Abstract
The Iowa Center for Research on Botanical Dietary Supplements seeks to optimize Echinacea, Hypericum, and Prunella botanical supplements for human-health benefit, emphasizing antiviral, anti-inflammatory and anti-pain activities. This mini-review reports on ongoing studies on Hypericum. The Center uses the genetically diverse, well-documented Hypericum populations collected and maintained at the USDA-ARS North Central Regional Plant Introduction Station (NCRPIS), and the strength of research in synthetic chemistry at Iowa State University to tap natural diversity, to help discover key constituents and interactions among constituents that impact bioactivity and toxicity. The NCRPIS has acquired more than 180 distinct populations of Hypericum, with a focus on Hypericum perforatum L. (Hypericaceae), representing about 13% of currently recognized taxa. Center chemists have developed novel synthetic pathways for key flavones, acyl phloroglucinols, hyperolactones and a tetralin that have been found in Hypericum, and these compounds are used as standards and for bioactivity studies. Both light-dependent and light-independent anti-viral activities have been identified by using bioactivity-guided fractionation of H. perforatum and a HIV-1 infection test system. Our Center has focused on light-independent activity, potentially due to novel chemicals, and polar fractions are undergoing further fractionation. Anti-inflammatory activity has been found to be light-independent, and fractionation of a flavonoid-rich extract revealed four compounds (amentoflavone, chlorogenic acid, pseudohypericin and quercetin) that interacted in the light to inhibit lipopolysaccharide-induced prostaglandin E2 activity. The Center continues to explore novel populations of H. perforatum and related species to identify constituents and interactions of constituents that contribute to potential health benefits related to infection.
Keywords: Hypericum, infection, anti-viral, anti-inflammatory, phloroglucinols, flavonoids
Introduction: Center organization
The Iowa Botanical Center is unique among Office of Dietary Supplements (ODS) Botanical Supplements Research Centers in that we have demonstrated the capacity to harness interdisciplinary research on the genetic, developmental, and biochemical diversity of Echinacea, Hypericum, and Prunella species to improve our understanding of the key constituents that contribute to their bioactivity. The range of variation in these plants, when systematically analyzed, provides a strong foundation on which to develop the strategies and tools needed to produce the most efficacious products for a growing body of consumers. This mini-review will focus on some of our work with Hypericum.
Our general approach is to identify chemical profiles and the key compounds that define these plants’ bioactivity, and to provide a context that can be used to reinterpret past studies and, in turn, serve as the basis for future research. The primary goal of our Center is to improve our understanding of the characteristics that contribute to health benefits and optimize these supplements for study in future clinical trials. Our Center is comprised of three projects focused on anti-viral, anti-inflammatory and anti-pain activities, respectively, and three cores that provide key services in support of these projects: Germplasm and Phytochemical Profiling; Separations/Structure/Bioavailability; and Administration, Data Management, Statistics and Bioinformatics.
The overarching objectives of our Center are to:
identify the compounds that contribute to the anti-viral, anti-inflammatory and pain-control effects, and those contributing to their toxicity;
assess the influence of plant species and population on the bioactive constituents in these genera;
understand the mechanisms of action of bioactive constituents –in particular the cellular-signaling pathways and receptors that are critical for their bioactivity– and the effects of their interactions;
assess the bioavailability of key constituents of these supplements and the effects of their complex chemical profiles on bioavailability.
Given the long and diverse history of traditional medicinal uses of H. perforatum in Europe, including its use as an agent to reduce inflammation and promote healing (Bombardelli & Morazzoni, 1995), this species fits especially well with our ongoing research.
Genetic resources and production of well-characterized plant material
A unique resource of the Center is our collection of a genetically diverse set of well-documented plant populations of Echinacea, Hypericum, and Prunella being conserved at the USDA-ARS North Central Regional Plant Introduction Station (NCRPIS). The NCRPIS, located at Iowa State University, is one of the main active genebanks in the US National Plant Germplasm System (NPGS) (McCoy, 2007) with significant collections of medicinal-plant germplasm. Efforts to assemble the NCRPIS Echinacea collection were described by Widrlechner and McKeown (2002), and a recent overview of this important collection and its relationship to the Center was presented by Birt et al. (2008). The NCRPIS Prunella collection is a recent development, resulting from the inclusion of this genus in research projects incorporated into the Center’s 2007 Project Renewal, while the more extensive NCRPIS Hypericum collection is described in detail below. These resources provide Center researchers with control over the production of known-source plant materials and a common basis for studies in genomic analysis and broad-based plant metabolic profiling, enhancing our ability to integrate complex datasets by using bioinformatics and other statistical tools.
