Abstract
This analysis describes the detection of urinary pesticide metabolites for Latino farmworkers across the agricultural season. Two hundred and eighty four farmworkers were recruited from 44 camps in eastern North Carolina in 2007. Data were collected at one month intervals for a total of 939 data points. The OP insecticide metabolites 3,5,6-trichloropyridinol (46.2%), malathion dicarboxylic acid (27.7%), and para-nitrophenol (97.4%); the pyrethroid metabolite 3-phenoxybenzoic acid (56.4%); and the herbicides 2,4-D (68.1%), acetochlor (29.2%), and metolachlor (16.9%) were found in sizable percentages of the samples. The percentage of farmworkers for whom metabolites were detected varied across the agricultural season. None of the farmworker characteristics were significantly associated with the detection of any pesticide metabolite. Seasonality overrides the effects of other farmworker characteristics in predicting detection of pesticide urinary metabolites. Future research needs to collect multiple exposure measures at frequent intervals over an extended period to characterize factors associated with exposure.
Keywords: farmworker, agriculture, occupational health, pesticide exposure, biomonitoring
Migrant and seasonal farmworkers in the United States, the vast majority of whom are Latino, are a vulnerable population who experience significant health risks in their work environments.1 Exposure to pesticides is one major occupational risk for farmworkers.2–4 Substantial research has begun to document the occupational pesticide exposure of farmworkers. For example, Fenske and colleagues5 found high concentrations of dimethylthiophosphate, a dialkylphosphate (DAP) metabolite of organophosphorus (OP) insecticides, in multiple urine samples collected from 20 apple thinners working in Washington State. Coronado and colleagues6 found that among 218 farmworkers in Washington State, those who worked in pome fruit (apples and pears) had greater concentrations of the dimethyl urinary metabolites of OP insecticides than those working in non-pome fruit. Salvatore and colleagues7 report that among 73 strawberry farmworkers wearing recommended clothing and following recommended hygiene procedures, lower levels of urinary OP insecticide metabolites were observed. In addition to these studies of farmworker occupational exposure, a series of studies have documented the residential pesticide exposure of farmworker families,8,9 the pesticide dose of women, infants, and children in farmworker homes,10,11,12 and the effectiveness of interventions to reduce pesticide exposure among the children of farmworkers.13
Research on farmworker pesticide exposure and dose has begun to document the frequency of exposure and the factors related to exposure; however, this research has several limitations. Most of this research has examined general DAP urinary metabolites of OP insecticides. Although these metabolites are markers for 75% of the OP insecticides, they provide no insight into the specific OP insecticides or the other pesticides to which workers are exposed. Most research has collected data on exposure and dose at a single time, a significant limitation since OP insecticides generally are metabolized within 72 hours of exposure.14 Research that has collected repeated measures of exposure is generally limited to small numbers of participants, and it has been designed to evaluate specific safety procedures,5,7 not to understand pesticide exposure or dose in the general farmworker population.
The goal of this analysis is to develop a better description of farmworker exposure to pesticides across the agricultural season. To achieve this goal longitudinal data obtained from 284 Latino farmworkers employed in North Carolina during the 2007 agricultural season are analyzed to accomplish two aims. The first aim is to describe the frequencies with which specific pesticide urinary metabolites are detected for Latino farmworkers in eastern North Carolina across the agricultural season. The second aim is to delineate the personal, occupational, and exposure characteristics of farmworkers associated with the detection of each pesticide urinary metabolite.
METHODS
Data for this analysis were collected as part of an ongoing translational research program addressing the health of Latino farmworkers and their families in eastern North Carolina. The primary partners for this community-based participatory project are Wake Forest University School of Medicine (WFUSM) and the North Carolina Farmworkers Project (NCFP); other participating agencies are Greene County Health Care, Inc., and Columbus County Community Health Center, Inc. The study protocol was approved by the WFUSM Institutional Review Board.
Location
Data collection was completed in 11 North Carolina counties: Brunswick, Columbus, Cumberland, Greene, Harnett, Johnston, Lenoir, Pitt, Sampson, Wayne, and Wilson Counties. Participants included migrant farmworkers, with and without H2A visas, and seasonal farmworkers. A migrant farmworker is an individual whose principal employment is in agriculture on a seasonal basis and who establishes a temporary residence for the purpose of employment. An H2A visa allows an individual to enter the United States to work in agriculture for a specified period of time for a particular farmer; the employer is obligated to provide workers an average of 35 hours of work per week, a specific hourly wage, and inspected housing; the employer must also meet all safety requirements. All farmworkers with an H2A visa are migrant farmworkers. A seasonal farmworker is an individual whose principal employment is in agriculture on a seasonal basis, but who does not migrate.
Estimates for 2007 by the North Carolina Employment Security Commission for the 11 eleven counties studied put the number of migrant farmworkers without H2A visas at 13,675, which is 36.2% of all migrant farmworkers without H2As visas employed in North Carolina. The number of migrant farmworkers with H2A visas in the study counties is 2,995 (34.3%), and the number of seasonal farmworkers is 5,800 (22.8%). The agricultural production in these counties varies, but the major hand-cultivated and hand-harvested crops include tobacco, sweet potatoes, and cucumbers.
Sample
Participants were selected using a two-stage procedure. The three partnering agencies prepared lists of farmworker camps that they served. Camps were approached in order until each agency recruited a minimum number of participants. Each camp that was approached agreed to participate. Forty-four farmworker camps participated in the study. NCFP recruited 23 camps, Greene County Health Care, Inc., recruited 11 camps, and Columbus County Community Health Center, Inc. recruited 10 camps.
Residents in each camp were recruited. In camps with seven or fewer residents, all farmworkers were invited to participate. In camps with more than seven residents, eight to ten farmworkers were recruited. During the first round of data collection, 261 farmworkers were recruited to participate, with an additional 27 farmworkers recruited in the second round to replace participants who were lost to follow-up, for a total sample of 288. However, four participants who completed a single interview but who did not provide a urine sample are not included in this analysis. Forty-one participants were lost to follow-up during the second round of data collection, 20 were lost in the third round, and 12 were lost in the fourth round. Four rounds of data collection were completed with 197 farmworkers, three rounds with 27, two rounds with 14, and one round with 50. Thirteen farmworkers approached by interviewers chose not to participate, for a participation rate of 95.7%. All participants gave signed informed consent.
Data Collection
The study used a longitudinal design in which data were collected from participants up to four times at monthly intervals. Data collection was completed from May through September, 2007. Detailed interviews completed with participants at each round of data collection included items on participant personal characteristics, including age, educational attainment, living conditions, and recent risk factors for pesticide exposure, such as workplace activities and behaviors. The questionnaire was developed in English and translated by an experienced translator who was a native Spanish speaker familiar with Mexican and farmworker vocabulary. It was back-translated to English to ensure that translated items maintained their meaning. Finally, it was reviewed by four fluent Spanish speakers familiar with farm work, pre-tested with 16 Spanish-speaking farmworkers, and revised as needed.
At the end of each interview participants were given urine collection containers with labels attached. They were instructed to fill the containers with their first void upon rising the next morning, and to only provide their urine in the containers, not that of any other workers in the camp. They were asked not to put any other fluid or chemicals in the urine containers. Participants placed their urine containers in a cooler with blue ice that was provided to them. Each morning project interviewers stopped by the camps and retrieved the containers, transported them to the nearest of the three collaborating community partners, aliquoted the samples into labeled containers, and placed them in a laboratory freezer where they were stored at −20°C. These samples were analyzed for pesticide metabolites. Participants were given an incentive valued at $20 when they completed data collection for each round.
