Abstract
Background
Extracellular pressure alterations in infection, inflammation, or positive pressure ventilation may influence macrophage phagocytosis. We hypothesized that pressure modulates β1-integrins to stimulate phagocytosis.
Methods
We assayed fibroblast phagocytosis of fluorescent latex beads at ambient or 20mmHg increased pressure, and macrophage integrin phosphorylation by Western blot.
Results
Pressure did not alter phagocytosis in β1-integrin-null GD25-fibroblasts, but stimulated phagocytosis in fibroblasts expressing wild type β1-integrin. In PMA-differentiated THP-1 macrophages, pressure stimulated β1-integrin T788/789 phosphorylation, but not S785 phosphorylation. Furthermore, pressure stimulated phagocytosis in cells expressing an inactivating S785A point mutation or a T788D substitution to mimic a constitutively phosphorylated threonine, but not in cells expressing an inactivating TT788/9AA mutation.
Conclusion
The effects of pressure on phagocytosis are not limited to macrophages but generalize to other phagocytic cells. These results suggest that pressure stimulates phagocytosis via increasing β1-integrin T789 phosphorylation. Interventions that target β1-integrin threonine 789 phosphorylation may modulate phagocytic function.
Keywords: inflammation, integrin, macrophage, mechanotransduction, phosphorylation, pressure, signaling
Introduction
Monocytes and macrophages are often recruited to sites of infection and inflammation. Interstitial tissue pressures are increased in closed space infections such as abscesses or rapidly dissecting fascial infections, while tissue pressure may decrease in otherwise edematous infected tissues [1–3]. Mechanical stimuli such as changes in extracellular pressure can influence diverse cell types [4–6], including phagocytic macrophages [7]. We have previously reported that increased extracellular pressure stimulates phagocytosis in PMA-differentiated THP-1 macrophages and primary monocytes [8].
We now sought to identify the membrane proteins that mediate pressure-stimulated phagocytic cell adhesion to the phagocytic target. Macrophages and non-professional phagocytic cells such as fibroblasts, osteoblasts, and epithelial cells can bind to their targets by a variety of receptors including the IgG FcRs (FcγR), complement receptors, and integrin heterodimers. [9–11]. β1 and β2 integrin proteins are of particular interest as mediators of complement receptor-mediated phagocytosis [9, 12]. In particular, β1-integrin has been linked to macrophage phagocytosis in regards to microbial pathogens.[19] Integrins are heterodimeric proteins that .may be activated after binding to an external target by integrin clustering, talin association and phosphorylation of the cytoplasmic tail [14, 16]. intracellular signals may influence integrin binding affinity for extracellular ligands prior to binding [15] Mechanical strain can stimulate conformational activation of integrins [17] as well as β1-integrin clustering [18].
Having previously shown that increased extracellular pressure promotes cancer cell adhesion by stimulating β1-integrin binding affinity [14], we now hypothesized that pressure stimulation of phagocytosis involves a similar effect, although cancer cells respond to pressure by different signals [9, 14]. We compared the effects of increased extracellular pressure on phagocytosis by β1-integrin-null GD-25 fibroblasts and GD-25 subclones expressing wild type β1-integrin subunit or various β1-integrin subunit point mutants that either mimic constitutive phosphorylation or constitutive dephosphorylation to determine whether β1-integrin-binding contributes to pressure stimulated phagocytosis, and to determine the specific phosphorylation sites on the β1-integrin subunit that might mediate this effect. We evaluated β1-integrin subunit phosphorylation in differentiated THP-1 macrophages to confirm our findings,.
Material and Methods
Cells and Reagents
The β1- integrin-null GD25 murine fibroblast line and its transfected β1- integrin-expressing derivatives GD25-β1A, GD25-β1A,S785A, GD25-β1A,T788/9A (a gift of Dr. M. Mulevey, University of Utah), and GD25-β1A,T788D (a gift of Dr. S. Johansson, Uppsala University) were cultured in a conditioned medium containing DMEM plus 10% FBS [20]. Stable transfection was maintained with 10µg/ml puromycin (Sigma-Aldrich). The THP-1 human monocytic cell line was obtained from the American Type Culture Collection (Rockville, MD), and maintained in RPMI-1640 (Gibco, Grand Island, NY) with 10% FBS (Sigma, St. Louis, MO), L-glutamine (200 mM) and 2-mercapto-ethanol (5 ×10−5M) (Sigma). THP-1 cells (5 × 105 cells per 35 mm dish) were differentiated by stimulation with PMA (50 ng/ml, final concentration) for 3 days to obtain a macrophage-like phenotype that resembles human monocyte-derived macrophages as previously reported [4, 21]. The fluorescent-labeled latex beads (2.0µ) used for phagocytosis were obtained from Polysciences, Inc. (Warrington, PA).
