Abstract
Viruses that infect the lung are a significant cause of morbidity and mortality in animals and humans worldwide. Coronaviruses are being associated increasingly with severe diseases in the lower respiratory tract. Alveolar epithelial cells are an important target for coronavirus infection in the lung, and infected cells can initiate innate immune responses to viral infection. In this overview, we describe in vitro models of highly differentiated alveolar epithelial cells that are currently being used to study the innate immune response to coronavirus infection. We have shown that rat coronavirus infection of rat alveolar type I epithelial cells in vitro induces expression of CXC chemokines, which may recruit and activate neutrophils. Although neutrophils are recruited early in infection in several coronavirus models including rat coronavirus. However, their role in viral clearance and/or immune-mediated tissue damage is not understood. Primary cultures of differentiated alveolar epithelial cells will be useful for identifying the interactions between coronaviruses and alveolar epithelial cells that influence the innate immune responses to infection in the lung. Understanding the molecular details of these interactions will be critical for the design of effective strategies to prevent and treat coronavirus infections in the lung.
Keywords: innate immunity, pneumocyte, SARS, neutrophil recruitment, lung infection, CXC chemokine
Introduction
Coronaviruses of humans and animals are increasingly being recognized as significant pathogens in the lower respiratory tract. Animals and poultry of agricultural importance, including cows, pigs, and chickens, are infected by coronavirus strains that cause respiratory and enteric diseases of varying severity. In 2002–2003, SARS-CoV emerged from wildlife to cause an epidemic with a 10% case fatality ratio. Since then, two previously unknown HCoV, NL63 and HKU1, were discovered and found to cause respiratory disease worldwide. New molecular technologies for concurrently screening clinical specimens for a large number of viruses have allowed investigators to associate these newly identified coronaviruses with a wide range of respiratory diseases, from mild upper respiratory tract infections to severe pneumonia. Primary epithelial cell cultures derived from conducting airways have been studied as targets for several respiratory viruses including SARS-CoV. It is also important to understand the role of alveolar epithelial cells in initiating and regulating local immune responses to viral infection in the alveoli through the expression of cytokines and chemokines. Until recently, the responses of alveolar epithelial cells to virus infection were studied in continuous human or animal cell lines derived from the lung. However, these cell lines do not maintain the differentiated phenotypes of alveolar cells and thus, are not optimal models for the highly specialized cell types of the alveolar epithelium. Cell culture techniques that maintain the differentiated phenotypes of primary alveolar epithelial cells permit studies on the virus/host interactions that influence immune responses to alveolar infection. Understanding the molecular details of these interactions will be critical for designing effective strategies for the prevention and treatment of respiratory virus infections.
RESPIRATORY CORONAVIRUS INFECTIONS
The repiratory and enteric tracts are common targets for coronaviruses that infect animals and poultry, including pigs, cows, dogs, rodents, and chickens. Porcine respiratory coronavirus infects the epithelial cells of the lung, and disease ranges from subclinical infection to moderate bronchointerstitial pneumonia, depending on the virus strain [1, 2]. Bovine coronavirus causes disease in the enteric tract and the upper and lower respiratory tracts, which has been associated with shipping fever [3]. Infectious bronchitis virus causes a highly infectious respiratory disease in the upper respiratory tract and bronchi of chickens that is especially severe in chicks [3]. Canine respiratory coronavirus was discovered in 2003 and is prevalent worldwide in populations of kenneled dogs; however, its pathogenesis and contribution to kennel cough in dogs are incompletely understood [4, 5]. The murine coronavirus MHV-1 causes fatal interstitial pneumonitis in the A/J strain of inbred mice [6]. RCoV strains cause respiratory diseases with differing degrees of severity, depending on the viral strain and the age, strain, and immune status of the animal [7,8,9].
Five HCoV cause respiratory infections with various degrees of severity. HCoV-229E and HCoV-OC43, which were discovered in the 1960s, are a significant cause of common colds and can cause severe lower respiratory tract disease in elderly, infant, and immunocompromised patients [10,11,12,13]. In 2003, SARS-CoV was identified as the causative agent of the epidemic of SARS [14, 15]. Subsequently, two additional HCoV, HCoV-NL63 and HCoV-HKU1, were discovered to cause respiratory disease in patients worldwide [16,17,18,19]. HCoV-NL63 is associated with mild upper respiratory tract infections, laryngotracheitis (croup), and bronchiolitis and pneumonia in children [17,18,19]. HCoV-HKU1 is also associated with upper respiratory tract infections in children and pneumonia in elderly patients with underlying diseases [10, 20]. Because of these findings, it is important to understand the mechanisms of coronaviral pathogenesis in the lung.
Most coronaviruses, with the notable exception of bovine coronavirus, infect and cause disease in one species or a limited number of related species [21]. Although there are several animal models for SARS-CoV infection [22], there are no animal models for respiratory diseases caused by the other four HCoV [23, 24]. Therefore, it is important to study these coronaviruses in differentiated human alveolar epithelial cells in vitro. However, it is desirable to study respiratory coronaviruses for which in vitro studies in differentiated alveolar cells can be correlated with pulmonary infection in vivo. Therefore, we are studying RCoV infection in its natural host as a model for pathogenesis of respiratory coronaviruses. The study of RCoV infection in rats provides an excellent model for understanding the innate immune responses of the alveoli to infection by a respiratory coronavirus of its natural host.
