Abstract
In nerve-smooth muscle preparations β-nicotinamide adenine dinucleotide (β-NAD) has emerged as a novel extracellular substance with putative neurotransmitter and neuromodulator functions. Thus, β-NAD is released along with noradrenaline and adenosine 5'-triphosphate (ATP) upon firing of action potentials in blood vessels, urinary bladder and large intestine. At present it is unclear whether noradrenaline, ATP and β-NAD are stored in and released from common populations of synaptic vesicles. This matter is unattainable to study in complex systems such as nerve-smooth muscle preparations. Adrenal chromaffin cells are used here as a single-cell model to examine mechanisms of concomitant neurosecretion. Using high-performance liquid chromatography techniques with electrochemical and fluorescence detections we simultaneously evaluated secretion of dopamine (DA), ATP, adenosine 5'-diphosphate, adenosine 5'-monophosphate, adenosine, and β-NAD and its immediate metabolites ADP-ribose and cyclic ADP-ribose in superfused nerve growth factor-differentiated rat pheochromocytoma PC12 cells. β-NAD, DA, and ATP were released constitutively and upon stimulation with high-K+ solution or nicotine. Botulinum neurotoxin A tended to increase the spontaneous secretion of all substances and abolished the high K+-evoked release of β-NAD and DA but not of ATP. Subcellular fractionation by continuous glycerol and sucrose gradients along with immunoblot analysis of the vesicular marker proteins synaptophysin and secretogranin II revealed that β-NAD, ATP and DA are stored in both small synaptic-like vesicles and large dense-core-like vesicles. Yet, the three substances appear to have different preferential sites of release upon membrane depolarization including sites associated with SNAP-25 and sites non-associated with SNAP-25.
Keywords: neurosecretion, adrenal, neurotransmitter storage, large dense-core vesicles (LDCV), small synaptic vesicles (SSV)
Introduction
In the peripheral sympathetic nervous system noradrenaline (NA) and adenosine 5'-triphosphate (ATP) are assumed to be primary cotransmitters (Burnstock, 1990; Todorov et al., 1996). We have recently found that β-nicotinamide adenine dinucleotide (β-NAD) is also released along with NA and ATP upon nerve stimulation in blood vessels (Smyth et al., 2004), urinary bladder (Smyth et al., 2004; Breen et al., 2006), and large intestine (Mutafova-Yambolieva et al., 2007). In these nerve-smooth muscle preparations the release of β-NAD correlates with neural activity and requires intact neural fast Na+ channels, N-type voltage-operated calcium channels, and the 25-kDa synaptosomal associated protein SNAP-25 (Smyth et al., 2004; Smyth et al., 2006b; Breen et al., 2006; Mutafova-Yambolieva et al., 2007; Smyth et al., 2009). Exogenous β-NAD reduces the release of NA in blood vessels (Smyth et al., 2004), inhibits spontaneous contractions in the bladder (Breen et al., 2006), induces vasodilatation or vasoconstriction in the mesenteric vasculature (Smyth et al., 2009) and causes membrane hyperpolarization and relaxation in the murine colon (Mutafova-Yambolieva et al., 2007). Therefore, β-NAD is likely a novel neurotransmitter and a novel neuromodulator. Moreover, in some systems β-NAD, but not ATP, may be the purine neurotransmitter (Mutafova-Yambolieva et al., 2007) and hence, the release of ATP and β-NAD might occur at different sites or be mediated by different mechanisms. Understanding these mechanisms will forward our knowledge of the roles of extracellular β-NAD in the context of adrenergic-purinergic cotransmission and may suggest new possibilities for synaptic regulation. At present only a few studies in nerve-smooth muscle preparations provide fairly indirect information about possible co-storage and co-release of β-NAD with either NA or ATP (i.e., Bobalova and Mutafova-Yambolieva, 2006; Mutafova-Yambolieva et al., 2007; Smyth et al., 2009).
Nerve-smooth muscle preparations contain multiple cell types including smooth muscle cells, nerve endings, endothelial cells, and fibroblasts that make studies on vesicular storage and release of neurotransmitters in smooth muscles a challenging task. Instead, we employed cultured rat pheochromocytoma PC12 cells, a single cell-type model commonly utilized for elucidating basic mechanisms of neurosecretion. These cells phenotypically resemble sympathetic neurons and contain both small vesicles and dense core granules (Greene and Tischler, 1976). ATP is stored together with catecholamines (mainly dopamine, DA) in secretory vesicles and the contents are released by Ca2+-dependent exocytosis (Wagner, 1985). To the best of our knowledge no information is available about storage and secretion of β-NAD in adrenal chromaffin cells. Here we show that in nerve growth factor-differentiated PC12 cells β-NAD, ATP, and DA are all present in small synaptic-like vesicles (SSVs) and large dense-core-like vesicles (LDCVs). All compounds, including β-NAD, are subject to spontaneous and regulated release. Yet, disruption of SNAP-25 by botulinum neurotoxin A (BoNT/A) abolished the high K+-evoked release of β-NAD and DA, but not of ATP, suggesting that the two purines β-NAD and ATP might have different preferential sites of release upon membrane depolarization.
Materials and Methods
Cell culture
PC12 (rat adrenal pheochromocytoma) cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA; #CRL-1721) and grown on 225 cm2 collagen-coated cell culture flasks in F12K medium (ATCC, #30-2004) supplemented with 2 mM L-glutamine, 5% fetal bovine serum (FBS), 10% horse serum, 100 units/ml penicillin, and 100 units/ml streptomycin until 75–80% confluence. To induce differentiation, cells were incubated in F12K medium supplemented with 0.1% FBS and 50 ng/ml murine nerve growth factor (NGF, 2.5S, G514, Promega Corporation, Madison, WI): incubation continued for 7 days with change of culture medium every second day.
Overflow experiments
PC12 cells (3×106) were applied under suction onto Whatman GF/B filter paper (3 mm diameter) which was placed in a 0.45 μm Cameo 3N syringe filter serving as a perfusion chamber (modification of a method described by Todorov et al., 1996). The cells in the chamber were perfused with a superfusion solution at a rate of 0.8 ml/min. The composition of the superfusion solution was as follows (mM): NaCl, 140; KCl, 4.7; MgCl2, 1.2; CaCl2, 2.5; dextrose, 11; HEPES, 10, pH 7.4. After an equilibration period of 30 min, 800 μl of superfusate were collected as pre-stimulation samples. The cells were then stimulated for 20 min with 60 mM KCl. The high-potassium-containing solution was prepared by substituting NaCl for an equal amount of KCl to maintain isotonicity. Samples of superfusate (approximately 800 μl) were collected at 1', 2', 5', 10' and 20' time intervals in ice-cold Eppendorf tubes. Each sample was divided into two parts for measuring the content of catecholamines and purines as previously described (Mutafova-Yambolieva et al., 2003; Smyth et al., 2004). In some experiments stimulation with either 25 mM KCl or 100 μM nicotine was also performed.
Treatment with BoNT/A
Cells grown to 80% confluence were exposed to 30 nM botulinum neurotoxin A (BoNT/A, List Biological Laboratories, Campbell, CA) for 16 h before performing either overflow experiments or western immunoblot analysis of SNAP-25. BoNT/A was dissolved in BSA (1 mg/ml) as recommended by the manufacturer and further diluted in cell culture medium.