Pressure Box Model
Surrounding pressure is controlled by a specifically designed apparatus that was used in previous studies [5, 8, 14, 22]. The cells are placed into a Lucite chamber, using thumb screws and an O- ring an airtight seal is achieved. The chamber contains a gas inlet and an outlet valve, a pressure manometer is used to periodically monitor the internal pressure. In order to prevent fluctuations of pressure caused by variance of temperature, the box is pre-warmed to 37°C for 1 hour prior to each study. This method allows for tight control of temperature, pressure osmolarity, pH, and pO2 [5].
Phagocytosis Assay
Fluorescent labeled latex beads (2.0µ) were opsonized with 10% FBS for 60 minutes at 37°C prior to the experiments. 3 × 105 fibroblast cells were plated in a 60 mm Petri dish, in 3 milliliter of conditioned tissue culture medium. Opsonized latex beads were added at a multiplicity ratio of 1:20 and incubated for 2 hours. One set of dishes was placed into the calibrated pressure box apparatus. The pressure inside the box was set at 20 mmHg above ambient, and the box was incubated at 37°C. The control dishes were placed under ambient pressure inside the same incubator. After 2 hours, the fibroblast monolayers were washed three times with phosphate buffered saline (PBS) to remove any extracellular beads, methanol-fixed for 10 minutes, and counterstained with methylene blue. The now engulfed intracellular fluorescent latex particles were counted using a fluorescence microscope (Olympus America Inc, Center Valley, PA). The phagocytic index was calculated as the number of cells with at least one bead as a percentage of the total number of cells counted. Six different fields per plate, with three plates for control and three for pressure [4].
Western Blotting
THP-1 macrophages were incubated under either ambient or increased pressure (20 mmHg) [20] conditions for 30 minutes. The cells were rinsed and lysed and protein was assayed in the lysates by bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL). [4] Equal protein aliquots of cell lysates were resolved by 10% SDS-PAGE, transferred to nitrocellulose, blocked with 5% bovine serum albumin in tris-buffered saline (TBS) with 0.1% Tween 20, and subjected to Western blot with antibodies against activated or total forms of the protein in question, peroxidase-conjugated anti-rabbit IgG, and ECL Plus Western Blotting Detection kits. (GE Healthcare, Buckinghamshire, UK). The blots were studied along the linear range of exposure from chemiluminescent images captured using a Kodak Digital Science Image Station 440CF (Rochester, NY) [4].
