Abstract
Persons with severe chronic obstructive pulmonary disease (COPD) and similar levels of forced expiratory volume in 1 second (FEV1), exercise capacity, and dyspnea have a wide range of health-related quality of life (HRQL). We identified the independent determinants of HRQL in persons with COPD. Comprehensive assessments of physiological, psychosocial, and clinical variables from the National Emphysema Treatment Trial were used. HRQL was assessed by the Medical Outcomes Study 36-Item Short Form Physical Component Summary (PCS) and Mental Component Summary (MCS) scores and the St. George’s Respiratory Questionnaire total score (SGRQ-TS). In multivariate linear regression models, exercise capacity, dyspnea, age, single-breath diffusing capacity of the lung for carbon monoxide percent predicted, and self-report of being disabled were significant determinants of PCS score. Dyspnea, depression, antidepressant use, daytime sleepiness, and education were significant determinants of MCS score. Prior participation in pulmonary rehabilitation, supplemental oxygen use, and oral corticosteroid use were significant determinants of SGRQ-TS. Although FEV1, 6-minute walk test distance, and dyspnea significantly correlated with HRQL, their effects on HRQL were reduced when other variables were considered. Greater exercise capacity, prior participation in pulmonary rehabilitation, and use of supplemental oxygen were significantly associated with better HRQL. Self-perception of being disabled, depression, dyspnea, oral corticosteroid use, and daytime sleepiness were associated with worse HRQL. To optimize HRQL, clinicians should pay attention to a number of clinical and physiological factors.
Keywords: chronic obstructive pulmonary disease, COPD, disability, dyspnea, emphysema, exercise capacity, health-related quality of life, multivariate regression models, National Emphysema Treatment Trial, pulmonary rehabilitation
INTRODUCTION
Health-related quality of life (HRQL) is patient-centered and integrates the complex physiological and psychosocial effects of chronic obstructive pulmonary disease (COPD) on a person’s life [1]. HRQL has been shown to predict healthcare resource utilization and mortality in COPD [2–3]. Currently, the medical management of severe COPD emphasizes treating airflow obstruction with pharmacologic agents and treating exercise intolerance and dyspnea with pulmonary rehabilitation, with the ultimate goal of optimizing HRQL [4]. However, relationships among HRQL, exercise capacity, lung function, and dyspnea are complex. Persons who have similar reductions in forced expiratory volume in 1 second (FEV1) and exercise capacity and similar levels of dyspnea have a wide range of HRQL, suggesting that other variables contribute to HRQL [5–14].
The National Emphysema Treatment Trial (NETT) was a multicenter randomized clinical trial that compared outcomes of lung-volume-reduction surgery (LVRS) to medical therapy among patients with severe airflow obstruction [15–18]. Now that it is completed, the NETT provides a large and comprehensive database of standardized assessments of HRQL, exercise capacity, lung function, dyspnea, psychosocial status, and demographic information in persons with Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages III and IV COPD [4]. The NETT database provides the unique opportunity to examine the many physiological, psychosocial, demographic, and clinical variables that may affect HRQL in severe COPD.
Kaplan et al. previously examined univariate correlations between physiological measures and HRQL in baseline data of the 1,218 subjects randomized in the NETT [14]. They found that HRQL, as measured by the Medical Outcomes Study 36-Item Short Form (MOS SF-36) Physical Component Summary (PCS) and Mental Component Summary (MCS) scores, St. George’s Respiratory Questionnaire total score (SGRQ-TS), and Self-Administered Quality-of-Well-Being Scale (QWB-SA) total score, was significantly correlated with FEV1 and 6-minute walk test (6MWT) distance. The simple correlations did not examine psychological, demographic, and clinical variables that may contribute to HRQL, and the authors did not “adjust for” multiple explanatory variables in the same model. In this article, we extend this analysis to model the complex nature of HRQL and identify its independent predictors. Unlike the previous analysis, we (1) considered psychosocial, demographic, and clinical variables in addition to the physiological variables; (2) used multivariate linear regression models; and (3) used data from 1,621 participants screened for the NETT. We hypothesized that when considered together with psychosocial, demographic, and clinical variables in multivariate models FEV1, 6MWT distance, and dyspnea would have less impact on HRQL in severe COPD than in univariate models.
METHODS
Patient Selection
The inclusion criteria for the NETT [18] are reviewed briefly and include (1) age ≥40 years, (2) smoking history ≥10 pack-years, (3) postbronchodilator FEV1/forced vital capacity ratio <0.70, and (4) postbronchodilator FEV1 percent predicted ≤40 percent. All patients provided written informed consent, and the study was approved by the institutional review board at each clinic participating in the NETT. Since the purpose of our investigation was to assess determinants of HRQL in COPD, we did not limit the analysis to persons who participated in the trial and included data from subjects screened for the NETT. Persons with a significant bronchodilator (BD) response, as evidenced by an increase in postbronchodilator FEV1 of ≥200 mL, were excluded from participation in the NETT. Therefore, subjects with the presence of BD reversibility were excluded from these retrospective analyses. Although persons with COPD and a BD response have been shown to have better HRQL as measured by the SGRQ-TS [19], it is unlikely that the presence or absence of a BD response would influence our analyses. Subjects with missing HRQL scores were also excluded from these analyses. Our inclusion criteria were met by 1,657 subjects, and 36 of these subjects were further excluded because they were missing 4 of the variables of interest. For 302 subjects (missing 3 variables), missing values were imputed using the mean value (if normally distributed) or the mode value (if skewed) from the available data. Results of regression analyses were similar in imputed and unimputed data sets; we present the results from analyses of the imputed data set to maximize the ability to consider multivariate models. In summary, our analyses include data from 1,621 subjects—1,036 who were randomized in the NETT and 585 who did not meet eligibility criteria for randomization but for whom entry data were collected.
Health-Related Quality of Life
HRQL was assessed with the MOS SF-36, a general measure of health status that includes eight health concepts: physical functioning, role-physical, bodily pain, general health perceptions, vitality, social functioning, role-emotional, and mental health [20]. We used the PCS and MCS scores from the MOS SF-36. Higher scores indicate better health status. The SGRQ assessed respiratory-specific HRQL [21]. The SGRQ is a standardized 50-item instrument that assesses symptoms, activity, and effects on daily life. The SGRQ-TS is calculated from the three component scores. We used SGRQ-TSs, with lower scores indicating better health status. In addition, we used the QWB-SA as another measure of general HRQL [22]. The QWB-SA combines preference-weighted values for symptoms and functioning.
