions were prepared by repeated subcapsular injection of complete culture media (high glucose phenol red-free DMEM supplemented with 10% FBS, 1% penicillin-streptomycin, 1 mM sodium pyruvate, 2 mM L-glutamine, 10 mM HEPES, 0.1 mM nonessential amino acids, and 50 μM β-ME). Following hypotonic lysis of RBCs, splenocytes were loaded into 96-well plates at 2.5 × 105 cells/well. In our initial studies examining the dose-dependence of Tc1 cytokine secretion, various concentrations of OVA or α2M*-OVA were added to each well, and cells were incubated for 24 h or 48 h at 37°C in a humidified 5% CO2 incubator. Positive controls included Con A (Sigma-Aldrich) at 5 μg/ml and SIINFEKL peptide (AnaSpec, San Jose, CA, USA) at 0.5 μM. Negative controls included PBS, amine-activated α2M* containing no antigen, and BSA647 (Molecular Probes, Eugene, OR, USA). Cell supernatants were then harvested and stored at −20°C until IFN-γ and IL-2 could be quantified using DUO-ELISA kits (R&D Systems, Minneapolis, MN, USA). Assays comparing the cytokine response with fluorescently labeled OVA versus unlabeled OVA found the biologic effect to be comparable (data not shown). Therefore, throughout this manuscript, OVA and α2M*-OVA refer to the fluorescent species.
In subsequent studies of antigen uptake, splenocytes were prepared as above, but cells were treated with 0.25 μM OVA or α2M*-OVA for 30 min–8 h, washed, and then incubated for an additional 48 h. Supernatants were then harvested, and IFN-γ secretion was measured by ELISA as above.
IFN-γ ELISPOT
The number of cells stimulated to produce IFN-γ was examined using the Becton Dickinson mouse IFN-γ ELISPOT kit (BD Biosciences PharMingen, San Diego, CA, USA). Briefly, OT-1 splenocytes were added at 5 × 105 cells/well to the 96-well ELISPOT plate, which was precoated with an anti-IFN-γ capture antibody. The splenocytes were incubated with antigen (0.0125–0.05 μM α2M*-OVA or free OVA) for 24 h or 48 h at 37°C in a humidified 5% CO2 incubator. Positive controls included Con A at 5 μg/ml and SIINFEKL peptide at 0.5 μM. Negative controls included PBS, 0.02 μM amine-activated α2M* containing no antigen, and 0.05 μM BSA647. After several washes to remove cells, the plate was incubated with a biotinylated anti-IFN-γ detection antibody for 2 h at room temperature and then washed again. The ELISPOT plate was then incubated for 1 h with Streptavidin-HRP and washed, and color development was achieved using the 3-amino-9-ethylcarbazole substrate reagent kit. Following the development of spots, the reaction was stopped with water, the plate was air-dried, and spots were counted using a CTL-ImmunoSpot® plate reader (CTL Analyzers, Cleveland, OH, USA).
Rate of antigen uptake
TG-elicited murine peritoneal macrophages were harvested from C57BL/6 mice by peritoneal lavage and plated at 2.5 × 105 cells/well in complete media in a 96-well plate. Cells were incubated at 37°C for 2 h and washed to remove nonadherent cells, which were treated with 0.05 μM OVA or α2M*-OVA and incubated at 37°C for 5 min–2 h. Cells were then washed four times with ice-cold PBS to remove unbound antigen. Uptake of antigen was measured directly by fluorescence imaging using an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA).
Tetramer binding
Single cell suspensions of C57BL/6 and OT-1 splenocytes were prepared as above and combined at a 20:1 (C57BL/6:OT-1) ratio. These diluted OT-1 cell suspensions were treated with OVA or α2M*-OVA (0.001–0.05 μM) for 4 h, washed, and then incubated at 37°C for a total of 4 days. Cells were stained with iTAg™ MHC Tetramer H-2Kb SIINFEKL-PE (Beckman Coulter, Fullerton, CA, USA) and Caltag™ FITC-conjugated rat anti-mouse CD8a antibody (Invitrogen Corp., Carlsbad, CA, USA) at room temperature for 30 min and then fixed with 0.5% paraformaldehyde and stored light-protected at 4°C until flow cytometric analysis (Guava EasyCyte Plus system, Guava Technologies, Hayward, CA, USA).
Tetramer staining of mouse PBLs was performed after collecting ∼200 μl whole blood from each mouse by puncturing the submandibular vein with a 4-mm animal lancet (Medipoint, Mineola, NY, USA). Staining with tetramer and anti-CD8a antibody was performed as above. RBCs were lysed according to the manufacturer’s directions using the iTag MHC tetramer lyse reagent supplemented with iTag MHC tetramer fixative reagent (Beckman Coulter). Cells were then washed, fixed with 0.5% paraformaldehyde, and stored light-protected at 4°C until flow cytometric analysis.
[3H]Thymidine proliferation assay
OT-1 splenocytes were pulsed for 4 h with 0.001–0.05 μM OVA, free or encapsulated within α2M*. As controls, cells were pulsed with Con A (5 μg/ml) or α2M* containing no antigen (0.02 μM; equivalent to the highest concentration of α2M*-OVA). Cells were then washed, loaded at 5 × 105 cells/well onto a 96-well flat-bottom plate, and cultured 4 days at 37°C in a humidified 5% CO2 incubator. Cultured cells were treated with 1 μCi/well [methyl-3H]thymidine (PerkinElmer, Waltham, MA, USA) 24 h prior to harvesting. [3H]Thymidine incorporation was measured using a Tri-Carb 2100 timed-resolved liquid scintillation counter (PerkinElmer).