Statistical Analysis
Data were analyzed by Student's t-test or paired t-test seeking 95% confidence as appropriate.
Results
Pressure stimulates phagocytosis by fibroblasts expressing β1-integrin subunit
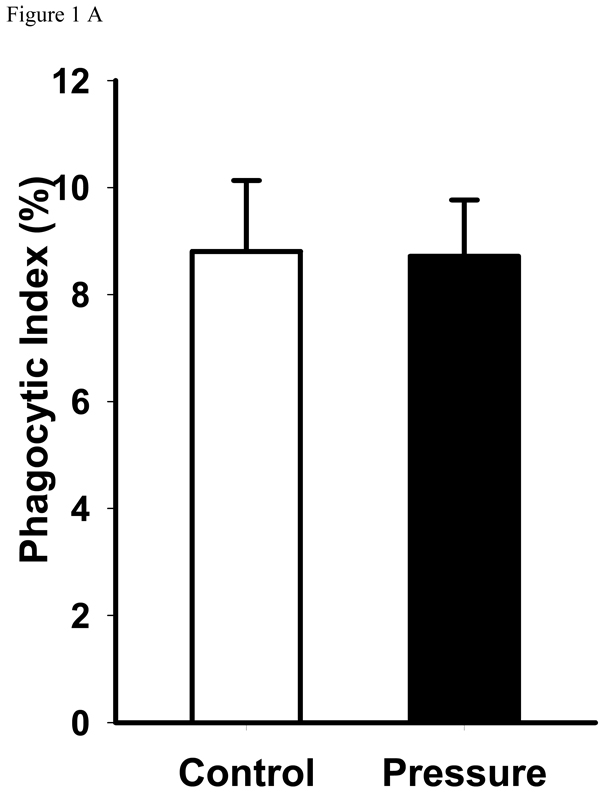
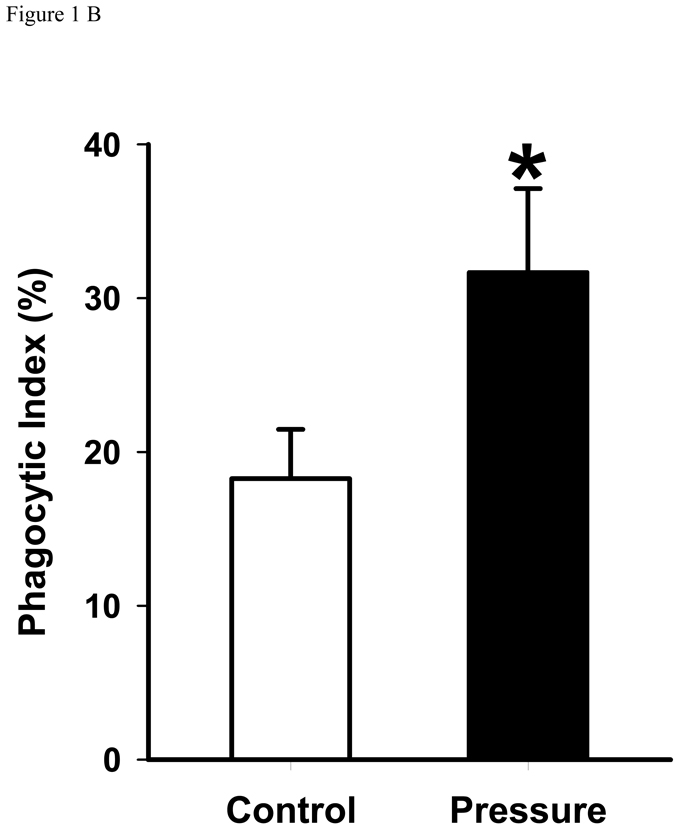
Exposure to increased pressure for two hours increased phagocytosis of latex beads by GD25 cells containing wild type β1-integrin compared with phagocytosis at ambient pressure (18.3±1.9 vs. 31.7±3.5%, n=6, p<0.01). (Figure 1B) In contrast, the GD25 fibroblasts that did not express the β1-integrin subunit did not display increased phagocytosis in response to increased extracellular pressure. (Figure 1A)
Figure 1. Pressure effect on phagocytosis.
A: Pressure effect on phagocytosis by GD25-β1A null fibroblast cells. Null cells were incubated with serum-opsonized fluorescent-labeled latex beads under ambient pressure, or 20 mmHg increased pressure conditions for 2 hours, non-adherent beads were washed away, the cells were fixed, and the number of intracellular latex beads was counted. Data were expressed as percent phagocytosis as detailed in the Methods section. Pressure did not affect phagocytosis by GD25-β1A null fibroblast cells.
B: Pressure effect on GD25-β1A WT fibroblast cells phagocytosis. GD25-β1A WT fibroblasts were incubated with serum-opsonized fluorescent-labeled latex beads under ambient pressure, or 20 mmHg increased pressure conditions for 2 hours, non-adherent beads were washed away, the cells were fixed, and the number of intracellular latex beads was counted. Data were expressed as percent phagocytosis as detailed in the Methods section. Pressure increased GD25-β1A WT fibroblast (* p<0.01, n=6).
Pressure specifically stimulates β1-integrin threonine 788/789 phosphorylation
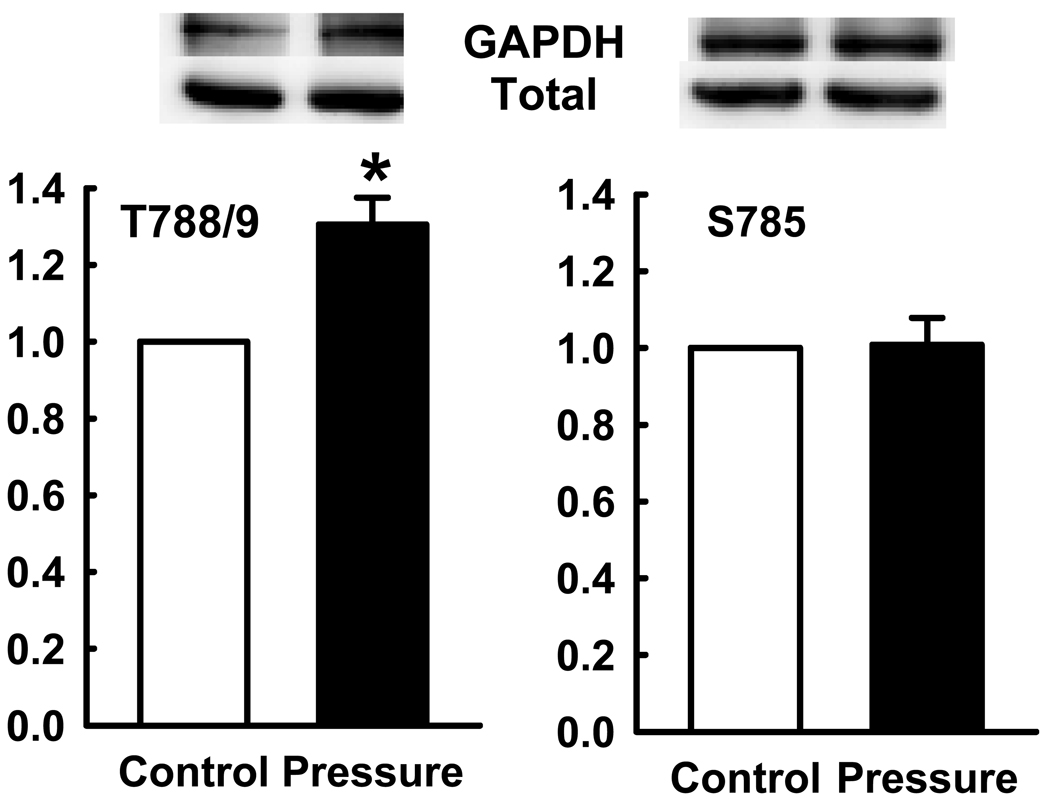
Since the β1-integrin subunit seemed important for the stimulation of phagocytosis by increased extracellular pressure, we examined specifically the effects of pressure on the T788/789 and S785 phosphorylation of the β1-integrin subunit by Western blot. In PMA-differentiated THP-1 macrophages, pressure stimulated β1-integrin T788/789 phosphorylation by 30.5±7.8% (n=6, p<0.05). However, S785 phosphorylation did not change significantly in response to extracellular pressure increases. (Figure 2)
Figure 2. Studies of T788 and S785 and pressure.