Fractionation of synaptic vesicles by glycerol and sucrose gradient centrifugation
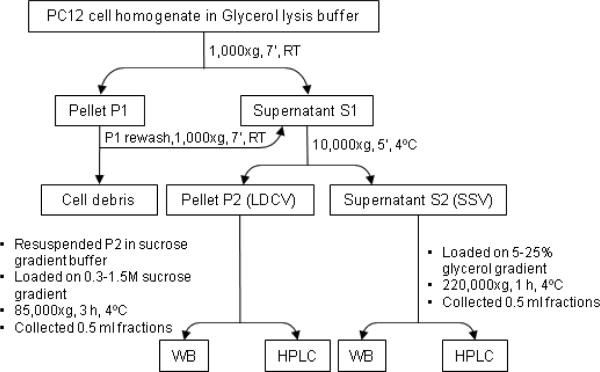
For velocity sedimentation, we applied a modified method by Clift-O'Grady et al., 1990 and Melikian and Buckley, 1999 summarized in Fig. 1. Differentiated cells were scraped into homogenization buffer A containing (mM): NaCl, 150; HEPES 10, pH 7.4; EGTA 1.0; MgCl2 0.1, pelleted (300xg, 5 min, room temperature) and resuspended in 2 ml buffer A containing protease inhibitors (1.0 mM PMSF and 1.0 μg/ml each leupeptin, aprotinin, and pepstatin). The cells were then homogenized (20 strokes up-and-down) in a teflon/glass homogenizer and postnuclear supernatant was obtained after centrifugation at 1000xg for 7 min at 4°C. The pellet was rewashed and centrifuged again, and the two postnuclear supernatants were pooled together. The sample was centrifuged at 10,000xg for 5 min at 4°C to obtain LDCVs in the P2 pellet, and SSVs in the S2 supernatant. S2 supernatant was then layered onto a continuous 5–25% glycerol gradient over a 1-ml 60% (1.75 M) sucrose pad, and SSVs were fractionated by centrifugation at 220,000xg for 1 hour at 4°C (SW41 Ti rotor, on a Beckman L8-70M Ultracentrifuge, Beckman Coulter, Inc., Fullerton, CA). P2 pellet was resuspended in 1 ml buffer containing 10 mM HEPES/KOH, pH 7.4, 0.3 M sucrose, and protease inhibitors, and layered onto a continuous 0.3–1.5M sucrose gradient over a 1-ml 60% (1.75 M) sucrose pad. LDCVs were fractionated by centrifugation at 85,000xg for 3 hours at 4°C (SW41 Ti rotor, on a Beckman L8-70M Ultracentrifuge, Beckman Coulter, Inc., Fullerton, CA). After centrifugation, tubes were punctured with 18-gauge syringe needles just above the sucrose pads, and 0.5-ml fractions were collected from the glycerol and sucrose gradients. Each sample was sonicated 3×15 sec, vortexed and then centrifuged at 15,000xg for 15 min at 4°C to remove insoluble material. Glycerol and sucrose concentrations were determined by measuring the refractive index of each fraction and converting to percentage glycerol or moles sucrose using a glycerol or sucrose standard/calibration curves. Four hundred μl aliquots were processed for HPLC analysis of purines (80 μl), catecholamines (60 μl) and HPLC fractions (260 μl) as described in Preparation of samples for purine detection, HPLC assay of etheno-purines, HPLC fraction analysis, Preparation of samples for catecholamine detection, and HPLC assay of catecholamines, The remaining samples (~ 100 μl) were for analysis of vesicular markers by immunoblotting.
Figure 1. Protocol for isolation of SSVs and LDCVs from cultured NGF-differentiated rat PC12 cells.
Different velocities of cold ultracentrifugation and glycerol and sucrose gradient purification were used to obtain fractions enriched in SSVs or LDCVs. RT, room temperature; WB, Western blot analysis; HPLC, high performance liquid chromatography; LDCV, large debse-cord-like vesicles; SSV, small synaptic-like vesicles.
Preparation of samples for purine detection
A modified method of Levitt et al., 1984 was employed to detect 1,N6-etheno-derivatives of the endogenous purines present in the cell superfusates as described previously (i.e., Bobalova et al., 2002). Briefly, 100 μl of a citrate phosphate buffer (pH 4.0) was added to 200 μl of the superfusate sample in Eppendorf tubes. Ten μl of 2-chloroacetaldehyde was added to the samples in a fume hood; the samples were then heated for 40 min at 80°C in a dry bath incubator (Fisher Scientific, USA). Using this procedure endogenous ATP, ADP, AMP, and adenosine (ADO) are derivatized to 1,N6-etheno-ATP (eATP), 1,N6-etheno-ADP (eADP), 1,N6-etheno-AMP (eAMP), and 1,N6-etheno-ADO (eADO), respectively (Bobalova et al., 2002). The endogenous β-NAD, ADP-ribose (ADPR) and cyclic ADPR (cADPR) are derivatized to 1,N6-etheno-ADP-ribose (eADPR) as described previously (Smyth et al., 2004). The reactions were stopped by placing samples on ice.
HPLC assay of etheno-purines
The HP1100 liquid chromatography module system (Agilent Technologies, Wilmington, DE) was used throughout this study and has been previously described (Bobalova et al., 2002). The mobile phase consisted of 0.1 mol/L KH2PO4 (pH 6.0) as eluent A; eluent B contained of 35 % methanol and 65 % eluent A. Gradient elution was employed according to the following linear program: time 0, 0 % eluent B; 18 min, 100 % eluent B. Flow rate was 1 ml/min and run time was 20 min. Column temperature was ambient while the autosampler temperature was 4°C. The fluorescent detector recorded signals at an excitation wavelength of 230 nm and emission wavelength of 420 nm, which are the optimum conditions for detecting etheno-derivatives of nucleotides and nucleosides (Bobalova et al., 2002). The amounts of purines in each sample were calculated from calibration curves of purine standards run simultaneously with every set of unknown samples. Results were normalized for sample volume and cell count and the overflow of purines was expressed in pmol/106 cells.
Sample concentration and HPLC fraction analysis
As aforementioned β-NAD, ADPR and cADPR elute as a single peak of eADPR at 11.2 min. To identify the ratio of β-NAD:ADPR:cADPR forming eADPR we carried out an HPLC fraction analysis as described previously (Smyth et al., 2004). Briefly, in some overflow experiments 2.4-ml cell superfusate samples containing either 5 mM KCl (pre-stimulation sample) or 60 mM KCl for 5 min (stimulation sample) were collected in Eppendorf tubes and were immediately immersed in liquid N2. Control samples from solutions containing either 5 mM KCl or 60 mM KCl with no contact with cells were also collected. The samples were then concentrated by Speed Vacuum (Savant SVC100, Thermo Electron Corp., Westmont, IL) to 1 ml volume. Nine hundred μl of each concentrated sample were injected into the HPLC system and 400 μl-fractions corresponding to the retention times of cADPR (7.0–7.4 min, “7.2-min fraction”), ADPR (8.3–8.7 min, “8.5-min fraction”), and β-NAD (10.3–10.7 min, “10.5-min fraction”) were collected in Eppendorf tubes containing 180 μl citric buffer. The exact retention times for the three nucleotides were determined by injecting authentic β-NAD, cADPR and ADPR standards (20 nmol/injection) in the same sequence prior to the concentrated superfusate samples. The HPLC fractions were further subjected to etheno-derivatization with 20 μl 2-chloroacetaldehyde as described in Preparation of samples for purine detection. The derivatized samples were injected into the HPLC and analyzed for eADPR content. HPLC-fraction analysis was also performed with samples collected during glycerol and sucrose fractionation. Since these experiments were carried out with larger number of cells (>50×106 cells) there was no need of concentrating the samples prior collecting HPLC fractions. For these experiments 240 μl of each glycerol or sucrose fraction was injected in the HPLC system and the samples were processed further as described above.
Preparation of samples for catecholamine detection
One hundred and fifteen μl of superfusate solution was acidified to pH 2.6 with 3 μl 1 N perchloric acid and the samples were filtered through 0.22 μm Cameo 3N syringe filters.
HPLC assay of catecholamines
The overflow of catecholamines was assayed as described previously (Mutafova-Yambolieva et al., 2003). Briefly, 115-μl aliquots from the samples were acidified with 3 μl 1 M perchloric acid to pH 2.6 and injected (70 μl) into an isocratic HP1100 HPLC system equipped with an HP1049A electrochemical detector (Agilent Technologies, Wilmington, DE, USA) and a MD-150 column (ESA Inc., Chelmsford, MA, USA). The mobile phase for separation consisted of the following (mmol/L): 50 Na2PO4; 0.2 EDTA; 3.0 l-heptanesulfonic acid, 10 LiCl, and methanol 3 % v/v in deionized water (pH 2.6). The HPLC systems were controlled, and data collected, by a Dell computer equipped with HP ChemStation (A.10.02) software from Agilent Technologies (Wilmington, DE, USA). The amounts of DA in each sample were calculated from calibration curves of catecholamine standards run simultaneously with every set of unknown samples. Results were normalized for sample volume and cell count and the overflow of DA was expressed in pmol/106 cells.
Western immunoblot analysis of SNAP-25 in total PC12 cell extracts
PC12 cells from control and BoNT/A-treated groups were washed 3×5 ml cell culture medium, scraped, solubilized in 200 μl RIPA buffer, sonicated 3×30 s, incubated on ice, vortexed, and centrifuged at 15,000xg for 20 min at 4°C. Total protein concentration of the supernatant was determined by the Bradford assay (BioRad kit, Hercules, CA) using bovine serum albumin (BSA) for standards. Cell homogenates were reduced with Laemmli sample buffer and equal amounts of total protein (10 μg) were resolved by SDS-PAGE (15% acrylamide) and transferred onto nitrocellulose membranes for 1.5 hours at 24V and 4°C (Genie blotter, Idea Scientific Company, Minneapolis, MN). Membranes were blocked for 1 hour with LI-COR blocking buffer (LI-COR, Inc., Lincoln, NE) and probed for 18 hours at 4°C with a SNAP-25 primary mouse monoclonal antibody (Sternberger Monoclonals Inc., Lutherville, MD), diluted 1000-fold in LI-COR buffer. After removal of excess primary antibody, membranes were incubated for 45 min at room temperature with a secondary mouse antibody coupled to IR800 infrared marker (emission wavelength 800 nm, Rockland Immunochemicals, PA), diluted 100,000-fold in LI-COR buffer. Images were obtained with an infrared Odyssey scanner (LI-COR, Inc., Lincoln, NE). For positive controls on immune blots we used 30 μg of total protein, obtained from rat brain tissue homogenized in RIPA buffer.