In 2008 and 2009, project staff located and met with project participants. Project staff reported individual pesticide urinary metabolite results to participants at these meetings. Project staff also conducted pesticide safety training with groups of participants and other camp residents at these meetings.
Laboratory Analysis
The frozen urine samples were shipped overnight on dry ice to a laboratory of the Centers for Disease Control and Prevention in Atlanta, Georgia, for analysis. Samples were analyzed using a modification of the method of Olsson et al.15 Briefly, 2 mL urine samples were hydrolyzed by enzymes to liberate the glucuronide- or sulfate-bound conjugated metabolites. Hydrolysates were extracted using a mixed mode solid phase extraction cartridge. Concentrated extracts were analyzed using high performance liquid chromatography-tandem mass spectrometry. Two precursor/product ion pairs were analyzed per analyte, one for quantification and one for confirmation. Analyte concentrations were quantified using isotope dilution calibration. The acronyms, analytes, parent chemicals, and limits of detection for the 19 metabolites are reported in Table 1. Approximately 10% of the samples tested were positive and negative quality control samples.
TABLE 1.
Pesticide Urinary Metabolites included in the Analysis with Analyte, Parent Chemical, and Limit of Detection
| Pesticide Urinary Metabolites |
Analyte | Parent Chemical | Limit of Detection ng/mL |
|---|---|---|---|
| Organophosphorus insecticides | |||
| TCPy | 3,5,6-trichloro-2-pyridinol | chlorpyrifos; chlorpyrifos methyl |
0.2 |
| CMHC | 3-chloro-4-methyl-7-hydroxycoumarin | coumaphos | 0.2 |
| IMPY | 2-isopropyl-4-methyl-6-hydroxypyrimidinol | diazinon | 0.7 |
| CIT | 5-chloro-1,2-dihydro-1-isopropyl-[3H]-1,2,4-triazol- 3-one |
isazaphos | 1.5 |
| MDA | malathion dicarboxylic acid | malathion | |
| PNP | para-nitrophenol | parathion; methyl parathion |
0.1 |
| DEAMPY | 2-diethylamino-6-methyl pyrimidin-4-ol | primiphos methyl | 0.2 |
| Pyrethroid insecticides | |||
| 4F3PBA | 4-fluoro-3-phenoxybenzoic acid | cyfluthrin | 0.2 |
| DBCA | cis-2,2-(dibromo)-2-dimethylvinylcyclopropane carboxylic acid |
deltamethrin | 0.1 |
| CCC | cis-2,2-(dichloro)-2-dimethylvinylcyclopropane carboxylic acid |
permethrin; cypermethrin; cyfluthrin |
0.2 |
| CDCA | cis-3-(2,2-dimethylvinyl)-2,2-dimethylcyclopropane dicarboxylic acid |
allethrin; resmethrin | 0.3 |
| 3PBA | 3-phenoxybenzoic acid | general pyrethroid metabolite |
0.1 |
| TCC | trans-2,2-(dichloro)-2-dimethylvinylcyclopropane carboxylic acid |
permethrin; cypermethrin; cyfluthrin |
0.4 |
| Herbicides | |||
| 2,4-D | 2,4-Dichlorophenoxyacetic acid | same | 0.2 |
| 2,4,5-T | 2,4,5-trichlorophenoxyacetic acid | same | 0.1 |
| ACE | acetochlor mercapturate | acetochlor | 0.1 |
| ALA | alachlor mercapturate | alachlor | 0.1 |
| ATZ | atrazine mercapturate | atrazine | 0.3 |
| MET | metolachlor mercapturate | metolachlor | 0.2 |
Measures
Outcome measures for this analysis are the detection of each of 19 pesticide urinary metabolites (Table 1). These include seven urinary OP pesticide metabolites, six urinary pyrethroid insecticide metabolites, and six urinary herbicide metabolites. Caution is required in the interpretation of study results as small changes in the limits of detection (LODs) could affect the number of farmworkers for whom a metabolite was detected. In addition, since method LODs are not based upon any biological or exposure factor but rather are an instrumental value that has no biological meaning, our ability to understand exposure predictors using these data may be limited.
Detection of the urinary pesticide metabolites are reported for all samples (n = 939) collected across the agricultural season and for each of four periods across the agricultural season. Period 1 is May 1 to June 8 (n = 250), period 2 is June 9 to July 7 (n = 233), period 3 is July 8 to August 5 (n = 229), and period 4 is August 6 to September 4 (n = 227). These periods roughly correspond to the major phases of eastern North Carolina agriculture; the major activities are: planting tobacco and sweet potatoes in period 1; harvesting cucumbers, topping tobacco, and planting sweet potatoes in period 2; topping and harvesting tobacco in period 3; and harvesting and curing tobacco in period 4.
Selection of predictors of pesticide exposure is guided by an established framework,4 and these predictors fall into three categories. First, farmworker personal characteristics are sex, age (18 to 24 years, 25 to 29 years, 30 to 39 years, 40 or more years), educational attainment (0 to 6 years, 7 or more years), speaking an indigenous language, time spent in US agriculture (1 year or less, 2 to 7 years, 8 or more years), and worker type (migrant worker without H2A visa, migrant worker with H2A visa, seasonal worker). Second, farmworker work characteristics are whether the participant had worked in tobacco, cucumbers, or sweet potatoes in any of the three days before the interview and whether the participant planted, cultivated, topped tobacco, or harvested in any of the three days before the interview. The final work characteristic is total hours that the participant had worked in the three days before the interview (did not work, 1 to 20 hours, more than 20 hours). Third, farmworker exposure characteristics include self-reported pesticide safety training (no training or understood some or none of training in current year, training in current year and understood most or all). Other exposure characteristics are housing type (house, barracks, trailer); people in camp per showerhead (fewer than 4, 4 to 7.9, 8 or more); pesticides applied in room, pesticides applied in camp; whether the participant had mixed, loaded, or applied pesticides on any of the three days before the interview; whether the participant had worked in fields in which pesticides had been applied in last seven days on any of the three days before the interview; and the average number of times the participant washed hands per day in the three days before the interview (0 to 2, 3 or more).
Statistical Analysis
Analyses were completed to describe the associations of specific farmworker characteristics and the detection of specific metabolites. All data analyses accounted for the study’s clustered longitudinal design. A generalized estimating equation (GEE) approach was employed to model the presence of specific metabolites. For the following series of analyses, alternating logistic regression models were used to account for the multiple clustering levels. Addressing the first aim of the paper – to describe the frequencies with which specific pesticide urinary metabolites are detected farmworkers across the agricultural season – began with a basic model to evaluate whether the agricultural period influenced the pattern of detections. This model included only agricultural period as a predictor, and comparisons were made to test the differences between the periods. Separate basic models were developed to address the second aim of the paper: to delineate the personal, occupational, and exposure characteristics of farmworkers associated with the detection of each pesticide urinary metabolite. The models included the main effect for the specific variable of interest (for example, age, pesticide safety training) and time, as well as the interaction of the variable of interest with time. A small number of interactions appeared significant in these models. However, the resulting odds ratios had wide confidence limits due to the small cell sizes (usually less than 5 subjects) used to estimate these parameters. Therefore, the estimates of these interaction terms were considered statistically unreliable and insignificant. The frequencies of detections were plotted over the four time periods for selected farmworker characteristics for illustration. Next, the results of the basic models were used to develop multivariate models. The first of these models was specific to the metabolite and the second was a combination of predictors used in the first model and was used for all metabolites. All data analyses were performed by SAS 9.1 (SAS Institute, Cary, NC) and p-values of less than 0.05 were considered statistically significant.