Since the late 1990s, the NCRPIS has acquired more than 180 distinct populations (or accessions) of Hypericum, currently representing about 60 taxa, or ~13% of currently recognized taxa (Robson, 2003), with an emphasis on H. perforatum and its close relatives. Because representation of the 400+ species of Hypericum (Robson, 2003) is currently incomplete, efforts will be taken to improve coverage, both for those species most closely related to H. perforatum and for a broad, stratified sample of other taxonomic sections within the genus. Dr. Norman Robson, an expert in Hypericum systematics, has agreed to serve as an advisor on both our acquisition and characterization efforts. New collections are being obtained both through exchange relationships with other institutions and through targeted field collections. For example, an NPGS-funded collection trip to the Republic of Georgia focusing on medicinal plants was conducted in August-September 2007. The Republic of Georgia was selected due to the high degree of Hypericum biodiversity represented in the region.
The genetic integrity of each Hypericum accession is preserved by regenerating seed samples under controlled conditions (systems described by Widrlechner et al., 1997 and Brenner & Widrlechner, 1998) in screened field cages with insect pollinators or, in the greenhouse, in mesh tents under controlled-temperature conditions to reduce the possibility of infection by the debilitating fungal pathogen, Colletotrichum gloeosporioides (Hildebrand & Jensen, 1991). Because this destructive anthracnose pathogen can be seedborne, we have developed a Colletotrichum blotter test to screen germinating seeds for fungal contamination, with 61% of our Hypericum collection now screened. All germination and pathogen data collected have been entered in the Germplasm Resources Information Network (GRIN) database (US Department of Agriculture, 2007).
During seed regeneration, taxonomic identities are verified and populations are being characterized for phenotypic traits following a standardized descriptor list. Detailed passport data describing each accession are available from the GRIN database. Phenotypic descriptors along with images of the accessions are currently being assembled. Seeds of all available accessions are distributed for research and educational purposes at no cost to the user and can be accessed via the GRIN database.
Hypericum samples used by Center researchers have typically been produced from NCRPIS accessions collected from a variety of sources, including both wild populations and commercial varieties. By using these accessions produced under known conditions, we minimize both the genetic and environmental components of biochemical variation in the resulting products, which increases the overall repeatability of bioassays in our Center’s projects. Replicated field plantings of four accessions of H. perforatum were established in 2003 and expanded the following year to include three more accessions by Center researchers, Drs. Kathleen Delate and Fredy Romero, to optimize production of above-ground vegetative material. These plots were maintained through 2005, when a Colletotrichum infection was identified.
Fifty-six Hypericum accessions representing 11 species were supplied to Dr. Jonathan Wendel’s laboratory for genetic-diversity studies and to identify species-specific Amplified Fragment Length Polymorphism (AFLP) markers for taxonomic identification (Percifield et al., 2007). Similarly, 76 Hypericum samples were supplied to Dr. Eve Wurtele’s laboratory to develop chemical fingerprints for various accessions and identify bioactive compounds of interest. Percifield et al. (2007) recently identified 17 AFLP markers present in all 42 H. perforatum accessions evaluated; of these, two were unique to H. perforatum. The unique markers may be valuable for raw-material verification. All of the materials provided for these two projects were collected, taxonomically verified, and propagated by Center researchers, Drs. Joe-Ann McCoy and Mark Widrlechner. After production and processing, all plant samples for Center use are inventoried with a standardized coding system, and unextracted dried plant materials are packaged in nitrogen and stored frozen at -20°C.
