Abstract
Ovarian carcinomas are a heterogeneous group of neoplasms. Pathologists currently employ a morphology-based classification system to divide ovarian carcinomas into major subgroups based on degree (tumor grade) and type of differentiation (e.g., serous, endometrioid, clear cell or mucinous). Molecular studies have shown that specific genetic defects are likely to be present in certain histologic types of ovarian carcinomas, and unlikely to be present in others. Within the serous and endometrioid carcinomas, the molecular defects in low-grade versus high-grade tumors also appear to be largely distinct. Recently, mouse models of ovarian carcinoma have been developed that recapitulate many of the morphological features and biological behavior of selected subtypes of ovarian cancer. It is expected that these mouse models will yield new insights into ovarian cancer pathogenesis and prove useful for pre-clinical testing of novel strategies for ovarian cancer treatment.
INTRODUCTION
When surgical pathologists evaluate an ovarian carcinoma and assign it a particular histopathologic type, grade and stage, our intent is to convey important information about how that tumor is likely to behave, whether it will to respond to therapy, and ultimately, how well the patient is likely to fare. As part of our evaluation, we usually classify a given ovarian carcinoma into one of four major histopathologic subtypes: serous, endometrioid, mucinous or clear cell. However, it is somewhat disconcerting that this information is largely ignored by our clinical colleagues when treating the patient. Once a carcinoma of any histologic subtype has spread beyond the ovaries, standard first-line therapy consists of surgical debulking followed by a systemic chemotherapy regimen that includes a platinum-based drug (carboplatin or cisplatin) and a taxane (paclitaxel or docitaxel)1. Although most ovarian carcinomas initially respond to first-line therapy, recurrence with drug-resistant disease is typical and the majority of patients eventually succumb to their disease. Like histopathologic typing, standard grading of invasive ovarian carcinomas as well, moderately, or poorly differentiated, currently offers clinicians little guidance for therapy decisions. It is noteworthy that for carcinomas of the endometrium, clinicians recognize that behavior of endometrioid adenocarcinoma is quite different from that of clear cell or serous carcinoma. Accordingly, the latter two are treated differently, and more aggressively, than the former 2.
This review will draw upon selected older and more recent studies to show how molecular tools that weren’t available a few short years ago have not only enriched our understanding of ovarian cancer pathogenesis, but also impacted our approach to morphology-based tumor classification. As we learn about the molecular alterations in tumors from individual patients, we can not only improve upon our existing tumor classification schemes, but also start to develop personalized therapies using drugs that target specific defects in an individual patient’s tumor cells.
OVARIAN CARCINOMA SUBTYPES ARE LARGELY DISTINGUISHABLE BASED ON SPECIFIC GENE MUTATIONS AND GLOBAL GENE EXPRESSION PATTERNS
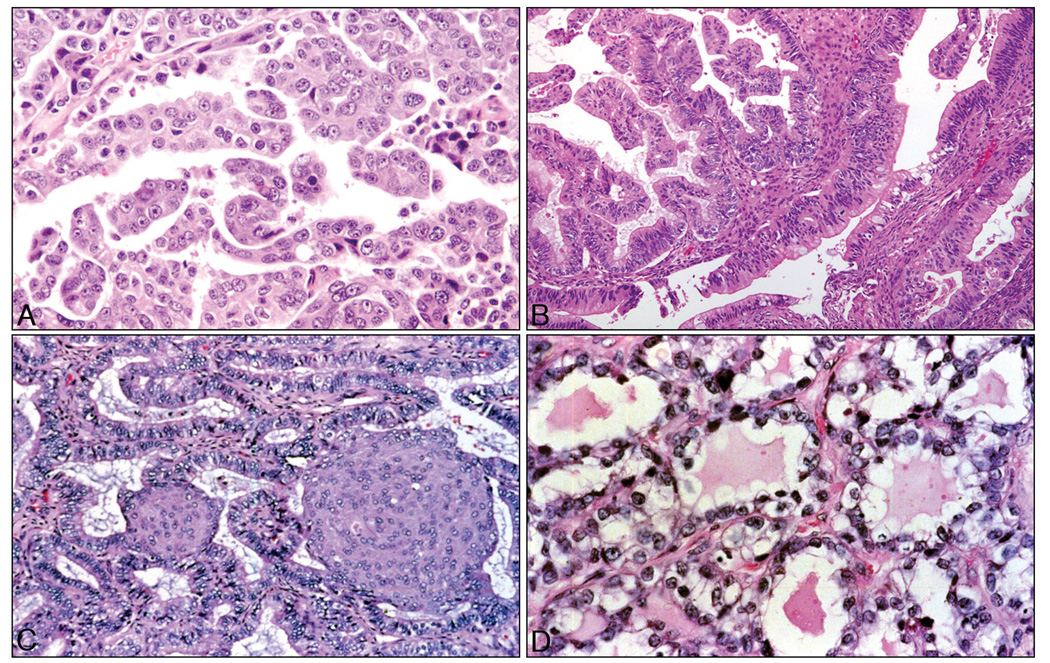
An important take home message of this review is that pathologists’ traditional morphology-based classification allows prediction of specific molecular genetic alterations likely to be present in a given ovarian tumor. Several recent papers have reviewed the molecular pathology of ovarian cancer 3–5. While not intended to be an exhaustive compilation of all the mutations in ovarian carcinomas reported to date, Figures 1A–1D show that each of the four major histopathologic types of ovarian carcinoma are characterized by rather distinctive, though not necessarily unique, genetic abnormalities. For example, TP53 gene mutations are extremely common in serous carcinomas, while mucinous adenocarcinomas have a high prevalence of KRAS mutations 6–9. Mutations of CTNNB1, the gene encoding β-catenin, are common in endometrioid adenocarcinomas but rare in serous, mucinous and clear cell carcinomas 10,11. Mutations of PIK3CA, which encodes the catalytic subunit of PI3K (phosphoinositide 3-kinase), are observed most frequently in clear cell carcinomas 12. The point is that specific genetic defects are likely to be present in certain histologic types of ovarian carcinomas, and unlikely to be present in others.