Antigen-specific cell-mediated cytotoxicity
Specific lysis of OVA-presenting cells was assayed using the Guava Cell Toxicity kit (Guava Technologies). OT-1 splenocytes were pulsed for 4 h with 0.01 μM OVA or α2M*-OVA. Following treatment with antigen, splenocytes were washed and cultured at 5 × 105 cells/well in a flat-bottom 96-well plate for 48 h at 37°C in a humidified 5% CO2 incubator. OVA-expressing MO5 tumor cells were used as targets for this assay. The MO5 cell line, an OVA-transfected subclone of B16 melanoma, was a kind gift from Dr. Kenneth. Rock (University of Massachusetts Medical School, Worcester, MA, USA). Target cells were stained with CFSE and added to the OT-1 effector cells to achieve E:T ratios between 1:1 and 20:1. Cells were centrifuged at 50 g for 2 min and then incubated at 37°C for 4 h. Cells were then incubated with 7-amino-actinomycin for 10 min to stain dead cells. The percentage of killed target cells was determined using the Guava EasyCyte Plus system (Guava Technologies). To determine the precise proportion of killed MO5 targets, the background percentage of dead MO5 cells in suspension (when not exposed to effector cells) was subtracted from the total percentage of dead MO5 cells.
Intradermal immunizations and tumor challenge
C57BL/6 mice were immunized by intradermal injection into the right ear pinna with 10 μl antigen or PBS, with or without the addition of α2M* or CpG 1826, 5′-TCCATGACGTTCCTGACGTT-3′ (Midland Certified Reagent Co., Midland, TX, USA). The treatment groups (n=5; each receiving 1.35 μg OVA/injection) included the following: OVA alone; OVA administered with 10 μg CpG 1826; α2M*-OVA; and α2M*-OVA administered with 10 μg CpG 1826. Mice were subsequently boosted at Days 35 and 63. Control groups (n=5) received intradermal injections of PBS, CpG 1826 alone, or α2M* containing no antigen (6 μg; equivalent to the amount of α2M* present in the α2M*-OVA preparations). Serum anti-OVA IgG was monitored every 2 weeks by ELISA. Mice were injected s.c. in the left flank with 104 MO5 tumor cells in Matrigel™ basement membrane matrix (BD Biosciences PharMingen) at Week 14. Tetramer staining of mouse PBLs (as described above) was performed 2 weeks following tumor implantation. Tumor diameters were measured using digital calipers, and tumor volume was calculated using the equation V = 0.4ab2, where a and b are the longest and shortest diameters, respectively. Mice were killed when tumor volume reached ∼2 cm3.
Statistical analysis
For the in vitro studies, the Student’s t-test was performed to determine P values and ascertain statistical significance between OVA and α2M*-OVA treatments. For the in vivo studies (antibody titers, tetramer staining, and tumor growth), ANOVA was performed, followed by multiple comparison procedures (Tukey) to determine differences between groups. Significance between Kaplan-Meier survival curves was determined by log-rank Mantel-Cox analysis. The level of significance used was 0.05.
RESULTS
Encapsulation of OVA into α2M*
Native and SDS-PAGE of α2M*-OVA complexes demonstrated successful incorporation of OVA into α2M* as well as effective removal of free OVA (Fig. 1B). α2M dissociates into disulfide-linked dimers under nonreducing conditions and can be observed migrating primarily as a single band. Under SDS-nonreducing conditions, the direct infrared fluorescence scan demonstrates the Alexa Fluor® 647 label comigrating with the α2M* dimers (MW 360 kDa) rather than in the location of free OVA (MW 45 kDa). OVA remains covalently associated with α2M* under SDS-reducing conditions. Fluorescence quantification of complexes revealed a molar ratio of incorporation of 2.5–3.5:1 (OVA:α2M*). Consistent with α2M* incorporation ratios observed for other unlabeled as well as radiolabeled proteins [19,20,21, 23], this finding demonstrates that α2M* encapsulation of the full-length OVA protein was achieved with high efficiency.
α2M* encapsulation of OVA enhances dose-dependent IFN-γ and IL-2 secretion
To better understand the impact of α2M* encapsulation on CTL immunity, we investigated the secretion of Tc1 cytokines (IFN-γ and IL-2) in response to α2M*-OVA. IFN-γ is produced by activated Tc1 CD8+ T cells as well as Th1-differentiated CD4+ T cells, progenitor Th0 cells, DC, and NK cells [26, 27]. IFN-γ performs numerous functions that enhance adaptive immunity, including the up-regulation of antigen processing and presentation by APCs [28], and is critical for the propagation of CD8+ CTLs. Therefore, measurements of IFN-γ secretion and IFN-γ-secreting cells are often used as indicators of CTL activity [29]. IL-2, another Th1/Tc1 cytokine, is produced primarily by activated CD4+ and CD8+ T cells, functioning to support the clonal expansion and differentiation of CTLs [30].
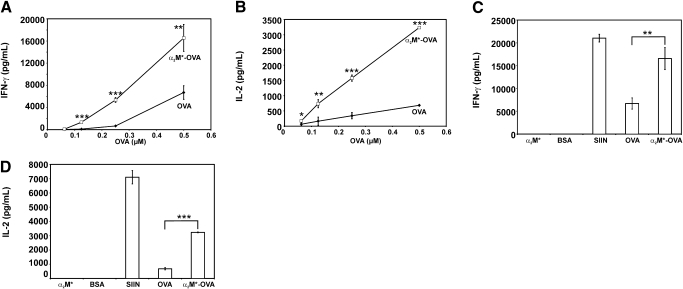
Splenocytes from OT-1 mice were incubated with OVA or α2M*-OVA for 24 h or 48 h. Various concentrations of antigen were studied, and IFN-γ secretion was measured by ELISA. At 24 h and 48 h, incubation with OVA and α2M*-OVA demonstrated dose-dependent increases in IFN-γ. However, at either time, IFN-γ responses rose above baseline levels at lower concentrations of α2M*-OVA compared with OVA alone. At 48 h, a twofold higher concentration of OVA, 0.5 μM, was required to elicit approximately the same response as that stimulated by 0.25 μM α2M*-OVA, suggesting more efficient immune stimulation with α2M* encapsulation than with antigen alone. In addition, the IFN-γ response at 48 h ranged from three- to 13-fold greater with α2M*-OVA compared with OVA alone (Fig. 2A). Although detectable enhancement of IFN-γ secretion was small at the lowest tested concentration, 0.0625 μM OVA, the enhancement seen with α2M*-mediated delivery of OVA, achieved statistical significance at all other tested concentrations.