Western immunoblot analysis of vesicular protein markers and SNAP-25 in glycerol and sucrose gradient fractions
Cell fractions were prepared as described in Fractionation of synaptic vesicles by glycerol and sucrose gradient centrifugation. Proteins from equal fraction volumes were resolved by SDS-PAGE and transferred onto nitrocellulose membranes. Membranes were blocked and incubated with mouse anti-synaptophysin (Chemicon International, Tamecula, CA, MAB368, dilution 1:1000) as an SSV marker (Jahn et al., 1985; Cutler and Cramer, 1990), rabbit anti-secretogranin II (Santa Cruz Biotechnologies, Santa Cruz, CA, sc-50290, dilution 1:100) as a LDCV marker (Fischer-Colbrie et al., 1995; Cutler and Cramer, 1990), and mouse anti-SNAP25 (Sternberger Monoclonals Inc., Lutherville, MD, SMI-81, dilution 1:1000) as a marker of plasma membranes (Sollner et al., 1993; McMahon and Sudhof, 1995; Sorensen, 2005) and were further processed as described in the Western immunoblot analysis of SNAP-25 in total PC12 cell extracts section.
Statistics
Data are presented as means ± SEM. One-way ANOVA with Bonferroni's multiple comparison post test was performed using GraphPadPrism v. 3 for Windows (GraphPad Software, San Diego, CA) when three or more groups of data were compared. Unpaired two-tailed Student's t-test was performed using the same software when two groups of data were compared. A probability value of less than 0.05 was considered significant.
Results
Spontaneous secretion of catecholamines and purines in NGF-treated PC12 cells
Differentiated PC12 cells perfused with normal K+ solution (5 mM KCl) spontaneously secreted DA (1.93±0.48 pmol/106 cells), ATP (0.106±0.03 pmol/106 cells), ADP (1.023±0.16 pmol/106 cells), AMP (0.752±0.134 pmol/106 cells), and ADO (0.467±0.075 pmol/106 cells), n=13–21. Figs. 2 and 3 show the values of spontaneously secreted DA and purines (at KCl 5 mM) in the controls for BoNT/A-treated cells, discussed below. Spontaneous release of a mixture of β-NAD, ADPR and cADPR was also detected; due to etheno-derivatization at 80°C, the components of this cocktail elute as one peak of eADPR with a retention time of 11.2 min (Fig. 3A, KCl 5 mM). Thus, the superfusates collected before stimulation contained 0.410±0.061 pmol/106 cells, n=21, of the mixture; these amounts were calculated based on the assumption that β-NAD is the major component of the peak (see results from fraction analysis shown in Distribution of β-NAD, ADPR and cADPR in cell superfusates determined by HPLC fraction analysis). Total purines (ATP+ADP+AMP+ADO+β-NAD+ADPR+cADPR) secreted in the superfusates were 3.00±0.42 pmol/106 cells, n=21. Noradrenaline was not detected in the samples collected during perfusion of the PC12 cells with 5 mM KCl solution, likely due to concentrations of noradrenaline below detection limits.
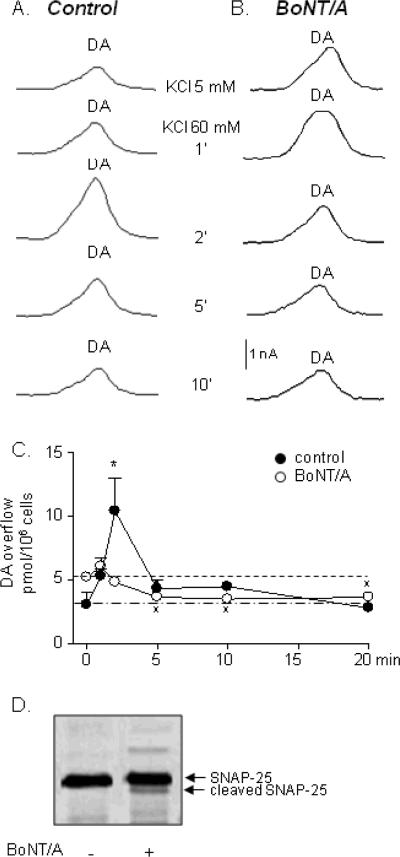
Figure 2. Spontaneous and high-K+-evoked secretion of DA in NGF-treated PC12 cells.
(A) PC12 cells secrete DA in a solution containing 5 mM KCl. Superfusion of the cells with 60 mM KCl evokes additional secretion of DA, which is maximum at 2 minutes of superfusion. (B) Pretreatment of cells with BoNT/A (30 nM for 16 h) reduces the evoked release of DA. Note also that DA amounts in superfusates are significantly reduced at 5–20' below the initial levels in non-stimulated cells. (C) Quantified data from 4–5 experiments expressed as the mean ± SEM. *P<0.05 vs. controls at 0' (KCl 5 mM), xP<0.05 vs. the highest value in BoNT/A group at 1'. One-way ANOVA followed by Bonferroni's multiple comparison tests. (D) Immunoblot analysis of SNAP-25 shows a single band at 25 kDa in homogenates from control PC12 cells. An additional 24-kDa band appears in BoNT/A-treated cells indicating cleavage of SNAP-25 induced by BoNT/A.
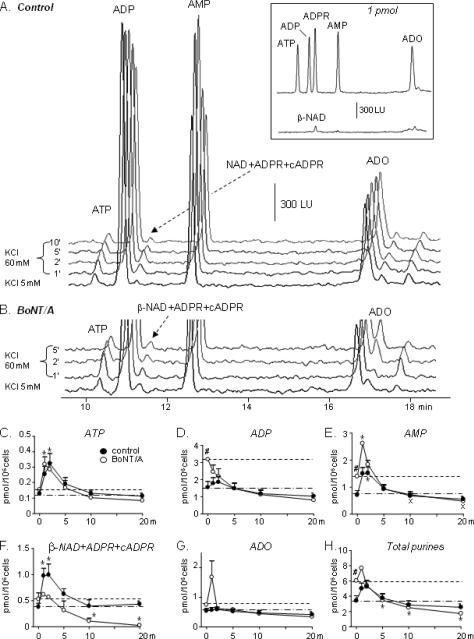
Figure 3. Spontaneous and high-K+-evoked release of adenine purines in NGF-treated PC12 cells.
(A) Original chromatograms from samples collected during perfusion of the PC12 cells with either 5 mM KCl (pre-stimulation sample) or in the presence of 60 mM KCl for different time periods. KCl 60 mM evoked overflow of ATP, ADP, AMP, adenosine (ADO) and a mixture of β-NAD, cADPR, and ADPR. Inset: chromatographic peaks generated by etheno-derivatized purine standards (1 pmol each). Note that β-NAD has significantly lower fluorescence coefficient in generating eADPR than ATP, ADP, ADPR, AMP and ADO in generating corresponding e-purines. (B) Excerpts of original chromatograms of superfusates from BoNT/A-pretreated cells with an emphasis on ATP and the mixture of β-NAD, ADPR, and cADPR. (C) Quantified data from 4–5 experiments expressed as the mean ± SEM. Data are averaged for each individual purine as well as for the sum of all purines (total purines). *P<0.05 vs. controls at 0' (KCl 5 mM). One-way ANOVA followed by Bonferroni's multiple comparison tests. #P<0.05 vs. 0' in controls. Unpaired Student's t-test. BoNT/A increased the spontaneous release of ADP, AMP, and total purines. The evoked release of most purines, except for ATP, was significantly reduced by BoNT/A. In BoNT/A-treated cells secretion of AMP, β-NAD+ADPR+cADPR, and total purines at 5–20' was below the initial purine levels.