Chemical synthesis of key phytochemicals
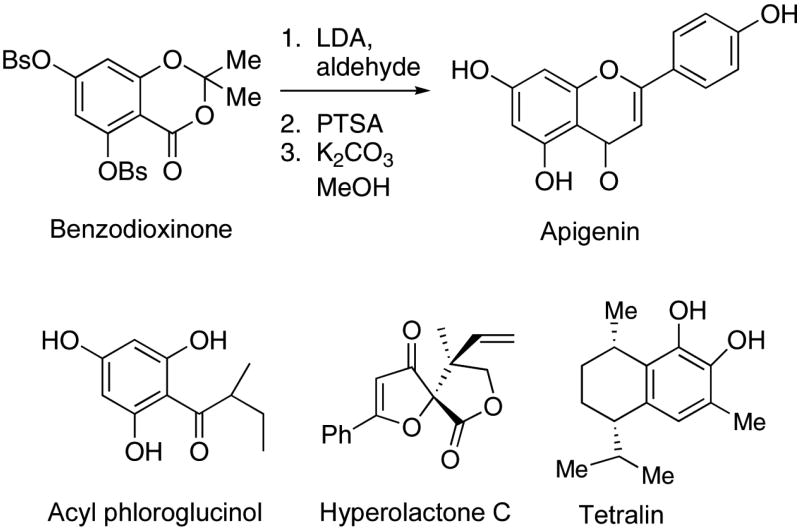
As part of a multidisciplinary effort to identify new bioactive constituents from Echinacea and Hypericum, we required flexible synthetic routes to produce the key flavones and acyl phloroglucinols present in Hypericum. Access to highly pure standards is important for both chemical profiling among species and for biological evaluation. Although synthetic routes to individual flavones have been reported (Marais et al., 2005), no synthesis of flavones or acyl phloroglucinols from benzodioxinones had been reported.
Apigenin is a natural antioxidant flavone that occurs in a number of Hypericum species (Kartnig et al., 1996). It has been shown to promote cell-cycle arrest and apoptosis in various malignant cell lines and is also a potent inhibitor of glucosyltransferase activity (Viola et al., 1995). Our methodology has been applied to a direct synthesis of apigenin. Unexpectedly, the reaction of the enolate of para-methoxyacetophenone returned starting materials. As shown in Fig. 1, the lithium diisopropylamide-derived enolate of para-benzenesulfonyloxyacetophenone reacted efficiently with the benzodioxinone to produce the β-diketone which was cyclized and deprotected to provide apigenin in 40% overall yield. The proton and 13C NMR spectra of our synthetic material matched the spectra of an authentic sample (Kraus & Wei, 2004).
Figure 1.
Chemical standards from Hypericum.
Acyl phloroglucinols, a diverse class of natural products that exhibit antibacterial activity, anticancer activity, and antitubercular activity, are broadly distributed among plant families. Several species of Hypericum contain biologically active acyl phloroglucinols (Gibbons et al., 2005). We recently synthesized the acyl phloroglucinol shown in Fig. 1 from the benzodioxinone. This compound can serve as an intermediate to more complex acyl phloroglucinols.
The hyperolactones constitute a growing class of novel metabolites found in Hypericum species (Aramaki et al., 1995). The structure of hyperolactone C is depicted in Fig. 1. In the context of a study of Hypericum metabolites through bioassay-guided fractionation, we required an authentic sample of hyperolactone C, whose extended conjugation and resemblance to known antiviral agents made it a metabolite of interest. Kinoshita and coworkers reported interesting syntheses of hyperolactone C from chiral precursors (Ichinari et al., 1997). Although their synthesis defined the absolute stereochemistry, a more direct synthetic approach was needed to support biological studies. We synthesized hyperolactone C in four steps from commercially available starting materials (Kraus & Wei, 2004), via a tandem Claisen/lactonization reaction.
A novel tetralin (Fig. 1) was isolated from H. elodeoides Choisy (Mathela et al., 1984). We prepared this tetralin from 3-methylcatechol by way of a stereoselective electrophilic cyclization onto a protected catechol (Kraus & Jeon, 2006).