Figure 2.
(A and B) Splenocyte cytokine production in response to OVA and α2M*-OVA challenge is shown. Splenocytes from OT-1 mice, which express a transgenic MHC class I-restricted TCR that recognizes the SIINFEKL (OVA257–264) peptide, were incubated for 48 h with varying concentrations of OVA or α2M*-OVA. Secretion of (A) IFN-γ and (B) IL-2 was measured by ELISA. (C and D) Splenocyte cytokine responses to BSA647 (BSA), α2M*, and SIINFEKL (SIIN) are shown. For reference, cytokine responses to OVA, alone or incorporated into α2M*, are shown for the highest concentration tested in A and B, 0.5 μM. Secretion of IFN-γ and IL-2 are shown in C and D, respectively. Each concentration was assayed in triplicate; values are mean ± sd. Results are representative of three experiments. Where sd bars are not evident, it is because the sd was too small to be seen on the scale of the graph. Data for 24 h incubation are not shown. *, P < 0.05; **, P < 0.005; ***, P < 0.0001, between α2M*-OVA and OVA responses, as calculated by the Student’s t-test.
OVA and α2M*-OVA also produced dose-dependent increases in splenocyte IL-2 production at 24 h and 48 h. Similar to the IFN-γ studies, an increase in IL-2 secretion was seen with lower concentrations of α2M*-OVA compared with OVA alone. At 48 h, a greater than fourfold concentration of OVA was required to elicit a similar degree of IL-2 secretion as that seen with α2M*-OVA (Fig. 2B). Increases in IL-2 production at 48 h ranged from nearly threefold up to fivefold with α2M*-OVA compared with free OVA. This enhancement of IL-2 secretion with α2M* delivery of OVA was found to be statistically significant at all tested OVA concentrations. These data suggest significant amplification of the CTL response, particularly at low antigen concentrations, with α2M* encapsulation of OVA. Of note, similar cytokine data were obtained with MHC class II-restricted, OVA-specific TCR transgenic mice (termed DO11.10), which express CD4+ TCRs specific for OVA323–339 (data not shown).
To ensure that any observed differences in cytokine secretion were the result of α2M* receptor-mediated antigen delivery and not the immunogenicity of the fluorescent label or the α2M* molecule itself, splenocytes were also incubated with α2M* containing no antigen and BSA647. The concentration of BSA647 used was equivalent to the highest concentration of OVA tested, 0.5 μM. The concentration tested of α2M* containing no antigen was equivalent to the amount of α2M* present, 0.2 μM, at the highest concentration of α2M*-encapsulated OVA, 0.5 μM. As a positive control, splenocytes were treated with the OVA257–264, the SIINFEKL peptide (0.5 μM). Unlike native OVA, the SIINFEKL peptide can bind directly to surface MHC class I molecules without internalization or further processing by APCs. As expected, these cells exhibited the greatest degree of Tc1 cytokine secretion. Treatment with BSA647 or α2M* alone, however, resulted in no detectable increase in IFN-γ or IL-2 secretion (Fig. 2, C and D). These findings demonstrate that the enhanced Tcl cytokine secretion observed with α2M*-OVA is not related to the immunogenicity of α2M* or the fluorescent species.
α2M* encapsulation of antigen enhances the number of IFN-γ-producing Tc1 cells
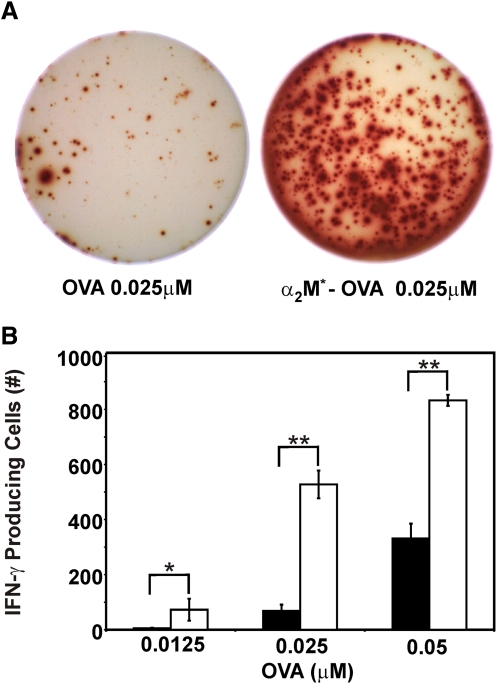
In addition to demonstrating enhanced secretion of Tc1 cytokines with α2M* encapsulation, we quantified the reactive cells responsible for secretion. By ELISPOT assays, we observed that the frequency of activated cells producing IFN-γ increased ∼15-fold with α2M*-OVA compared with OVA alone (Fig. 3). At 48 h, significantly increased numbers of IFN-γ-producing T cells were observed at low concentrations of α2M*-OVA, 0.0125 μM; twofold higher concentrations of OVA, 0.025 μM, were required to elicit similar counts of IFN-γ-producing cells. The increased frequency of IFN-γ-producing cells with α2M* encapsulation was statistically significant at all tested concentrations of antigen. Similar results were also observed at 24 h (data not shown). As seen with the ELISA studies, treatment with BSA647 or α2M* containing no antigen failed to stimulate a detectable IFN-γ response. In summary, the number of IFN-γ-producing cells was observed to be dose-dependent, and the magnitude of enhancement of IFN-γ-producing cells observed with α2M*-OVA compared with OVA was similar to that seen in the ELISA studies. Therefore, we conclude that the increase in Tc1 cytokine secretion observed with α2M* encapsulation is primarily the result of an increased number of stimulated cytokine-producing T cells.