Physiological Testing
Exercise capacity was assessed with the 6MWT, and we used maximal distance walked in these analyses [23]. Subjects underwent cardiopulmonary exercise testing (CPET) with cycle ergometry, supplemented with oxygen if needed, and we used maximum workload, measured in watts, in these analyses [15]. Pulmonary function was assessed with the postbronchodilator FEV1 percent predicted, residual volume (RV)/total lung capacity (TLC) ratio, and the single-breath diffusing capacity of the lung for carbon monoxide (DLCO) percent predicted [15].
Clinical Assessment
Data on demographics, medication use, and clinical history were available (Table 1). Subjects had been asked, “Are you currently disabled?” and “How often are you troubled by sleepiness in the daytime or during working hours?” Prior participation in a pulmonary rehabilitation program and alcohol use had been assessed by medical history. Dyspnea was quantified with the University of California, San Diego Shortness of Breath Questionnaire (UCSD SOBQ) [24]. We used the total score, with lower scores indicating less dyspnea. We also analyzed dyspnea scores assessed with the modified Borg score for breathlessness (Borg) at the completion of the 6MWT and CPET [25]. Depression assessment was made with the Beck Depression Inventory (Beck) [26]. The radiographic severity of emphysema was determined from high-resolution computed tomography scans of the chest [15]. Left ventricular ejection fraction was assessed by echocardiography, and the partial pressure of arterial carbon dioxide was assessed by arterial blood gas analysis.
Table 1.
Subject characteristics and candidate variables for multivariate regression models (n = 1,621).
| Variable | Mean ± SD or n (%) |
|---|---|
| Age | 66 ± 6 |
| Sex: Male | 976 (60) |
| Race: White | 1,523 (94) |
| Marital Status: Married | 1,039 (64) |
| Education: Completed High School or Higher | 1,301 (80) |
| BMI | 25 ± 4 |
| Disabled | 1,101 (68) |
| Prior Pulmonary Rehabilitation | 1,013 (62) |
| Daytime Sleepiness | 888 (55) |
| Cigarette Smoking (pack-years) | 64 ± 31 |
| Alcohol Use: Never | 628 (39) |
| Current Oral Corticosteroid Use | 535 (33) |
| Antidepressant Use | 279 (17) |
| Antianxiety Medication Use | 190 (12) |
| Oxygen Use | 1,226 (76) |
| Beck Total Score | 10 ± 6 |
| FEV1 % Predicted, After BD | 26 ± 6 |
| RV/TLC Ratio, After BD | 0.66 ± 0.08 |
| DLCO, % Predicted | 27 ± 10 |
| PaCO2 | 43 ± 6 |
| CT Emphysema Score | 16 ± 4 |
| LVEF, ≥45% | 1,566 (97) |
| MOS SF-36 PCS Score (range 8–56) | 28 ± 7 |
| MOS SF-36 MCS Score (range 12–76) | 52 ± 10 |
| SGRQ-TS (range 17–96) | 57 ± 13 |
| 6MWT Distance (m) (range 35–750) | 335 ± 98 |
| Maximal CPET Workload (W) | 33 ± 20 |
| UCSD SOBQ Total Score (range 8–120) | 66 ± 19 |
| 6MWT Borg | 5 ± 2 |
| CPET Borg | 6 ± 2 |
6MWT = 6-minute walk test; BD = bronchodilator; Beck = Beck Depression Inventory; BMI = body mass index; Borg = Borg score for breathlessness; CPET = cardiopulmonary exercise testing; CT = computed tomography; DLCO = single-breath diffusing capacity of the lung for carbon monoxide; FEV1 = forced expiratory volume in 1 second; LVEF = left ventricular ejection fraction; MCS = Mental Component Summary; MOS SF-36 = Medical Outcomes Study 36-Item Short Form; PaCO2 = partial pressure of arterial carbon dioxide; PCS = Physical Component Summary; RV = residual volume; SD = standard deviation; SGRQ-TS = St. George’s Respiratory Questionnaire total score; TLC = total lung capacity; UCSD SOBQ = University of California, San Diego Shortness of Breath Questionnaire.
Statistical Analysis
We assessed measures of exercise capacity, dyspnea, and lung function as the main candidate determinants of HRQL. In addition, covariates (Table 1) that may affect HRQL were selected a priori based on our clinical experience caring for persons with severe COPD. All analyses were performed with the SAS statistical software package (version 9.1, SAS Institute, Inc; Cary, North Carolina). Since HRQL scores are continuous data, linear regression methods (PROC GLM) were used to assess the relationship of each independent variable of interest with PCS score, MCS score, and SGRQ-TS [27]. In univariate models, a single independent variable was assessed and the “unadjusted coefficient” indicates the impact of the independent variable on the HRQL outcome without other potential explanatory variables in the regression model. Independent variables, which were significant at the 0.10 level in the univariate analyses, were assessed together as a single block in multivariate regression models, and variables significant below the 0.05 level were retained in the multivariate models. Therefore, in the multivariate models, the terminology “adjusted coefficient” indicates the effect of each independent variable on HRQL in the context of (or adjusting for) all other independent variables. To check for collinearity, we assessed correlations between the continuous variables and HRQL with Pearson correlation coefficients. Model diagnostics revealed no outliers or influential points, and linear regression model assumptions were examined and satisfied. Residual plots were examined for goodness of fit.