RESULTS
Farmworker Characteristics
Personal characteristics of farmworkers who participated in this study are presented in Table 2. Work and exposure characteristics of participants are presented in Table 3.
TABLE 2.
Personal Characteristics of Farmworkers, eastern North Carolina, 2007 (N = 284)
| Personal Characteristics | n | % |
|---|---|---|
| Sex | ||
| Male | 258 | 90.9 |
| Female | 25 | 8.8 |
| Age | ||
| 18 to 24 years | 60 | 21.1 |
| 25 to 29 years | 55 | 19.4 |
| 30 to 39 years | 91 | 32.0 |
| 40 or more years | 77 | 27.1 |
| Educational attainment | ||
| 0 to 6 years | 146 | 51.4 |
| 7 or more years | 137 | 48.2 |
| Speaks indigenous language | 64 | 22.5 |
| Seasons in US agriculture | ||
| 1 year or less | 48 | 16.9 |
| 2 to 7 years | 129 | 45.4 |
| 8 or more years | 105 | 37.0 |
| Worker type/visa status | ||
| Migrant without H2A visa | 105 | 37.0 |
| Migrant with H2A visa | 146 | 51.4 |
| Seasonal | 32 | 11.3 |
TABLE 3.
Work and Exposure Characteristics for Four Periods across the Agricultural Season for Farmworkers, eastern North Carolina, 2007
| Period | ||||||||
|---|---|---|---|---|---|---|---|---|
| Work and Exposure Characteristics | 5/1–6/8 (n = 250) |
6/9–7/7 (n = 233) |
7/8–8/5 (n = 229) |
8/6–9/4 (n = 227) |
||||
| n | % | n | % | n | % | n | % | |
| Work Characteristics | ||||||||
| Previous 3 Days Worked in: | ||||||||
| Tobacco | 82 | 32.8 | 112 | 48.1 | 171 | 74.7 | 190 | 83.7 |
| Cucumbers | 9 | 3.6 | 29 | 12.4 | 15 | 6.6 | 0 | 0 |
| Sweet potatoes | 76 | 30.4 | 55 | 23.6 | 16 | 7.0 | 16 | 7.0 |
| Previous 3 Days: | ||||||||
| Planted | 99 | 39.6 | 46 | 19.7 | 5 | 2.2 | 1 | 0.4 |
| Cultivated | 84 | 33.6 | 50 | 21.5 | 13 | 5.7 | 10 | 4.4 |
| Topped tobacco | 0 | 0 | 68 | 29.2 | 106 | 46.3 | 5 | 2.2 |
| Harvested | 60 | 24.0 | 59 | 25.3 | 76 | 33.2 | 118 | 52.0 |
| Total Hours worked in Previous 3 Days | ||||||||
| Did not work | 9 | 3.6 | 7 | 3.0 | 13 | 5.7 | 8 | 3.5 |
| 1–20 | 108 | 43.2 | 107 | 45.9 | 104 | 45.4 | 96 | 42.3 |
| More than 20 | 133 | 53.2 | 119 | 51.1 | 112 | 48.9 | 123 | 54.2 |
| Exposure Characteristics | ||||||||
| Pesticide Safety Training | ||||||||
| No Training or Understood Some or None of Training in Current Year |
129 | 51.6 | 129 | 55.4 | 118 | 51.5 | 109 | 48.0 |
| Training in Current Year and Understood Most or All |
121 | 48.4 | 104 | 44.6 | 111 | 48.5 | 118 | 52.0 |
| Housing Type | ||||||||
| House | 109 | 43.6 | 100 | 42.9 | 93 | 40.6 | 86 | 37.9 |
| Barracks | 49 | 19.6 | 48 | 20.6 | 43 | 18.8 | 42 | 18.5 |
| Trailer | 92 | 36.8 | 85 | 36.5 | 92 | 40.2 | 99 | 43.6 |
| People in Camp per Showerhead | ||||||||
| Fewer than 4 | 57 | 22.8 | 47 | 20.2 | 50 | 21.8 | 90 | 39.6 |
| 4 – 7.9 | 127 | 50.8 | 123 | 52.8 | 129 | 56.3 | 96 | 42.3 |
| 8 or more | 66 | 26.4 | 63 | 27.0 | 50 | 21.8 | 41 | 18.1 |
| Pesticides Applied In Room | 35 | 14.0 | 48 | 20.6 | 23 | 10.0 | 25 | 11.0 |
| Pesticides Applied In Camp | 62 | 24.8 | 34 | 14.6 | 12 | 5.2 | 13 | 5.7 |
| In Previous 3 Days | ||||||||
| Mix, Load, or Apply Pesticides | 26 | 10.4 | 10 | 4.3 | 13 | 5.7 | 2 | 0.9 |
| Worked in Fields in which Pesticides Had Been Applied in Last 7 Days |
85 | 34.0 | 58 | 24.9 | 48 | 21.0 | 26 | 11.5 |
| Average Number of Times Washed Hands | ||||||||
| Per Days Worked | ||||||||
| 0–2 | 40.5 | 56.8 | 125 | 53.6 | 119 | 52.0 | 92 | 40.5 |
| More than 2 | 59.5 | 43.2 | 108 | 46.4 | 110 | 48.0 | 135 | 59.5 |
Detection of Urinary Pesticide Metabolite across the Agricultural Season
The proportion of farmworkers for whom the urinary OP pesticide metabolites were detected at any point in the season varied from 97.4% for para-nitrophenol (PNP) to 0.9% for 5-chloro-1,2-dihydro-1-isopropyl-[3H]-1,2,4-triazol-3-one (CIT) (Table 4). We detected 3,5,6-trichloro-2-pyridinol (TCPy) (46.2%) and malathion dicarboxylic acid (MDA) (27.7%) in a sizeable proportion of farmworkers. Several of the urinary OP pesticide metabolites were detected in a small proportion of farmworkers, including 3-chloro-4-methyl-7-hydroxycoumarin (CMHC) (3.3%), 2-isopropyl-4-methyl-6-hydroxypyrimidinol (IMPY) (3.4%), and 2-diethylamino-6-methyl pyrimidin-4-ol (DEAMPY) (6.0%).
TABLE 4.