Figure 1.
Representative photomicrographs of the four major histopathologic types of ovarian carcinoma. A) Serous carcinomas comprise approximately 70% of ovarian carcinomas and frequently harbor TP53 mutations. B) Mucinous carcinomas are rarest (approximately 3%) and often harbor KRAS mutations. C) Endometrioid carcinomas (≈10–15%) have relatively frequent mutations of CTNNB1, PIK3CA, KRAS and TP53. D) Clear cell carcinomas (≈10%) have the highest frequency of PIK3CA mutations. All sections stained with hematoxylin & eosin; original magnification X200.
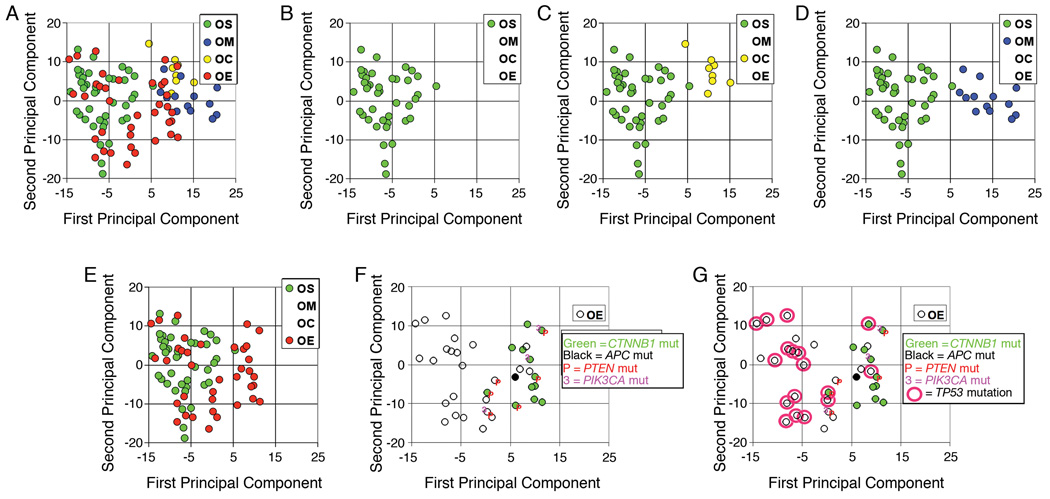
Practicing pathologists and researchers alike are accustomed to thinking about molecular alterations in tumors on a gene-by-gene basis. Indeed, pathologists routinely use immunohistochemical staining, with one antibody at a time, to evaluate altered expression of individual gene products in tumor sections. Just a few years ago, the notion of comprehensively and simultaneously evaluating expression or DNA copy number of thousands of genes in a single assay seemed rather far-fetched. Nevertheless, using technologies developed during the past decade, reams of information can now be collected routinely from individual tumor samples based on single experiments. A previously published study using oligonucleotide microarrays to evaluate gene expression in a sizeable series of primary ovarian carcinomas is illustrative 10,13. In this study, Affymetrix U-133A oligonucleotide microarrays were used to simultaneously interrogate expression of approximately 14,500 well characterized genes in 99 primary ovarian carcinomas (mostly serous or endometrioid with fewer clear cell and mucinous carcinomas). A statistical method called principal component analysis (PCA) was used to compare global gene expression in each tumor by distilling variances in expression across the entire set of genes down to a two-dimensional plot, in which tumors that fall closer together on the plot are more similar in their global gene expression than tumors that are farther apart. Figure 2A shows PCA data from each of the 99 primary tumors. When viewed separately, serous carcinomas (each represented by a green circle, Figure 2B) are mostly clustered together toward the left side of the two-dimensional PCA plot. Note that clear cell and mucinous carcinomas (yellow and blue circles, respectively) are quite distinct from serous carcinomas with respect to gene expression pattern, as shown by their own clustering profiles (Figures 2C and 2D). The divergent gene expression profiles observed amongst serous, clear cell, and mucinous carcinomas likely reflect, at least in part, readily apparent differences in the histopathologic features of these tumor types. The gene expression profiles of the endometrioid adenocarcinomas are more problematic. While a number of these tumors appear to be quite distinct from the serous carcinomas, others overlap directly into the serous carcinoma gene expression space (Figure 2E). These findings prompted an investigation of the basis for the apparent heterogeneity of endometrioid tumors, because each of the four histological groups was originally expected to have clearly distinct gene expression profiles. Specifically, each endometrioid tumor was annotated with information on the mutational status of genes previously shown to be frequently altered in both ovarian and endometrial endometrioid adenocarcinomas.