Figure 3.
IFN-γ ELISPOT responses elicited by OVA and α2M*-OVA. Splenocytes harvested from OT-1 mice were incubated for 48 h with varying concentrations of OVA or α2M*-OVA. (A) Representative wells are shown. (B) Quantified spot number, i.e., cells stimulated to produce IFN-γ, is represented as a function of antigen concentration, where cells treated with OVA are represented by solid bars, and cells treated with α2M*-OVA are represented by open bars. All conditions were assayed in triplicate and are represented as mean ± sd. Results are representative of three experiments. *, P < 0.005; **, P < 0.0001, between α2M*-OVA and OVA responses, as calculated by the Student’s t-test.
Rapid uptake of α2M*-encapsulated antigen enhances the CTL response
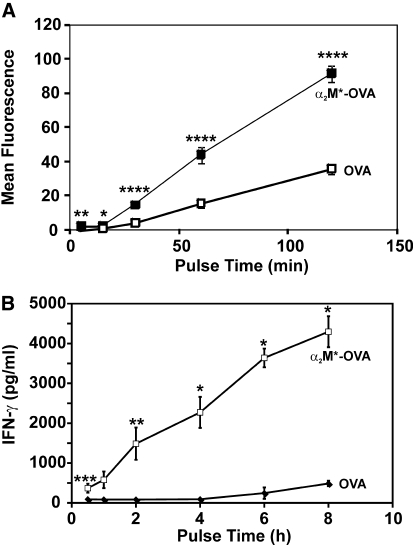
Previous studies have demonstrated that the high-capacity LRP-1/CD91 receptor allows rapid uptake of α2M*-encapsulated antigens, even at low antigen concentrations [8, 12, 19]. Consistent with earlier published studies, we observed significantly greater uptake of α2M*-OVA/unit time compared with free OVA (Fig. 4A). We, therefore, hypothesized that the in vitro enhancement of CTL responses to α2M* encapsulation would be even more pronounced following brief antigen exposure. OT-1 splenocytes were again incubated with OVA (0.25 μM), free or encapsulated within α2M*; cells were then washed and placed in fresh media after 30 min–8 h and cultured for an additional 48 h. As expected, IFN-γ secretion was increased (up to 25-fold) in the cells treated with α2M*-OVA (Fig. 4B). This enhancement was statistically significant for all time-points. Consistent with previous findings, this result supports the hypothesis that the rapid receptor-mediated uptake of α2M*-encapsulated antigens results in enhanced adaptive immune responses in conditions of low antigen concentration or brief antigen exposure, as would often be expected physiologically. Based on these findings, all subsequent studies were performed with 4-h pulses of antigen.
Figure 4.
Rapid uptake of α2M*-encapsulated antigen contributes to the enhanced biological response. (A) Murine TG-elicited peritoneal macrophages were treated with OVA or α2M*-OVA for durations of 5 min–2 h. Cells were then washed, and the quantity of OVA taken up by cells was measured directly by fluorescent imaging. (B) OT-1 splenocytes were pulsed for 30 min–8 h with OVA or α2M*-OVA. After 48 h of subsequent incubation at 37°C, IFN-γ secretion was measured by ELISA. Each time-point was assayed in quadruplicate in A and triplicate in B; values are mean ± sd. Results are representative of three experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, between α2M*-OVA and OVA responses, as calculated by the Student’s t-test.
Expansion of antigen-specific CD8+ T cells with α2M* encapsulation
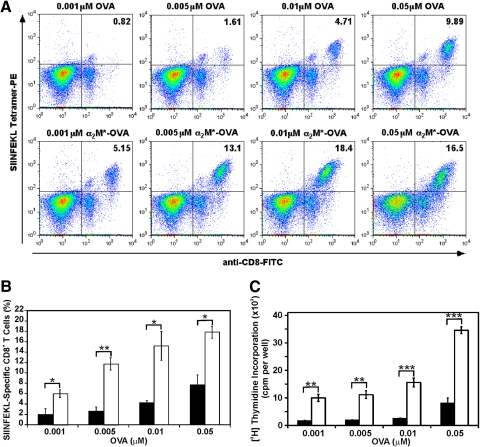
Although the cytokine ELISA and ELISPOT data suggest an enhanced CTL response with α2M*-encapsulated antigen, direct evidence of antigen-specific CD8+ T cell expansion was also obtained by a MHC class I tetramer assay (Fig. 5, A and B). As OT-1 CD8+ T cells are already specific for H-2Kb-SIINFEKL, we diluted OT-1 splenocytes 1:20 with SIINFEKL-naïve C57BL/6 splenocytes to increase our ability to detect significant expansion of antigen-specific T cells, which were then pulsed for 4 h with various concentrations of OVA or α2M*-OVA, washed, and cultured for an additional 4 days. Splenocytes pulsed with α2M*-OVA demonstrated up to eightfold enhancement of tetramer binding compared with cells pulsed with the equivalent amount of free OVA. Furthermore, when averaged over three separate experiments, this increased tetramer binding with α2M*-OVA compared with OVA was found to be statistically significant for all tested antigen exposures. Thus, in addition to enhancing secretion of Tc1 cytokines, α2M* encapsulation results in a greater expansion of antigen-specific CD8+ T cells available to mount a CTL response.
Figure 5.
Expansion of antigen-specific CD8+ T cells with OVA and α2M*-OVA. Suspensions containing a 20:1 ratio of C57BL/6:OT-1 splenocytes were incubated for 4 h with OVA, free or encapsulated within α2M*. Cells were then washed to remove remaining antigen and incubated for another 4 days at 37°C. (A) Comparison of antigen-specific CD8+ T cell expansion with OVA and α2M*-OVA for a representative experiment. (B) The concentration dependence of antigen-specific T cell expansion, as determined by pooling the results of three experiments. Cells treated with OVA are represented by solid bars, and cells treated with α2M*-OVA are represented by open bars. (C) Cell proliferation stimulated by OVA and α2M*-OVA. OT-1 splenocytes were incubated for 4 h with various concentrations of OVA (solid bars) or α2M*-OVA (open bars). After 4 days of culture, cell proliferation was measured by [3H]thymidine incorporation. Each condition was assayed in triplicate; values are mean ± sd. Results are representative of three experiments. *, P < 0.01; **, P < 0.001; ***, P < 0.0001, between α2M*-OVA and OVA responses, as calculated by the Student’s t-test.