VIRAL INFECTION OF THE ALVEOLAR EPITHELIUM
The alveolar epithelium consists of two morphologically and functionally distinct cell types [25]. Ninety-eight percent of the surface area of the alveolar epithelium is made up of AT1 cells, which are large, flattened, nondividing cells that function in gas exchange and fluid homeostasis [26, 27]. AT1 cells are identified in lung tissue by their morphology, specific binding to Ricinus communis 1 lectin, and expression of T1α and aquaporin-5 [26]. AT2 cells are cuboidal, dividing cells and are progenitors for replacement of damaged AT1 cells [28]. AT2 cells produce surfactant lipids and proteins that keep the alveoli open and contribute to innate defense of the lung [29]. AT2 cells are distinguished in situ by binding to Maclura pomifera lectin, the presence of lamellar bodies, and expression of SP-A, SP-B, and SP-C [26, 29]. Infection of alveolar epithelial cells in vivo by respiratory viruses, including respiratory syncytial virus, influenza A virus, and SARS-CoV, can have significant effects on respiratory functions in the alveoli. Infection of AT1 cells can impair gas exchange and removal of fluid from the lung. In addition, infection of AT2 cells can compromise repair of the damaged alveolar epithelium and innate defense of the alveoli.
In autopsy specimens from SARS patients, immunohistochemistry detected SARS-CoV antigens in AT1 or AT2 cells, or both cell types, as well as in alveolar macrophages and bronchial and bronchiolar epithelial cells [30,31,32,33]. Differences in the cell types that contain viral antigen in different patients may reflect the age of the patient and/or the time after infection when the patient died. Studies on SARS-CoV infection in primate, murine, feline, and ferret models have also demonstrated infection of alveolar epithelial cells. SARS-CoV antigens were detected in AT1 cells of cynomolgus macaques 4 days after inoculation with SARS-CoV, at which time, there was diffuse alveolar damage and neutrophil infiltration in the lung [34]. van den Brand et al. [35] found SARS-CoV antigen predominantly in AT1 and AT2 cells of cats and AT2 cells of ferrets 4 days after inoculation with SARS-CoV, when all animals had diffuse alveolar damage with infiltrating neutrophils and macrophages. Although cats had no clinical signs of infection with SARS-CoV, ferrets inoculated with SARS-CoV were lethargic, and one of four ferrets died 4 days after inoculation. In aged mouse and mouse-adapted models of SARS, viral antigens were detected in alveolar epithelial cells without distinguishing AT1 from AT2 cells [36,37,38]. In contrast to inoculation of young mice, inoculation of aged mice with SARS-CoV causes clinical signs of disease, lymphocyte infiltration, and alveolar damage 3–9 days after inoculation [37]. Despite these clinical and histopathological signs of disease, aged mice recover from infection. The mouse-adapted SARS-CoV (MA15) isolated by Roberts et al. [36] causes lethal infection in BALB/c mice, characterized by viral antigens in bronchial and alveolar epithelial cells with cellular necrosis and infiltration of mononuclear cells. A second mouse-adapted SARS isolate (F-musX-VeroE6) causes clinical signs of disease in BALB/c mice, with a 30% mortality rate [38]. Inoculation of adult mice with this virus results in viral antigen in alveolar epithelial cells, diffuse alveolar damage, and infiltration of macrophages, lymphocytes, and neutrophils into the alveoli. Porcine respiratory coronavirus antigen has been identified by immunofluorescence in epithelial cells of the alveoli, bronchi, and bronchioli, as well as alveolar macrophages 2–6 days after inoculation of infant pigs, resulting in subclinical interstitial pneumonia [1]. RCoV infection of adult rats results in an influx of neutrophils, followed by lymphocytes and monocytes, into the respiratory tract [7, 39]. The interactions between respiratory viruses and alveolar epithelial cells can mediate the innate immune response to virus infection in the lung. Primary cultures of differentiated alveolar epithelial cells are a valuable model for studying these interactions.
CORONAVIRUS ISOLATION OFTEN REQUIRES DIFFERENTIATED HOST CELLS
Human respiratory coronaviruses could not be isolated from patients with colds in continuous human cell lines, but instead, virus isolation required serial blind passage in human diploid fibroblasts or human fetal tracheal organ cultures [40,41,42,43,44]. HCoV-229E and HCoV-OC43 caused only mild cytopathic effects in these cells, but these viruses could be used to infect human volunteers to study the pathogenesis of coronavirus infection of the upper respiratory tract [41, 45]. HCoV-NL63 is also difficult to isolate from clinical specimens [46] and can be isolated most readily in primary, differentiated human airway epithelial cells [47]. Infectious HCoV-HKU1 has not yet been propagated in any cell culture, although its entire genome sequence has been determined [16]. In contrast, SARS-CoV could be readily isolated from patients in the Vero E6 line of monkey kidney cells or in fetal rhesus kidney cells [14, 48, 49]. The reasons for the fastidious requirements of most human respiratory coronaviruses for differentiated human respiratory epithelial cells are not yet understood.
CORONAVIRUS INFECTION IN PRIMARY DIFFERENTIATED RESPIRATORY EPITHELIAL CELLS
The respiratory tract is lined with epithelial cells that have different functions in the upper respiratory tract (nasal and sinusoidal epithelium), conducting airways (tracheal and bronchial epithelium), and alveoli (alveolar epithelial cells), and all of these are susceptible to infection with a variety of respiratory viruses. Winther et al. [50] demonstrated the susceptibility of primary cultures of ciliated nasal epithelial cells to infection by HCoV-229E without cytopathic effects. Polarized cultures of differentiated, ciliated human conducting airway epithelia have also been used to study infection and the polarity of entry and release by HCoV-229E, HCoV-NL63, and SARS-CoV [47, 51,52,53,54,55]. These studies emphasize the importance of the differentiation state of ciliated cells for susceptibility to coronavirus infection [52, 53]. Recent advances in the cultivation of differentiated alveolar epithelial cells now allow analysis of these important cell types in virus infection.