Figure 2.
Gene expression profiling of primary ovarian carcinomas. A) Principal component analysis (PCA) of 99 ovarian carcinomas using all probe sets on the U133A array; the first two principal components are shown. Individual tumors are annotated with histopathologic type as indicated (green-OS, serous; blue-OM, mucinous; yellow-OC, clear cell; and red-OE, endometrioid); B) Same PCA plot showing only serous carcinomas; C) Same PCA plot showing only serous and clear cell carcinomas; D) Same PCA plot showing only serous and mucinous carcinomas; E) Same PCA plot showing only serous and endometrioid carcinomas; F) Same PCA plot showing only endometrioid carcinomas annotated with mutational status of CTNNB1, APC, PTEN and PIK3CA as indicated; G) Same PCA plot showing only endometrioid carcinomas annotated with mutational status of CTNNB1, APC, PTEN, PIK3CA, and TP53 as indicated. ****Figure (modified version) reprinted from Cancer Cell, 11:321–333, 2007 (R Wu et al., Mouse Model of Human Ovarian Endometrioid Adenocarcinoma Based on Somatic Defects in the Wnt/β-Catenin and PI3K/Pten Signaling Pathways)10 with permission from Elsevier.
CHARACTERISTIC SIGNALING PATHWAY DEFECTS ARE PRESENT IN A SUBSET OF OVARIAN ENDOMETRIOID ADENOCARCINOMAS
Like other cancers, ovarian carcinomas arise through a multi-step process in which clonal selection acts on cells with somatic mutations and altered gene expression to allow outgrowth of progeny with increasingly aggressive growth properties. The genes mutated in cancer are not selected randomly, but frequently encode proteins that function in highly conserved signaling pathways. Hence, it is important to think about mutant genes in the context of their functions in pathways that regulate important cellular processes such as growth and differentiation. This can be viewed as a “good news – bad news” scenario. The bad news is that a single genetic mutation can fundamentally alter a critical pathway comprised of many other signaling molecules. The good news is that abnormal function of the mutant protein can potentially be countered by targeting other molecules in the pathway, particularly those further downstream.
The canonical Wnt signaling pathway, which is frequently deregulated in endometrioid adenocarcinomas in the endometrium and ovary, as well as other types of human cancers, illustrates this point. Wnts are a conserved family of secreted proteins with roles in diverse cellular processes, including regulation of cell fate, proliferation, motility, and survival 14,15. Wnts bind specific receptors on the cell surface and mediate intracellular signaling events via several different pathways 16. In the “canonical” and best characterized pathway, β-catenin is a key effector that is stabilized as a consequence of the binding of selected Wnts to their surface receptors. A substantial fraction of the cellular β-catenin pool is bound to the cytoplasmic domain of the E-cadherin cell adhesion protein at the cell membrane. Levels of “free” cytosolic β-catenin are tightly regulated by a multi-protein complex including Apc, Axin, and GSK3β. In the absence of Wnt signals, this multi-protein complex promotes degradation of free β-catenin via GSK3β-mediated phosphorylation of specific β-catenin residues; phosphorylated β-catenin is subsequently ubiquitinated and degraded by the proteasome. When the pathway is activated by Wnts, GSK3β activity and β-catenin degradation are inhibited. Stabilized β-catenin translocates to the nucleus where it binds T cell factor (Tcf) [also known as lymphoid enhancer factor (Lef)] transcription regulator proteins. The β-catenin/Tcf complex affects transcription of target genes. To summarize, when the Wnt pathway is activated, free β-catenin is stabilized, translocates to the nucleus, and activates transcription of a number of downstream target genes. Although Wnt ligands can also exert effects independent of β-catenin, deregulation of the canonical pathway has been most clearly implicated in cancer pathogenesis15.