RESULTS
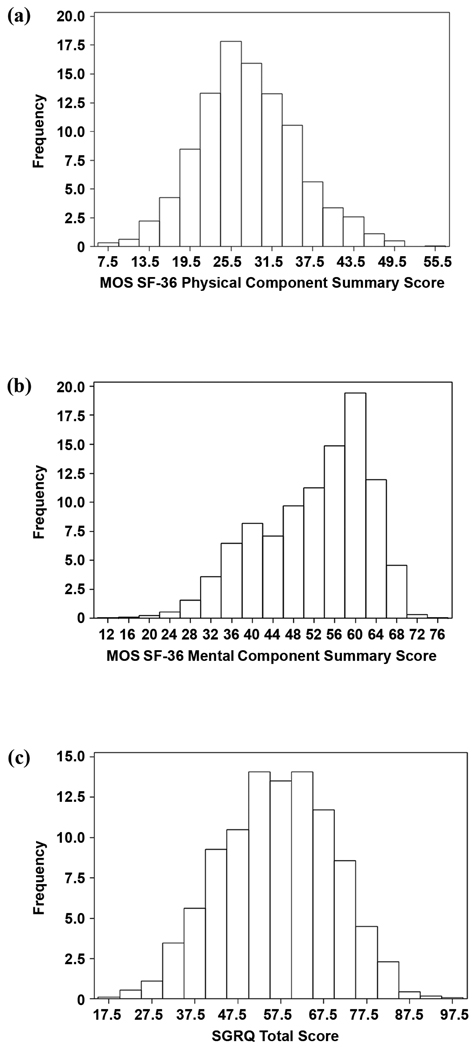
The 1,621 subjects, 60 percent of whom were male, had a mean age of 66 ± 6 years (all data are presented as mean ± standard deviation unless otherwise noted) (Table 1). Subjects had severe airways obstruction with a mean post bronchodilator FEV1 of 0.73 ± 0.22 L (26 ± 6% of predicted values). HRQL was reduced with mean PCS, MCS, and SGRQ-TS scores of 28 ± 7, 52 ± 10, and 57 ± 13, respectively. Exercise capacity was severely limited with a mean maximal distance walked on the 6MWT of 335 ± 98 m and a mean maximal workload achieved on cycle ergometry of 33 ± 20 W. Mean UCSD SOBQ total score was 66 ± 19. Lung function, dyspnea, psychosocial, demographic, and clinical variables are summarized in Table 1. Twenty-two percent of the subjects had at least mild depression defined as a Beck total score ≥14, and seventeen percent reported antidepressant use. Daytime sleepiness was reported by 55 percent of subjects. Sixty-eight percent of subjects had self-perception of being disabled. Although all subjects had severely reduced FEV1, a wide range of PCS, MCS, and SGRQ-TS scores was found (Figure).
Figure 1.
Distribution of health-related quality of life scores: (a) Medical Outcomes Study 36-Item Short Form (MOS SF-36) Physical Component Summary, (b) MOS SF-36 Mental Component Summary, and (c) St. George’s Respiratory Questionnaire (SGRQ) total score.
Univariate Models
In univariate regression models, all measures of exercise capacity, dyspnea, and lung function were significant determinants of PCS score and SGRQ-TS (Table 2). FEV1 percent predicted was not a significant determinant of MCS score, while all the other measures of exercise capacity, dyspnea, and lung function were significant determinants of MCS score. These results are similar to the previous relationships reported by Kaplan et al. in the persons randomized in the NETT [14]. UCSD SOBQ total score explained the greatest variance in HRQL, accounting for 22 percent of the variance in PCS score, 12 percent of the variance in MCS score, and 46 percent of the variance in SGRQ-TS. Age, oral corticosteroid use, alcohol use, self-report of being disabled, daytime sleepiness, antidepressant use, supplemental oxygen use, and Beck total score were significant determinants of all three measures of HRQL (Table 3). Being underweight or obese, compared with those who had a normal body mass index (BMI), was consistently associated with worse HRQL as measured by PCS, MCS, and SGRQ-TS (Table 3).
Table 2.
Univariate models* of exercise capacity, dyspnea, and lung function as determinants of health-related quality of life.
| Variable | MOS SF-36 PCS Score† |
MOS SF-36 MCS Score† |
SGRQ-TS‡ |
|||
|---|---|---|---|---|---|---|
| Unadjusted Coefficient (95% CI) |
p-Value | Unadjusted Coefficient (95% CI) |
p-Value | Unadjusted Coefficient (95% CI) |
p-Value | |
| 6MWT Distance | 0.02 | <0.001 | 0.02 | <0.001 | −0.04 | <0.001 |
| (0.01 to 0.02) | (0.01 to 0.02) | (−0.05 to −0.04) | ||||
| Maximal CPET Workload | 0.08 | <0.001 | 0.08 | <0.001 | −0.18 | <0.001 |
| (0.06 to 0.09) | (0.05 to 0.10) | (−0.21 to −0.15) | ||||
| UCSD SOBQ Total Score | −0.18 | <0.001 | −0.19 | <0.001 | 0.46 | <0.001 |
| (−0.19 to −0.16) | (−0.22 to −0.17) | (0.44 to 0.48) | ||||
| 6MWT Borg | −0.75 | <0.001 | −0.52 | <0.001 | 1.7 | <0.001 |
| (−0.92 to −0.58) | (−0.76 to −0.27) | (1.4 to 2.0) | ||||
| CPET Borg | −0.47 | <0.001 | −0.33 | 0.0045 | 1.1 | <0.001 |
| (−0.63 to −0.31) | (−0.56 to −0.10) | (0.80 to 1.4) | ||||
| FEV1 % Predicted, After BD | 0.14 | <0.001 | 0.02 | NS | −0.33 | <0.001 |
| (0.08 to 0.19) | (−0.06 to 0.10) | (−0.42 to −0.23) | ||||
| RV/TLC Ratio, After BD | −9.5 | <0.001 | −11 | <0.001 | 21 | <0.001 |
| (−14 to −5.1) | (−18 to −4.9) | (13 to 29) | ||||
| DLCO, % Predicted | 0.06 | 0.002 | 0.07 | 0.01 | −0.16 | <0.001 |
| (0.02 to 0.09) | (0.02 to 0.12) | (−0.22 to −0.09) | ||||
Example of univariate model: MOS SF-36 PCS Score = (0.02) × (6MWT Distance).
Higher score indicates better health status.
Lower score indicates better health status.
6MWT = 6-minute walk test; BD = bronchodilator; Borg = Borg score for breathlessness; CI = confidence interval; CPET = cardiopulmonary exercise testing; DLCO = single-breath diffusing capacity of the lung for carbon monoxide; FEV1 = forced expiratory volume in 1 second; MCS = Mental Component Summary; MOS SF-36 = Medical Outcomes Study 36-Item Short Form; NS = nonsignificant; PCS = Physical Component Summary; RV = residual volume; SGRQ-TS = St. George’s Respiratory Questionnaire total score; TLC = total lung capacity; UCSD SOBQ = University of California, San Diego Shortness of Breath Questionnaire.
Table 3.