CULTIVATION AND CORONAVIRUS INFECTION OF PRIMARY DIFFERENTIATED ALVEOLAR EPITHELIAL CELLS
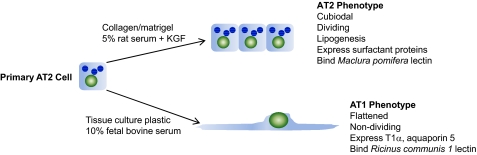
As continuous cell lines derived from alveolar epithelium do not maintain their differentiated characteristics, primary cultures must be used to study differentiated AT1 and AT2 cells in vitro. Primary AT1 cells are difficult to isolate to a high yield and purity and propagate [26]. However, AT2 cells can be readily isolated from lung tissue, and under special culture conditions, they maintain their AT2 phenotype. Under different culture conditions, AT2 cells transdifferentiate into an AT1 cell phenotype (Fig. 1). This process of transdifferentiation occurs during repair of the alveolar epithelium in vivo and can be replicated in vitro [56,57,58,59,60,61]. Primary rat AT2 cells maintain their differentiated phenotype when they are cultured on collagen/matrigel in medium containing 5% rat serum and KGF [62, 63]. The AT2 phenotype is characterized by a cuboidal shape, lipogenesis (evident by the presence of lamellar bodies) and expression of surfactant proteins. When rat AT2 cells are cultured for 3–5 days in 10% FBS without KGF, they lose properties of AT2 cells and transdifferentiate into an AT1 cell phenotype (tAT1 cell, also called AT1-like cell) [56, 58, 59]. These cells are flattened, express markers characteristic of AT1 cells in vivo (T1α, aquaporin-5, and caveolin-1), and react with AT1 cell-specific antibodies and lectins [26, 59, 64,65,66,67,68]. As the markers used to distinguish AT1 cells in situ are present in tAT1 cells in vitro, tAT1 cells are a practical alternative to AT1 cell isolation for the study of AT1 cells in vitro. As with any in vitro model, the biological relevance of such studies must ultimately be confirmed in vivo.
Figure 1.
Schematic representation of culture conditions for primary differentiated alveolar epithelial cells. AT2 cells are isolated from rat lung and are cultured to maintain an AT2 phenotype or transdifferentiate into a tAT1 cell phenotype.
We showed that the differentiation status of primary alveolar epithelial cells is critical in determining susceptibility to SARS-CoV infection [69]. Human alveolar epithelial cells that were maintained with the AT2 phenotype supported infection by SARS-CoV, whereas cells from the same donor that were transdifferentiated in vitro to a tAT1 cell phenotype were resistant to infection. In contrast to SARS-CoV, HCoV-229E replicates and causes cytopathic effects in cloned AT2 cells, which resemble an AT1 cell phenotype [70]. Our studies using RCoV infection of primary differentiated rat alveolar epithelial cells were the first to demonstrate coronavirus infection in tAT1 and AT2 cells in vitro [71, 72].
INNATE IMMUNE RESPONSES OF ALVEOLAR EPITHELIAL CELLS TO VIRUS INFECTION
In the lung, the roles of AT2 cells and alveolar macrophages in initiating and regulating an immune response have been studied extensively. AT2 cells produce inflammatory mediators upon exposure to inhaled microbes or particles and regulate the functions of immune cells, including macrophages, dendritic cells, and lymphocytes in the lung [29, 73,74,75]. In vitro, human bronchial epithelial cells and AT2 cells produce cytokines and chemokines in response to infection with viruses including RSV, influenza A virus, and SARS-CoV [76,77,78,79,80]. The innate immune functions of AT1 cells have only been recognized recently. Expression of chemokines by primary differentiated tAT1 cells in vitro is increased by exposure to IL-1α, IL-1β, or LPS [64, 65, 81, 82]. In primary cultures of murine tAT1 cells, influenza A virus induces expression of CCL2 and CCL5, resulting in transmigration of monocytes [83].
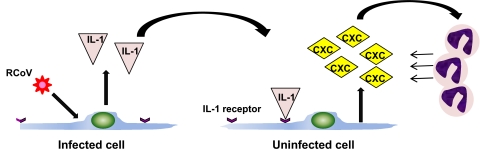
We have shown that rat tAT1 cells in vitro express cytokines and chemokines upon infection with RCoV [71]. This study was the first to show that virus infection of primary tAT1 cells induces a proinflammatory response. RCoV infection of tAT1 cells induces expression of cytokines, including GM-CSF, IFN-γ, and TNF-α, and chemokines, predominantly those of the CXC family [71]. The primary functions of CXC chemokines are to recruit and activate neutrophils. We hypothesize that RCoV infection in the lung induces CXC chemokine expression by AT1 cells, which in turn, recruits neutrophils to the lung. The role of neutrophils during RCoV infection of the lung is unknown, but these cells may contribute to viral clearance and immunopathology. Rat tAT1 cells are a valuable model in which to study the virus/host interactions that regulate this response. Using this model, we showed that like RCoV infection, UV-inactivated RCoV induces CXC chemokine expression in rat tAT1 cells [71], so virus replication is not required to induce the chemokine response in rat tAT1 cells. Dual immunolabeling of viral antigen and CXC chemokines in rat tAT1 cells showed that CXC chemokines are expressed predominantly from uninfected cells in the culture [71]. Therefore, expression of CXC chemokines during RCoV infection of tAT1 cells may be mediated by a paracrine mechanism. We found that RCoV-infected rat tAT1 cells treated with IL-1Ra had markedly decreased expression of CXC chemokines relative to cells without IL-1Ra [71]. Treatment with soluble TNFR protein did not affect chemokine expression by RCoV-infected tAT1 cells. Thus, signaling through the IL-1R likely mediates CXC chemokine expression by rat tAT1 cells during RCoV infection (Fig. 2). As IL-1α and IL-1β signal through the IL-1R, either or both of these cytokines may contribute to CXC chemokine expression during RCoV infection of tAT1 cells. Manzer et al. [65, 82] showed that rIL-1α and rIL-1β induce expression of CXC chemokines by rat tAT1 cells in vitro. Rat tAT1 cells are a valuable model for investigating the early events in innate immune responses to respiratory coronavirus infections.
Figure 2.
Model of RCoV-induced expression of CXC chemokines in primary rat tAT1 cells. RCoV infection induces expression of IL-1α and/or IL-1β, which signal through the IL-1R on uninfected cells to induce expression of CXC chemokines, likely recruiting neutrophils to the site of infection.