In endometrioid adenocarcinomas, the Wnt pathway is often constitutively activated, usually via missense mutations of the gene (CTNNB1) that encodes β-catenin itself. These mutations typically alter residues phosphorylated by GSK3β, and hence, β-catenin protein cannot be targeted for degradation. In colorectal carcinomas, the Wnt signaling pathway is more often deregulated by a different mechanism, i.e., inactivating mutations of APC. When Apc is inactivated, the multi-protein complex that promotes degradation of free cytosolic β-catenin is dysfunctional and β-catenin protein is stabilized. In either case, stabilized β-catenin protein translocates to the nucleus and activates transcription of downstream target genes, at least some of which are likely important for neoplastic transformation and/or tumor progression. It follows that immunohistochemical staining for β-catenin can be used to determine whether or not the Wnt signaling pathway is likely to be activated in a given tumor sample; those tumors with deregulated Wnt signaling caused by activating CTNNB1 mutations or inactivating APC mutations are expected to show nuclear accumulation of β-catenin protein, while those tumors with intact Wnt signaling show membrane-bound β-catenin staining.
The PI3K/Akt pathway is another signaling pathway that is frequently altered in human cancers, including endometrial and ovarian endometrioid adenocarcinomas. This signaling pathway plays important roles in cell cycle progression, cell survival, response to nutrient availability, cell motility, and angiogenesis17,18. Signaling via the PI3K pathway is initiated by interaction of specific ligands with plasma membrane-spanning receptor tyrosine kinases, such as EGFR (epidermal growth factor receptor), c-kit, and insulin-like growth factor receptor 1. The ligand/receptor interaction leads to the recruitment and activation of PI3K, which converts PIP2 (phosphatidylinositol-4,5 biphosphate) to PIP3 (phosphatidylinositol-3,4,5 triphosphate). PIP3 in turn transmits growth and survival signals by recruiting certain kinases such as the protein kinase B/Akt family of kinases and phosphoinositide-dependent kinase 1 (PDK1) to the membrane. In part through phosphorylation by PDK1, Akt is activated and phosphorylates specific downstream targets, many of which play key roles in the regulation of important cellular functions such as proliferation, cell size, apoptosis, response to nutrients and DNA damage. The lipid phosphatase Pten removes the D3 phosphate from PIP3, inactivating the signaling cascade and regenerating PIP2. In endometrioid adenocarcinomas, the PI3K/Akt pathway is often deregulated via inactivating mutations of PTEN or through activating mutations of PIK3CA, which encodes the catalytic subunit of PI3K.
In an attempt to understand the heterogeneity of ovarian endometrioid adenocarcinomas highlighted by differences in their global gene expression profiles (Figure 2E), a series of 72 primary tumors, including all of those with gene expression profile data, were analyzed for mutational defects in the canonical Wnt and PI3K/Akt signaling pathways10. Based on β-catenin immunostaining and CTNNB1 and APC mutational analyses, Wnt signaling pathway defects were identified in 26% of ovarian endometrioid adenocarcinomas, almost always due to missense mutation of CTNNB1. Each tumor with documented mutation of a Wnt pathway gene showed the expected nuclear staining pattern, whereas those tumors with intact Wnt signaling had the expected membrane staining pattern. Notably, Wnt pathway defects were significantly associated with low tumor grade and stage, and were often observed in tumors showing prominent squamous differentiation. Mutational analyses for both PTEN and PIK3CA were performed in the same group of ovarian endometrioid adenocarcinomas, and mutations predicted to deregulate PI3K/Akt signaling were found in nearly 15% of tumors, many of which also harbored Wnt signaling pathway defects. Statistical analysis confirmed that dysregulation of both the Wnt and PI3K/Akt pathways occurred disproportionately frequently in a subset of endometrioid adenocarcinomas, suggesting that defects in these two pathways cooperate in their pathogenesis. When endometrioid adenocarcinomas profiled in the earlier gene expression analysis were annotated with mutational status of genes affecting these pathways (Figure 2F), it was discovered that ovarian endometrioid carcinomas with deregulated Wnt and/or PI3K/Akt signaling were the ones readily separable from the serous carcinomas.