Univariate models* of candidate determinants of Medical Outcomes Study 36-Item Short Form (MOS SF-36) Physical Component Summary (PCS), MOS SF-36 Mental Component Summary (MCS), and St. George’s Respiratory Questionnaire total score (SGRQ-TS).
| Variable | MOS SF-36 PCS Score† |
MOS SF-36 MCS Score† |
SGRQ-TS‡ |
|||
|---|---|---|---|---|---|---|
| Unadjusted Coefficient (95% CI) |
p-Value | Unadjusted Coefficient (95% CI) |
p-Value | Unadjusted Coefficient (95% CI) |
p-Value | |
| Age | 0.17 | <0.001 | 0.13 | 0.002 | −0.45 | <0.001 |
| (0.12 to 0.23) | (0.05 to 0.21) | (−0.55 to −0.35) | ||||
| Current Oral Corticosteroid Use | −2.0 | <0.001 | −1.4 | 0.009 | 5.2 | <0.001 |
| (ref = no use) | (−2.7 to −1.2) | (−2.5 to −0.36) | (3.8 to 6.5) | |||
| Alcohol Use | 2.1 | <0.001 | 1.8 | <0.001 | −4.0 | <0.001 |
| (ref = never use) | (1.3 to 2.8) | (0.74 to 2.8) | (−5.3 to −2.7) | |||
| Disabled | −3.00 | <0.001 | −1.8 | 0.001 | 6.8 | <0.001 |
| (ref = nondisabled) | (−3.75 to −2.25) | (−2.9 to −0.74) | (5.4 to 8.1) | |||
| Daytime Sleepiness | –1.1 | <0.001 | −3.3 | <0.001 | 4.0 | <0.001 |
| (ref = never sleepy during day) | (−1.8 to −0.40) | (−4.3 to −2.3) | (2.7 to 5.2) | |||
| Antidepressant Use | –1.0 | 0.04 | −4.6 | <0.001 | 4.8 | <0.001 |
| (ref = no use) | (−1.9 to −0.06) | (−6.0 to −3.3) | (3.2 to 6.5) | |||
| Oxygen Use | −1.6 | <0.001 | –1.3 | 0.04 | 3.2 | <0.001 |
| (ref = no use) | (−2.4 to −0.78) | (−2.4 to −0.072) | (1.8 to 4.7) | |||
| Beck Total Score | −0.22 | <0.001 | −0.97 | <0.001 | 1.01 | <0.001 |
| (−0.28 to −0.17) | (−1.04 to −0.90) | (0.92 to 1.1) | ||||
| BMI (ref = BMI 18–25, n = 836) | ||||||
| BMI <18 | −1.8 | 0.06 | −2.3 | 0.09 | 4.2 | 0.02 |
| (Underweight, n = 61) | (−3.8 to 0.05) | (−5.1 to 0.39) | (0.80 to 7.55) | |||
| BMI 25–30 | 0.02 | NS | 1.1 | 0.05 | −0.14 | NS |
| (Overweight, n = 547) | (−0.77 to 0.81) | (−0.011 to 2.2) | (−1.5 to 1.3) | |||
| BMI >30 | −1.7 | 0.004 | −0.91 | NS | 2.8 | 0.01 |
| (Obese, n = 177) | (−2.9 to −0.55) | (−2.6 to 0.79) | (0.67 to 4.9) | |||
| Sex | −0.24 | NS | −1.4 | 0.008 | 0.46 | NS |
| (ref = male) | (−0.96 to 0.49) | (−2.5 to −0.37) | (−0.83 to 1.8) | |||
| Education | 0.51 | NS | 4.6 | <0.001 | −4.7 | <0.001 |
| (ref = not high school graduate) | (−0.39 to 1.4) | (3.3 to 5.8) | (−6.3 to −3.2) | |||
| Race | −0.11 | NS | −1.3 | NS | −0.67 | NS |
| (ref = white) | (−1.61 to 1.39) | (−3.4 to 0.84) | (−3.3 to 2.0) | |||
| Marital Status | −0.16 | NS | −1.2 | 0.03 | 1.2 | 0.07 |
| (ref = married) | (−0.90 to 0.58) | (−2.2 to −0.09) | (−0.086 to 2.6) | |||
| Prior Rehabilitation | 0.17 | NS | 0.19 | NS | −0.36 | NS |
| (ref = no rehabilitation) | (−0.57 to 0.91) | (−0.87 to 1.24) | (−1.7 to 0.95) | |||
| Cigarette Smoking | −0.001 | NS | −0.021 | 0.02 | 0.028 | 0.008 |
| (−0.013 to 0.011) | (−0.037 to −0.004) | (0.007 to 0.049) | ||||
| Antianxiety Medication Use | −0.75 | NS | −3.3 | <0.001 | 4.08 | <0.001 |
| (ref = no use) | (−1.86 to 0.36) | (−4.9 to −1.7) | (2.12 to 6.04) | |||
| LVEF | −0.80 | NS | 3.2 | 0.03 | −0.33 | NS |
| (ref = 45%) | (−2.77 to 1.17) | (0.38 to 6.02) | (−3.8 to 3.2) | |||
| PaCO2 | −0.02 | NS | −0.099 | 0.03 | 0.076 | NS |
| (−0.08 to 0.04) | (−0.19 to −0.008) | (−0.036 to 0.19) | ||||
| CT Emphysema Score | −0.005 | NS | 0.039 | NS | 0.12 | NS |
| (−0.095 to 0.085) | (−0.09 to 0.17) | (−0.04 to 0.28) | ||||
Example of univariate model: MOS SF-36 PCS Score = (0.17) × (Age).
Higher score indicates better health status.
Lower score indicates better health status.
Beck = Beck Depression Inventory, BMI = body mass index, CI = confidence interval, CT = computed tomography, LVEF = left ventricular ejection fraction, NS = nonsignificant, PaCO2 = partial pressure of arterial carbon dioxide, ref = reference.
Collinearity between variables was assessed. Weak correlations were found among the continuous variables that were significant determinants of PCS, MCS, or SGRQ-TS in the univariate regression models and that were later considered in multivariate models. Pairs of variables with Pearson correlation coefficients ≥0.50 were 6MWT distance and maximal CPET workload (r = 0.57), RV/TLC and maximal CPET workload (r = −0.53), and RV/TLC and FEV1 percent predicted (r = −0.50).