Anti-Viral screening using HIV-1
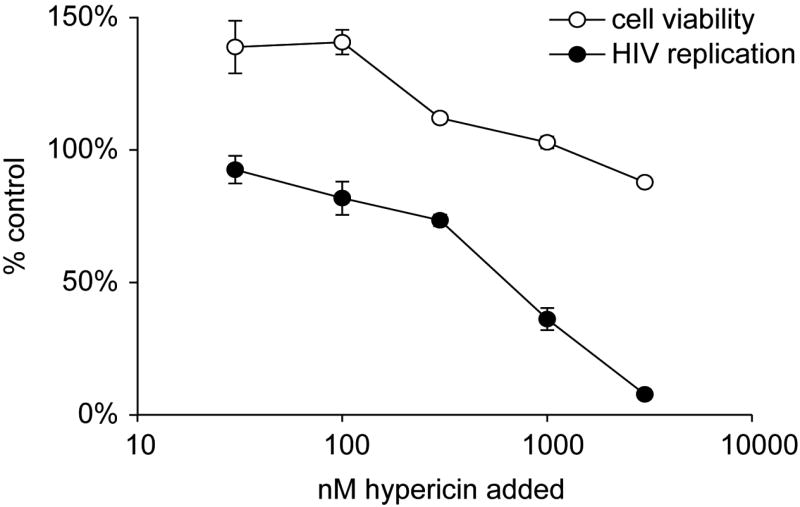
Light-dependent antiviral activities of H. perforatum have been extensively explored by others (Hudson et al., 1991; Jacobson et al., 2001; Laurent et al., 2005; Naesens et al., 2006). This light-dependent, antiviral activity against various enveloped viruses, but not non-enveloped viruses, has been ascribed to the H. perforatum naphthodianthrones, hypericin, and pseudohypericin (Kraus et al., 1990; Lopez-Bazzocchi et al., 1991; Degar et al., 1992; Carpenter et al., 1994; Lavie et al., 1995; Vyas, 1995; Farnet et al., 1998; Park et al., 1998). Hypericin, when exposed to light, displays multiple modes of antiviral activity, including inhibition of budding of new virions (Meruelo et al., 1988), cross-linking of capsids preventing viral uncoating (Degar et al., 1992), and inhibition of protein kinase activity required for replication of a number of viruses (De Witte et al., 1993; Agostinis et al., 1995). The observation that hypericin specifically inhibits enveloped viruses strongly implicates membrane-associated events as central to the inhibition. Consistent with this, light-sensitized hypericin is directly viricidal to these viruses (Kuhn, 1995; Lavie et al., 1995; Vyas, 1995). We evaluated the ability of hypericin to inhibit HIV-1 replication in a short term infectivity assay. Consistent with other reports, a dose-dependent inhibition of HIV-1 expression was observed when hypericin was added at the time of infection under ambient light conditions (Fig. 2). However, the light-dependent nature of the antiviral activity of hypericin reduces the potential applicability of this compound for in vivo studies.
Figure 2.
Ability of hypericin to inhibit HIV-1 infection. The HIV-1 molecular clone, pNL4-3 (multiplicity of infection (MOI) = 0.005), was incubated with permissive HeLa37 cells that express CD4 and CXCR4. Three-fold serial dilutions of hypericin were added to the infections. The mixture was incubated under ambient light conditions within the laboratory for 10 min prior to incubation at 37°C in a tissue culture incubator. Cells were fixed at 40 h following infection and immunostained for HIV-1 antigens as previously described (Reed-Inderbitzin & Maury, 2003). HIV antigen-positive cells were enumerated. The number of HIV-1 positive cells observed in the presence of hypericin was divided by the number of HIV-1 positive cells found in the absence of hypericin, shown as % control values. In parallel, the cytotoxicity of the extract was evaluated in HeLa37 cells in an ATP Lite assay (Packard Instruments) and those values are shown as the number of cells that are viable in the presence of hypericin divided by the number of viable cells in the presence of vehicle alone.