To demonstrate further that α2M* delivery enhances T cell proliferation, we also performed a tritiated thymidine incorporation assay (Fig. 5C). Consistent with the above tetramer studies, cells treated with α2M*-OVA demonstrated significantly enhanced proliferation compared with OVA-treated cells. More than tenfold greater concentrations of free OVA were required to elicit the same degree of proliferation as that seen with α2M*-OVA. At any given OVA concentration investigated, a four- to sixfold enhancement of proliferation was observed with α2M*-OVA compared with OVA alone. This increase in cell proliferation with α2M* delivery of antigen was found to be statistically significant at all OVA concentrations tested. This finding further supports our previous observation that α2M* delivery of antigen results in enhanced antigen-specific T cell proliferation.
Enhanced antigen-specific, cell-mediated cytotoxicity with α2M* encapsulation
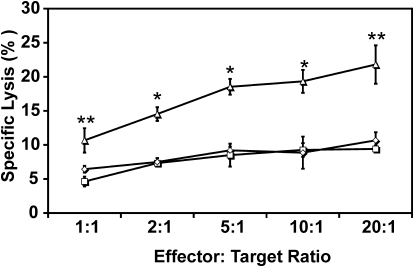
Although the previous data clearly indicate increased expansion of antigen-specific CD8+ T cells with α2M*-OVA, they do not demonstrate improved cytolytic activity directly. To evaluate the functionality of these expanded CTLs, we performed a flow cytometric cytotoxicity assay (Fig. 6) and found that OT-1 splenocytes pulsed with α2M*-OVA demonstrate significantly enhanced cytotoxicity against OVA-expressing MO5 tumor cells compared with OVA-pulsed cells. For each of the conditions examined, treatment with α2M*-OVA stimulated approximately twofold greater lysis by effector cells compared with treatment with OVA alone. Furthermore, lysis observed with OVA treatment did not differ significantly from treatment with PBS. No significant cell lysis was observed under any tested condition against B16 melanoma cells, which do not present OVA. These findings verify the suggestion of previous results that α2M*-mediated antigen delivery increases antigen-specific cell lysis.
Figure 6.
Enhancement of antigen-specific cytotoxicity with α2M*-OVA. OT-1 splenocytes, pulsed with 0.01 μM OVA (□) or α2M*-OVA (▵), were incubated for 4 h with MO5 cells. Splenocytes pulsed with PBS only are represented by ⋄. Antigen-specific cell lysis was measured as the percentage of dead target MO5 cells. Each condition was assayed in triplicate; values are mean ± sd. Results are representative of three experiments. *, P < 0.01; **, P < 0.001, between α2M*-OVA and OVA responses, as calculated by the Student’s t-test.
Immunization with α2M*-encapsulated antigen induces protection against antigen-presenting tumor
Although the previous data establish an enhanced in vitro CTL response with α2M*-mediated antigen delivery, it is important to determine if a similar response can be observed in vivo. Toward this end, groups of C57BL/6 mice (n=5) were immunized with OVA or α2M*-OVA, alone or in combination with CpG 1826, in PBS. Control groups were immunized with PBS, α2M*, or CpG in the absence of the OVA antigen. Mice were subsequently challenged at Week 14 by s.c. implantation of MO5 tumors.
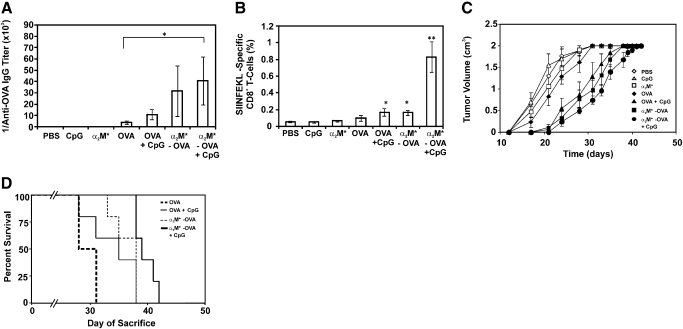
Although the focus of our work is on the development of CTL immunity, we found it pertinent to monitor the development of anti-OVA antibody titers prior to tumor implantation to validate our work in comparison with previous reports of humoral immune responses to α2M*-encapsulated antigen. The development of anti-OVA IgG antibody was observed in treated mice as early as 8 weeks following initial injection. End-point titers (antibody titers at the time of tumor implantation; Week 14) are shown in Figure 7A. Positive antibody titers were observed for each treatment group. Consistent with previous studies of humoral immunity and α2M*-encapsulated antigen [18, 22, 23], mice treated with a combination of α2M*-OVA and a secondary adjuvant (i.e., CpG 1826) developed the strongest antibody titers, which were significantly greater (more than tenfold) than mice treated with OVA alone.
Figure 7.
Immunization with α2M*-encapsulated antigen induces protection against antigen-presenting tumor. (A) End-point titer for each mouse was determined by ELISA and averaged by group. IgG antibody titer was defined as the highest dilution of sera that produced an A450 of 0.125. (B) Tetramer staining of PBLs from each mouse 2 weeks following implantation of MO5 tumors, averaged by treatment group. (C) Tumor growth over days following implantation observed in each mouse, averaged by treatment group. Mice were killed at a tumor volume of ∼2 cm3. (A–C) Values indicate mean ± sem (n=5/group), and ANOVA was performed for each assay, followed by multiple pairwise comparisons (Tukey) to detemine significant differences between groups. In the absence of brackets, P value (*, P<0.05; **, P<0.005) indicates difference between respective treatment group and each of the three control groups (PBS, CpG, α2M*). The presence of brackets indicates a difference between the indicated treatment groups. (D) “Survival” data presented as Kaplan-Meier plot. As survival of control groups (PBS, CpG, and α2M*-treated mice) was not significantly different from that of the OVA treatment group, survival data for these groups are omitted from this plot for simplicity. Statistical significance between survival curves was determined by Mantel-Cox log-rank analysis and is discussed in the text.