Increase in T788 phosphorylation in PMA-differentiated THP-1 macrophages by pressure. The top panel represents typical Western blots for phosphorylated T788 and S785 sites, each stripped and reprobed for GAPADH as a loading control. The graph summarizes densitometric results expressed as mean ± se of the ratio of phosphorylated T788/789 to total T788/789 and the the ratio of phosphorylated S785 to total S785, normalized to control. Open bars represent ambient pressure and closed bars increased pressure. Increased pressure stimulated T788/789 phosphorylation (*p<0.05, n=5).
Effect of pressure on phagocytosis by fibroblasts transfected with β1-integrin subunit point mutants
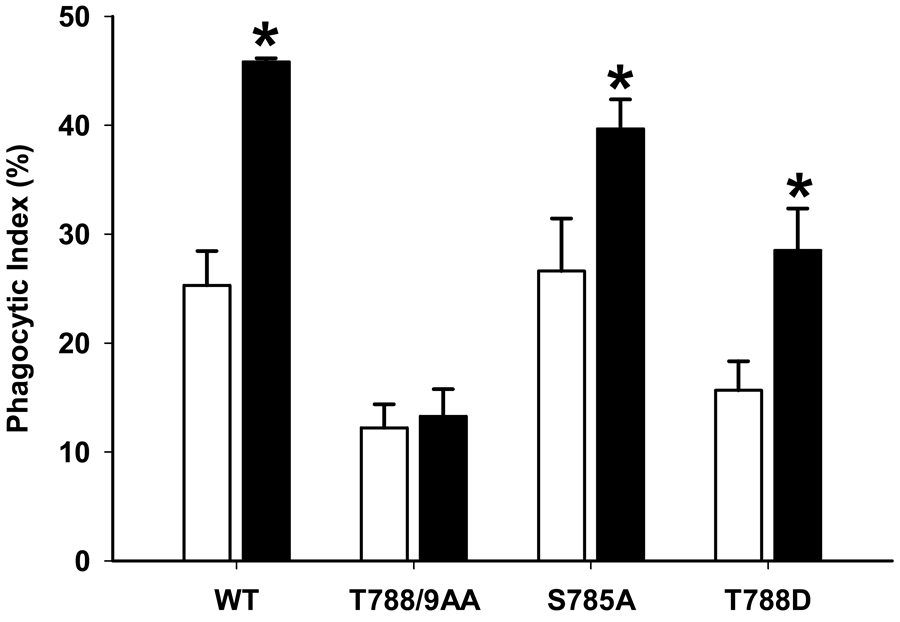
Because β1-integrin seemed important for pressure-stimulated phagocytosis and because β1-integrin phosphorylation seemed specifically modulated by pressure in phagocytic cells, we next compared the effects of pressure on phagocytosis in GD25 fibroblasts stably transfected with various β1-integrin point mutants. β1-integrin S785 phosphorylation regulates and enhances cell attachment [23, 24], while mutations that replace both threonine residues in T788 and T789 in β1-integrin with alanine residues disrupts fibroblast attachment [25]. In contrast the T788D substitution, acts as a continuously phosphorylated active site that significantly enhances cell adhesion [16]. Cells expressing β1-integrin with the inactivating S785A point mutation, where the serine residue has been replaced with an unphosphorylatable alanine, displayed a substantial increase in pressure stimulated phagocytosis compared to the WT (26.2±3.3 vs. 39.7±2.0%, n=6, p<0.01), as these cells still have T788 and T789 sites available for phosphorylation. Expression of the T788D substitution, which mimics constitutively phosphorylated threonine, did not alter basal phagocytosis significantly but permitted increased phagocytic activity under pressure conditions similarly to WT cells (15.7± 2.7 vs. 28.5±3.9%, n=6, p<0.01). In contrast, expression of the TT788/9AA mutation, in which both the T788 and T789 sites are rendered unphosphorylatable and presumably inactive, due to the threonine replacement with alanine, show reduced basal phagocytosis (25.3± 3.1 vs. 12.2±2.1%, n=6, p<0.01) and abolished the increase phagocytosis in response to pressure. (Figure 3) Thus, lack of phagocytosis in these cells correlates with inability to be phosphorylated or constitutively mimicking phosphorylation depending upon the substitution.