Because TP53 mutations are also known to be prevalent in ovarian endometrioid adenocarcinomas 19, the same group of tumors was analyzed for TP53 mutation using a combination of immunohistochemical staining and sequence analysis. TP53 encodes a protein (p53) that acts as a central mediator of the cellular response to both genotoxic and non-genotoxic stress. Under conditions of cell stress, p53 activates a number of genes that induce growth arrest and activate DNA repair functions so that repair can occur before damaged DNA is replicated. Alternatively, if damage is severe, p53 can drive cells into apoptosis. Mutations that lead to loss of p53 function result in unrepaired genetic damage and increased chromosomal instability 20. For many tumor types, p53 mutation is an independent marker of poor prognosis 21. About 80% of TP53 gene mutations are missense mutations that result in single amino acid substitutions in the p53 protein. The mutant protein accumulates in the cell due to delayed/impaired degradation, and immunostains of tumors with missense mutations typically show strong and diffuse nuclear accumulation of p53 protein. Most of the remaining TP53 mutations are frameshift or nonsense, and result either in absent or truncated versions of the protein. Tumors with these mutations usually lack detectable expression of p53 protein. Roughly 80% of TP53 mutations occur in exons 5 through 8, which encode p53’s functionally crucial DNA binding domain. It is this portion of the gene that is usually examined in routine mutational analyses. Tumors lacking mutations in exons 5–8 could show overexpression of p53 consistent with a presumptive missense mutation outside of the region sequenced.
Based on a combination of immunostaining and sequencing of exons 5–8, documented or presumptive TP53 mutations were identified in roughly half of the ovarian endometrioid tumors analyzed for mutations in the Wnt and PI3K/Akt pathways. TP53 mutations correlated negatively with Wnt and PI3K/Akt pathway defects, and not surprisingly, were observed primarily in high-grade, high-stage tumors. When endometrioid adenocarcinomas profiled in the gene expression analysis were annotated with mutational status of TP53 (Figure 2G), it was discovered that endometrioid tumors with mutant p53 were mostly the ones with gene expression overlapping that of the serous carcinomas. Hence, the molecular findings support the subdivision of ovarian endometrioid adenocarcinomas into two “molecular” subgroups, which correlate with tumor grade and stage – and by extension, clinical outcome. Low-grade (FIGO grade 1) tumors are characterized by mutations that deregulate the canonical Wnt/β-cat and PI3K/Pten signaling pathways and typically lack TP53 mutations. High-grade (FIGO grade 2 and 3) tumors often harbor mutations of TP53 and lack Wnt/β-cat or PI3K/Pten signaling pathway defects. Whether some of the FIGO grade 3 endometrioid tumors in this series would have been more appropriately classified as high grade serous carcinomas remains unresolved. Overlap of both morphological and molecular features between high-grade endometrioid and high-grade serous carcinomas has led some pathologists to default the vast majority of gland forming or near-solid cytologically high-grade ovarian carcinomas to the serous category, to the degree that “true” high-grade endometrioid carcinomas are considered by some to be rare 22.
NEW CONCEPTS OF OVARIAN TUMOR CLASSIFICATION
This “two pathway” scheme for ovarian endometrioid carcinoma pathogenesis is reminiscent of one previously proposed for ovarian serous carcinomas 23,24. Low grade serous carcinomas typically show micropapillary architecture, and often arise in association with recognizable precursors – specifically, serous borderline tumors (serous tumors of low malignant potential; atypical proliferative serous tumors). The low grade serous carcinomas characteristically have mutations of KRAS or BRAF, but TP53 mutations are uncommon in these tumors. Mutations of KRAS or BRAF lead to constitutive activation of the MAPK (mitogen-activated protein kinase) signaling pathway. MAP kinases are serine/threonine-specific protein kinases that respond to extracellular stimuli (mitogens) and regulate various cellular activities, such as gene expression, mitosis, differentiation, and cell survival/apoptosis. Although the low grade serous carcinomas tend to behave in a more indolent fashion than their high grade counterparts, some investigators have noted poor response of low-grade serous carcinomas to platinum-based therapeutic regimens 25. The great majority of serous carcinomas are high-grade and precursor lesions for these tumors remain poorly defined. Possible sites of origin include the ovarian surface epithelium, surface epithelial inclusion cysts, and the distal fallopian tube. High grade serous carcinomas have a high prevalence of TP53 gene mutations, while mutations of KRAS or BRAF are rare.