NEUTROPHILS IN RESPIRATORY VIRUS INFECTIONS
Neutrophils infiltrate tissues early after viral infection and, through the expression of proinflammatory cytokines and chemokines, can direct the subsequent recruitment of monocytes and lymphocytes. For example, in infants with RSV bronchiolitis, neutrophils accounted for 93% and 76% of inflammatory cells in the upper and lower airways, respectively [84, 85]; however, the specific functions of neutrophils during RSV infection are unclear [86]. Neutrophils infiltrate the respiratory tract by 18 h after inoculation of mice with influenza A virus, and increased numbers of neutrophils have been associated with highly pathogenic influenza virus infections in mice [87, 88]. Depletion of neutrophils from mice exacerbated infection with a highly pathogenic recombinant influenza virus strain containing the hemagglutinin and neuraminidase genes of the 1918 influenza virus [87]. Thus, neutrophils can play a role in protection from virulent influenza virus infection. In infection with less virulent strains of influenza A virus, neutrophils can have a protective effect [89, 90] or no effect [91] on viral replication and pathogenesis. Thus, with different virus strains, neutrophils can have different functions in the innate immune response to respiratory infections.
Neutrophils also infiltrate tissues infected by coronaviruses, including SARS-CoV, RCoV, and MHV. A high neutrophil count in the blood of SARS patients at the time of hospital admission was associated with a poor prognosis [92, 93]. A mouse-adapted isolate of SARS-CoV (F-musX-VeroE6) causes lethal infection in adult, but not young, mice [38]. The disease severity in adult mice correlates with increased pulmonary inflammation consisting predominantly of neutrophils, which are also the predominant cell type detected in the nasal exudates from chickens infected with infectious bronchitis virus and are believed to contribute to disease pathology [94]. These findings suggest that neutrophils can contribute to immune-mediated pathology in some coronavirus infections. Infection of rats with RCoV results in infiltration of neutrophils to the respiratory tract early after inoculation, followed by the recruitment of macrophages and lymphocytes [7, 8, 39, 95]. Infection of mice with a neurotropic murine coronavirus, MHV-JHM, results in infiltration of neutrophils into the brain by 1 day after inoculation, which then promotes the recruitment of other types of inflammatory cells into the brain, likely through loss of the blood brain barrier [96]. Despite the presence of neutrophils in coronavirus-infected tissues, their role in the clearance and/or immunopathology of coronavirus infections is largely unknown. Future studies on the responses of neutrophils to RCoV-infected tAT1 cells in vitro may elucidate the role of neutrophils in the pathogenesis of respiratory coronavirus infections.
Acknowledgments
T. A. M. is supported by grant number P20 RR015587 from the National Center for Research Resources (NCRR), a component of the NIH. This work was supported by NIH grants AI-059576 and AI-25231. We are grateful to Drs. Robert Mason, Joel Funk, Jieru Wang, Rizwan Manzer, and Emily Travanty for their collaboration on RCoV research, to Drs. Samuel Dominguez, Zhaohui Qian, and Kejun Guo for review of the manuscript, and to Ms. Anoria Haick for assistance with the manuscript.
Footnotes
Abbreviations: AT1/2 cell=alveolar type I and II cell, HCoV=human coronavirus, IL-1Ra=IL-1R antagonist, KGF=keratinocyte growth factor, MHV=mouse hepatitis virus, NIH=National Institutes of Health, RCoV=rat coronavirus, RSV=respiratory syncytial virus, SARS=severe acute respiratory syndrome, SARS-CoV=SARS-associated coronavirus, SP=surfactant protein, tAT1 cell=in vitro transdifferentiated AT1-like cell, TNFR=TNF receptor
References
- Cox E, Hooyberghs J, Pensaert M B. Sites of replication of a porcine respiratory coronavirus related to transmissible gastroenteritis virus. Res Vet Sci. 1990;48:165–169. doi: 10.1016/S0034-5288(18)30984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbur P G, Opriessnig T, Pallares F J, Vaughn E M, Paul P S. Pathogenicity of three isolates of porcine respiratory coronavirus in the USA. Vet Rec. 2003;152:358–361. doi: 10.1136/vr.152.12.358. [DOI] [PubMed] [Google Scholar]
- Saif L J. Animal coronaviruses: what can they teach us about the severe acute respiratory syndrome? Rev Sci Tech. 2004;23:643–660. doi: 10.20506/rst.23.2.1513. [DOI] [PubMed] [Google Scholar]
- Erles K, Brownlie J. Canine respiratory coronavirus: an emerging pathogen in the canine infectious respiratory disease complex. Vet Clin North Am Small Anim Pract. 2008;38:815–825. doi: 10.1016/j.cvsm.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K, Toomey C, Brooks H W, Brownlie J. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology. 2003;310:216–223. doi: 10.1016/S0042-6822(03)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Albuquerque N, Baig E, Ma X, Zhang J, He W, Rowe A, Habal M, Liu M, Shalev I, Downey G P, Gorczynski R, Butany J, Leibowitz J, Weiss S R, McGilvray I D, Phillips M J, Fish E N, Levy G A. Murine hepatitis virus strain 1 produces a clinically relevant model of severe acute respiratory syndrome in A/J mice. J Virol. 2006;80:10382–10394. doi: 10.1128/JVI.00747-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt P N, Jacoby R O. Experimental infection of adult axenic rats with Parker’s rat coronavirus. Arch Virol. 1977;54:345–352. doi: 10.1007/BF01314779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcinski Z W, Percy D H. Sialodacryoadenitis virus-associated lesions in the lower respiratory tract of rats. Vet Pathol. 1986;23:278–286. doi: 10.1177/030098588602300308. [DOI] [PubMed] [Google Scholar]