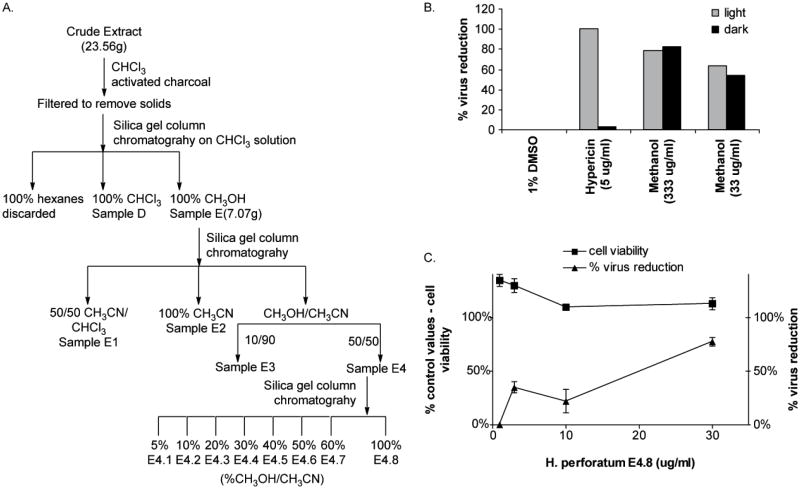
Additional anti-viral studies performed at the Iowa Center for Research on Botanical Dietary Supplements found that H. perforatum extracts also contain light-independent anti-HIV-1 activity. Chloroform extracts of H. perforatum that lacked detectable levels of naphthodianthrones were used as the starting material in bioassay-guided fractionation studies to identify light-independent anti-HIV-1 activity. Extracts were fractionated through a series of three successive column chromatography experiments, and the fractions obtained were assayed for anti-HIV-1 activity (Fig. 3A). At each step, the most polar fractions were found to contain constituents that inhibited HIV infection in a dose-dependent, but light-independent manner. As early as the initial fractionation, material eluted in methanol had significant anti-HIV-1 activity in both light and dark conditions (Fig. 3B). Further purification provided evidence of anti-HIV-1 activity in the absence of cell cytotoxicity (Fig. 3C). Continued fractionation of the most advanced active fractions (E4.7 and E4.8) must be conducted to identify the botanical constituents that confer this novel antiviral activity.
Figure 3.
Fractionation of light-independent anti-HIV-1 activity of H. perforatum extracts. (A) Bioactivity-guided fractionation protocol. (B) Methanol extracted fractions had equivalent ability to inhibit HIV infection in light and dark. Chloroform extracts of H. perforatum were extracted with hexane, chloroform, or methanol and the methanol fraction was analyzed for anti-HIV activity. The HIV-1 molecular clone, pNL4-3, was incubated with HeLa37 cells in the presence of 10-fold dilutions of the methanol fraction under ambient light or low light conditions for 10 min. Hypericin was evaluated in parallel and served as the light-dependent control. At 40 h following infection, cells were fixed and immunostained for HIV antigens. Shown is the percent reduction of HIV positive cells in the presence of hypericin or extract compared to the number of HIV positive cells in the presence of vehicle alone. (C) The ability of increasing concentrations of subfraction E4.8 to inhibit HIV infectivity. Subfraction concentrations were incubated with HIV-1 and HeLa37 cells over a 40 h period. Cells were acetone fixed and evaluated for HIV positivity. The cytotoxicity of increasing concentrations of the subfraction to HeLa37 cells is also shown as the cell viability relative to control.
Anti-inflammatory screening using RAW264.7 macrophages
The primary approach taken to assess the anti-inflammatory activity of Hypericum was to measure the influence of accessions of H. perforatum on lipopolysaccharide (LPS)-induced prostaglandin E2 (PGE2) production by RAW264.7 mouse macrophages. Anti-inflammatory activity of different accessions of H. perforatum was studied in Soxhlet chloroform and Soxhlet ethanol extracts at the highest concentrations, and all accessions inhibited PGE2 production in LPS-induced RAW264.7 macrophages (Hammer et al., 2007). Dilution studies suggest that the constituents that account for the anti-inflammatory activity of H. perforatum accessions are extracted by either chloroform or ethanol, indicating that hypericin and related compounds that would only be present in the ethanol extract do not account for this bioactivity. The most active accession, Ames 27452 ‘Medizinal’, marketed under the name Elixir™, was developed using conventional plant breeding for higher concentrations of putative bioactive compounds, which was reflected in its relatively high concentration of total flavonoids in a multi-accession evaluation (Gaudin et al., 2003), and its more potent anti-inflammatory activity as reported by Hammer et al. (2007), supporting the success of this strategy.