Figure 3. Pressure effect on phagocytosis in cells expressing point-mutated b1 integrin constructs.
Pressure effect on phagocytosis by GD25 fibroblasts expressing wild type (WT) β1A subunit compared to fibroblasts expressing three different point mutated constructs: GD25-β1A T788A, GD25-β1A T788/9AA, GD25-β1A S785A. All cell lines were incubated with serum-opsonized fluorescent-labeled latex beads under ambient pressure (open bars), or 20 mmHg increased pressure conditions (shaded bars) for 2 hours, non-adherent beads were washed away, the cells were fixed, and the number of intracellular latex beads was counted. Data were expressed as percent phagocytosis as detailed in the Methods section Pressure increased phagocytosis in GD25-β1A WT fibroblast (* p<0.01, n=6), GD25-β1A T788D (* p<0.01, n=6) fibroblast and GD25-β1A S785A (* p<0.01, n=6) fibroblast cells but did not increase in GD25-β1A T788/9AA fibroblasts.
Discussion
Normal interstitial tissue pressure is 20 to 30 mm Hg [2, 26–28], but the interstitial extracellular pressure will decrease in infected or inflamed tissue [2, 27, 28]. Edema formation in unconstrained infected or inflamed tissues decreases connective tissue interstitial fluid pressures by as much as 150 mm Hg [2]. However, in closed compartments or abscesses, infection or edema can increase tissue pressure by 5 to 80 mm Hg [1, 3]. We have previously reported that an 20mmHg above ambient increase in extracellular pressure stimulates the phagocytosis of serum-opsonized latex beads by PMA-stimulated THP-1 macrophages, primary isolated human peripheral monocyes and monocyte-derived macrophages [4, 8]. Fibroblasts increase adhesion to matrix proteins under similar conditions[5], and many matrix adhesion receptors can also serve as phagocytic receptors. This study demonstrates that extracellular pressure also enhances phagocytosis in fibroblast cells, so the stimulation of phagocytosis by increased extracellular pressure extends beyond macrophages and monocytes to non-professional phagocytic cells such as fibroblasts. Furthermore, pressure-stimulated phagocytosis appears mediated by β1 integrin heterodimers, and in particular requires phosphorylation of β1 integrin at the T788 site which may in turn facilitate engagement of the phagocytic target.
Fibroblasts and many other non-professional phagocytic cells play an important role in phagocytosis in various settings. For instance, fibroblasts and epithelial cells have been found to internalize B. anthracis in its spore and vegetative state [29]. In addition, fibroblasts play an important role at various stages of tissue repair. In the early phases of tissue repair, granulation tissue invasion, wound contraction, and fibroblast proliferation and migration are all involved [30]. Fibroblasts are responsible for invading the temporary clot in a wound, and forming contractile granulation tissue that allows the wound margins to come together. As the wound clots, activated fibroblasts are present in large numbers, and are responsible for synthesizing a new collagen-rich matrix [30].
Phagocytosis requires initial binding of the phagocytic cell to its target. This binding can occur by many different receptors, depending upon the target. Receptors commonly involved in phagocytosis include the IgG FcRs (FcγR), complement receptors, integrin heterodimers, toll-like receptors, and mannose receptors[9–11]. Cellular receptors can also recognize patterns on membranes of forigen particles. For example, the mannose receptor recognizes mannan, while scavanger receptors will recognize surface components such as lipopolysaccharide [9]. The phagocytosis of an apoptotic cell is via complement receptors. Integrin heterodimers are an example of complement receptors [9]. For instance, B1 integrin has specifically been identified as an important receptor for the internalization of S.aureus in a Src-dependent manner [31]. β1 integrin is known to be important for internalization of pathogens [31]. β1 integrin is also responsible for phagocytosis by malignant human breast cancer cells. β2 integrin also mainly acts a complement receptor [32] and plays an active role in wound healing [33]. Many phagocytic receptors serve dual functions by mediating both phagocytosis and cell adhesion, so it may not be surprising that integrins, originally described as cell-matrix or cell-cell adhesion molecules, are also involved in phagocytosis.
Although not itself crucial for phagocytosis, β1-integrin plays an important role in increasing the rate of phagocytosis. Our present results suggest that the phagocytosis that is stimulated by increased extracellular pressure is β1-integrin-mediated, because cells that do not express the β1-integrin subunit do not display this response and cells that express mutated constitutively activated or inactivated β1-integrin subunit exhibit predictably altered responses.