Multivariate Models
In multivariate regression models, 6MWT distance, self-report of being disabled, UCSD SOBQ total score, 6MWT Borg, age, and DLCO percent predicted were significant determinants of PCS score (model R2 = 0.24) (Table 4). Beck total score, antidepressant use, UCSD SOBQ total score, education, and daytime sleepiness were significant determinants of MCS score (model R2 = 0.35) (Table 4). The determinants of PCS and MCS scores were essentially the same as those for SGRQ-TS, except that education contributed to MCS score, while race contributed to SGRQ-TS (Table 4). In addition, prior participation in pulmonary rehabilitation and supplemental oxygen use were independently associated with better SGRQ-TS, while current corticosteroid use was independently associated with worse SGRQ-TS (Table 4). We also examined the performance of the significant determinants of PCS and MCS score in multivariate models using the QWB-SA total score as the outcome. Table 5 shows that all the determinants except age were significant or trended toward significance in determining the QWB-SA total score.
Table 4.
Significant determinants of Medical Outcomes Study 36-Item Short Form (MOS SF-36) Physical Component Summary (PCS) score (model R2 = 0.24 ), MOS SF-36 Mental Component Summary (MCS) score (model R2 = 0.35), and St. George’s Respiratory Questionnaire total score (SGRQ-TS) (model R2 = 0.55) in multivariate models* (n = 1,621).
| Determinant | MOS SF-36 PCS Score† |
MOS SF-36 MCS Score† |
SGRQ-TS‡ |
|||
|---|---|---|---|---|---|---|
| Adjusted Coefficient (95% CI) |
p-Value | Adjusted Coefficient (95% CI) |
p-Value | Adjusted Coefficient (95% CI) |
p-Value | |
| 6MWT Distance | 0.0042 | 0.02 | 0.0025 | 0.29 | −0.006 | 0.02 |
| (0.0005 to 0.0080) | (−0.0022 to 0.0072) | (−0.011 to −0.001) | ||||
| UCSD SOBQ Total Score | −0.16 | <0.001 | −0.07 | <0.001 | 0.37 | <0.001 |
| (−0.18 to −0.14) | (−0.10 to −0.04) | (0.34 to 0.39) | ||||
| 6MWT Borg | −0.22 | 0.007 | — | — | 0.24 | 0.03 |
| (−0.38 to −0.06) | (0.024 to 0.46) | |||||
| Age | 0.08 | 0.006 | — | — | −0.19 | <0.001 |
| (0.02 to 0.13) | (−0.27 to −0.12) | |||||
| Beck Total Score | — | — | –0.82 | <0.001 | 0.48 | <0.001 |
| (−0.89 to −0.74) | (0.40 to 0.55) | |||||
| Antidepressant Use | — | — | −1.6 | 0.004 | ||
| (ref = no use) | (−2.7 to −0.50) | |||||
| DLCO, % predicted | −0.05 | 0.008 | — | — | 0.07 | 0.008 |
| (−0.08 to −0.01) | (0.02 to 0.11) | |||||
| Education | — | — | 2.3 | <0.001 | — | — |
| (ref = not high school graduate) | (1.2 to 3.3) | |||||
| Daytime Sleepiness | — | — | −1.3 | 0.002 | 1.5 | <0.001 |
| (ref = never sleepy during day) | (−2.2 to −0.49) | (0.65 to 2.4) | ||||
| Disabled | −1.1 | 0.002 | — | — | 1.8 | <0.001 |
| (ref = nondisabled) | (−1.9 to −0.41) | (0.77 to 2.8) | ||||
| Race | — | — | — | — | −2.6 | 0.005 |
| (ref = white) | (−4.4 to −0.79) | |||||
| Current Oral Corticosteroid Use | — | — | — | — | 2.2 | <0.001 |
| (ref = no use) | (1.3 to 3.1) | |||||
| Oxygen Use | — | — | — | — | −1.4 | 0.01 |
| (ref = no use) | (−2.5 to −0.34) | |||||
| Prior Rehabilitation | — | — | — | — | −1.2 | 0.008 |
| (ref = no rehabilitation) | (−2.1 to −0.33) | |||||
Note: Dash indicates variable not included in the model.
Example of multivariate model: MOS SF-36 PCS score = ((0.0042) × 6MWT distance + (−0.16) UCSD SOBQ total score + (−0.22) 6MWT Borg + (0.08) × age + (−0.05) × DLCO % predicted + (−1.1) × (disabled).
Higher score indicates better health status.
Lower score indicates better health status.
6MWT = 6-minute walk test; Beck = Beck Depression Inventory; Borg = Borg score for breathlessness; CI = confidence interval; DLCO = single-breath diffusing capacity of the lung for carbon monoxide; ref = reference; UCSD SOBQ = University of California, San Diego Shortness of Breath Questionnaire.
Table 5.
Performance of significant determinants of Medical Outcomes Study 36-Item Short Form Physical Component Summary and Mental Component Summary scores in multivariate model with Self-Administered Quality-of-Well-Being Scale* score as outcome (n = 1,617; model R2 = 0.29).
| Determinant | Adjusted Coefficient (95% CI) | p-Value |
|---|---|---|
| 6MWT Distance | 0.00017 (0.0001 to 0.0002) | <0.001 |
| UCSD SOBQ Total Score | −0.0017 (−0.0020 to −0.0014) | <0.001 |
| 6MWT Borg | −0.0029 (−0.0055 to −0.0003) | 0.03 |
| Age | 0.000072 (−0.00082 to 0.00097) | NS |
| DLCO, % Predicted | −0.00054 (−0.0011 to 0.000022) | 0.06 |
| Disabled (ref = nondisabled) | −0.011 (−0.023 to 0.00068) | 0.06 |
| Beck Total Score | −0.0050 (−0.0060 to −0.0041) | <0.001 |
| Antidepressant Use (ref = no use) | −0.014 (−0.028 to −0.00024) | 0.05 |
| Education (ref = not high school graduate) | −0.012 (−0.025 to 0.00067) | 0.06 |
| Daytime Sleepiness (ref = never sleepy during day) | −0.023 (−0.033 to −0.012) | <0.001 |
Higher score indicates better health status.
6MWT = 6-minute walk test; Beck = Beck Depression Inventory; Borg = Borg score for breathlessness; CI = confidence interval; DLCO = single-breath diffusing capacity of the lung for carbon monoxide; NS = nonsignificant; ref = reference; UCSD SOBQ = University of California, San Diego Shortness of Breath Questionnaire.