Several constituents of Hypericum, including hyperforin, hypericin, pseudohypericin, quercetin, quercitrin, isoquercitrin, rutin, amentoflavone, and chlorogenic acid, were studied for their impact on LPS-induced PGE2 production (Hammer et. al., 2007). Based on the concentrations of these chemicals in the extracts and fractions, none of these constituents alone could account for the anti-inflammatory activity. However, it is possible that concentrations of specific flavonoids, such as amentoflavone, that would not inhibit PGE2 production in their pure forms, were effective in the fractions and extracts in the presence of other compounds.
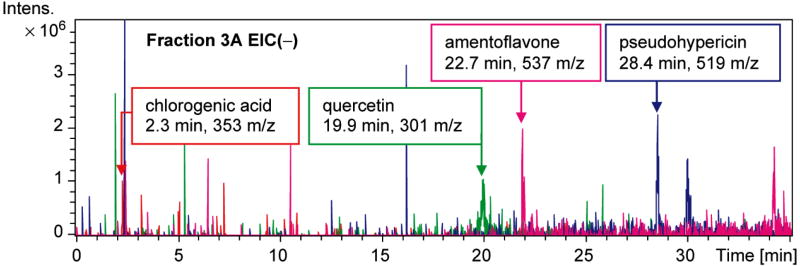
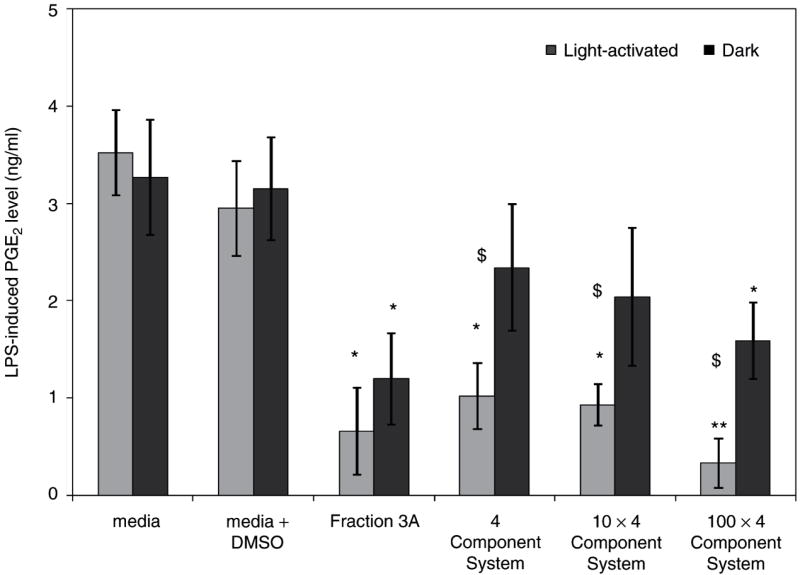
Because of published evidence that flavonoids possess anti-inflammatory activity (Middleton, Jr et al., 2000), and from the similar anti-inflammatory activity of the ethanol and chloroform extracts, which would both contain flavonoids, H. perforatum was fractionated to generate flavonoid-rich fractions (sequential hexane, ethanol then chloroform extracts). The fraction with the greatest concentrations of flavonoids from the first round of fractionation was the most anti-inflammatory. Further fractionation by column chromatography with a gradient from chloroform to 50:50 acetonitrile/methanol yielded a fraction with similar anti-inflammatory activity. Subsequently, subfractionation of this second-round active fraction yielded fraction 3A where four constituents (amentoflavone, chlorogenic acid, pseudohypericin and quercetin) were identified and quantified by liquid chromatography – mass spectrometry (Fig. 4) (Hammer et al., 2008). A proportional combination of these constituents was then used for assessment in the LPS-induced PGE2 assay. In the light, the 1× 4 component system that contained the four identified constituents in the amounts found in fraction 3A inhibited LPS-induced production of PGE2 by nearly as much as fraction 3A (Fig. 5). However, it was clear that no one constituent was present at a high enough concentration to account for this degree of inhibition (Table 1). For example, amentoflavone was found at 0.08 μM in fraction 3A while pure amentoflavone was only effective at concentrations of ≥10 μM. However, these data also highlight the possibility that unknown and/or unidentified compounds significantly contribute to the light-independent activity of fraction 3A.