- Parker J C, Cross S S, Rowe W P. Rat coronavirus (RCV): a prevalent, naturally occurring pneumotropic virus of rats. Arch Gesamte Virusforsch. 1970;31:293–302. doi: 10.1007/BF01253764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S K P, Woo P C Y, Yip C C Y, Tse H, Tsoi H W, Cheng V C C, Lee P, Tang B S F, Cheung C H Y, Lee R A, So L Y, Lau Y L, Chan K H, Yuen K Y. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol. 2006;44:2063–2071. doi: 10.1128/JCM.02614-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G, Campanini G, Rovida F, Percivalle E, Sarasini A, Marchi A, Baldanti F. Genetic variability of human coronavirus OC43-, 229E-, and NL63-like strains and their association with lower respiratory tract infections of hospitalized infants and immunocompromised patients. J Med Virol. 2006;78:938–949. doi: 10.1002/jmv.20645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick D M, Petric M, Skowronski D M, Guasparini R, Booth T F, Krajden M, McGeer P, Bastien N, Gustafson L, Dubord J, Macdonald D, David S T, Srour L F, Parker R, Andonov A, Isaac-Renton J, Loewen N, McNabb G, McNabb A, Goh S H, Henwick S, Astell C, Guo J P, Drebot M, Tellier R, Plummer F, Brunham R C. An outbreak of human coronavirus OC43 infection and serological cross-reactivity with SARS coronavirus. Can J Infect Dis Med Microbiol. 2006;17:330–336. doi: 10.1155/2006/152612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham J D, Cadena A, Lin J, Piedra P A, Glezen W P, Greenberg S B, Atmar R L. Respiratory viral infections in patients with chronic, obstructive pulmonary disease. J Infect. 2005;50:322–330. doi: 10.1016/j.jinf.2004.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T G, Erdman D, Goldsmith C S, Zaki S R, Peret T, Emery S, Tong S, Urbani C, Comer J A, Lim W, Rollin P E, Dowell S F, Ling A E, Humphrey C D, Shieh W J, Guarner J, Paddock C D, Rota P, Fields B, DeRisi J, Yang J Y, Cox N, Hughes J M, LeDuc J W, Bellini W J, Anderson L J. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Tsui P T, Kwok M L, Yuen H, Lai S T. Severe acute respiratory syndrome: clinical outcome and prognostic correlates. Emerg Infect Dis. 2003;9:1064–1069. doi: 10.3201/eid0909.030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P C, Lau S K, Chu C M, Chan K H, Tsoi H W, Huang Y, Wong B H, Poon R W, Cai J J, Luk W K, Poon L L, Wong S S, Guan Y, Peiris J S, Yuen K Y. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79:884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Hoek L, Pyrc K, Jebbink M F, Vermeulen-Oost W, Berkhout R J, Wolthers K C, Wertheim-van Dillen P M, Kaandorp J, Spaargaren J, Berkhout B. Identification of a new human coronavirus. Nat Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier R A, Hartwig N G, Bestebroer T M, Niemeyer B, de Jong J C, Simon J H, Osterhaus A D. A previously undescribed coronavirus associated with respiratory disease in humans. Proc Natl Acad Sci USA. 2004;101:6212–6216. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrc K, Berkhout B, van der Hoek L. The novel human coronaviruses NL63 and HKU1. J Virol. 2007;81:3051–3057. doi: 10.1128/JVI.01466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P C, Lau S K, Tsoi H W, Huang Y, Poon R W, Chu C M, Lee R A, Luk W K, Wong G K, Wong B H, Cheng V C, Tang B S, Wu A K, Yung R W, Chen H, Guan Y, Chan K H, Yuen K Y. Clinical and molecular epidemiological features of coronavirus HKU1-associated community-acquired pneumonia. J Infect Dis. 2005;192:1898–1907. doi: 10.1086/497151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M M C, Holmes K V. Coronaviridae: the viruses and their replication. Knipe D M, Howley P M, editors. Lippincott Williams & Wilkins: Philadelphia, PA, USA; 2001:1163–1185. [Google Scholar]
- Roberts A, Lamirande E W, Vogel L, Jackson J P, Paddock C D, Guarner J, Zaki S R, Sheahan T, Baric R, Subbarao K. Animal models and vaccines for SARS-CoV infection. Virus Res. 2008;133:20–32. doi: 10.1016/j.virusres.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth D E, Tresnan D B, Turner B C, Lerman I R, Bullis B, Hemmila E M, Levis R, Shapiro L H, Holmes K V. Cells of human aminopeptidase N (CD13) transgenic mice are infected by human coronavirus-229E in vitro, but not in vivo. Virology. 2005;335:185–197. doi: 10.1016/j.virol.2005.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassnig C, Sanchez C M, Egerbacher M, Walter I, Majer S, Kolbe T, Pallares P, Enjuanes L, Muller M. Development of a transgenic mouse model susceptible to human coronavirus 229E. Proc Natl Acad Sci USA. 2005;102:8275–8280. doi: 10.1073/pnas.0408589102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo J D, Barry B E, Gehr P, Bachofen M, Weibel E R. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis. 1982;126:332–337. doi: 10.1164/arrd.1982.126.2.332. [DOI] [PubMed] [Google Scholar]
- Williams M C. Alveolar type I cells: molecular phenotype and development. Annu Rev Physiol. 2003;65:669–695. doi: 10.1146/annurev.physiol.65.092101.142446. [DOI] [PubMed] [Google Scholar]
- Johnson M D, Bao H F, Helms M N, Chen X J, Tigue Z, Jain L, Dobbs L G, Eaton D C. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci USA. 2006;103:4964–4969. doi: 10.1073/pnas.0600855103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson I Y, Bowden D H. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest. 1974;30:35–42. [PubMed] [Google Scholar]