Both adhesion and phagocytic receptors have the ability to activate and inhibit the function of the other. For example the attachement of a ligand to a fibronectin receptor (α5β1 integrin) at the surface of a monocyte allow the cell to activate an otherwise inactive complement receptor (αMβ2 integrin) that is responsible for mediating phagocytosis [34, 35] Thus, it also remains possible as an alternate explanation that activation of the β1-integrin may modulate an otherwise inactive αMβ2 integrin complement receptor via such crosstalk. Distinguishing whether ligand binding during phagocytosis is specifically β1-integrin mediated or involves cross-activation of β2-integrins awaits further study beyond this manuscript.
Functional regulation of integrin activity during cell adhesion has been previously associated with the phosphorylation of the β-subunit cytoplasmic domain of α/β-integrin heterodimers.[36–38] The cytoplasmic domain of all β-subunits excluding β4 and β8 subunits contains a unique NPXY/F-motifs and a serine-threonine cluster. [39] The serine-threonine cluster can be subdivided into five potential phosphorylation regulatory sites: Y783, Y795, S785, T788, and T789 [24, 40]. We particularly focused on three major phosphorylation sites S785, T788, and T789.
β1-Integrin S785 phosphorylation regulates and enhances cell attachment, but inhibits cell motility. [23, 24]. Our results demonstrate that the phophorylation of the S785 shows no change in phagocytosis under increased extracellular pressure. When the S785 site is rendered inactive via alanine substitution, there is an increase in pressure stimulated phagocytosis. Thus showing that the S785 site in not a vital site for pressure induced phagocytosis. One of the remaining sites, presumably the T788 or T789 site may be responsible for this increase. Mutations that replace both threonine residues in T788 and T789 in β1-integrin with alanine residues prevent T788/789 phosphorylation and disrupt fibronectin fibril formation and the attachment to fibronectin substrates[25]. This mutation causes a conformational change in the extracelllar domain and it prevents ligand binding [25]. Our present results demonstrate that β1-integrin subunit T788/789 phosphorylation is stimulated by extracellular pressure increases and that preventing T788/789 phosphorylation by point mutation prevents the stimulation of phagocytosis by increased pressure. In contrast, the T788D substitution replaces the threonine with an aspartic acid rather than an alanine. The aspartic acid mimics a continuously phosphorylated active site, and enhances cell adhesion, in particular to fibronectin [16]. However, our study shows that mimicking constitutive phosphorylation of this threonine does not alter basal or pressure stimulated phagocytic activity. Taken together, these results suggest that while phosphorylation of the threonine 788 residue may be critical for cell adhesion to matrix substrates, the threonine 789 residue of the β1 integrin subunit is critical in the stimulation of phagocytosis by extracellular pressure. Although specific siRNA knockdown of beta-1 integrins were beyond the scope of the current manuscript, β1 integrin expression was completely ablated in the control cell populations studied here by introduction of a null mutation via homologous recombination, allowing for stable and specific elimination of the β1 integrin subunit [16, 25].
Phagocytic cells could respond differently to physical forces within the complex neurohumoral in vivo environment, but the in vitro studies performed here permitted us to isolate and identify β1 integrins as critical for the phagocytic response to pressure, and threonine 789 phosphorylation of the β1 integrin subunit as a critical mediator of this effect. Although the identity of the pressure-activated kinase that phosphorylates the β1 integrin subunit at this threonine or the pressure-inhibited phosphatase that no longer degrades it as effectively awaits further study, these results identify β1 integrin threonine 789 phosphorylation as a potential target for pharmacologic intervention to manipulate phagocytosis in settings in which extracellular pressure is altered.
Acknowledgments
Supported in part by NIH RO1 DK060771 and a VA Merit Research Award (each MDB)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Summary
Changes in extracellular pressure as may occur in conditions of infection or inflammation can influence macrophage phagocytosis, but the pressure-stimulated receptor by which phagocytes interact with their targets has not been identified. These results demonstrate that non-professional phagocytes such as fibroblasts display the same phenomenon and suggest that increases in extracellular pressure stimulate phagocytosis by modulating β1-integrins threonine 789 phosphorylation.