Frequencies of Detections for Organophosphorus Insecticide, Pyrethroid Insecticide, and Herbicide Urinary Metabolites for All Samples and for Four Periods of the Agricultural Season for Farmworkers in eastern North Carolina, 2007
| All (n = 939) |
Period | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metabolites | 5/1–6/8 (n = 250) |
6/9–7/7 (n = 233) |
7/8–8/5 (n = 229) |
8/6–l9/4 (n = 227) |
||||||
| n | % | n | % | n | % | n | % | n | % | |
| Organophosphorus Insecticide Urinary Metabolites |
||||||||||
| TCPy | 434 | 46.2 | 50 | 20.0 | 113 | 48.5 | 136 | 59.4 | 135 | 59.5 |
| CMHC | 31 | 3.3 | 6 | 2.4 | 3 | 1.3 | 13 | 5.7 | 9 | 4.0 |
| IMPY | 32 | 3.4 | 10 | 4.0 | 4 | 1.7 | 1 | 0.4 | 17 | 7.5 |
| CIT | 9 | 0.9 | 3 | 1.2 | 0 | 6 | 2.6 | 0 | ||
| MDA | 260 | 27.7 | 38 | 15.2 | 93 | 39.9 | 74 | 32.3 | 55 | 24.2 |
| PNP | 915 | 97.4 | 243 | 97.2 | 227 | 97.4 | 220 | 96.1 | 225 | 99.1 |
| DEAMPY | 56 | 6.0 | 18 | 7.2 | 12 | 5.1 | 4 | 1.7 | 22 | 9.7 |
| Pyrethroid Insecticide Urinary Metabolites |
||||||||||
| 4F3PBA | 3 | 0.3 | 1 | 0.4 | 1 | 0.4 | 0 | 1 | 0.4 | |
| DBCA | 2 | 0.2 | 1 | 0.4 | 1 | 0.4 | 0 | 0 | ||
| CCC | 2 | 0.2 | 1 | 0.4 | 0 | 1 | 0.4 | 0 | ||
| CDCA | 22 | 2.3 | 1 | 0.4 | 18 | 7.7 | 2 | 0.9 | 1 | 0.4 |
| 3PBA | 530 | 56.4 | 127 | 50.8 | 104 | 44.6 | 138 | 60.2 | 161 | 70.9 |
| TCC | 26 | 2.8 | 3 | 1.2 | 1 | 0.4 | 11 | 4.8 | 11 | 4.8 |
| Herbicide Urinary Metabolites |
||||||||||
| 2,4-D | 639 | 68.1 | 175 | 70.0 | 134 | 57.5 | 160 | 69.9 | 170 | 74.9 |
| 2,4,5-T | 7 | 0.7 | 1 | 0.4 | 0 | 5 | 2.2 | 1 | 0.4 | |
| ACE | 274 | 29.2 | 6 | 2.4 | 6 | 2.6 | 113 | 49.3 | 149 | 65.6 |
| ALA | 0 | 0 | 0 | 0 | 0 | |||||
| ATZ | 11 | 1.2 | 3 | 1.2 | 1 | 0.4 | 4 | 1.7 | 3 | 1.3 |
| MET | 159 | 16.9 | 33 | 13.2 | 12 | 5.2 | 60 | 26.2 | 54 | 23.8 |
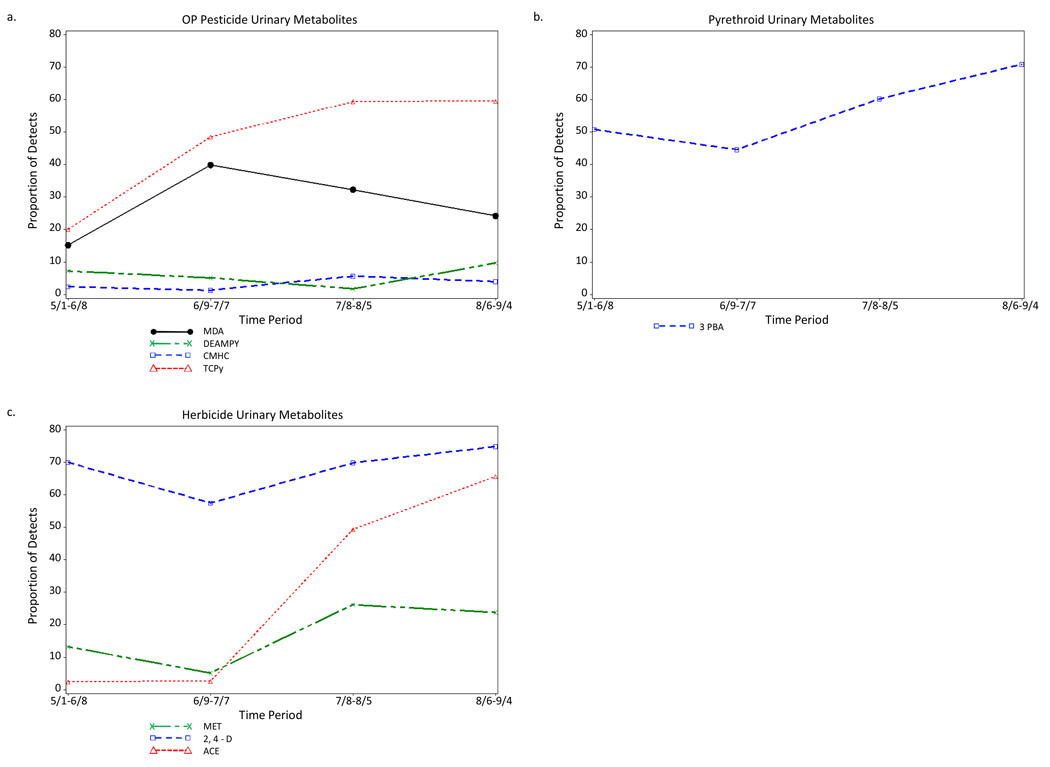
The percent of farmworkers with detectable levels for the OP urinary pesticide metabolites CMHC, CIT, IMPY and PNP changed little across the four periods of the agricultural season. Detections of 2-diethylamino-6-methyl pyrimidin-4-ol (DEAMPY) varied across the four periods, with a decrease in the number of detection during the third period (Figure 1a). The increase in the proportion of farmworkers who had measureable levels of TCPy from the first period (20.0%) to the second (48.5%), third (59.4%), and fourth (59.5%) periods was statistically significant (Table 5). Similarly, the increased detections of MDA from the first period (15.2%) to the second (39.9%), third (32.3%), and fourth (24.2%) periods was statistically significant, as was the decrease from the second to the fourth periods.
Figure 1.
Proportion of Farmworkers with Measureable Levels of Selected Urinary (a) Organophosphorus Insecticide Metabolites, (b) Pyrethroid Insecticide Metabolites, and (c) Herbicide Metabolites for Each of Four Periods Across the Agricultural Season, Eastern North Carolina, 2007.
TABLE 5.