Collectively, studies of the type described above have led to the proposal of a new model for classifying ovarian carcinomas - in which the surface epithelial tumors can be divided into two broad categories designated Type I and Type II tumors based on their pattern of tumor progression and molecular genetic changes 26,27. Importantly, in this model, Type I and Type II refer to tumorigenic pathways and are not specific histopathologic diagnostic terms. Type I tumors include low-grade serous carcinoma, low-grade endometrioid carcinoma, mucinous carcinoma, and a subset of clear cell carcinomas, which develop in a stepwise fashion from well-recognized precursors, in most cases, borderline tumors. The borderline tumors, in turn, appear to develop from the ovarian surface epithelium or inclusion cysts in the case of serous and mucinous tumors and from endometriosis in the case of endometrioid and clear cell tumors. Most Type I tumors are slow growing as evidenced by the observation that they are generally large and often confined to the ovary at diagnosis. In contrast, the Type II tumors are high-grade and almost always have spread beyond the ovaries at presentation. Type II carcinomas include high-grade serous carcinoma, high-grade endometrioid carcinoma, undifferentiated carcinoma, probably some clear cell carcinomas, and malignant mixed mesodermal tumor (carcinosarcoma). Other than their association with endometriosis (in keeping with the Type I pathway), the clinicopathologic and molecular features allowing distinction of Type I from Type II clear cell carcinomas are yet to be defined. Type II carcinomas presumably evolve rapidly, disseminate early in their clinical course and are highly aggressive. In contrast to Type I tumors, Type II tumors are rarely associated with morphologically recognizable precursor lesions; however, Type II tumors may arise from “dysplasia” in inclusion cysts or serous intraepithelial carcinoma in the fallopian tubes 28–30. These precursor lesions may be difficult to recognize because they presumably undergo rapid transit from the occult lesion to a clinically diagnosed carcinoma. Type I and Type II tumors have very different molecular profiles. Chromosomal instability levels, as reflected by genome-wide changes in DNA copy number, are much higher in Type II tumors than in Type I tumors. Type I tumors often harbor somatic mutations of genes encoding protein kinases including KRAS, BRAF, PIK3CA and ERRB2, and other signaling molecules including CTNNB1 and PTEN. In contrast, Type II tumors generally lack these mutations but are characterized by a high frequency of TP53 mutations which are rare in Type I tumors.
The division of ovarian cancer into two broad groups, Type I and Type II, continues to emphasize the heterogeneity of ovarian cancers, but also provides a morphological and molecular framework for future studies aimed at improving our understanding of ovarian cancer pathogenesis and developing more effective strategies for their early detection. Previous attempts to improve early diagnosis of ovarian cancer were based on the assumption that ovarian cancer represents one disease when, from a pathogenetic view, it is at least two - with each having different implications for early detection. Current strategies are largely aimed at detecting low stage tumors. These strategies are most suitable for detecting tumors belonging to the Type I group, which often present as large tumor masses without dissemination at the time of diagnosis. A more useful endpoint for early detection of Type 2 ovarian carcinomas may be low tumor volume rather than low tumor stage. Importantly, it is well recognized that the most important prognostic indicator for Type 2 tumors is not stage at diagnosis, but the volume of residual disease following cytoreductive surgery 31,32.
The studies described above should prompt practicing pathologists to strongly consider the merits of a two-grade system for assigning tumor grade to ovarian carcinomas, rather than the traditional three-grade system used for most tumors. Indeed, two-tiered systems for grading serous carcinoma, the most common type of ovarian carcinoma, have already been proposed 33,34. Although uncertainties remain regarding the specific criteria that should be used to distinguish Type 1 from Type 2 ovarian carcinomas, careful analysis of both morphological and molecular features should help in determining which criteria are most appropriate.
THE NEXT STEP: MOUSE MODELS TO TEST NEW APPROACHES TO OVARIAN CANCER THERAPY
As described above, specific histologic subtypes of ovarian carcinomas have characteristic signaling pathway defects. For example, there is a high likelihood that a low grade endometrioid adenocarcinoma will have deregulated Wnt and/or PI3K/Akt signaling. Similarly, low grade (micropapillary) serous carcinomas are highly likely to have deregulated MAPK signaling. This molecular information may prove useful for designing new treatment regimens that target specific molecular defects in a given patient’s tumor cells and offer the means with which to improve upon or eventually replace currently available treatment modalities. Already, many drugs have been developed that target components of the PI3K/Akt signaling pathway, including some that have been tested in clinical trials. However, getting a new drug from the bench to the bedside is a complex and expensive undertaking and there are limited numbers of patients willing and able to participate in clinical trials aimed at testing their safety and efficacy. For many in the cancer research community, a key focus has been to develop animal models in which pre-clinical testing of drug dose, schedule, and combination can be used to help design clinical trials with greatest chance of success in patients.
Historically, most animal models of ovarian cancer were based on xenografting human ovarian cancer cells into immunodeficient mice. Xenograft models have limitations, including incomplete recapitulation of tumor-host interactions and inability to replicate early stages of tumor development. Recently described genetically engineered mouse models of ovarian cancer appear to overcome some weaknesses of the xenograft models, as tumors arise in the appropriate location in immunologically intact animals and more closely mimic the behavior of human ovarian cancers. In light of the data identifying frequent co-occurrence of canonical Wnt and PI3K/Akt signaling defects in a subset of human ovarian endometrioid adenocarcinomas, Wu and colleagues attempted to develop a mouse model of endometrioid adenocarcinoma by inactivating the Pten and Apc tumor suppressor genes (and hence deregulating these two signaling pathways) in the murine ovarian surface epithelium 10. The “Cre/lox” system was used to conditionally inactivate the two genes. The Cre/lox system is a genetic tool that can be used to control site-specific recombination events in genomic DNA. The system allows researchers to manipulate genetically modified mice to delete undesired DNA sequences and, hence, alter expression and function of targeted genes. In the Cre/lox system, a site-specific DNA recombinase called Cre catalyzes recombination of DNA between specific sites in a DNA molecule. These sites, known as loxP sequences, contain specific binding sites for Cre that surround a directional core sequence where recombination can occur. When Cre recombinase is expressed in cells with strategically engineered loxP sites, DNA sequences between the loxP sites are excised, and the two DNA strands are rejoined.