In multivariate models, FEV1 percent predicted was not a significant determinant of PCS, MCS, or SGRQ-TS. Although 6MWT distance remained a significant determinant of PCS, MCS, and SGRQ-TS in multivariate models, its effect on HRQL decreased by a factor of ~10 compared with univariate models, as assessed by the change in value of the unadjusted to the adjusted regression coefficients (Table 2 and Table 4). Similarly, the effect of dyspnea, measured by the 6MWT Borg, on PCS and SGRQ-TS was reduced by at least a factor of three in the multivariate models compared with the univariate models. Based on the adjusted regression coefficients from the multivariate models (Table 4), the effects of self-report of being disabled, prior participation in pulmonary rehabilitation, antidepressant use, daytime sleepiness, current oral corticosteroid use, and supplemental oxygen use on PCS score, MCS score, or SGRQ-TS were generally of similar magnitude or greater than the effects of mean 6MWT distance or Borg on PCS, MCS, or SGRQ-TS.
DISCUSSION
Persons with FEV1 <40 percent predicted (GOLD stages III and IV COPD) and with limited exercise capacity have a wide range of HRQL scores, suggesting that other clinical variables contribute to HRQL. This is the first analysis, to our knowledge, to incorporate multiple psychological, demographic, and clinical variables along with physiological variables in multivariate models to identify the significant determinants of HRQL. Our results indicate that FEV1 percent predicted is not an independent determinant of HRQL in severe COPD but measures of exercise capacity and dyspnea are strongly and independently associated with HRQL.
The finding that exercise capacity and dyspnea are independent determinants of HRQL confirms prior observations; however, our analyses are the first to include them with psychosocial, demographic, and clinical variables in the same multivariate models that adjust for the effects of other factors. Our multivariate models demonstrate that the absolute effects of 6MWT distance and Borg on HRQL are reduced compared with their effects in the univariate models.
We identified other less well-recognized clinical and psychosocial parameters that independently contribute to HRQL. Given that the effects of these parameters on unit change in HRQL are of similar magnitude or greater (higher absolute values of adjusted regression coefficients) than the effects of FEV1, 6MWT distance, or dyspnea on HRQL, our results suggest that these variables also deserve attention in optimizing therapies that might lead to an improvement in HRQL in patients with severe COPD. For example, depression is an independent determinant of HRQL. We found that 22 percent of the subjects had at least mild depression, defined as a Beck total score ≥14, and 17 percent reported antidepressant use. Daytime sleepiness, reported by 55 percent of subjects, was also significantly associated with HRQL. Self-perception of being disabled was significantly associated with worse HRQL. Prior participation in pulmonary rehabilitation, supplemental oxygen use, and use of oral corticosteroids were significant determinants of HRQL as assessed by SGRQ-TS. These data also show that BMI is associated with HRQL. Low BMI, a marker for the systemic manifestations of COPD, has been previously shown to have weak, independent predictive ability for increased mortality in COPD [28]. The current result is the first to demonstrate that being obese versus having normal BMI is associated with worse HRQL in severe COPD.
In the final model (PCS score = [0.0042] × 6MWT distance + [−0.16] UCSD SOBQ total score + [−0.22] 6MWT Borg + [0.08] × age + [−0.05] × DLCO percent predicted + [−1.1] × [disabled]), the absolute values of the adjusted coefficients are low (Table 4). For example, a change in 6MWT distance of 238 m results in a 1-point change in PCS score when adjusting for the other explanatory variables in the model. However, we found that small changes in PCS and MCS scores were clinically meaningful and were associated with differences in healthcare resource utilization. Patients who reported that they had not had a doctor’s office visit, emergency room visit, or hospitalization in the 3 months prior to NETT entry had average PCS or MCS scores that were 1.1 to 3.1 points higher than those patients who reported having had at least one use of medical resources. Similarly, the multivariate model for SGRQ-TS shows that a change of 167 m in 6MWT distance results in a 1-point change in SGRQ-TS when adjusting for the other explanatory variables in the model (Table 4). Again, we found that small changes in SGRQ-TS were associated with differences in healthcare resource utilization. Subjects who reported that they had not had a doctor’s office visit, emergency room visit, or hospitalization in the 3 months prior to NETT entry had mean SGRQ-TSs 3.0 to 5.1 points lower than those patients who had reported at least one use of medical resources, which is consistent with the 4-point change in SGRQ-TS that is reported in the literature as a clinically significant change [29].
Several associations deserve discussion, and we speculate as to the mechanisms to explain these associations. We acknowledge that these cross-sectional analyses offer no insight into the causal mechanisms. For example, greater age was independently associated with better HRQL, assessed by both the MOS SF-36 and the SGRQ-TS. These results suggest that subjects may adapt to their disability and limitations with age. Conversely, better HRQL in older subjects in this cohort willing to consider LVRS may have resulted from a survivor effect if worse HRQL in younger subjects was a marker for greater mortality. Supplemental oxygen use was independently associated with better SGRQ-TS, while oral corticosteroid use was associated with worse SGRQ-TS. These results suggest that the many side effects of oral corticosteroid use may outweigh its beneficial effects as a COPD therapy with respect to its final impact on SGRQ-TS.
The models also show that higher DLCO percent predicted is independently associated with worse HRQL, assessed by both the PCS and SGRQ-TS; a result that is physiologically difficult to explain. Plots of the relationship between DLCO and PCS and SGRQ-TS show that very small changes in HRQL exist across the ranges of DLCO seen in this cohort, suggesting that the negative coefficient reflects an unstable estimate. Statistical check of collinearity did not reveal high correlations between DLCO percent predicted and the other continuous variables, making it unlikely that the effect of DLCO percent predicted on PCS and SGRQ-TS is being altered by the other covariates.
That the independent determinants of PCS score and MCS score were the same as those for SGRQ-TS offers evidence for the validity of the models. This evidence is further supported by the finding that pulmonary-specific variables such as prior participation in pulmonary rehabilitation, supplemental oxygen use, and oral corticosteroid use were independent determinants of respiratory disease-specific HRQL measured by the SGRQ-TS but not of general health status measured by the MOS SF-36. The validity of our model is further supported by the performance of the same variables in independently determining HRQL measured by the QWB-SA.
Our cross-sectional results demonstrate that, in addition to exercise capacity and dyspnea, level of depression, daytime sleepiness, oxygen use, pulmonary rehabilitation, oral corticosteroid use, and BMI are important factors that determine HRQL at a particular point in time for a given individual patient and these factors should be routinely assessed in the clinical setting. It is interesting that despite the comprehensive assessment of physiological, psychosocial, clinical, and demographic status, only 24 to 55 percent of the variance in HRQL was explained by these models. We speculate that other clinical factors not assessed in the NETT, such as cumulative free-living physical activity, may be important determinants of HRQL in COPD. Further study of such factors among persons with all severities of COPD is warranted.