Changes in Detections for Selected Pesticide Urinary Metabolites across the Four Periods of the Agricultural Season
| Metabolites | Comparisons | OR | 95% CI | p |
|---|---|---|---|---|
| TCPy | Period 1 vs Period 2 | 0.26 | 0.15, 0.45 | <.0001 |
| Period 1 vs Period 3 | 0.16 | 0.09, 0.29 | <.0001 | |
| Period 1 vs Period 4 | 0.16 | 0.08, 0.31 | <.0001 | |
| Period 2 vs Period 3 | 0.63 | 0.35, 1.11 | 0.1074 | |
| Period 2 vs Period 4 | 0.61 | 0.34, 1.11 | 0.1072 | |
| Period 3 vs Period 4 | 0.98 | 0.56, 1.72 | 0.9456 | |
| MDA | Period 1 vs Period 2 | 0.26 | 0.12, 0.54 | 0.0003 |
| Period 1 vs Period 3 | 0.36 | 0.16, 0.81 | 0.0130 | |
| Period 1 vs Period 4 | 0.54 | 0.30, 0.97 | 0.0402 | |
| Period 2 vs Period 3 | 1.39 | 0.78, 2.47 | 0.2576 | |
| Period 2 vs Period 4 | 2.07 | 1.26, 3.40 | 0.0039 | |
| Period 3 vs Period 4 | 1.49 | 0.77, 2.87 | 0.2349 | |
| DEAMPY | Period 1 vs Period 2 | 1.42 | 0.37, 5.45 | 0.6056 |
| Period 1 vs Period 3 | 4.26 | 0.81, 22.38 | 0.0871 | |
| Period 1 vs Period 4 | 0.70 | 0.24, 1.99 | 0.4992 | |
| Period 2 vs Period 3 | 2.99 | 0.60, 14.87 | 0.1809 | |
| Period 2 vs Period 4 | 0.49 | 0.15, 1.61 | 0.2397 | |
| Period 3 vs Period 4 | 0.16 | 0.03, 0.84 | 0.0297 | |
| 3PBA | Period 1 vs Period 2 | 1.30 | 0.71, 2.40 | 0.3970 |
| Period 1 vs Period 3 | 0.68 | 0.31, 1.48 | 0.3272 | |
| Period 1 vs Period 4 | 0.43 | 0.21, 0.87 | 0.0198 | |
| Period 2 vs Period 3 | 0.52 | 0.27, 0.98 | 0.0443 | |
| Period 2 vs Period 4 | 0.33 | 0.19, 0.57 | <.0001 | |
| Period 3 vs Period 4 | 0.63 | 0.39, 1.02 | 0.0629 | |
| 2,4-D | Period 1 vs Period 2 | 1.60 | 0.99, 2.59 | 0.0540 |
| Period 1 vs Period 3 | 0.94 | 0.58, 1.52 | 0.7968 | |
| Period 1 vs Period 4 | 0.72 | 0.36, 1.43 | 0.3482 | |
| Period 2 vs Period 3 | 0.59 | 0.38, 0.91 | 0.0187 | |
| Period 2 vs Period 4 | 0.45 | 0.21, 0.98 | 0.0447 | |
| Period 3 vs Period 4 | 0.77 | 0.37, 1.57 | 0.4673 | |
| ACE | Period 1 vs Period 2 | 0.93 | 0.11, 7.93 | 0.9460 |
| Period 1 vs Period 3 | 0.02 | 0.00, 0.15 | <.0001 | |
| Period 1 vs Period 4 | 0.01 | 0.00, 0.07 | <.0001 | |
| Period 2 vs Period 3 | 0.03 | 0.01, 0.08 | <.0001 | |
| Period 2 vs Period 4 | 0.01 | 0.00, 0.04 | <.0001 | |
| Period 3 vs Period 4 | 0.50 | 0.20, 1.26 | 0.1410 | |
| MET | Period 1 vs Period 2 | 2.97 | 1.19, 7.37 | 0.0193 |
| Period 1 vs Period 3 | 0.43 | 0.25, 0.75 | 0.0029 | |
| Period 1 vs Period 4 | 0.46 | 0.25, 0.85 | 0.0137 | |
| Period 2 vs Period 3 | 0.14 | 0.06, 0.35 | <.0001 | |
| Period 2 vs Period 4 | 0.16 | 0.06, 0.38 | <.0001 | |
| Period 3 vs Period 4 | 1.08 | 0.65, 1.79 | 0.7615 |
Note: N=937 due to missing data in covariate measures.
One of the six urinary pyrethroid insecticide metabolites, 3-phenoxybenzoic acid (3PBA), was frequently detected (56.4%) across all of the samples (Figure 1b). The percentages of detections were not significantly different from the first (50.8%) to the second (44.6%) and third (60.2%) periods, although the increases from the first and second periods to the fourth period (70.9%) were significant (Table 5). Although the overall frequencies of detections were low for cis-3-(2,2-dimethylvinyl)-2,2-dimethylcyclopropane dicarboxylic acid (CDCA) (2.3%) and trans-2,2-(dichloro)-2-dimethylvinylcyclopropane carboxylic acid (TCC) (2.8%), most of the detections for CDCA were in the second period, and most of the detections for TCC were in the third and fourth periods (Table 4).
Three of the six herbicide urinary metabolites were detected in a sizeable proportion of the total samples: 2,4-Dichlorophenoxyacetic acid (2,4-D) (68.1%), acetochlor mercapturate (ACE) (29.2%), and metolachlor mercapturate (MET) (16.9%). The detections of each of these three herbicide urinary metabolites varied across the agricultural periods (Figure 1c). Detection of 2,4-D declined from 70.0% in the first period to 57.5% in the second, but then increased to 69.6% and 74.9% in the third and fourth periods, respectively. ACE was detected in 2.4% and 2.6% of farmworkers for the first two periods, but increased to 49.3% and 65.6% in the third and fourth periods. Detection of MET declined from 13.2% to 5.2% from the first to the second period, but then increased to 26.2% and 23.8% in the third and fourth periods.
Farmworker Characteristics Associated with the Detection of Pesticide Urinary Metabolites
None of the farmworker personal, work, or exposure characteristics was associated with the detection of any pesticide urinary metabolite. Bivariate and multivariate analyses did uncover a few statistically significant associations of specific farmworker characteristics with the frequencies of detection for specific metabolites. However, these analyses did not find any consistent associations of farmworker characteristics across the pesticide urinary metabolites. Nor did analyses find consistent associations of farmworker characteristics with specific pesticide urinary metabolites across the agricultural periods. The large number of comparisons and the small number of statistically significant associations indicates that the associations between characteristics and metabolites must be evaluated with considerable caution.