Figure 4.
Extracted ion chromatographs (EIC) from liquid chromatography-mass spectrometry analysis (negative ion mode) of fraction 3A showed that chlorogenic acid, quercetin, amentoflavone, and pseudohypericin were present, based on matching retention times and mass-to-charge ratios (from data published in Hammer et al., 2008). The fractions were analyzed with an Agilent Technologies Ion Trap 1100 LC-ESI-MS (Ganzera et al., 2002).
Figure 5.
Fraction 3A was compared with 1, 10, and 100× dilutions (four-component system) of the four identifiable constituents (amentoflavone, chlorogenic acid, pseudohypericin, and quercetin) in fraction 3A (from data published in Hammer et al., 2008). Data are presented as LPS-induced PGE2 production (ng/mL) as mean + standard error; *p < 0.05 as compared to media + dimethylsulfoxide (DMSO) control using the Tukey–Kramer test for multiple comparisons. n = 4 for each treatment. Each fraction/four-component system was assayed in both light-activated and dark treatments and differences between light and dark treatments in PGE2 levels are noted by $ (p < 0.05).
Table 1.
Comparison of concentration of chlorogenic acid, quercetin, amentoflavone, and pseudohypericin in fraction 3A with the concentrations of pure chemicals that inhibit LPS-induced PGE2.
| Constituent | Amount detected in fraction 3A (μM) | Amount of pure constituent used in assay (μM) | % Inhibition of LPS-induced PGE2 by constituenta |
|---|---|---|---|
| Chlorogenic acid | 0.1 | 0.1, 1, 5, 10, 20, 40 | 0 |
| Quercetin | 0.07 | 0.07, 0.2, 2 | 0 |
| 5 | 66* | ||
| 10 | 87* | ||
| 20 | 93* | ||
| 40 | 96* | ||
| Amentoflavone | 0.08 | 0.08 | 0 |
| 1 | 38 | ||
| 5 | 53 | ||
| 10 | 77* | ||
| Pseudohypericin (light-activated) | 0.03 | 0.02, 0.03 | 0 |
| 0.2 | 2 | ||
| 0.5 | 3 | ||
| 1 | 53* | ||
| 2 | 61* |
Anti-inflammatory activity (amean % inhibition of LPS-induced PGE2 levels as compared to media + LPS + DMSO control (95% confidence intervals published in Hammer et al., 2007)) of pure compounds identified within H. perforatum extracts on RAW264.7 macrophage cells. Data represent light-activated and dark treatments combined, except for pseudohypericin which is just presented under light-activated conditions, as there was no significant difference between light-activated and dark treatments for the other constituents. n = 8 for chlorogenic acid, quercetin, and amentoflavone treatments; n = 4 for pseudohypericin treatments.
p-value < 0.05 as compared to control.