CONCLUSIONS
Although FEV1, 6MWT distance, and dyspnea are significantly correlated with HRQL in univariate analyses, their effects on HRQL are reduced when considered with other physiological, psychosocial, and clinical variables in multivariate models. Greater exercise capacity, prior participation in pulmonary rehabilitation, and use of supplemental oxygen are significantly associated with a better HRQL. Self-perception of being disabled, depression, dyspnea, oral corticosteroid use, and daytime sleepiness are associated with a worse HRQL. These results suggest that in order to optimize HRQL in subjects with severe COPD, clinicians should pay attention to a number of physiological, psychosocial, and clinical variables.
ACKNOWLEDGMENTS
Funding/Support: This material was based on work supported by the Department of Veterans Affairs (VA), Veterans Health Administration, Rehabilitation Research and Development Service through a VA Career Development Award to Dr. Moy and the National Institutes of Health (NIH) National Institute of Child Health and Human Development (grant NIH/NICHD RO1 HD42141 to Dr. Garshick). The NETT was supported by the NIH National Heart, Lung, and Blood Institute (grants N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119); the Centers for Medicare and Medicaid Services; and the Agency for Healthcare Research and Quality.
Participant Follow-Up: The authors do not plan on notifying the study subjects of the publication of this article because of a lack of contact information.
Abbreviations
- 6MWT
6-minute walk test
- BD
bronchodilator
- Beck
Beck Depression Inventory
- BMI
body mass index
- Borg
Borg score for breathlessness
- COPD
chronic obstructive pulmonary disease
- CPET
cardiopulmonary exercise testing
- DLCO
single-breath diffusing capacity of the lung for carbon monoxide
- FEV1
forced expiratory volume in 1 second
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- HRQL
health-related quality of life
- LVRS
lung-volume-reduction surgery
- MCS
Mental Component Summary
- MOS SF-36
Medical Outcomes Study 36-Item Short Form
- NETT
National Emphysema Treatment Trial
- NIH
National Institutes of Health
- PCS
Physical Component Summary
- QWB-SA
Self-Administered Quality-of-Well-Being Scale
- RV
residual volume
- SGRQ-TS
St. George’s Respiratory Questionnaire total score
- TLC
total lung capacity
- UCSD SOBQ
University of California, San Diego Shortness of Breath Questionnaire
- VA
Department of Veterans Affairs
Footnotes
Clinical Trial Registration: National Emphysema Treatment Trial. National Clinical Trials Registration ID: NCT00000606 (in http://ClinicalTrials.gov/).
Author Contributions:
Study concept and design: M. L. Moy, J. J. Reilly.
Acquisition of data: M. L. Moy, J. J. Reilly, A. L. Ries, Z. Mosenifar, R. M. Kaplan.
Analysis and interpretation of data: M. L. Moy, J. J. Reilly, R. M. Kaplan, R. Lew, E. Garshick.
Drafting of manuscript: M. L. Moy, E. Garshick.
Critical revision of manuscript for important intellectual content: J. J. Reilly, A. L. Ries, Z. Mosenifar, R. M. Kaplan.
Statistical analysis: M. L. Moy, R. Lew, E. Garshick.
Obtained funding: J. J. Reilly.
Study supervision: J. J. Reilly, E. Garshick
Financial Disclosures: The authors have declared that no competing interests exist.
References
- 1.Jones PW. Issues concerning health-related quality of life in COPD. Chest. 1995;107 5 Suppl:187S–193S. doi: 10.1378/chest.107.5_supplement.187s. [PMID: 7743825] DOI:10.1378/chest.107.5_Supplement.187S. [DOI] [PubMed] [Google Scholar]
- 2.Fan VS, Curtis JR, Tu SP, McDonell MB, Fihn SD. Ambulatory Care Quality Improvement Project Investigators. Using quality of life to predict hospitalization and mortality in patients with obstructive lung diseases. Chest. 2002;122(2):429–436. doi: 10.1378/chest.122.2.429. [PMID: 12171813] DOI:10.1378/chest.122.2.429. [DOI] [PubMed] [Google Scholar]
- 3.Sprenkle MD, Niewoehner DE, Nelson DB, Nichol KL. The Veterans Short Form 36 questionnaire is predictive of mortality and health-care utilization in a population of veterans with a self-reported diagnosis of asthma or COPD. Chest. 2004;126(1):81–89. doi: 10.1378/chest.126.1.81. [PMID: 15249446] DOI:10.1378/chest.126.1.81. [DOI] [PubMed] [Google Scholar]
- 4.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverly P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, Van Weel C, Zielinski J. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [PMID: 17507545] DOI:10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 5.Weaver TE, Narsavage GL. Physiological and psychological variables related to functional status in chronic obstructive pulmonary disease. Nurs Res. 1992;41(5):286–291. [PMID: 1523109] DOI:10.1097/00006199-199209000-00006. [PubMed] [Google Scholar]
- 6.Kim HF, Kunik ME, Molinari VA, Hillman SL, Lalani S, Orengo CA, Petersen NJ, Nahas Z, Goodnight-White S. Functional impairment in COPD patients: The impact of anxiety and depression. Psychosomatics. 2000;41(6):465–471. doi: 10.1176/appi.psy.41.6.465. [PMID: 11110109] DOI:10.1176/appi.psy.41.6.465. [DOI] [PubMed] [Google Scholar]
- 7.Felker B, Katon W, Hedrick SC, Rasmussen J, McKnight K, McDonnell MB, Fihn SD. The association between depressive symptoms and health status in patients with chronic pulmonary disease. Gen Hosp Psychiatry. 2001;23(2):56–61. doi: 10.1016/s0163-8343(01)00127-x. [PMID: 11313071] DOI:10.1016/S0163-8343(01)00127-X. [DOI] [PubMed] [Google Scholar]
- 8.Hajiro T, Nishimura K, Tsukino M, Ikeda A, Oga T. Stages of disease severity and factors that affect the health status of patients with chronic obstructive pulmonary disease. Respir Med. 2000;94(9):841–846. doi: 10.1053/rmed.2000.0804. [PMID: 11001074] DOI:10.1053/rmed.2000.0804. [DOI] [PubMed] [Google Scholar]