In order to deregulate PI3K/Akt and canonical Wnt signaling in the mouse ovarian surface epithelium, genetically engineered mice with loxP sites in selected introns of the Pten and Apc genes were crossbred so that both copies of both genes contained the appropriate loxP sites. Cre was delivered to the ovarian surface epithelium by injecting the ovarian bursa with a replication deficient adenovirus expressing Cre recombinase.
The injected ovaries uniformly and rapidly developed tumors with similar morphology to human ovarian endometrioid cancers. Tumors were often accompanied by hemorrhagic ascites and metastases to the peritoneal surfaces. Disease progression was rapid, with all mice dead of disease within 19 weeks after injection of AdCre. In addition, the mouse tumors exhibited gene expression patterns similar to their human tumor counterparts with comparable signaling pathway defects. When tumor-bearing mice were treated with Rapamycin, a drug that inhibits a downstream effector of Akt called mTOR, growth inhibition of the tumors was observed. The mice have been further engineered to allow use of bioluminescence imaging to non-invasively monitor tumor growth in living animals. It is expected that mouse models such as these will yield new insights into ovarian cancer pathogenesis and prove useful for pre-clinical testing of novel therapeutics that target specific molecular defects in ovarian tumor cells.
CONCLUSION
Traditional morphology-based classification of ovarian carcinomas by surgical pathologists has provided an indispensable guide to understanding the molecular pathogenesis of ovarian carcinomas. Molecular pathology has returned the favor by providing diagnostic pathologists a framework with which to improve ovarian tumor classification in a fashion that will allow us to focus on categories that more effectively convey information about predicted behavior and ultimately, response to therapy.
ACKNOWLEDGMENTS
I wish to thank Dr. Daniel Visscher for providing editorial assistance during preparation of this manuscript. Studies described herein were supported by grants from the National Institutes of Health/National Cancer Institute (RO1 CA94172) and the Department of Defense Ovarian Cancer Research Program (W81XWH-08-1-0453 and W81WH-04-1-0211).
Footnotes
Presented in part at the New Frontiers in Pathology: An Update for Practicing Pathologists meeting, Ann Arbor, Michigan, September 18, 2008.
REFERENCE LIST
- 1.Markman M. Pharmaceutical management of ovarian cancer: current status. Drugs. 2008;68(6):771–789. doi: 10.2165/00003495-200868060-00004. [DOI] [PubMed] [Google Scholar]
- 2.NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network, Inc. 2006 v.1. 2006 [Google Scholar]
- 3.Cho KR, Shih IM. Ovarian Cancer. Annu Rev Pathol Mech Dis. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanseverino F, D'Andrilli G, Petraglia F, Giordano A. Molecular pathology of ovarian cancer. Anal Quant Cytol Histol. 2005;27(3):121–124. [PubMed] [Google Scholar]
- 5.Bell DA. Origins and molecular pathology of ovarian cancer. Mod Pathol. 2005;18 Suppl 2:S19–S32. doi: 10.1038/modpathol.3800306. [DOI] [PubMed] [Google Scholar]
- 6.Singer G, Stohr R, Cope L, et al. Patterns of p53 mutations separate ovarian serous borderline tumors and low- and high-grade carcinomas and provide support for a new model of ovarian carcinogenesis: a mutational analysis with immunohistochemical correlation. Am J Surg Pathol. 2005;29(2):218–224. doi: 10.1097/01.pas.0000146025.91953.8d. [DOI] [PubMed] [Google Scholar]
- 7.Salani R, Kurman RJ, Giuntoli R, 2nd, et al. Assessment of TP53 mutation using purified tissue samples of ovarian serous carcinomas reveals a higher mutation rate than previously reported and does not correlate with drug resistance. Int J Gynecol Cancer. 2008;18(3):487–491. doi: 10.1111/j.1525-1438.2007.01039.x. [DOI] [PubMed] [Google Scholar]
- 8.Enomoto T, Weghorst CM, Inoue M, Tanizawa O, Rice JM. K-ras activation occurs frequently in mucinous adenocarcinomas and rarely in other common epithelial tumors of the human ovary. Am J Pathol. 1991;139(4):777–785. [PMC free article] [PubMed] [Google Scholar]
- 9.Gemignani ML, Schlaerth AC, Bogomolniy F, et al. Role of KRAS and BRAF gene mutations in mucinous ovarian carcinoma. Gynecol Oncol. 2003;90(2):378–381. doi: 10.1016/s0090-8258(03)00264-6. [DOI] [PubMed] [Google Scholar]