Bibliography
- 1.Schaser KD, Zhang L, Haas NP, Mittlmeier T, Duda G, Bail HJ. Temporal profile of microvascular disturbances in rat tibial periosteum following closed soft tissue trauma. Langenbecks Arch Surg. 2003;388(5):323–330. doi: 10.1007/s00423-003-0411-5. [DOI] [PubMed] [Google Scholar]
- 2.Wiig H, Rubin K, Reed RK. New and active role of the interstitium in control of interstitial fluid pressure: potential therapeutic consequences. Acta Anaesthesiol Scand. 2003;47(2):111–121. doi: 10.1034/j.1399-6576.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- 3.Schnall SB, Vu-Rose T, Holtom PD, Doyle B, Stevanovic M. Tissue pressures in pyogenic flexor tenosynovitis of the finger. Compartment syndrome and its management. J Bone Joint Surg Br. 1996;78(5):793–795. [PubMed] [Google Scholar]
- 4.Shiratsuchi H, Basson MD. Activation of p38 MAPKalpha by extracellular pressure mediates the stimulation of macrophage phagocytosis by pressure. Am J Physiol Cell Physiol. 2005;288(5):C1083–C1093. doi: 10.1152/ajpcell.00543.2004. [DOI] [PubMed] [Google Scholar]
- 5.Basson MD, Yu CF, Herden-Kirchoff O, et al. Effects of increased ambient pressure on colon cancer cell adhesion. J Cell Biochem. 2000;78(1):47–61. doi: 10.1002/(sici)1097-4644(20000701)78:1<47::aid-jcb5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Vouyouka AG, Jiang Y, Rastogi R, Basson MD. Ambient pressure upregulates nitric oxide synthase in a phosphorylated-extracellular regulated kinase- and protein kinase C-dependent manner. J Vasc Surg. 2006;44(5):1076–1084. doi: 10.1016/j.jvs.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Craig DH, Owen CR, Conway WC, Walsh MF, Downey C, Basson MD. Colchicine inhibits pressure-induced tumor cell implantation within surgical wounds and enhances tumor-free survival in mice. J Clin Invest. 2008;118(9):3170–3180. doi: 10.1172/JCI34279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiratsuchi H, Basson MD. Extracellular pressure stimulates macrophage phagocytosis by inhibiting a pathway involving FAK and ERK. Am J Physiol Cell Physiol. 2004;286(6):C1358–C1366. doi: 10.1152/ajpcell.00553.2003. [DOI] [PubMed] [Google Scholar]
- 9.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 10.Tamaki Y, Takakubo Y, Goto K, et al. Increased Expression of Toll-like Receptors in Aseptic Loose Periprosthetic Tissues and Septic Synovial Membranes Around Total Hip Implants. J Rheumatol. 2009 doi: 10.3899/jrheum.080390. [DOI] [PubMed] [Google Scholar]
- 11.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 12.Sengelov H. Complement receptors in neutrophils. Crit Rev Immunol. 1995;15(2):107–131. [PubMed] [Google Scholar]
- 13.Singh S, D'Mello V, van Bergen en Henegouwen P, Birge RB. A NPxY-independent beta5 integrin activation signal regulates phagocytosis of apoptotic cells. Biochem Biophys Res Commun. 2007;364(3):540–548. doi: 10.1016/j.bbrc.2007.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig DH, Gayer CP, Schaubert KL, et al. Increased extracellular pressure enhances cancer cell integrin-binding affinity through phosphorylation of beta1-integrin at threonine 788/789. Am J Physiol Cell Physiol. 2009;296(1):C193–C204. doi: 10.1152/ajpcell.00355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim M, Carman CV, Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science. 2003;301(5640):1720–1725. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson S, Kaniowska D, Brakebusch C, Fassler R, Johansson S. Threonine 788 in integrin subunit beta1 regulates integrin activation. Exp Cell Res. 2006;312(6):844–853. doi: 10.1016/j.yexcr.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Katsumi A, Naoe T, Matsushita T, Kaibuchi K, Schwartz MA. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J Biol Chem. 2005;280(17):16546–16549. doi: 10.1074/jbc.C400455200. [DOI] [PubMed] [Google Scholar]
- 18.Knies Y, Bernd A, Kaufmann R, Bereiter-Hahn J, Kippenberger S. Mechanical stretch induces clustering of beta1-integrins and facilitates adhesion. Exp Dermatol. 2006;15(5):347–355. doi: 10.1111/j.0906-6705.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang QQ, Li H, Oliver T, Glogauer M, Guo J, He YW. Integrin beta 1 regulates phagosome maturation in macrophages through Rac expression. J Immunol. 2008;180(4):2419–2428. doi: 10.4049/jimmunol.180.4.2419. [DOI] [PubMed] [Google Scholar]
- 20.Eto DS, Jones TA, Sundsbak JL, Mulvey MA. Integrin-mediated host cell invasion by type 1-piliated uropathogenic Escherichia coli. PLoS Pathog. 2007;3(7):e100. doi: 10.1371/journal.ppat.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auwerx J. The human leukemia cell line, THP-1: a multifacetted model for the study of monocyte-macrophage differentiation. Experientia. 1991;47(1):22–31. doi: 10.1007/BF02041244. [DOI] [PubMed] [Google Scholar]