To observe expansion of the antigen-specific CD8+ T cell population in these mice, tetramer staining of PBLs was performed 2 weeks following tumor implantation (Fig. 7B). The α2M*-OVA without CpG and the OVA with CpG treatment groups demonstrated a significant expansion of the OVA-specific CD8+ T cell population compared with the control groups. However, as with the antibody titer data, mice treated with a combination of α2M*-OVA and CpG demonstrated by far the strongest response (>17-fold greater than control groups).
The above antibody titer and tetramer data demonstrate an enhancement of humoral and cell-mediated immunity with α2M*-mediated antigen delivery. However, observations of MO5 tumor growth are necessary to understand whether these responses actually represent effective anti-tumor activity. Tumor growth for each mouse treatment group is demonstrated in Figure 7C. Observable growth of MO5 tumors was delayed in each of the treatment groups. Survival curves for each group following tumor implantation were calculated by the Kaplan-Meier method (Fig. 7D), and statistical significance was determined by log-rank Mantel-Cox analysis. Each of the α2M*-encapsulated and CpG treatment groups demonstrated improved survival (i.e., prolonged time before tumor volume reached 2 cm3) compared with the PBS, CpG, and α2M* control groups. Survival of α2M*-OVA-treated mice was not significantly different from that of mice treated with OVA and CpG. Consistent with the antibody and tetramer data, the treatment group that combined α2M*-OVA with CpG demonstrated the strongest anti-tumor response and the longest survival; this improvement in survival was statistically significant compared with all other groups (P values ranging from 0.0018 to 0.029). We propose that this anti-tumor response is largely the result of CTL activity; however, given the significantly enhanced humoral response reported above, we cannot rule out a contribution from antibody-dependent, cell-mediated cytotoxicity at this time.
DISCUSSION
Given the necessity of potent CTL responses for the prevention and treatment of numerous human diseases, including viral infections and cancers, the development of practical and effective adjuvants or antigen-delivery systems that promote CTL immunity is imperative. α2M incorporates a wide variety of nonproteolytic antigens when coincubated with such antigens during proteolytic activation [20]. Antigens incorporated in this manner demonstrate enhanced MHC class II presentation to antigen-specific T cells in vitro and increased antibody production in rabbits in vivo [18, 19]. A nonproteolytic method of antigen incorporation developed and used in subsequent studies has shown increased antibody titers to HBsAg and HIV envelope gp120 C4-V3 peptide with α2M*-antigen complexes relative to antigen alone [22, 23]. These studies, however, were limited to measurements of humoral immune responses.
Here, we demonstrate that α2M*-antigen complexes also amplify CTL responses. When OVA is incorporated into α2M*, Tc1 cytokine (IFN-γ and IL-2) production is enhanced up to 25-fold compared with OVA alone. Detectable CTL responses are observed with antigen doses of α2M*-OVA two- to tenfold less than those needed with OVA alone. In addition to demonstrating a quantifiable enhancement of Tc1 cytokine production, we show an ∼15-fold increase in the number of IFN-γ-secreting Tc1 cells with α2M*-OVA and that these cells, as shown by tetramer assay and [3H]thymidine incorporation, represent an expansion of the antigen-specific CD8+ T cell population. This CTL expansion is also associated with increased antigen-specific cellular cytotoxicity. Furthermore, these findings were validated in vivo, as mice immunized with α2M*-encapsulated antigen demonstrated enhanced protection against antigen-presenting tumors.
The anti-tumor response observed with α2M*-mediated antigen delivery was comparable with that of an accepted vaccine adjuvant (CpG 1826) and as expected, was improved further by combining α2M* delivery with that adjuvant. This finding is consistent with previous work that found α2M* delivery to elicit humoral responses comparable with or better than CFA or monophosphoryl lipid A-squalene emulsion with GM-CSF, and codelivery of these adjuvants with α2M* complexes yields the strongest response [18, 22, 23]. This finding validates the investigation of α2M* as a delivery system capable of enhancing immunity by a magnitude similar to other investigative adjuvants. Furthermore, as α2M* has been shown to elicit even more robust responses when combined with a secondary adjuvant, vaccine formulations containing combinations of α2M*-encapsulated antigen with other adjuvants may have an improved success rate.
Amplification of CTL responses is historically very difficult to achieve using vaccine adjuvants that are safe and cost-effective. Some adjuvants that are known to elicit potent Th1/Tc1 and Th2/Tc2 responses, such as CFA, are too toxic for human use. Others, including liposomal adjuvants, appear to enhance humoral and cellular immunity safely but suffer from significant stability and manufacturing issues [31]. However, no toxic effects of α2M occur during in vivo animal studies. This result is not surprising, given its high concentration in plasma [3]. The wide availability of α2M, from large-scale protein fractionation or cloning, and its stability in solution at room temperature further increase its appeal as an antigen-delivery vehicle, especially lyophilized and stored for use in developing nations [18, 22, 23].
The observation that α2M*-mediated antigen delivery is capable of enhancing specific CTL responses provides new support for the use of α2M* as an in vivo antigen-delivery vehicle. Although originally thought to promote only MHC class II presentation [18], it now appears evident that α2M*-mediated antigen delivery also promotes the cross-presentation of exogenous antigen onto MHC class I. This finding, evidenced by the enhanced antigen-specific CTL responses described here, suggests that α2M*, given its ability to incorporate diverse antigen, may be quite useful for the delivery of subunit vaccines. Furthermore, these results suggest that α2M* may serve as a helpful resource for the study of cross-presentation and cross-priming.