- Mason R J. Biology of alveolar type II cells. Respirology. 2006;11:S12–S15. doi: 10.1111/j.1440-1843.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- Ye J, Zhang B, Xu J, Chang Q, McNutt M A, Korteweg C, Gong E, Gu J. Molecular pathology in the lungs of severe acute respiratory syndrome patients. Am J Pathol. 2007;170:538–545. doi: 10.2353/ajpath.2007.060469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao C H, Wu M Z, Chen C L, Hsueh P R, Hsieh S W, Yang P C, Su I J. Evolution of pulmonary pathology in severe acute respiratory syndrome. J Formos Med Assoc. 2005;104:75–81. [PubMed] [Google Scholar]
- Shieh W J, Hsiao C H, Paddock C D, Guarner J, Goldsmith C S, Tatti K, Packard M, Mueller L, Wu M Z, Rollin P, Su I J, Zaki S R. Immunohistochemical, in situ hybridization, and ultrastructural localization of SARS-associated coronavirus in lung of a fatal case of severe acute respiratory syndrome in Taiwan. Hum Pathol. 2005;36:303–309. doi: 10.1016/j.humpath.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls J M, Butany J, Poon L L, Chan K H, Beh S L, Poutanen S, Peiris J S, Wong M. Time course and cellular localization of SARS-CoV nucleoprotein and RNA in lungs from fatal cases of SARS. PLoS Med. 2006;3:e27. doi: 10.1371/journal.pmed.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagmans B L, Kuiken T, Martina B E, Fouchier R A, Rimmelzwaan G F, van Amerongen G, van Riel D, de Jong T, Itamura S, Chan K H, Tashiro M, Osterhaus A D. Pegylated interferon-α protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med. 2004;10:290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Brand J M, Haagmans B L, Leijten L, van Riel D, Martina B E, Osterhaus A D, Kuiken T. Pathology of experimental SARS coronavirus infection in cats and ferrets. Vet Pathol. 2008;45:551–562. doi: 10.1354/vp.45-4-551. [DOI] [PubMed] [Google Scholar]
- Roberts A, Deming D, Paddock C D, Cheng A, Yount B, Vogel L, Herman B D, Sheahan T, Heise M, Genrich G L, Zaki S R, Baric R, Subbarao K. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog. 2007;3:e5. doi: 10.1371/journal.ppat.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Paddock C, Vogel L, Butler E, Zaki S, Subbarao K. Aged BALB/c mice as a model for increased severity of severe acute respiratory syndrome in elderly humans. J Virol. 2005;79:5833–5838. doi: 10.1128/JVI.79.9.5833-5838.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata N, Iwata N, Hasegawa H, Fukushi S, Harashima A, Sato Y, Saijo M, Taguchi F, Morikawa S, Sata T. Mouse-passaged severe acute respiratory syndrome-associated coronavirus leads to lethal pulmonary edema and diffuse alveolar damage in adult but not young mice. Am J Pathol. 2008;172:1625–1637. doi: 10.2353/ajpath.2008.071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby R O, Bhatt P N, Jonas A M. Pathogenesis of sialodacryoadenitis in gnotobiotic rats. Vet Pathol. 1975;12:196–209. doi: 10.1177/030098587501200305. [DOI] [PubMed] [Google Scholar]
- Hamre D, Procknow J J. A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med. 1966;121:190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- Kapikian A Z. The coronaviruses. Dev Biol Stand. 1975;28:42–64. [PubMed] [Google Scholar]
- Almeida J D, Tyrrell D A. The morphology of three previously uncharacterized human respiratory viruses that grow in organ culture. J Gen Virol. 1967;1:175–178. doi: 10.1099/0022-1317-1-2-175. [DOI] [PubMed] [Google Scholar]
- Tyrrell D A, Bynoe M L. Cultivation of a novel type of common-cold virus in organ cultures. BMJ. 1965;1:1467–1470. doi: 10.1136/bmj.1.5448.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K, Dees J H, Becker W B, Kapikian A Z, Chanock R M. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci USA. 1967;57:933–940. doi: 10.1073/pnas.57.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bende M, Barrow I, Heptonstall J, Higgins P G, Al-Nakib W, Tyrrell D A, Akerlund A. Changes in human nasal mucosa during experimental coronavirus common colds. Acta Otolaryngol. 1989;107:262–269. doi: 10.3109/00016488909127507. [DOI] [PubMed] [Google Scholar]
- Schildgen O, Jebbink M F, de Vries M, Pyrc K, Dijkman R, Simon A, Müller A, Kupfer B, van der Hoek L. Identification of cell lines permissive for human coronavirus NL63. J Virol Methods. 2006;138:207–210. doi: 10.1016/j.jviromet.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banach S B, Orenstein J M, Fox L M, Randell S H, Rowley A H, Baker S C. Human airway epithelial cell culture to identify new respiratory viruses: coronavirus NL63 as a model. J Virol Methods. 2009;156:19–26. doi: 10.1016/j.jviromet.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C, Gunther S, Preiser W, van der Werf S, Brodt H R, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier R A, Berger A, Burguiere A M, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra J C, Muller S, Rickerts V, Sturmer M, Vieth S, Klenk H D, Osterhaus A D, Schmitz H, Doerr H W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Peiris J S M, Lai S T, Poon L L M, Guan Y, Yam L Y C, Lim W, Nicholls J, Yee W K S, Yan W W, Cheung M T, Cheng V C C, Chan K H, Tsang D N C, Yung R W H, Ng T K, Yuen K Y. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winther B, Gwaltney J M, Hendley J O. Respiratory virus infection of monolayer cultures of human nasal epithelial cells. Am Rev Respir Dis. 1990;141:839–845. doi: 10.1164/ajrccm/141.4_Pt_1.839. [DOI] [PubMed] [Google Scholar]