- 10.Wu R, Hendrix-Lucas N, Kuick R, et al. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/B-catanin and PI3K/Pten signaling pathways. Cancer Cell. 2007;11(4):321–333. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Wright K, Wilson P, Morland S, et al. Beta-catenin mutation and expression analysis in ovarian cancer: exon 3 mutations and nuclear translocation in 16% of endometrioid tumours. Int J Cancer. 1999;82(5):625–629. doi: 10.1002/(sici)1097-0215(19990827)82:5<625::aid-ijc1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Kuo KT, Mao TL, Jones S, et al. Frequent Activating Mutations of PIK3CA in Ovarian Clear Cell Carcinoma. Am J Pathol. 2009 May;174(5):1597–1601. doi: 10.2353/ajpath.2009.081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz DR, Kardia SL, Shedden KA, et al. Gene expression in ovarian cancer reflects both morphology and biological behavior, distinguishing clear cell from other poor-prognosis ovarian carcinomas. Cancer Res. 2002;62(16):4722–4729. [PubMed] [Google Scholar]
- 14.Miller JR. The Wnts. Genome Biol. 2002;3 doi: 10.1186/gb-2001-3-1-reviews3001. REVIEWS3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 16.Dale TC. Signal transduction by the Wnt family of ligands. Biochem J. 1998;329(pt2):209–223. doi: 10.1042/bj3290209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24(50):7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 18.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22(14):2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 19.Kolasa IK, Rembiszewska A, Janiec-Jankowska A, et al. PTEN mutation, expression and LOH at its locus in ovarian carcinomas. Relation to TP53, K-RAS and BRCA1 mutations. Gynecol Oncol. 2006;103(2):692–697. doi: 10.1016/j.ygyno.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Tomasini R, Mak TW, Melino G. The impact of p53 and p73 on aneuploidy and cancer. Trends Cell Biol. 2008;18(5):244–252. doi: 10.1016/j.tcb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Petitjean A, Achatz MI, Borresen-Dale AL, Hainaut P, Olivier M. TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene. 2007;26(15):2157–2165. doi: 10.1038/sj.onc.1210302. [DOI] [PubMed] [Google Scholar]
- 22.McCluggage WG. My approach to and thoughts on the typing of ovarian carcinomas. J Clin Pathol. 2008;61(17):152–163. doi: 10.1136/jcp.2007.049478. [DOI] [PubMed] [Google Scholar]
- 23.Care A, Felicetti F, Meccia E, et al. HOXB7: a key factor for tumor-associated angiogenic switch. Cancer Res. 2001;61(17):6532–6539. [PubMed] [Google Scholar]
- 24.Singer G, Kurman RJ, Chang HW, Cho SK, Shih IeM. Diverse tumorigenic pathways in ovarian serous carcinoma. Am J Pathol. 2002;160(4):1223–1228. doi: 10.1016/s0002-9440(10)62549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith-Sehdev AE, Sehdev PS, Kurman RJ. Noninvasive and invasive micropapillary (low-grade) serous carcinoma of the ovary: a clinicopathologic analysis of 135 cases. Am J Surg Pathol. 2003;27(6):725–736. doi: 10.1097/00000478-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Shih IeM, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164(5):1511–1518. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shih IeM, Kurman RJ. Molecular pathogenesis of ovarian borderline tumors: new insights and old challenges. Clin Cancer Res. 2005;11(20):7273–7279. doi: 10.1158/1078-0432.CCR-05-0755. [DOI] [PubMed] [Google Scholar]
- 28.Lee Y, Miron A, Drapkin R, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211(1):26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 29.Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19(1):3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- 30.Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31(2):161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs IJ, Skates SJ, MacDonald N, et al. Screening for ovarian cancer: a pilot randomised controlled trial. Lancet. 1999;353(9160):1207–1210. doi: 10.1016/S0140-6736(98)10261-1. [DOI] [PubMed] [Google Scholar]
- 32.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 33.Malpica A, Deavers MT, Lu K, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28(4):496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Seidman JD, Horkayne-Szakaly I, Cosin JA, et al. Testing of two binary grading systems for FIGO stage III serous carcinoma of the ovary and peritoneum. Gynecol Oncol. 2006;103(2):703–708. doi: 10.1016/j.ygyno.2006.05.030. [DOI] [PubMed] [Google Scholar]