The findings presented here are consistent with previous reports of enhanced CTL immunity with α2M*-antigen complexes [32, 33]. These reports demonstrated that α2M*-bound peptides stimulate IFN-γ secretion by cells that express the α2M* receptor, CD91, and that mice immunized with these α2M*-bound peptides exhibit relative protection against antigen-presenting tumors. In contrast to these reports, here, we demonstrate that a full-length protein, rather than a peptide, may be incorporated and delivered by α2M*, processed by APCs, and cross-presented to stimulate CTL immunity. We have also further characterized the impact of α2M*-mediated antigen delivery on CTL immunity by examining the activation and expansion of antigen-specific CD8+ CTLs by ELISPOT and in vitro and in vivo tetramer assays. Furthermore, we believe that the high efficiency by which α2M* incorporates the whole OVA protein in this study (2.5-3.5:1 OVA:α2M*), compared with the relatively low incorporation of peptide presented in the above-mentioned studies (∼0.05:1 peptide:α2M*), represents an improved model for examining the biological potential of using α2M* as an antigen-delivery vehicle.
In conclusion, we demonstrate in this study that receptor-mediated delivery of antigen by α2M* promotes cross-presentation and stimulation of antign-specific CTL responses. When considered in the context of previous publications, these results suggest that α2M* is an effective antigen-delivery system that enhances humoral and cell-mediated immunity. Other characteristics of α2M*, including its favorable safety profile, cost-effectiveness, and flexibility to incorporate small ligands and large proteins efficiently, further support its potential use for vaccines based on weakly immunogenic subunits.
AUTHORSHIP
E. V. B. conducted the majority of the experiments described in this manuscript. J. J. H. conducted preliminary experiments that provided the foundation for this work. J. E. B. assisted in conducting several of the experiments reported here. G. J. C. is a co-investigator who assisted in experimental design and the interpretation of results. S. V. P. is the principal investigator who oversaw this study.
ACKNOWLEDGMENTS
This work was supported by grant #HL-24066 from the National Heart, Lung, and Blood Institute. We thank Dr. K. Rock for his kind gift of the MO5 tumor cell line. We also thank the Duke Human Vaccine Institute for use of the ELISPOT plate reader. Finally, many thanks to Sturgis Payne, Yvonne Mowery, Steve Conlon, and Marie Thomas for their contributions to this work.
Footnotes
Abbreviations: α2M=α2-macroglobulin, α2M*=receptor-recognized/activated a2M, α2M*-OVA=α2M*-encapsulated OVA, BSA647=Alexa Fluor® 647-conjugated BSA, CpG 1826=5′-TCCATGACGTTCCTGACGTT-3′, DC=dendritic cell(s), HBsAg=hepatitis B surface antigen, HEL=hen egg lysozyme, LRP-1=low-density lipoprotein receptor-related protein 1 (or CD91), OVA257–264=H2-Kb-restricted CTL epitope of OVA (SIINFEKL peptide), Tc1=type 1 CD8+ CTL, TG=thioglycollate
References
- Rock K L, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol Rev. 2005;207:166–183. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- Swenson R P, Howard J B. Structural characterization of human α2-macroglobulin subunits. J Biol Chem. 1979;254:4452–4456. [PubMed] [Google Scholar]
- Chu C T, Pizzo S V. α 2-Macroglobulin, complement, and biologic defense: antigens, growth factors, microbial proteases, and receptor ligation. Lab Invest. 1994;71:792–812. [PubMed] [Google Scholar]
- Barrett A J, Brown M A, Sayers C A. The electrophoretically “slow” and “fast” forms of the α 2-macroglobulin molecule. Biochem J. 1979;181:401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A J, Starkey P M. The interaction of α 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973;133:709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James K. Interactions between cytokines and α 2-macroglobulin. Immunol Today. 1990;11:163–166. doi: 10.1016/0167-5699(90)90067-j. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L, Petersen T E, Magnusson S. Trypsin-induced activation of the thiol esters in α 2-macroglobulin generates a short-lived intermediate (“nascent” α 2-M) that can react rapidly to incorporate not only methylamine or putrescine but also proteins lacking proteinase activity. FEBS Lett. 1981;128:123–126. doi: 10.1016/0014-5793(81)81096-4. [DOI] [PubMed] [Google Scholar]
- Anderson R B, Cianciolo G J, Pizzo S V. α2-Macroglobulin binds CpG oligodeoxynucleotides and enhances their immunostimulatory properties by a receptor-dependent mechanism. J Leukoc Biol. 2008;83:381–892. doi: 10.1189/jlb.0407236. [DOI] [PubMed] [Google Scholar]
- Kaplan J, Nielsen M L. Analysis of macrophage surface receptors. II. Internalization of α-macroglobulin. trypsin complexes by rabbit alveolar macrophages. J Biol Chem. 1979;254:7329–7335. [PubMed] [Google Scholar]
- Kaplan J, Nielsen M L. Analysis of macrophage surface receptors. I. Binding of α-macroglobulin. protease complexes to rabbit alveolar macrophages. J Biol Chem. 1979;254:7323–7328. [PubMed] [Google Scholar]
- Feldman S R, Sangha N D. Immunohistochemical localization of α 2-macroglobulin receptors in human skin. Acta Derm Venereol. 1992;72:331–333. [PubMed] [Google Scholar]