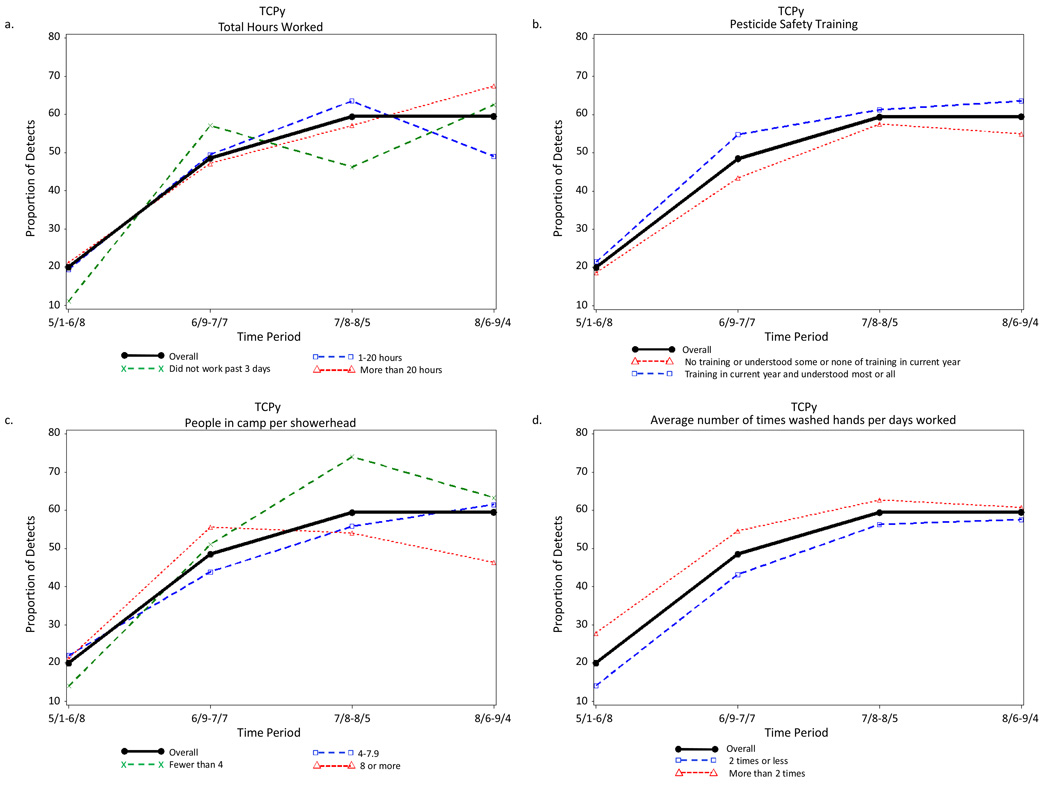
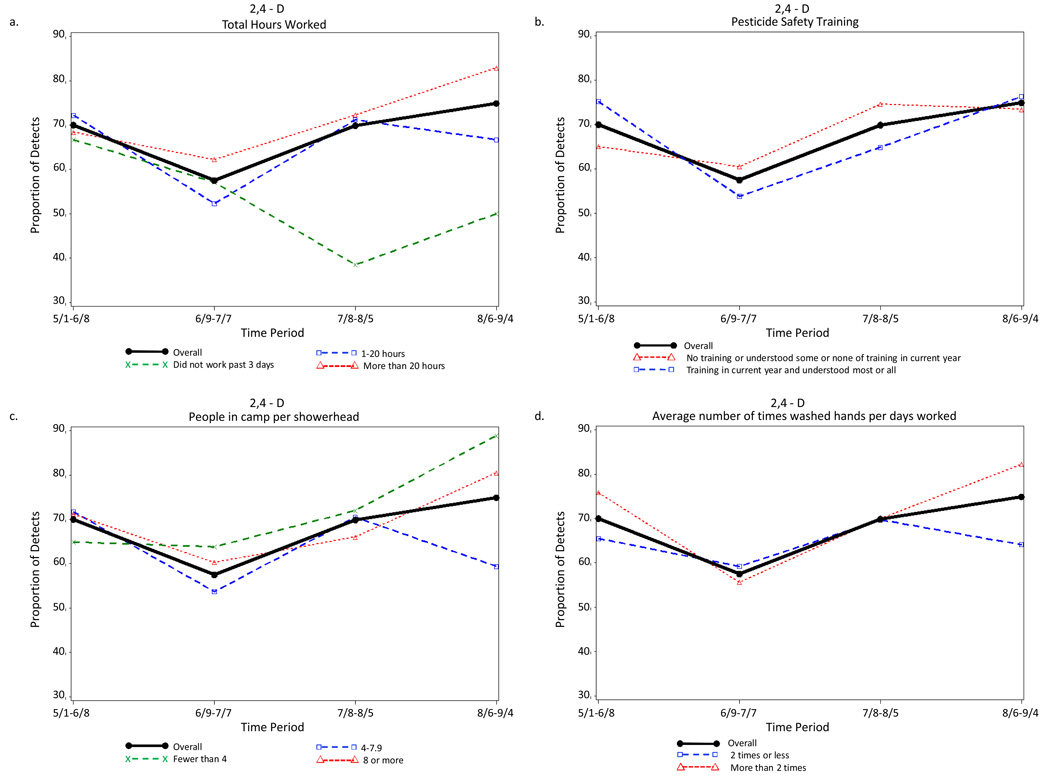
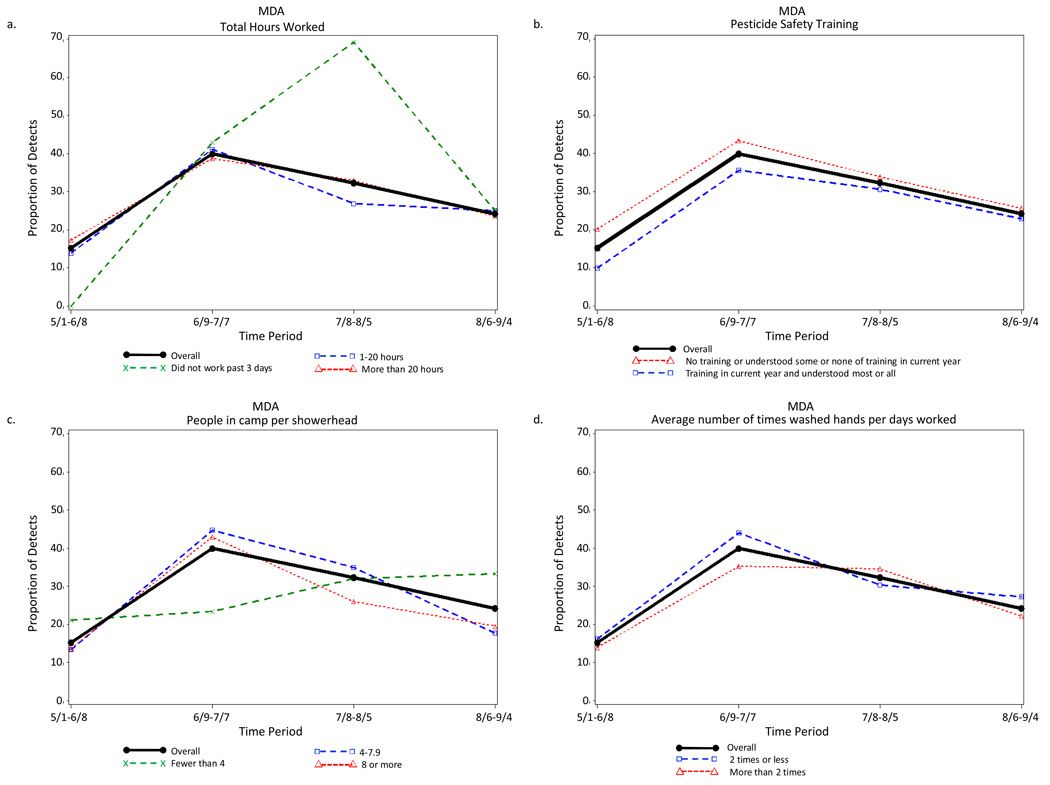
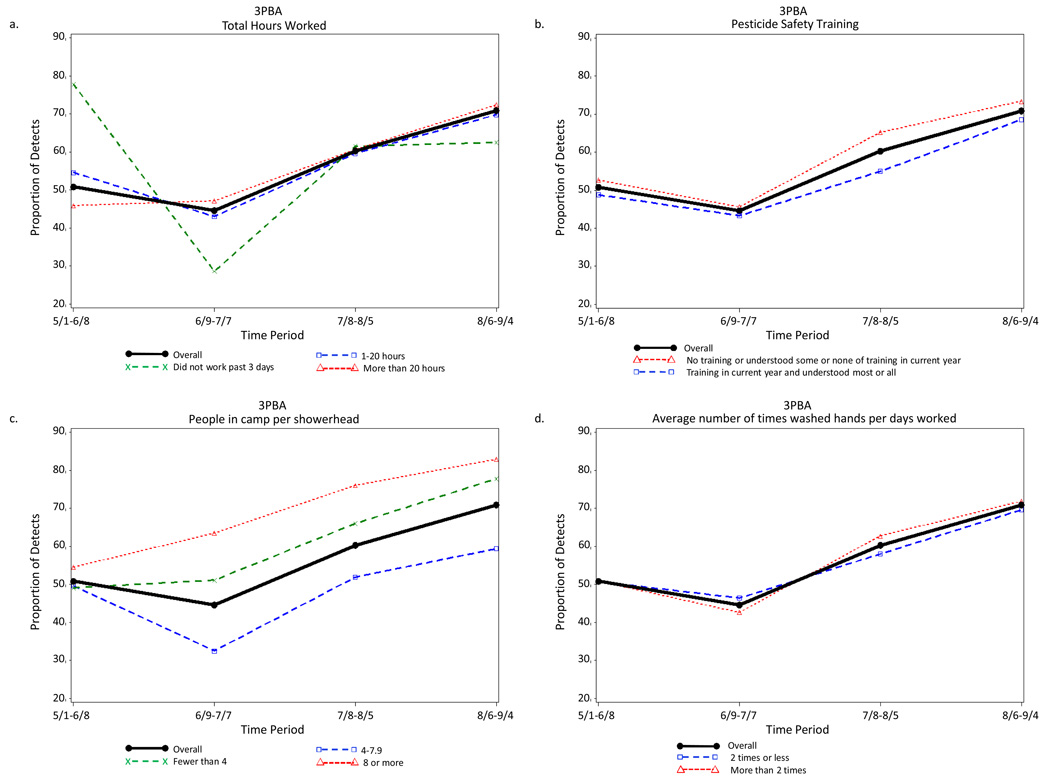
Period of the agricultural season was the strongest predictor of detection of each pesticide urinary metabolite. Examples of the indifference to various farmworker characteristics in the seasonal distribution of the detection of pesticide metabolites are illustrated in Figure 2 to Figure 5. The four farmworker characteristics were selected as examples because they include a work characteristic (total hours worked in previous 3 days) and three exposure characteristics (pesticide safety training, people in camp per shower head, and average number of times washed hands per days worked).