- 22.Thamilselvan V, Basson MD. Pressure activates colon cancer cell adhesion by inside-out focal adhesion complex and actin cytoskeletal signaling. Gastroenterology. 2004;126(1):8–18. doi: 10.1053/j.gastro.2003.10.078. [DOI] [PubMed] [Google Scholar]
- 23.Barreuther MF, Grabel LB. The role of phosphorylation in modulating beta 1 integrin localization. Exp Cell Res. 1996;222(1):10–15. doi: 10.1006/excr.1996.0002. [DOI] [PubMed] [Google Scholar]
- 24.Mulrooney JP, Hong T, Grabel LB. Serine 785 phosphorylation of the beta1 cytoplasmic domain modulates beta1A-integrin-dependent functions. J Cell Sci. 2001;114(Pt 13):2525–2533. doi: 10.1242/jcs.114.13.2525. [DOI] [PubMed] [Google Scholar]
- 25.Wennerberg K, Fassler R, Warmegard B, Johansson S. Mutational analysis of the potential phosphorylation sites in the cytoplasmic domain of integrin beta1A. Requirement for threonines 788–789 in receptor activation. J Cell Sci. 1998;111(Pt 8):1117–1126. doi: 10.1242/jcs.111.8.1117. [DOI] [PubMed] [Google Scholar]
- 26.Guyton AC, Granger HJ, Taylor AE. Interstitial fluid pressure. Physiol Rev. 1971;51(3):527–563. doi: 10.1152/physrev.1971.51.3.527. [DOI] [PubMed] [Google Scholar]
- 27.Koller ME, Woie K, Reed RK. Increased negativity of interstitial fluid pressure in rat trachea after mast cell degranulation. J Appl Physiol. 1993;74(5):2135–2139. doi: 10.1152/jappl.1993.74.5.2135. [DOI] [PubMed] [Google Scholar]
- 28.Ostgaard G, Reed RK. Increased negatively of interstitial fluid pressure in rat skin contributes to the edema formation induced by Zymosan. Microvasc Res. 1993;46(3):283–292. doi: 10.1006/mvre.1993.1053. [DOI] [PubMed] [Google Scholar]
- 29.Russell BH, Vasan R, Keene DR, Xu Y. Bacillus anthracis internalization by human fibroblasts and epithelial cells. Cell Microbiol. 2007;9(5):1262–1274. doi: 10.1111/j.1462-5822.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 30.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276(5309):75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 31.Fowler T, Johansson S, Wary KK, Hook M. Src kinase has a central role in in vitro cellular internalization of Staphylococcus aureus. Cell Microbiol. 2003;5(6):417–426. doi: 10.1046/j.1462-5822.2003.00290.x. [DOI] [PubMed] [Google Scholar]
- 32.Graham IL, Anderson DC, Holers VM, Brown EJ. Complement receptor 3 (CR3, Mac-1, integrin alpha M beta 2, CD11b/CD18) is required for tyrosine phosphorylation of paxillin in adherent and nonadherent neutrophils. J Cell Biol. 1994;127(4):1139–1147. doi: 10.1083/jcb.127.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sindrilaru A, Peters T, Schymeinsky J, et al. Wound healing defect of Vav3−/−mice due to impaired {beta}2-integrin dependent macrophage phagocytosis of apoptotic neutrophils. Blood. 2009 doi: 10.1182/blood-2008-07-166702. [DOI] [PubMed] [Google Scholar]
- 34.Pommier CG, Inada S, Fries LF, Takahashi T, Frank MM, Brown EJ. Plasma fibronectin enhances phagocytosis of opsonized particles by human peripheral blood monocytes. J Exp Med. 1983;157(6):1844–1854. doi: 10.1084/jem.157.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright SD, Craigmyle LS, Silverstein SC. Fibronectin and serum amyloid P component stimulate C3b- and C3bi-mediated phagocytosis in cultured human monocytes. J Exp Med. 1983;158(4):1338–1343. doi: 10.1084/jem.158.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Willigen G, Hers I, Gorter G, Akkerman JW. Exposure of ligand-binding sites on platelet integrin alpha IIB/beta 3 by phosphorylation of the beta 3 subunit. Biochem J. 1996;314(Pt 3):769–779. [PMC free article] [PubMed] [Google Scholar]
- 37.Dahl SC, Grabel LB. Integrin phosphorylation is modulated during the differentiation of F-9 teratocarcinoma stem cells. J Cell Biol. 1989;108(1):183–190. doi: 10.1083/jcb.108.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fagerholm SC, Hilden TJ, Gahmberg CG. P marks the spot: site-specific integrin phosphorylation regulates molecular interactions. Trends Biochem Sci. 2004;29(9):504–512. doi: 10.1016/j.tibs.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 39.OToole TE, Ylanne J, Culley BM. Regulation of integrin affinity states through an NPXY motif in the beta subunit cytoplasmic domain. J Biol Chem. 1995;270(15):8553–8558. doi: 10.1074/jbc.270.15.8553. [DOI] [PubMed] [Google Scholar]
- 40.Stroeken PJ, van Rijthoven EA, Boer E, Geerts D, Roos E. Cytoplasmic domain mutants of beta1 integrin, expressed in beta 1-knockout lymphoma cells, have distinct effects on adhesion, invasion and metastasis. Oncogene. 2000;19(9):1232–1238. doi: 10.1038/sj.onc.1203423. [DOI] [PubMed] [Google Scholar]