- Hart J P, Gunn M D, Pizzo S V. A CD91-positive subset of CD11c+ blood dendritic cells: characterization of the APC that functions to enhance adaptive immune responses against CD91-targeted antigens. J Immunol. 2004;172:70–78. doi: 10.4049/jimmunol.172.1.70. [DOI] [PubMed] [Google Scholar]
- Tobian A A, Harding C V, Canaday D H. Mycobacterium tuberculosis heat shock fusion protein enhances class I MHC cross-processing and -presentation by B lymphocytes. J Immunol. 2005;174:5209–5214. doi: 10.4049/jimmunol.174.9.5209. [DOI] [PubMed] [Google Scholar]
- Van Leuven F, Cassiman J J, Van den Berghe H. Uptake and degradation of α2-macroglobulin-protease complexes in human cells in culture. Exp Cell Res. 1978;117:273–282. doi: 10.1016/0014-4827(78)90141-6. [DOI] [PubMed] [Google Scholar]
- Maxfield F R, Willingham M C, Haigler H T, Dragsten P, Pastan I H. Binding, surface mobility, internalization, and degradation of rhodamine-labeled α 2-macroglobulin. Biochemistry. 1981;20:5353–5358. doi: 10.1021/bi00521a041. [DOI] [PubMed] [Google Scholar]
- Chu C T, Howard G C, Misra U K, Pizzo S V. α 2-Macroglobulin: a sensor for proteolysis. Ann N Y Acad Sci. 1994;737:291–307. doi: 10.1111/j.1749-6632.1994.tb44319.x. [DOI] [PubMed] [Google Scholar]
- Misra U K, Gonzalez-Gronow M, Gawdi G, Hart J P, Johnson C E, Pizzo S V. The role of Grp 78 in α 2-macroglobulin-induced signal transduction. Evidence from RNA interference that the low density lipoprotein receptor-related protein is associated with, but not necessary for, GRP 78-mediated signal transduction. J Biol Chem. 2002;277:42082–42087. doi: 10.1074/jbc.M206174200. [DOI] [PubMed] [Google Scholar]
- Chu C T, Oury T D, Enghild J J, Pizzo S V. Adjuvant-free in vivo targeting. Antigen delivery by α 2-macroglobulin enhances antibody formation. J Immunol. 1994;152:1538–1545. [PubMed] [Google Scholar]
- Chu C T, Pizzo S V. Receptor-mediated antigen delivery into macrophages. Complexing antigen to α 2-macroglobulin enhances presentation to T cells. J Immunol. 1993;150:48–58. [PubMed] [Google Scholar]
- Gron H, Pizzo S V. Nonproteolytic incorporation of protein ligands into human α 2-macroglobulin: implications for the binding mechanism of α 2-macroglobulin. Biochemistry. 1998;37:6009–6014. doi: 10.1021/bi973027c. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee G, Gron H, Pizzo S V. Incorporation of non-proteolytic proteins by murine α2-macroglobulin. Biochim Biophys Acta. 1999;1432:49–56. doi: 10.1016/s0167-4838(99)00072-2. [DOI] [PubMed] [Google Scholar]
- Cianciolo G J, Enghild J J, Pizzo S V. Covalent complexes of antigen and α(2)-macroglobulin: evidence for dramatically-increased immunogenicity. Vaccine. 2001;20:554–562. doi: 10.1016/s0264-410x(01)00361-9. [DOI] [PubMed] [Google Scholar]
- Liao H X, Cianciolo G J, Staats H F, Scearce R M, Lapple D M, Stauffer S H, Thomasch J R, Pizzo S V, Montefiori D C, Hagen M, Eldridge J, Haynes B F. Increased immunogenicity of HIV envelope subunit complexed with α2-macroglobulin when combined with monophosphoryl lipid A and GM-CSF. Vaccine. 2002;20:2396–2403. doi: 10.1016/s0264-410x(02)00090-7. [DOI] [PubMed] [Google Scholar]
- Robert J, Ramanayake T, Maniero G D, Morales H, Chida A S. Phylogenetic conservation of glycoprotein 96 ability to interact with CD91 and facilitate antigen cross-presentation. J Immunol. 2008;180:3176–3182. doi: 10.4049/jimmunol.180.5.3176. [DOI] [PubMed] [Google Scholar]
- Gron H, Thogersen I B, Enghild J J, Pizzo S V. Structural and functional analysis of the spontaneous re-formation of the thiol ester bond in human α 2-macroglobulin, rat α 1-inhibitor-3 and chemically modified derivatives. Biochem J. 1996;318:539–545. doi: 10.1042/bj3180539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenborn J R, Wilson C B. Regulation of interferon-γ during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- Suto A, Nakajima H, Tokumasa N, Takatori H, Kagami S, Suzuki K, Iwamoto I. Murine plasmacytoid dendritic cells produce IFN-γ upon IL-4 stimulation. J Immunol. 2005;175:5681–5689. doi: 10.4049/jimmunol.175.9.5681. [DOI] [PubMed] [Google Scholar]
- Schroder K, Hertzog P J, Ravasi T, Hume D A. Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Horton H, Russell N, Moore E, Frank I, Baydo R, Havenar-Daughton C, Lee D, Deers M, Hudgens M, Weinhold K, McElrath M J. Correlation between interferon-γ secretion and cytotoxicity, in virus-specific memory T cells. J Infect Dis. 2004;190:1692–1696. doi: 10.1086/424490. [DOI] [PubMed] [Google Scholar]
- Kristensen N N, Christensen J P, Thomsen A R. High numbers of IL-2-producing CD8+ T cells during viral infection: correlation with stable memory development. J Gen Virol. 2002;83:2123–2133. doi: 10.1099/0022-1317-83-9-2123. [DOI] [PubMed] [Google Scholar]
- Petrovsky N, Aguilar J C. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82:488–496. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- Binder R J, Karimeddini D, Srivastava P K. Adjuvanticity of α 2-macroglobulin, an independent ligand for the heat shock protein receptor CD91. J Immunol. 2001;166:4968–4972. doi: 10.4049/jimmunol.166.8.4968. [DOI] [PubMed] [Google Scholar]
- Binder R J, Kumar S K, Srivastava P K. Naturally formed or artificially reconstituted non-covalent α2-macroglobulin-peptide complexes elicit CD91-dependent cellular immunity. Cancer Immun. 2002;2:16. [PubMed] [Google Scholar]