Secretion of catecholamines and purines evoked by 60 mM KCl and effects of BoNT/A
Perfusion of PC12 cells with 25 mM KCl for 20 minutes caused no additional release of DA and purines (data not shown), suggesting that higher concentrations of KCl are necessary to evoke measurable effects in these cells. Therefore, in the majority of the study 60 mM KCl was used to stimulate the PC12 cells. The high-K+ (60 mM KCl) solution evoked additional release of DA and purines with greatest release occurring within 2 min of contact with the cells (Fig. 2A,C-control and Fig. 3A, C–H-control). Secretion of DA and purines returned to basal (pre-stimulation) levels by about the 5th minute of superfusion of 60 mM KCl. Thus, DA was 3.045±0.971 pmol/106 cells before stimulation, 10.391±2.615 pmol/106 cells at 2' (P<0.01, t=4.184, F=4.857), and 4.335±0.661 pmol/106 cells at 5' (P>0.05), one-way ANOVA, followed by Bonferroni's multiple comparison posttest, n=5. ATP was 0.128±0.026 pmol/106 cells before stimulation, 0.324±0.064 pmol/106 cells at 2' (P<0.05, t=3.363, F=4.021), and 0.192±0.041 pmol/106 cells at 5' of stimulation with high K+ solution (P>0.05, t=1.092), one-way ANOVA, followed by Bonferroni's test, n=5. ADP and AMP greatly exceeded the amounts of ATP in the superfusate samples collected before and during stimulation, which is in agreement with previously published results in bovine chromaffin cells (i.e. Todorov et al., 1996; Kasai et al., 1999). The chromatographic peaks formed by β-NAD+ADPR+cADPR appear small due to the very low fluorescence coefficient of β-NAD generating eADPR (see Fig. 3 inset). However, when compared to standards the amounts of β-NAD+ADPR+cADPR (which comprises mainly β-NAD, see below) is much greater than other purines, including ATP (Fig. 3C,F). Thus, the overflow of β-NAD was 0.379±0.071 pmol/106 cells before stimulation, 0.975±0.147 pmol/106 cells at 1' (P<0.05, t=3.351), 0.994±0.210 pmol/106 cells at 2' of stimulation (P<0.05, t=3.459), and 0.627±0.102 pmol/106 cells at 5' of stimulation with KCl 60 mM (P>0.05, t=1.457), one-way ANOVA, followed by Bonferroni's test. At 10' and 20' of KCl stimulation the overflow of ATP and β-NAD was not significantly different from pre-stimulation values (P>0.05), Fig. 3.
We next examined whether disruption of SNAP-25 with BoNT/A would affect the spontaneous and evoked by 60 mM KCl release of DA and purines. Immunoblot analysis of PC12 cells revealed a single immunoreactive band with molecular size of ~25 kDa, representing intact SNAP-25 (Fig. 2D, lane 1). Incubation of PC12 cells with BoNT/A produced a secondary immunoreactive band with apparent molecular size of ~24 kDa (lane 2), which was not found in control cell protein extracts (lane 1). The 24-kDa band likely represents degradation product(s) of SNAP-25 owing to cleavage by BoNT/A (Blasi et al., 1993). BoNT/A tended to increase the spontaneous secretion of DA and all purines (see pre-stimulation samples at KCl 5 mM in Figs. 2 and 3, controls vs. BoNT/A-treated, P<0.05 for ADP, AMP, and total purines, P>0.05 for DA, ATP, β-NAD and ADO, unpaired t-test, two-tailed. The high K+-evoked release of DA (Fig. 2C), ADP, AMP, ADO and the mixture of β-NAD, ADPR and cADPR (Fig. 3D–H) was significantly attenuated and shortened within 1 minute of perfusion with 60 mM KCl in BoNT/A-treated cells. The amount of released ATP was not significantly changed in BoNT/A-treated cells (Fig. 3B, C): thus, in BoNT/A-treated cells ATP overflow was 0.154±0.006 pmol/106 cells before stimulation and 0.318±0.049 pmol/106 cells at 1' of stimulation (P<0.05, t=3.387, one-way ANOVA, followed by Bonferroni's posttest). However, the overflow of β-NAD in the same samples was 0.528±0.123 pmol/106 cells before stimulation, 0.616±0.015 pmol/106 cells at 1'of stimulation (P>0.05, t=0.674), and 0.565±0.03 pmol/106 cells at 2' of stimulation with 60 mM KCl (P>0.05, t=0.284). Continuous stimulation of the PC12 cells for 10 minutes and 20 minutes led to reduced levels of DA. Thus, DA overflow was 3.673±0.028 pmol/106 cells at 5', 3.510±0.175 pmol/106 cells at 10', and 3.657±0.431 pmol/106 cells at 20' of stimulation, which was significantly lower than the overflow of DA at 1' of stimulation reaching 6.087±0.658 pmol/106 cells (P<0.05, one-way ANOVA, followed by Bonferroni's posttest). Likewise, in BoNT/A-treated cells the overflow of β-NAD, AMP, and total purines at 10' and 20' was significantly lower than the spontaneous overflow in pre-stimulation samples (Fig. 3 E,F,H).
Secretion evoked by nicotine 100 μM
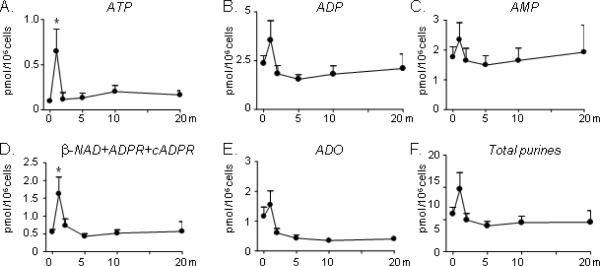
Similar to the effects of high-K+ solution, nicotine 100 μM transiently increased the secretion of both ATP and β-NAD (Fig. 4A, D). In some cases secretion of purines at 5–20 min was significantly lower than secretion at 1' of stimulation, which was not significantly different from pre-stimulation values. DA secretion was increased from 0.396±0.058 pmol/106 cells before nicotine to 0.583±0.060 pmol/106 cells at 1' (P>0.05, t=1.239), 0.858±0.16 pmol/106 cells at 2' (P <0.05, t=3.055, F=4.116), and 0.82±0.114 pmol/106 cells at 10' (P>0.05, t=2.823) of stimulation with nicotine, one-way ANOVA, followed by Bonferroni's test, n=8. Secretion of ATP was increased from 0.097±0.023 pmol/106 cells at 0' to 0.649±0.246 pmol/106 cells at 1' of stimulation (P<0.05, t=3.347, F=3.188, one-way ANOVA, followed by Bonferroni's test). Secretion of ATP at 2', 5', 10', and 20' was not significantly different from secretion of ATP before stimulation (P>0.05). Likewise, secretion of β-NAD was increased from 0.552±0.074 pmol/106 cells at 0' to 1.629±0.471 pmol/106 cells at 1' (P<0.05, t=3.187, F=3.411), whereas no significant increase was observed at 2–20' of stimulation with nicotine (P>0.05), one-way ANOVA, followed by Bonferroni's posttest. No significant increase of ADP, AMP, adenosine or total purines was observed at 1–20' of stimulation with nicotine, P>0.05 (Fig. 4). In general, the results with KCl 60 mM simulation and nicotine stimulation were qualitatively similar, although under these experimental conditions the effect of nicotine seemed more transient than the effect of KCl.
Figure 4. Spontaneous and nicotine (100 μM)-evoked release of adenine purines in NGF-treated PC12 cells.
Quantified data from 6–10 experiments expressed as the mean ± SEM. Data are averaged for each individual purine as well as for the sum of all purines (total purines). *P<0.05 vs. controls at 0' (KCl 5 mM). xP<0.05 vs. 1'. One-way ANOVA followed by Bonferroni's multiple comparison test. Note that nicotine induced a transient secretion of purines, which was statistically significant for ATP and β-NAD+ADPR+cADPR. Although secretion of ADO and total purines did not show statistically significant increase at 1', the values of these purines at 5–20' of stimulation showed significantly lower levels than at 1'.
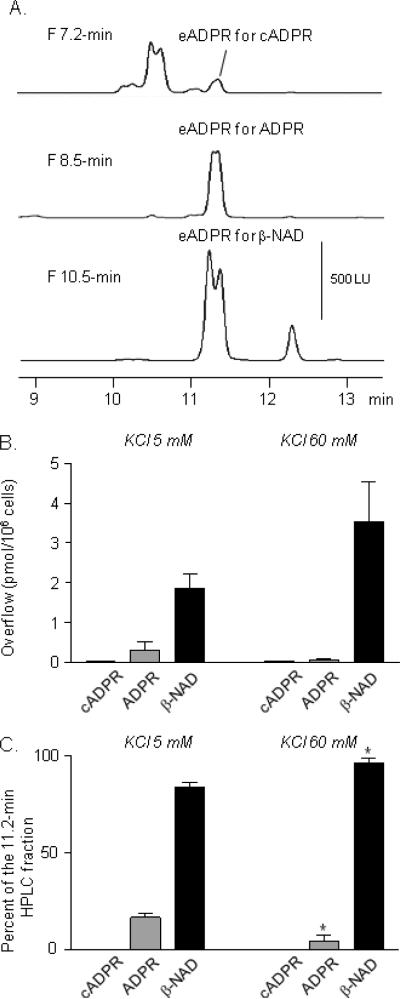
Distribution of β-NAD, ADPR and cADPR in cell superfusates determined by HPLC fraction analysis
β-NAD, ADPR and cADPR co-elute as 1,N6-etheno-ADPR at ~11.2 min due to conversion of β-NAD and cADPR to ADPR during the etheno-derivatization at 80°C (Smyth et al., 2004; Breen et al., 2006). HPLC fraction analysis of the 11.2-min peak showed that β-NAD is the predominant compound, comprising 83.56±2.47% of purines in the samples collected before stimulation (KCl 5 mM) (Fig. 5C) and 95.72±2.9% during stimulation (KCl 60 mM), n=4, P=0.02, t=3.143, df=6, unpaired t-test, two-tailed. ADPR comprised 16.34±2.47% and 4.277±2.93% of the mixture before and during stimulation, respectively (n=4, P=0.02, t=3.141, df=6, unpaired t-test, two-tailed). Thus, during stimulation with 60 mM KCl the release of β-NAD+ADPR+cADPR was increased (Fig. 3A, F) mainly due to increased secretion of β-NAD and not of ADPR or cADPR (Fig. 5B,C, KCl 60 mM). It is reasonable, therefore, to refer to the compound released during stimulation with high-K+ solution as β-NAD.