- Wang G, Deering C, Macke M, Shao J, Burns R, Blau D M, Holmes K V, Davidson B L, Perlman S, McCray P B., Jr Human coronavirus 229E infects polarized airway epithelia from the apical surface. J Virol. 2000;74:9234–9239. doi: 10.1128/jvi.74.19.9234-9239.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims A C, Baric R S, Yount B, Burkett S E, Collins P L, Pickles R J. Severe acute respiratory syndrome coronavirus infection of human ciliated airway epithelia: role of ciliated cells in viral spread in the conducting airways of the lungs. J Virol. 2005;79:15511–15524. doi: 10.1128/JVI.79.24.15511-15524.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H P, Look D C, Shi L, Hickey M, Pewe L, Netland J, Farzan M, Wohlford-Lenane C, Perlman S, McCray P B., Jr ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol. 2005;79:14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T, Rockx B, Donaldson E, Sims A, Pickles R, Corti D, Baric R. Mechanisms of zoonotic severe acute respiratory syndrome coronavirus host range expansion in human airway epithelium. J Virol. 2008;82:2274–2285. doi: 10.1128/JVI.02041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims A C, Burkett S E, Yount B, Pickles R J. SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium. Virus Res. 2008;133:33–44. doi: 10.1016/j.virusres.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borok Z, Hami A, Danto S I, Zabski S M, Crandall E D. Rat serum inhibits progression of alveolar epithelial cells toward the type I cell phenotype in vitro. Am J Respir Cell Mol Biol. 1995;12:50–55. doi: 10.1165/ajrcmb.12.1.7811470. [DOI] [PubMed] [Google Scholar]
- Shannon J M, Jennings S D, Nielsen L D. Modulation of alveolar type II cell differentiated function in vitro. Am J Physiol. 1992;262:L427–L436. doi: 10.1152/ajplung.1992.262.4.L427. [DOI] [PubMed] [Google Scholar]
- Dobbs L G. Isolation and culture of alveolar type II cells. Am J Physiol. 1990;258:L134–L147. doi: 10.1152/ajplung.1990.258.4.L134. [DOI] [PubMed] [Google Scholar]
- Borok Z, Lubman R L, Danto S I, Zhang X L, Zabski S M, King L S, Lee D M, Agre P, Crandall E D. Keratinocyte growth factor modulates alveolar epithelial cell phenotype in vitro: expression of aquaporin 5. Am J Respir Cell Mol Biol. 1998;18:554–561. doi: 10.1165/ajrcmb.18.4.2838. [DOI] [PubMed] [Google Scholar]
- Danto S I, Shannon J M, Borok Z, Zabski S M, Crandall E D. Reversible transdifferentiation of alveolar epithelial cells. Am J Respir Cell Mol Biol. 1995;12:497–502. doi: 10.1165/ajrcmb.12.5.7742013. [DOI] [PubMed] [Google Scholar]
- Wang J, Edeen K, Manzer R, Chang Y, Wang S, Chen X, Funk C J, Cosgrove G P, Fang X, Mason R J. Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro. Am J Respir Cell Mol Biol. 2007;36:661–668. doi: 10.1165/rcmb.2006-0410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R J, Gao B, Pan T, Jiang X, Eckart M, Neben S. Role of keratinocyte growth factor in regulating lipogenesis in alveolar type II cells: a gene-profiling approach. Chest. 2002;121:77S. doi: 10.1378/chest.121.3_suppl.77s. [DOI] [PubMed] [Google Scholar]
- Mason R J, Lewis M C, Edeen K E, McCormick-Shannon K, Nielsen L D, Shannon J M. Maintenance of surfactant protein A and D secretion by rat alveolar type II cells in vitro. Am J Physiol Lung Cell Mol Physiol. 2002;282:L249–L258. doi: 10.1152/ajplung.00027.2001. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang S, Manzer R, McConville G, Mason R J. Ozone induces oxidative stress in rat alveolar type II and type I-like cells. Free Radic Biol Med. 2006;40:1914–1928. doi: 10.1016/j.freeradbiomed.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Manzer R, Wang J, Nishina K, McConville G, Mason R J. Alveolar epithelial cells secrete chemokines in response to IL-1β and lipopolysaccharide but not to ozone. Am J Respir Cell Mol Biol. 2006;34:158–166. doi: 10.1165/rcmb.2005-0205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borok Z, Danto S I, Lubman R L, Cao Y, Williams M C, Crandall E D. Modulation of t1α expression with alveolar epithelial cell phenotype in vitro. Am J Physiol. 1998;275:L155–L164. doi: 10.1152/ajplung.1998.275.1.L155. [DOI] [PubMed] [Google Scholar]
- Danto S I, Zabski S M, Crandall E D. Reactivity of alveolar epithelial cells in primary culture with type I cell monoclonal antibodies. Am J Respir Cell Mol Biol. 1992;6:296–306. doi: 10.1165/ajrcmb/6.3.296. [DOI] [PubMed] [Google Scholar]
- Dobbs L G, Williams M C, Gonzalez R. Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated, type II cells. Biochim Biophys Acta. 1988;970:146–156. doi: 10.1016/0167-4889(88)90173-5. [DOI] [PubMed] [Google Scholar]
- Mossel E C, Wang J, Jeffers S, Edeen K E, Wang S, Cosgrove G P, Funk C J, Manzer R, Miura T A, Pearson L D, Holmes K V, Mason R J. SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells. Virology. 2008;372:127–135. doi: 10.1016/j.virol.2007.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D A, Mika-Johnson M, Phillips G, Douglas W H, Chapple P J. Infection of cultured human type II pneumonocytes with certain respiratory viruses. Infect Immun. 1979;26:621–629. doi: 10.1128/iai.26.2.621-629.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T A, Wang J, Holmes K V, Mason R J. Rat coronaviruses infect rat alveolar type I epithelial cells and induce expression of CXC chemokines. Virology. 2007;369:288–298. doi: 10.1016/j.virol.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T A, Wang J, Mason R J, Holmes K V. Rat coronavirus infection of primary rat alveolar epithelial cells. Adv Exp Med Biol. 2006;581:351–356. doi: 10.1007/978-0-387-33012-9_62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaseelan S, Manzer R, Young S K, Yamamoto M, Akira S, Mason R J, Worthen G S. Induction of CXCL5 during inflammation in the rodent lung involves activation of alveolar epithelium. Am J Respir Cell Mol Biol. 2005;32:531–539. doi: 10.1165/rcmb.2005-0063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanj R S, Kang J L, Castranova V. Interaction between primary alveolar macrophages and primary alveolar type II cells under basal conditions and after lipopolysaccharide or quartz exposure. J Toxicol Environ Health A. 2006;69:1097–1116. doi: 10.1080/14736480500360504. [DOI] [PubMed] [Google Scholar]
- Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res. 2001;2:33–46. doi: 10.1186/rr36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M C, Cheung C Y, Chui W H, Tsao S W, Nicholls J M, Chan Y O, Chan R W, Long H T, Poon L L, Guan Y, Peiris J S. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir Res. 2005;6:135. doi: 10.1186/1465-9921-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Luxon B A, Casola A, Garofalo R P, Jamaluddin M, Brasier A R. Expression of respiratory syncytial virus-induced chemokine gene networks in lower airway epithelial cells revealed by cDNA microarrays. J Virol. 2001;75:9044–9058. doi: 10.1128/JVI.75.19.9044-9058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Oberley-Deegan R, Wang S, Nikrad M, Funk C J, Hartshorn K L, Mason R J. Differentiated human alveolar type II cells secrete antiviral IL-29 (IFN-λ1) in response to influenza A infection. J Immunol. 2009;182:1296–1304. doi: 10.4049/jimmunol.182.3.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Hill T, Peters C J, Tseng C T. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J Virol. 2009;83:3039–3048. doi: 10.1128/JVI.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellow T E, Murphy P C, Carson J L, Noah T L, Zhang L, Pickles R J. The effect of respiratory synctial virus on chemokine release by differentiated airway epithelium. Exp Lung Res. 2004;30:43–57. doi: 10.1080/01902140490252812. [DOI] [PubMed] [Google Scholar]
- Nishina K, Zhang F, Nielsen L D, Edeen K, Wang J, Mason R J. Expression of CINC-2β is related to the state of differentiation of alveolar epithelial cells. Am J Respir Cell Mol Biol. 2005;33:505–512. doi: 10.1165/rcmb.2005-0113OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzer R, Dinarello C A, McConville G, Mason R J. Ozone exposure of macrophages induces an alveolar epithelial chemokine response through IL-1α. Am J Respir Cell Mol Biol. 2008;38:318–323. doi: 10.1165/rcmb.2007-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold S, von Wulffen W, Steinmueller M, Pleschka S, Kuziel W A, Mack M, Srivastava M, Seeger W, Maus U A, Lohmeyer J. Alveolar epithelial cells direct monocyte transepithelial migration upon influenza virus infection: impact of chemokines and adhesion molecules. J Immunol. 2006;177:1817–1824. doi: 10.4049/jimmunol.177.3.1817. [DOI] [PubMed] [Google Scholar]
- Everard M L, Swarbrick A, Wrightham M, McIntyre J, Dunkley C, James P D, Sewell H F, Milner A D. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child. 1994;71:428–432. doi: 10.1136/adc.71.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P K, Wang S Z, Dowling K D, Forsyth K D. Leukocyte populations in respiratory syncytial virus-induced bronchiolitis. J Paediatr Child Health. 2001;37:146–151. doi: 10.1046/j.1440-1754.2001.00618.x. [DOI] [PubMed] [Google Scholar]
- Collins P L, Graham B S. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82:2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumpey T M, Garcia-Sastre A, Taubenberger J K, Palese P, Swayne D E, Pantin-Jackwood M J, Schultz-Cherry S, Solorzano A, Van Rooijen N, Katz J M, Basler C F. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79:14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobasa D, Takada A, Shinya K, Hatta M, Halfmann P, Theriault S, Suzuki H, Nishimura H, Mitamura K, Sugaya N, Usui T, Murata T, Maeda Y, Watanabe S, Suresh M, Suzuki T, Suzuki Y, Feldmann H, Kawaoka Y. Enhanced virulence of influenza A viruses with the hemagglutinin of the 1918 pandemic virus. Nature. 2004;431:703–707. doi: 10.1038/nature02951. [DOI] [PubMed] [Google Scholar]
- Fujisawa H. Neutrophils play an essential role in cooperation with antibody in both protection against and recovery from pulmonary infection with influenza virus in mice. J Virol. 2008;82:2772–2783. doi: 10.1128/JVI.01210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate M D, Brooks A G, Reading P C. The role of neutrophils in the upper and lower respiratory tract during influenza virus infection of mice. Respir Res. 2008;9:57. doi: 10.1186/1465-9921-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareing M D, Shea A L, Inglis C A, Dias P B, Sarawar S R. CXCR2 is required for neutrophil recruitment to the lung during influenza virus infection, but is not essential for viral clearance. Viral Immunol. 2007;20:369–378. doi: 10.1089/vim.2006.0101. [DOI] [PubMed] [Google Scholar]
- Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt G M, Ahuja A, Yung M Y, Leung C B, To K F, Lui S F, Szeto C C, Chung S, Sung J J. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Manocha S, Walley K R, Russell J A. Severe acute respiratory distress syndrome (SARS): a critical care perspective. Crit Care Med. 2003;31:2684–2692. doi: 10.1097/01.CCM.0000091929.51288.5F. [DOI] [PubMed] [Google Scholar]
- Raj G D, Savage C E, Jones R C. Effect of heterophil depletion by 5-fluorouracil on infectious bronchitis virus infection in chickens. Avian Pathol. 1997;26:427–432. doi: 10.1080/03079459708419224. [DOI] [PubMed] [Google Scholar]
- Bihun C G, Percy D H. Morphologic changes in the nasal cavity associated with sialodacryoadenitis virus infection in the Wistar rat. Vet Pathol. 1995;32:1–10. doi: 10.1177/030098589503200101. [DOI] [PubMed] [Google Scholar]
- Zhou J, Stohlman S A, Hinton D R, Marten N W. Neutrophils promote mononuclear cell infiltration during viral-induced encephalitis. J Immunol. 2003;170:3331–3336. doi: 10.4049/jimmunol.170.6.3331. [DOI] [PubMed] [Google Scholar]