- 9.Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: Role of exercise capacity and health status. Am J Respir Crit Care Med. 2003;167(4):544–549. doi: 10.1164/rccm.200206-583OC. [PMID: 12446268] OI:10.1164/rccm.200206-583OC. [DOI] [PubMed] [Google Scholar]
- 10.Okubadejo AA, Jones PW, Wedzicha JA. Quality of life in patients with chronic obstructive pulmonary disease and severe hypoxaemia. Thorax. 1996;51(1):44–47. doi: 10.1136/thx.51.1.44. [PMID: 8658368] DOI:10.1136/thx.51.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones PW, Baveystock CM, Littlejohns P. Relationships between general health measured with the sickness impact profile and respiratory symptoms, physiological measures, and mood in patients with chronic airflow limitation. Am Rev Respir Dis. 1989;140(6):1538–1543. doi: 10.1164/ajrccm/140.6.1538. [PMID: 2604285] [DOI] [PubMed] [Google Scholar]
- 12.Oga T, Nishimura K, Tsukino M, Hajiro T, Ikeda A, Mishima M. Relationship between different indices of exercise capacity and clinical measures in patients with chronic obstructive pulmonary disease. Heart Lung. 2002;31(5):374–381. doi: 10.1067/mhl.2002.127941. [PMID: 12487016] OI:10.1067/mhl.2002.127941. [DOI] [PubMed] [Google Scholar]
- 13.Ries AL, Make BJ, Lee SM, Krasna MJ, Bartels M, Crouch R, Fishman AP. National Emphysema Treatment Trial Research Group. The effects of pulmonary rehabilitation in the National Emphysema Treatment Trial. Chest. 2005;128(6):3799–3809. doi: 10.1378/chest.128.6.3799. [PMID: 16354848] DOI:10.1378/chest.128.6.3799. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan RM, Ries AL, Reilly J, Mohsenifar Z. Measurement of health-related quality of life in the National Emphysema Treatment Trial. Chest. 2004;126(3):781–789. doi: 10.1378/chest.126.3.781. [PMID: 15364757] DOI:10.1378/chest.126.3.781. [DOI] [PubMed] [Google Scholar]
- 15.National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med. 2001;345(15):1075–1083. doi: 10.1056/NEJMoa11798. [PMID: 11596586] DOI:10.1056/NEJMoa11798. [DOI] [PubMed] [Google Scholar]
- 16.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood DE. National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059–2073. doi: 10.1056/NEJMoa030287. [PMID: 12759479] DOI:10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 17.Ramsey SD, Berry K, Etzioni R, Kaplan RM, Sullivan SD, Wood DE. National Emphysema Treatment Trial Research Group. Cost effectiveness of lung-volume-reduction surgery for patients with severe emphysema. N Engl J Med. 2003;348(21):2092–2102. doi: 10.1056/NEJMsa030448. [PMID: 12759480] DOI:10.1056/NEJMsa030448. [DOI] [PubMed] [Google Scholar]
- 18.Rationale and design of The National Emphysema Treatment Trial. A prospective randomized trial of lung volume reduction surgery. The National Emphysema Treatment Trial Research Group. Chest. 1999;116(6):1750–1761. doi: 10.1378/chest.116.6.1750. [PMID: 10593802] DOI:10.1378/chest.116.6.1750. [DOI] [PubMed] [Google Scholar]
- 19.Tashkin DP, Celli B, Decramer M, Liu D, Burkhart D, Cassino C, Kesten S. Bronchodilator responsiveness in patients with COPD. Eur Respir J. 2008;31(4):742–750. doi: 10.1183/09031936.00129607. [PMID: 18256071] DOI:10.1183/09031936.00129607. [DOI] [PubMed] [Google Scholar]
- 20.Ware JE. The SF-36 Health Survey. In: Spilker B, editor. Quality of life and pharmacoeconomics in clinical trials. 2nd ed. Philadelphia (PA): Lippincott-Raven; 1996. pp. 337–345. [Google Scholar]
- 21.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. doi: 10.1164/ajrccm/145.6.1321. [PMID: 1595997] [DOI] [PubMed] [Google Scholar]
- 22.Kaplan RM, Atkins CJ, Timms R. Validity of a quality of well-being scale as an outcome measure in chronic obstructive pulmonary disease. J Chronic Dis. 1984;37(2):85–95. doi: 10.1016/0021-9681(84)90050-x. [PMID: 6420431] DOI:10.1016/0021-9681(84)90050-X. [DOI] [PubMed] [Google Scholar]
- 23.Sciurba F, Criner GJ, Lee SM, Mohsenifar Z, Shade D, Slivka W, Wise RA National Emphysema Treatment Trial Research Group. Six-minute walk distance in chronic obstructive pulmonary disease: Reproducibility and effect of walking course layout and length. Am J Respir Crit Care Med. 2003;167(11):1522–1527. doi: 10.1164/rccm.200203-166OC. [PMID: 12615634] DOI:10.1164/rccm.200203-166OC. [DOI] [PubMed] [Google Scholar]
- 24.Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Validation of a new dyspnea measure: The UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest. 1998;113(3):619–624. doi: 10.1378/chest.113.3.619. [PMID: 9515834] DOI:10.1378/chest.113.3.619. [DOI] [PubMed] [Google Scholar]
- 25.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PMID: 7154893] DOI:10.1249/00005768-198205000-00012. [PubMed] [Google Scholar]
- 26.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [PMID: 13688369] [DOI] [PubMed] [Google Scholar]
- 27.Kleinbaum DJ, Kupper LL, Muller KE, Nizam A. Applied regression analysis and other multivariable methods. 3rd ed. Boston (MA): Duxbury Press; 1997. [Google Scholar]
- 28.Martinez FJ, Foster G, Curtis JL, Criner G, Weinmann G, Fishman A, DeCamp MM, Benditt J, Sciurba F, Make B, Mohsenifar Z, Diaz P, Hoffman E, Wise R NETT Research Group. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006;173(12):1326–1334. doi: 10.1164/rccm.200510-1677OC. [PMID: 16543549] DOI:10.1164/rccm.200510-1677OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones PW. St. George’s Respiratory Questionnaire: MCID, COPD. 2005;2(1):75–79. doi: 10.1081/copd-200050513. [PMID: 17136966] DOI:10.1081/COPD-200050513. [DOI] [PubMed] [Google Scholar]