Figure 5. HPLC fraction analysis of the mixture of β-NAD, ADPR and cADPR.
(A) Original chromatograms of the 7.2-min (cADPR-containing) fraction, the 8.5-min (ADPR-containing) fraction and the 10.5-min (β-NAD containing) fraction of cell superfusate samples collected in the first 5 min of superfusion with 60 mM KCl and etheno-derivatized with 2-chloroacetaldehyde at 80°C, pH 4.0 for 40 min. (B) Averaged data. The pre-stimulation sample (5 mM KCl) contained mainly β-NAD and small amounts of ADPR, but no detectable amounts of cADPR. Stimulation with 60 mM KCl caused increase in the β-NAD content in the cell superfusate. (C) Distribution of β-NAD, ADPR and cADPR in pre-stimulation (5 mM KCl) samples and in samples collected during stimulation with 60 mM KCl. In both samples β-NAD is the primary purine nucleotide in the 11.2-min fraction. The ratio β-NAD:ADPR increases in the samples collected during stimulation with a high-K+ solution. *P<0.05 vs. KCl 5 mM, unpaired t-test, two-tailed.
Fractionation of synaptic vesicles by glycerol gradient: neural markers, content of DA and purines, HPLC fraction analysis of β-NAD, ADPR, and cADPR
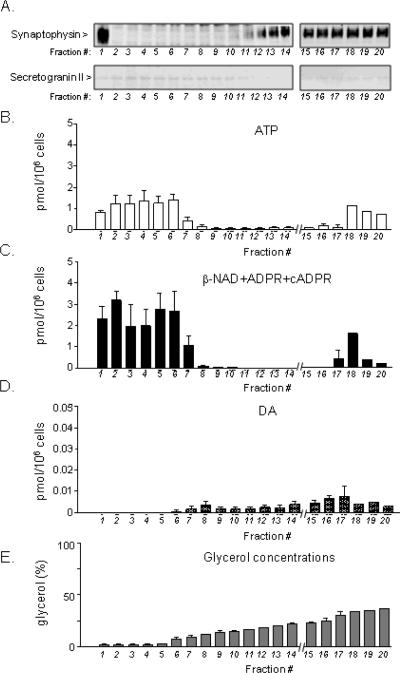
Aliquots of samples collected from glycerol gradient centrifugation were processed for Western immunoblot analysis of synaptophysin and secretogranin II. Aliquots of the samples were also processed for analysis of purine and catecholamine contents. Synaptophysin, an SV marker, labeled vesicle population in the fractions containing ~12–25% glycerol (Fig. 6A, top row, F11–F20), whereas the immunoreactive bands of secretogranin II, a LDCV marker, were negligible in all glycerol fractions (Fig. 6A, bottom row). Synaptophysin-like immunoreactivity was also present in F1, which may be due to the presence of synaptophysin in membranes of very small low-density vesicles such as small synaptic-like microvesicles (SLMV), which fractionate at the top of the gradient. Parallel HPLC measurements of content of neurotransmitter substances in the glycerol fractions showed that all fractions contained DA and purines, but in different proportions. Fractions with low glycerol (<6%) concentrations (e.g., F1–F6) contained the largest amounts of ATP and β-NAD+ADPR+cADPR. Then the concentrations of ATP and β-NAD+cADPR+ADPR gradually declined as the glycerol concentrations raised from ~ 6 % to ~ 20 % (F6–F14), and increased again at F17–19 (~22–25% glycerol). A similar pattern of distribution was observed with ATP, ADP, AMP, and adenosine (data not shown). An HPLC fraction analysis of the peak representing β-NAD+ADPR+cADPR in selected samples from the glycerol gradient samples showed that β-NAD is the prevailing compound in all fractions. Thus, F3 comprised of 99.49% β-NAD, 0.36% ADPR and 0.15% cADPR. Likewise, F9 was composed of 99.40% β-NAD, 0.32% ADPR and 0.29% cADPR. F13–20 contained 92% β-NAD, 8% ADPR and no detectable amounts of cADPR. DA was not detected in the low-glycerol fractions (F1–F5, Fig. 6D). However, DA was detected in fractions with higher glycerol levels and peaked in F15–F19.
Figure 6. Fraction separation of small synaptic vesicles (SSV) by glycerol gradient centrifugation.
(A) Fractionation of synaptic vesicles was evaluated by the immunoreactivity of the vesicular markers synaptophysin (for SSV and SLMV) and secretogranin II (for LDCV) using equal fraction volumes. Synaptophysin-immunoreactive SSVs (top row) were present mostly in fractions with 10–23% glycerol, F13–F20. Secretogranin II immunoreactivity was faint (bottom row) or absent, indicating lack of LDCV in the glycerol fractions. (B, C, D) Contents of ATP, β-NAD+ADPR+cADPR, and DA in fractions separated by glycerol gradient centrifugation. Averaged data (means ± SEM), n=4. (E) Concentrations of glycerol in fractions isolated by glycerol gradient centrifugation. Averaged data (means ± SEM), n=4.
Fractionation of synaptic vesicles by sucrose gradient: neural markers, content of DA and purines, HPLC fraction analysis of β-NAD, ADPR, and cADPR
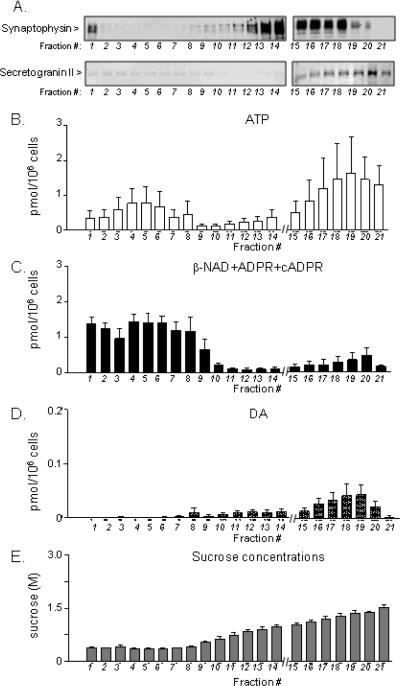
Aliquots of samples collected from sucrose gradient centrifugation (presumably containing LDCVs) were also processed for Western immunoblot analysis of synaptophysin and secretogranin II expression and for HPLC analysis of contents of purines and catecholamines. Synaptophysin labeled the fractions containing 0.4–1.0 M sucrose (F12–F18), whereas secretogranin II labeled mainly the fractions with 0.9–1.4 M sucrose (F16–F21), Fig. 7A. Similar to glycerol gradient fractions, F1 showed high expression of synaptophysin, possibly due to greater amounts of microvesicles in this fraction. Low-sucrose fractions (F1–F8) showed similar amounts of ATP (Fig. 7B). Likewise, the content of β-NAD+cADPR+ADPR was similar in F1–F8 fractions (Fig. 7C). Fractions F9–F15 showed declining concentrations of ATP and β-NAD+cADPR+ADPR, whereas the content of ATP and β-NAD+cADPR+ADPR increased again in the high-sucrose fractions and peaked in F17–F20, the fractions with highest expression of secretogranin II. HPLC fraction analysis showed that the distribution of β-NAD, ADPR and cADPR in F3 was 100% β-NAD and no ADPR and cADPR. F9 contained 94.7% β-NAD, 0% ADPR, and 5.3% cADPR, whereas F11-16 contained 98.2% β-NAD and 1.8% ADPR. F19–20 contained 94.7% β-NAD, 4.4% ADPR and 0.9% cADPR. Therefore, β-NAD was the major compound in all examined fractions. DA was almost absent in the low-sucrose fractions (F1–F9, Fig. 7D). However, DA was detected in fractions with higher sucrose levels and peaked in F16–F20.