Figure 2.
Proportion of Farmworkers with Measureable Levels of the Urinary Chlorpyrifos Metabolite 3,5,6-trichloropyridinol for Each of Four Periods Across the Agricultural Season Total and (a) Controlling for Pesticide Safety Training, (b) Total Hours Worked, (c) Average Number of Times Washed Hands per Days Worked, and (d) People in Camp per Showerhead, Eastern North Carolina, 2007.
Figure 5.
Proportion of Farmworkers with Measureable Urinary Levels of the Herbicide 2,4-D for Each of Four Periods Across the Agricultural Season Total and (a) Controlling for Pesticide Safety Training, (b) Total Hours Worked, (c) Average Number of Times Washed Hands per Days Worked, and (d) People in Camp per Showerhead, Eastern North Carolina, 2007.
Detection of some urinary pesticide metabolites did differ for a few farmworker characteristics. For example, for MDA, the frequency of detection for total hours worked during the third period for the value “did not work in the past 3 days” is 69.2%, which was very different from the value for the total sample (32.3%) and for the values “worked 1–20 hours” (26.9%) and “worked more than 20 hours” (33.0%) (Figure 3b). However, this difference was due to the instability in the small number of farmworkers, 13, who did not work during the three day prior to data collection.
Figure 3.
Proportion of Farmworkers with Measureable Levels of the Urinary Malathion Metabolite Malathion Dicarboxylic Acid for Each of Four Periods Across the Agricultural Season Total and (a) Controlling for Pesticide Safety Training, (b) Total Hours Worked, (c) Average Number of Times Washed Hands per Days Worked, and (d) People in Camp per Showerhead, Eastern North Carolina, 2007.
DISCUSSION
Although pesticide exposure is acknowledged as an occupational and environmental health risk for farmworkers, data documenting farmworker occupational pesticide exposure are limited.4 Detailed longitudinal data documenting the pesticide exposures of women residing in farmworker communities have been reported.11,12 Data for three DAP metabolites collected from two panels of farmworkers in 1999 and in 2003 have also been reported.13 However, the present is the first study of farmworker pesticide exposure in the United States to include a large sample (284) of farmworkers from whom multiple samples (four) were collected across a single agricultural season. It is also the first study to measure a large number of pesticide urinary metabolites (19) associated with specific OP insecticides, pyrethroid insecticides, and herbicides. The detections of several pesticide urinary metabolites are common among these farmworkers. These metabolites include the OP insecticide metabolites TCPy, MDA, and PNP; the pyrethroid insecticide 3PBA; and the herbicide metabolites 2,4-D, ACE, and MET.
The lack of other population studies documenting specific pesticide urinary metabolites for farmworkers forecloses the possibility for comparison. Most other biomarker research with farmworkers has reported the general DAP metabolites of OP insecticides.5,6 Comparison of detections for these DAP metabolites among participants in the current study with these other analyses is reported elsewhere.16 Salvatore and colleagues7 do report that 93.1% of urine samples collected from 73 California farmworkers contained MDA. The percentage of these California farmworkers for whom MDA was detected is much greater than the percentage of North Carolina farmworkers for whom MDA was detected (27.7%); however, the California farmworkers were not typical in that their urine samples were collected immediately after they had worked in malathion treated fields for which the 72 hour pre-harvest interval had just expired. Studies of farmers, such as the Agricultural Health Study, have not reported pesticide metabolite data.17
Eskenazi and colleagues12 report detections for MDA and TCPy, as well as for the general DAP metabolites, for pregnant women participating in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) study. These women, many of whom worked as farmworkers, lived in a California agricultural community and provided urine samples in 1999–2000. MDA was detected in urine samples for 32% of women during a first pregnancy and 25% of women during a second pregnancy; TCPy was detected in urine samples for 71% of women during first pregnancy and 82% of women during second pregnancy. In comparing our study with the CHAMACOS study, consideration should be given to differences in California and North Carolina agricultural systems and, therefore, in pesticides used in the two states. Further, chlorpyrifos, the parent chemical for TCPy, was banned for residential use in the US at the end of 2001, after the California data were collected. The number of women in the California study for whom MDA was detected was similar to the number of farmworkers in North Carolina. The number of women in the California study for whom TCPy was detected was greater than the number of farmworkers in North Carolina.
The number of farmworkers for whom pesticide urinary metabolites were detected varied across the agricultural season. The temporal patterns in the frequencies of detection differ for each of the pesticide urinary metabolites. However, it is difficult to see how the temporal patterns of detections are associated with agricultural production. For example, the number of detections for the metabolite TCPy increases across the agricultural season. TCPy is associated with the OP insecticides chlorpyrifos and chlorpyrifos methyl. However, the proportion of farmworkers engaged in tobacco production also increases across the agricultural season, and chlorpyrifos is reported to have very limited application to tobacco.18 Similarly, detections for the general pyrethroid insecticide metabolite 3PBA and the herbicide metabolites ACE and MET increased across the agricultural season, yet none of these is common in tobacco production. Personal, work, and exposure characteristics of the farmworkers were not consistently associated with the detection of any pesticide urinary metabolites. Rather, the temporal patterns in the frequencies of detection for each metabolite were largely reflected in the frequencies of detection related to each of the farmworker characteristics.