Figure 7. Fraction separation of large dense-core vesicles (LDCVs) by sucrose gradient centrifugation.
(A) Fractionation of LDCV was evaluated by the immunoreactivity of the vesicular markers synaptophysin (top row) and secretogranin II (bottom row) using equal fraction volumes. Synaptophysin focused in fractions (F13–18) with 0.7–1.3 M sucrose (E). Secretogranin II-labeled LDCVs in fractions with sucrose concentrations above 0.9M (F15–21), A and E. (B,C,D) Contents of ATP, β-NAD+ADPR+cADPR, and DA in fractions separated by sucrose gradient centrifugation. Averaged data (means ± SEM), n=3. (E) Concentrations of sucrose in fractions isolated by sucrose gradient centrifugation. Averaged data (means ± SEM), n=3.
Western immunoblot analysis of SNAP-25 in glycerol and sucrose gradient centrifugation fractions
Fig. 8 shows the presence of SNAP-25 in all fractions with increasing concentrations of glycerol (6–25%) and sucrose (0.4–1.4 M). It appears that the SNAP-25 bands are stronger in the sucrose gradient fractions (compare Fig. 8A and 8B) taking into account that equal fraction volumes were loaded in the immunoblots.
Figure 8. Western immunoblot analysis of SNAP-25 in fractions isolated by glycerol and sucrose gradient centrifugation.

Expression of SNAP-25 was analyzed by immunoblotting using equal fraction volumes. (A) SNAP-25 was expressed in fractions containing >5% glycerol and peaked in fractions containing 10–25% glycerol. (B) SNAP-25 was also expressed in fractions containing >0.32 M sucrose and peaked in fractions containing 0.7–1.12 M sucrose.
Discussion
Studies in rat tail artery suggest that ATP and NA are likely stored in the same vesicles within the sympathetic nerve varicosity and are released in parallel by vesicular exocytosis (Stjarne, 1989; Stjarne et al., 1994; Brock and Cunnane, 1999). Likewise, ATP and catecholamines appear released in parallel in adrenal chromaffin cells (Wagner, 1985; Njus et al., 1986; Todorov et al., 1996) and hence are likely released from the same vesicles. In contrast, functional and overflow studies in the rodent vas deferens have suggested that ATP and NA are released differentially (e.g., Trachte, 1985; Ellis and Burnstock, 1989; Ellis and Burnstock, 1990; Todorov et al., 1996) and thus may originate from different synaptic vesicles. These and other studies illustrate decades-long controversy about the sources of storage and release of cotransmitter substances. Understanding mechanisms of co-storage and co-release of neurotransmitters is important for understanding mechanisms of synaptic integration and plasticity. It may raise the potentials for selective external control of synaptic events and may thus have potential clinical significance. This becomes particularly important when a novel neurotransmitter substance is discovered.
Work in our laboratory has contributed to the recent notion that, in addition to its well-known role in intracellular processes, β-NAD is also an extracellular molecule involved in cell-to-cell communications (Ziegler and Niere, 2004; Billington et al., 2006) and may function as a novel neuromodulator (Smyth et al., 2004; Breen et al., 2006) and a novel neurotransmitter (Mutafova-Yambolieva et al., 2007). β-NAD is released concomitantly with NA and ATP in blood vessels, urinary bladder, vas deferens, large intestine (Smyth et al., 2004; Breen et al., 2006; Smyth et al., 2006a; Smyth et al., 2006b; Bobalova and Mutafova-Yambolieva, 2006; Mutafova-Yambolieva et al., 2007; Smyth et al., 2009), and brain (Mutafova-Yambolieva et al., unpublished observations). In a number of smooth muscles the release of NA and β-NAD is regulated in parallel (i.e., Bobalova and Mutafova-Yambolieva, 2006; Smyth et al., 2009), suggesting that the two substances may originate from the same synaptic vesicles. The release of ATP and β-NAD, however, often appear differentially regulated (i.e., Mutafova-Yambolieva et al., 2007; Smyth et al., 2009). Therefore, ATP and β-NAD may originate from different release sites including different nerves or different secretory vesicles. The present study provides a thorough analysis of simultaneous release of catecholamines and purines in NGF-differentiated PC12 cells. The major novel finding is that differentiated PC12 cells exhibit constitutive and evoked release of not only DA and ATP, but also of β-NAD. This represents a significant advance in knowledge over previously published works limited to secretion of catecholamines and ATP in chromaffin cells. β-NAD, ATP, and DA appear to be co-stored in multiple vesicles with no well-defined distinction in the primary distribution of these substances. Nonetheless, β-NAD, ATP, and DA appear to have different preferential sites of secretion.
We employed a small-volume superfusion assay for collecting neurotransmitters secreted at rest and upon stimulation with high-K+ solution or nicotine from NGF-differentiated rat PC12 cells. This technique allowed examining the time course of neurotransmitter release under constant flow. NGF-treated PC12 cells showed constitutive release of DA, ATP, ADP, AMP, adenosine, β-NAD, and ADPR. Stimulation of cells with high-K+ solution (and hence causing Ca2+ influx due to membrane depolarization) elicited release of DA and the purines listed above. Importantly, the maximum evoked release of all substances was achieved within the first 2 minutes of stimulation and returned to basal levels by the 5th minute of stimulation even though the stimulation lasted for 20 minutes. Thus, studies assaying mass release of neurotransmitters in PC12 cells during stimulation with high-K+ stimulation for longer than 5 minutes may underestimate the evoked release of neurotransmitters at the expense of measuring basal release and more degradation products. As aforementioned, differentiated PC12 cells exhibited constitutive and evoked release of β-NAD. Small amounts of ADPR were also found in tissue superfusates. ADPR is the major direct metabolite of β-NAD, since 98 % of β-NAD is degraded to ADPR by NAD glycohydrolase and ~ 2% of β-NAD is degraded to cADPR by ADP-ribosyl cyclase (Lee, 2001). In samples collected in normal-K+ solution the β-NAD:ADPR ratio was 6:1, whereas the high-K+ (60 mM)-evoked release of β-NAD:ADPR was 70:1. Activation of nicotinic acetylcholine receptors with nicotine also caused a transient increase in secretion of DA, ATP and β-NAD as did the high K+-solution.
Synaptic-like microvesicles (SLMVs) are present in PC12 cells (Liu and Edwards, 1997; Varoqui and Erickson, 1998) and the biogenesis and composition of SLMV is similar to the SSVs in presynaptic nerves in the central and peripheral nervous systems (Navone et al., 1989; Llona, 1995). Synaptophysin is a component of the SLMV and SSV membrane (Navone et al., 1986; Cutler and Cramer, 1990; Jahn and Sudhof, 1994; Llona, 1995) and to a smaller content is also present in the LDCV membranes (Marxen et al., 1997). To isolate SSVs and LDCVs, after removing nuclei, mitochondria, lysosomes, proxyosomes, and other large particles, we applied the samples onto continuous glycerol and sucrose gradients, respectively. In agreement with previous studies (Melikian and Buckley, 1999; Liu and Edwards, 1997;Varoqui and Erickson, 1998) synaptophysin labeled the SSV population in the fractions containing 10–23% glycerol. As shown earlier (Marxen et al., 1997), synaptophysin was present in fractions containing 0.7–1.3 M sucrose, presumably enriched in LDCVs. Synaptophysin was also present in membranes of SLMV, which fractionated at the top (F1) of the two gradients. To label LDCVs we used secretogranin II (Fischer-Colbrie et al., 1995; Cutler and Cramer, 1990). As anticipated secretogranin II fractionated through the sucrose gradient with maximum occurrence in fractions with sucrose concentrations above 0.9 M. These observations are also in agreement with previous findings (e.g., Melikian and Buckley, 1999; Liu and Edwards, 1997; Yao and Hersh, 2007). Secretogranin II did not label well the glycerol fractions suggesting that no major pool of LDCVs was present in these samples. We concluded, therefore, that the glycerol and sucrose fractionation centrifugations led to isolation of fractions enriched in SSVs/SLMVs and LDCVs, respectively.
We next determined the content of purines and catecholamines in SSVs/SLMVs and LDCVs isolated as described above. Previous studies have shown that ATP and catecholamines are stored in and concomitantly released from large secretory vesicles in chromaffin cells and PC12 cells (Wagner, 1985; Kasai et al., 2001), although these studies did not examine the role of small synaptic vesicles in the release of ATP and DA. In the present study we also found that ATP and DA are co-stored in LDCVs: the high-sucrose fractions that labeled for secretogranin II showed high amounts of ATP, β-NAD and DA. β-NAD comprised >95% of the mixture β-NAD+ADPR+cADPR in the high-sucrose fractions. Low-sucrose fractions, presumably representing the cytosol, contained large amounts of ATP and β-NAD, but almost no DA. Likewise, fractions with low glycerol concentrations contained the largest amounts of ATP and β-NAD, but no DA. Based on immunoblot analysis of the distribution of synaptophysin and secretogranin II we assume that these glycerol-free fractions largely represented the cytosol. Thus, a fraction of the neurotransmitter substances detected in F1–F6 may be the neurotransmitters synthesized in the cytoplasm “waiting” to be packaged into synaptic vesicles. Another fraction may comprise neurotransmitters already transported into SLMVs. The content of β-NAD is greater than ATP (and DA) in the glycerol-free fractions. Moderate increase in glycerol concentrations led to isolation of fractions containing low concentrations of ATP and β-NAD. However, the concentrations of ATP and β-NAD increased again in the fractions with highest glycerol concentrations, presumably containing SSVs. In all fractions β-NAD represented the major compound of the β-NAD+ADPR+cADPR mixture comprising 92–99% of the mixture. The high glycerol fractions also contained DA. Therefore, ATP, DA and β-NAD are likely stored not only in LDCVs but also in SSVs.
Neuronal release of noradrenaline and ATP is generally believed to occur via exocytosis. The release of β-NAD evoked by electrical field stimulation is severely disrupted by SNAP-25 cleavage in mesenteric blood vessels and large intestine, and hence, in these systems, β-NAD appears released primarily by vesicular exocytosis (Smyth et al., 2006b; Mutafova-Yambolieva et al., 2007). We next tested whether in differentiated PC12 cells, β-NAD, ATP, and DA are secreted by SNAP-25 mediated mechanisms. SNAP-25 is present in synaptic membranes and transport vesicles and is involved in the docking and fusion of synaptic vesicles (Sollner et al., 1993). Biochemical and cellular fractionation studies have suggested that SNAP-25 is a part of the SNARE complex, which represents a critical step in synaptic exocytosis (Sollner et al., 1993; McMahon and Sudhof, 1995). In support of this, in the present study SNAP-25 showed parallel distribution in the fractions from glycerol and sucrose centrifugation gradients. Interestingly, samples collected before stimulation of treated with BoNT/A PC12 cells contained higher levels of DA and purines than samples collected before stimulation of non-treated cells. Therefore, spontaneous release of neurotransmitters might be a regulated process that requires intact SNAP-25. Continuous stimulation with high-K+ solution caused a progressive decrease in the levels of secreted catecholamines and purines in the BoNT/A-treated cells, suggesting that disruption of SNAP-25 may lead to depletion of pool(s) of secretory vesicles that are available for release upon membrane depolarization and Ca2+ influx. This is in accordance with a previous study showing that in hippocampal slice cultures 5–10 min high-K+ stimulation causes progressive depletion of synaptic vesicles and redistribution of SNAP-25 from the plasma membrane to membrane-bound tubulo-vesicular structures in the axoplasm (Tao-Cheng et al., 2000). It should be pointed out that the high-K+-evoked release of ATP remained unchanged by BoNT/A, whereas the evoked release of β-NAD and DA was abolished. Therefore, despite similar distribution of ATP, β-NAD and DA in SSVs and LDCVs, there may be differences in the preferential sites of evoked secretion of neurotransmitter substances in the NGF-differentiated PC12 cells: the predominant release of β-NAD and DA appears to be from SNAP-25-associated sources, whereas the release of ATP seems to occur predominantly from sources not associated with SNAP-25, but still dependent on membrane depolarization. As some studies show, ATP can also be released through native or overexpressed hemichannels (Ebihara, 2003; Belliveau et al., 2006; Huang et al., 2007) or secretion of ATP can occur independently of vesicular exocytosis (Hussl et al., 2007). However, these mechanisms cannot explain the results of the present study since these mechanisms usually occur independently of membrane depolarization. Further studies are warranted to determine the exact sources of differential secretion of β-NAD, DA, and ATP in these cells.
In summary, we demonstrate here that β-NAD, a putative neurotransmitter and a neuromodulator, is subject to constitutive and regulated release in NGF-differentiated PC12 cells. β-NAD, ATP, and DA are present in both SSV and LDCV populations of secretory vesicles with no definite distinction in the primary distribution of the three substances. SNAP-25-mediated mechanisms may be involved in the constitutive release of catecholamines and purines. Membrane depolarization-evoked release of DA, β-NAD and ATP is differentially affected by disruption of SNAP-25, suggesting that the three neurotransmitter substances may have different preferential sites of release, including sites associated with SNAP-25 (i.e., DA and β-NAD) and sites non-associated with SNAP-25 (i.e., ATP).
Acknowledgments
This work was supported by a National Institutes of Health Grant R01 HL 60031 and by an American Heart Association/Western States Affiliate Grant-in-Aid to VM-Y.
Reference List
- 1.Belliveau DJ, Bani-Yaghoub M, McGirr B, Naus CCG, Rushlow WJ. Enhanced Neurite Outgrowth in PC12 Cells Mediated by Connexin Hemichannels and ATP. J.Biol.Chem. 2006;281:20920–20931. doi: 10.1074/jbc.M600026200. [DOI] [PubMed] [Google Scholar]
- 2.Billington RA, Bruzzone S, De Flora A, Genazzani AA, Koch-Nolte F, Ziegler M, Zocchi E. Emerging functions of extracellular pyridine nucleotides. Mol.Med. 2006;12:324–327. doi: 10.2119/2006-00075.Billington. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blasi J, Chapman ER, Yamasaki S, Binz T, Niemann H, Jahn R. Botulinum neurotoxin C1 blocks neurotransmitter release by means of cleaving HPC-1/syntaxin. EMBO J. 1993;12:4821–4828. doi: 10.1002/j.1460-2075.1993.tb06171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobalova J, Mutafova-Yambolieva VN. Activation of the adenylyl cyclase/protein kinase A pathway facilitates neural release of beta-nicotinamide adenine dinucleotide in canine mesenteric artery. Eur.J Pharmacol. 2006;536:128–132. doi: 10.1016/j.ejphar.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 5.Bobalova J, Bobal P, Mutafova-Yambolieva VN. High-Performance Liquid Chromatographic Technique for Detection of a Fluorescent Analogue of ADP-Ribose in Isolated Blood Vessel Preparations. Analytical Biochemistry. 2002;305:269–276. doi: 10.1006/abio.2002.5667. [DOI] [PubMed] [Google Scholar]
- 6.Breen LT, Smyth LM, Yamboliev IA, Mutafova-Yambolieva VN. {beta}-NAD is a novel nucleotide released on stimulation of nerve terminals in human urinary bladder detrusor muscle. Am J Physiol Renal Physiol. 2006;290:F486–F495. doi: 10.1152/ajprenal.00314.2005. [DOI] [PubMed] [Google Scholar]
- 7.Brock JA, Cunnane TC. Effects of Ca2+ concentration and Ca2+ channel blockers on noradrenaline release and purinergic neuroeffector transmission in rat tail artery. Br.J Pharmacol. 1999;126:11–18. doi: 10.1038/sj.bjp.0702256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnstock G. The fifth Heymans memorial lecture-Ghent, February 17, 1990. Co-transmission. Arch.Int.Pharmacodyn.Ther. 1990;304:7–33. [PubMed] [Google Scholar]
- 9.Clift-O'Grady L, Linstedt AD, Lowe AW, Grote E, Kelly RB. Biogenesis of synaptic vesicle-like structures in a pheochromocytoma cell line PC-12. J Cell Biol. 1990;110:1693–1703. doi: 10.1083/jcb.110.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cutler DF, Cramer LP. Sorting during transport to the surface of PC12 cells: divergence of synaptic vesicle and secretory granule proteins. J Cell Biol. 1990;110:721–730. doi: 10.1083/jcb.110.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebihara L. New Roles for Connexons. News Physiol Sci. 2003;18:100–103. doi: 10.1152/nips.01431.2002. [DOI] [PubMed] [Google Scholar]