Our results indicate that any examination of pesticide dose or exposure for farmworkers, or other agricultural workers, that uses a cross-sectional design or that relies on a single measure from each participant will provide an unreliable characterization of pesticide exposure. Variability in detection for each urinary metabolite within and across individuals indicates that any single measure of urinary metabolites cannot be considered a dependable indicator of exposure for an individual. Further, urinary metabolite exposure measures collected at a single time in an agricultural season are not a good indicator of population pesticide exposure. Seasonal variability indicates that samples need to be collected systematically across the agricultural season to get an indication of pesticide exposure in the population of farmworkers. Looking across measures for multiple individuals provides an indication of the level of pesticide exposure in the population of farmworkers. Variability in single measures of pesticide dose or exposure could explain the difficulty in evaluating interventions to reduce farmworker pesticide exposure.13
Research on pesticide dose or exposure for agricultural workers at the population level should include multiple measures of dose or exposure collected at frequent intervals (daily or weekly) over an extended period with close evaluation of risk factors for exposure. Repeated exposure measures will allow us to discern the trajectory of exposure and the factors that are associated with exposure.
Limitations of this study should be considered in evaluating its results. This research was conducted in a selected area of one state; other states may differ in their patterns of pesticide use and exposure. The sample was limited to the camps known to community partner organizations, and participants were limited to those living in the camps at the time of recruitment. We collected pesticide biomarker data for an extended part of the agricultural season; however, the period covered did not include workers harvesting cucumbers and sweet potatoes in September and October. The specific pesticide metabolites, the number of detections, and their concentrations were limited to the capabilities of the existing laboratory procedures. In addition, metabolites can also be derived from exposure to the preformed metabolites in the environment or from pesticide exposures in non-occupational pathways including in housing and food.19
Farmworkers in North Carolina show evidence of frequent exposure to OP insecticides, pyrethroid insecticides, and herbicides. This study provides a strong characterization of the population pattern of pesticide exposure. Seasonality in the detections of pesticide urinary metabolites overrides the effects of any personal, work, or exposure characteristics measured. Future research needs to collect multiple exposure measures at frequent intervals over an extended period to characterize the factors associated with exposure.
Figure 4.
Proportion of Farmworkers with Measureable Levels of the Urinary Pyrethroid Insecticide Metabolite 3PBA for Each of Four Periods Across the Agricultural Season Total and (a) Controlling for Pesticide Safety Training, (b) Total Hours Worked, (c) Average Number of Times Washed Hands per Days Worked, and (d) People in Camp per Showerhead, Eastern North Carolina, 2007.
Acknowledgments
This study was supported by a grant from the National Institute of Environmental Health Sciences, United States National Institutes of Health (R01-ES008739).
Footnotes
The opinions expressed in this manuscript are those of the authors and do not necessarily reflect the official opinion of the Centers for Disease Control and Prevention.
Disclosures: The authors disclose no conflicts of interest.
References
- 1.Villarejo D. The health of U.S. hired farm workers. Annu Rev Public Health. 2003;24:175–193. doi: 10.1146/annurev.publhealth.24.100901.140901. [DOI] [PubMed] [Google Scholar]
- 2.Calvert GM, Karnik J, Mehler L, et al. Acute pesticide poisoning among agricultural workers in the United States, 1998–2005. Am J Ind Med. 2008;51:833–898. doi: 10.1002/ajim.20623. [DOI] [PubMed] [Google Scholar]
- 3.McCauley LA, Anger KA, Keifer M, Langley R, Robson MG, Rohlman D. Studying health outcomes in farmworker populations exposed to pesticides. Environ Health Perspect. 2006;114:953–960. doi: 10.1289/ehp.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quandt S, Hernández-Valero MA, Grzywacz JG, Hovey JD, Gonzales M, Arcury TA. Workplace, household, and personal predictors of pesticide exposure and health outcomes for farmworkers. Environ Health Perspect. 2006;114:943–952. doi: 10.1289/ehp.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenske RA, Curl CL, Kissel JC. The effect of the 14-day agricultural restricted entry interval on azinphosmethyl exposures in a group of apple thinners in Washington State. Regul Toxicol Pharmacol. 2003;38:91–97. doi: 10.1016/s0273-2300(03)00073-4. [DOI] [PubMed] [Google Scholar]
- 6.Coronado GD, Vigoren EM, Thompson B, Griffith WC, Faustman EM. Organophosphate pesticide exposure and work in pome fruit: evidence for the take-home pesticide pathway. Environ Health Perspect. 2006;114:999–1006. doi: 10.1289/ehp.8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salvatore AL, Bradman A, Castornia R, et al. Occupational behaviors and farmworkers' pesticide exposure: findings from a study in Monterey County, California. Am J Ind Med. 2008;51:782–794. doi: 10.1002/ajim.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arcury TA, Quandt SA, Rao P, et al. Organophosphate pesticide exposure in farmworker family members in western North Carolina and Virginia: Case comparisons. Hum Organ. 2005;64:40–51. [PMC free article] [PubMed] [Google Scholar]
- 9.Quandt SA, Arcury TA, Rao P, et al. Agricultural and residential pesticides in wipe samples from farmworker family residences in North Carolina. Environ Health Perspect. 2004;112:382–387. doi: 10.1289/ehp.6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arcury TA, Grzywacz JG, Barr DB, Tapia J, Chen H, Quandt SA. Pesticide urinary metabolite levels of children in eastern North Carolina farmworker households. Environ Health Perspect. 2007;115:1254–1260. doi: 10.1289/ehp.9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradman A, Eskenazi B, Barr DB, et al. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living an agricultural community. Environ Health Perspect. 2005;113:1802–1807. doi: 10.1289/ehp.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eskenazi B, Marks AR, Bradman A, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson B, Coronado GD, Vigoren EM, et al. Oara Niños Saludables: A community intervention trial to reduce organophosphate pesticide exposure in children of farmworkers. Environ Health Perspect. 2008;116:687–694. doi: 10.1289/ehp.10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barr DB, Bravo R, Weerasekera G, et al. Concentrations of dialkylphosphate metabolites of organophosphorus pesticides in the US population. Environ Health Perspect. 2004;112:186–200. doi: 10.1289/ehp.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olsson AO, Baker SE, Nguyen JV, et al. A liquid chromatography-tandem mass spectrometry multiresidue method for quantification of specific metabolites of organophosphorus pesticides, synthetic pyrethroids, selected herbicides, and DEET in human urine. Anal Chem. 2004;76:2453–2461. doi: 10.1021/ac0355404. [DOI] [PubMed] [Google Scholar]
- 16.Arcury TA, Grzywacz JG, Chen H, et al. Variation across the agricultural season in organophosphorus pesticide urinary metabolite levels for Latino farmworkers in eastern North Carolina: project design and descriptive results. Am J Ind Med. 2009;52:539–550. doi: 10.1002/ajim.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alavanja MC, Sandler DP, McDonnell CJ, et al. Characteristics of pesticide use in a pesticide applicator cohort: The Agricultural Health Study. Environ Res. 1999;80(2 Pt 1):172–179. doi: 10.1006/enrs.1998.3888. [DOI] [PubMed] [Google Scholar]
- 18.Southern PS, Sorenson CE. 2008 North Carolina Agricultural Chemicals Manual. Raleigh, NC: College of Agriculture and Life Sciences, NC State University; 2008. Chapter 5. Insect Control. [Google Scholar]
- 19.Zhang X, Driver JH, Li Y, Ross JH, Krieger RI. Dialkylphosphates (DAPs) in fruits and vegetables may confound biomonitoring in organophosphorus insecticide exposure and risk assessment. J Agric Food Chem. 2008;56:10638–10650. doi: 10.1021/jf8018084. [DOI] [PubMed] [Google Scholar]