- 12.Ellis JL, Burnstock G. Angiotensin neuromodulation of adrenergic and purinergic co-transmission in the guinea-pig vas deferens. Br.J.Pharmacol. 1989;97:1157–1164. doi: 10.1111/j.1476-5381.1989.tb12574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis JL, Burnstock G. Modulation by prostaglandin E2 of ATP and noradrenaline co-transmission in the guinea-pig vas deferens. J.Auton.Pharmacol. 1990;10:363–372. doi: 10.1111/j.1474-8673.1990.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 14.Fischer-Colbrie R, Laslop A, Kirchmair R. Secretogranin II: molecular properties, regulation of biosynthesis and processing to the neuropeptide secretoneurin. Prog.Neurobiol. 1995;46:49–70. doi: 10.1016/0301-0082(94)00060-u. [DOI] [PubMed] [Google Scholar]
- 15.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc.Natl.Acad.Sci.U.S.A. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. PNAS. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hussl S, Kubista H, Boehm S. Autoregulation in PC12 cells via P2Y receptors: Evidence for non-exocytotic nucleotide release from neuroendocrine cells. Purinergic.Signal. 2007;3:367–375. doi: 10.1007/s11302-007-9062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jahn R, Schiebler W, Ouimet C, Greengard P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc.Natl.Acad Sci U.S.A. 1985;82:4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jahn R, Sudhof TC. Synaptic vesicles and exocytosis. Annu.Rev.Neurosci. 1994;17:219–246. doi: 10.1146/annurev.ne.17.030194.001251. [DOI] [PubMed] [Google Scholar]
- 20.Kasai Y, Ito S, Kitamura N, Ohta T, Nakazato Y. On-line measurement of adenosine triphosphate and catecholamine released from adrenal chromaffin cells. Comp Biochem.Physiol A Mol Integr.Physiol. 1999;122:363–368. doi: 10.1016/s1095-6433(99)00020-3. [DOI] [PubMed] [Google Scholar]
- 21.Kasai Y, Ohta T, Nakazato Y, Ito S. Release of dopamine and ATP from PC12 cells treated with dexamethasone, reserpine and bafilomycin A1. J Vet.Med.Sci. 2001;63:367–372. doi: 10.1292/jvms.63.367. [DOI] [PubMed] [Google Scholar]
- 22.Lee HC. Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu.Rev.Pharmacol.Toxicol. 2001;41:317–345. doi: 10.1146/annurev.pharmtox.41.1.317. [DOI] [PubMed] [Google Scholar]
- 23.Levitt B, Head RJ, Westfall DP. High-pressure liquid chromatographic-fluorometric detection of adenosine and adenine nucleotides: application to endogenous content and electrically induced release of adenyl purines in guinea pig vas deferens. Anal.Biochem. 1984;137:93–100. doi: 10.1016/0003-2697(84)90352-x. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Edwards RH. Differential localization of vesicular acetylcholine and monoamine transporters in PC12 cells but not CHO cells. J Cell Biol. 1997;139:907–916. doi: 10.1083/jcb.139.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Llona I. Synaptic like microvesicles: do they participate in regulated exocytosis? Neurochem.Int. 1995;27:219–226. doi: 10.1016/0197-0186(95)00005-s. [DOI] [PubMed] [Google Scholar]
- 26.Marxen M, Maienschein V, Volknandt W, Zimmermann H. Immunocytochemical localization of synaptic proteins at vesicular organelles in PC12 cells. Neurochem.Res. 1997;22:941–950. doi: 10.1023/a:1022414607385. [DOI] [PubMed] [Google Scholar]
- 27.McMahon HT, Sudhof TC. Synaptic core complex of synaptobrevin, syntaxin, and SNAP25 forms high affinity alpha-SNAP binding site. J Biol.Chem. 1995;270:2213–2217. doi: 10.1074/jbc.270.5.2213. [DOI] [PubMed] [Google Scholar]
- 28.Melikian HE, Buckley KM. Membrane Trafficking Regulates the Activity of the Human Dopamine Transporter. J.Neurosci. 1999;19:7699–7710. doi: 10.1523/JNEUROSCI.19-18-07699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM, Sanders KM. beta-Nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. PNAS. 2007;104:16359–16364. doi: 10.1073/pnas.0705510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mutafova-Yambolieva VN, Smyth L, Bobalova J. Involvement of cyclic AMP-mediated pathway in neural release of noradrenaline in canine isolated mesenteric artery and vein. Cardiovascular Research. 2003;57:217–224. doi: 10.1016/s0008-6363(02)00648-x. [DOI] [PubMed] [Google Scholar]
- 31.Navone F, Di Gioia G, Jahn R, Browning M, Greengard P, De Camilli P. Microvesicles of the neurohypophysis are biochemically related to small synaptic vesicles of presynaptic nerve terminals. J Cell Biol. 1989;109:3425–3433. doi: 10.1083/jcb.109.6.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navone F, Jahn R, Di Gioia G, Stukenbrok H, Greengard P, De Camilli P. Protein p38: an integral membrane protein specific for small vesicles of neurons and neuroendocrine cells. J Cell Biol. 1986;103:2511–2527. doi: 10.1083/jcb.103.6.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Njus D, Kelley PM, Harnadek GJ. Bioenergetics of secretory vesicles. Biochim.Biophys.Acta. 1986;853:237–265. doi: 10.1016/0304-4173(87)90003-6. [DOI] [PubMed] [Google Scholar]
- 34.Smyth LM, Breen LT, Yamboliev IA, Mutafova-Yambolieva VN. Novel localization of CD38 in perivascular sympathetic nerve terminals. Neuroscience. 2006a;139:1467–1477. doi: 10.1016/j.neuroscience.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 35.Smyth LM, Bobalova J, Mendoza MG, Lew C, Mutafova-Yambolieva VN. Release of {beta}-Nicotinamide Adenine Dinucleotide upon Stimulation of Postganglionic Nerve Terminals in Blood Vessels and Urinary Bladder. J.Biol.Chem. 2004;279:48893–48903. doi: 10.1074/jbc.M407266200. [DOI] [PubMed] [Google Scholar]
- 36.Smyth LM, Breen LT, Mutafova-Yambolieva VN. Nicotinamide adenine dinucleotide (NAD) is released from sympathetic nerve terminals via a botulinum neurotoxin A mediated mechanism in canine mesenteric artery. Am J Physiol Heart Circ Physiol. 2006b;290:H1818–H1825. doi: 10.1152/ajpheart.01062.2005. [DOI] [PubMed] [Google Scholar]
- 37.Smyth LM, Yamboliev IA, Mutafova-Yambolieva VN. N-type and P/Q-type calcium channels regulate differentially the release of noradrenaline, ATP and [beta]-NAD in blood vessels. Neuropharmacology. 2009;56:368–378. doi: 10.1016/j.neuropharm.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 39.Sorensen JB. SNARE complexes prepare for membrane fusion. Trends Neurosci. 2005;28:453–455. doi: 10.1016/j.tins.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Stjarne L. Basic mechanisms and local modulation of nerve impulse-induced secretion of neurotransmitters from individual sympathetic nerve varicosities. Rev.Physiol Biochem.Pharmacol. 1989;112:1–137. doi: 10.1007/BFb0027496. [DOI] [PubMed] [Google Scholar]
- 41.Stjarne L, Astrand P, Bao JX, Gonon F, Msghina M, Stjarne E. Spatiotemporal pattern of quantal release of ATP and noradrenaline from sympathetic nerves: consequences for neuromuscular transmission. Adv.Second Messenger Phosphoprotein Res. 1994;29:461–496. doi: 10.1016/s1040-7952(06)80030-3. [DOI] [PubMed] [Google Scholar]
- 42.Tao-Cheng JH, Du J, McBain CJ. Snap-25 is polarized to axons and abundant along the axolemma: an immunogold study of intact neurons. J Neurocytol. 2000;29:67–77. doi: 10.1023/a:1007168231323. [DOI] [PubMed] [Google Scholar]
- 43.Todorov LD, Mihaylova-Todorova S, Craviso GL, Bjur RA, Westfall DP. Evidence for the differential release of the cotransmitters ATP and noradrenaline from sympathetic nerves of the guinea-pig vas deferens. J.Physiol.(Lond.) 1996;496:731–748. doi: 10.1113/jphysiol.1996.sp021723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trachte GJ. The influence of prostaglandins on neurotransmission in the rabbit isolated vas deferens. Prostaglandins. 1985;29:47–59. doi: 10.1016/0090-6980(85)90150-9. [DOI] [PubMed] [Google Scholar]
- 45.Varoqui H, Erickson JD. The cytoplasmic tail of the vesicular acetylcholine transporter contains a synaptic vesicle targeting signal. J Biol.Chem. 1998;273:9094–9098. doi: 10.1074/jbc.273.15.9094. [DOI] [PubMed] [Google Scholar]
- 46.Wagner JA. Structure of catecholamine secretory vesicles from PC12 cells. J Neurochem. 1985;45:1244–1253. doi: 10.1111/j.1471-4159.1985.tb05549.x. [DOI] [PubMed] [Google Scholar]
- 47.Yao J, Hersh LB. The vesicular monoamine transporter 2 contains trafficking signals in both its N-glycosylation and C-terminal domains. J Neurochem. 2007;100:1387–1396. doi: 10.1111/j.1471-4159.2006.04326.x. [DOI] [PubMed] [Google Scholar]
- 48.Ziegler M, Niere M. NAD+ surfaces again. Biochem.J. 2004;382:e5–e6. doi: 10.1042/BJ20041217. [DOI] [PMC free article] [PubMed] [Google Scholar]