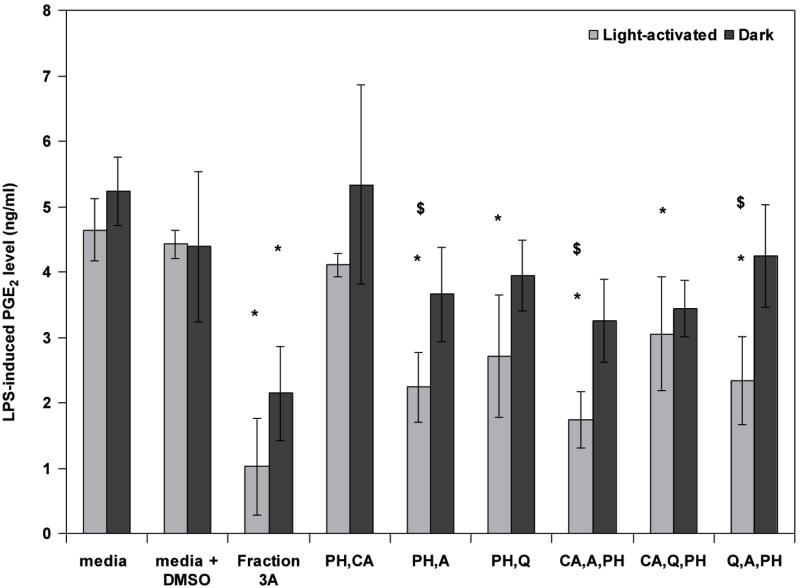
Further studies of the four constituents identified in the 4 component system showed that amentoflavone, chlorogenic acid, pseudohypericin and quercetin were effective in inhibiting LPS-induced PGE2 primarily with light activation and only when combined in groups of 2 or 3 chemicals if pseudohypericin was present. Further, the combinations of pseudohypericin with amentoflavone or quercetin, or all three of these constituents, were generally the most effective (Fig. 6). Current investigations are assessing the impact of these known constituents on gene expression and cellular-signaling pathways important in mediating inflammation in RAW264.7 mouse macrophages. In addition, studies on other mediators of inflammation suggest that distinct constituents of H. perforatum may alter mediators of inflammation beyond PGE2.
Figure 6.
Fraction 3A was compared with the four known constituents (amentoflavone, chlorogenic acid, pseudohypericin, and quercetin) combined in groups of two or three compounds at the concentrations detected in fraction 3A (from data published in Hammer et al., 2008). Data are presented as LPS-induced PGE2 production (ng/mL) as mean + standard error; *p < 0.05 as compared to media + DMSO control using the Tukey–Kramer test for multiple comparisons. n = 4 for each treatment. Combinations without pseudohypericin did not inhibit PGE2 production and are not shown. Each sample was assayed in both light-activated and dark treatments and differences between light and dark treatments in PGE2 levels are noted by $ (p < 0.05).
Research directions
Center scientists continue to screen populations of H. perforatum and related species to identify anti-viral and anti-inflammatory activities. Bioactivity-guided fractionation of active extracts is anticipated to yield novel compounds. We plan structural-elucidation studies and syntheses of identified key constituents with the objective of improving our understanding of those constituents of Hypericum with potential health benefits. As key bioactive constituents are identified, molecular and cellular mechanisms of action are being probed.
Acknowledgments
This publication was made possible by grant number P01 ES012020 from the National Institute of Environmental Health Sciences (NIEHS) and the Office of Dietary Supplements (ODS), and by grant 95P50AT004155 from the National Center of Complementary and Alternative Medicine (NCCAM) and ODS, NIH. Portions of this paper were also supported by the Iowa Agriculture and Home Economics Experiment Station, Ames, Iowa, with funding from the Hatch Act and State of Iowa. Mention of commercial brand names does not constitute an endorsement of any product by the U.S. Department of Agriculture or cooperating agencies. The contents of this paper are the responsibility of the authors and do not necessarily represent the views of the funding agencies.
Footnotes
Symposium: “Plants in the Service of Human Health: Continuing Search for Plant-based Therapies” - Society for Economic Botany 48th Annual Meeting in Chicago at Lake Forest College, June 4, 2007
References
- Agostinis P, Vandenbogaerde A, Donella-Deana A, Pinna LA, Lee KT, Goris J, Merlevede W, Vandenheede JR, De Witte P. Photosensitized inhibition of growth factor-regulated protein kinases by hypericin. Biochem Pharmacol. 1995;49:1615–1622. doi: 10.1016/0006-2952(95)00097-j. [DOI] [PubMed] [Google Scholar]
- Aramaki Y, Chiba K, Tada M. Spiro-lactones, hyperolactone A-D from Hypericum chinense. Phytochemistry. 1995;38:1419–1421. [Google Scholar]
- Birt DF, Widrlechner MP, LaLone CA, Wu L, Bae J, Solco AKS, Kraus GA, Murphy PA, Wurtele ES, Leng Q, Hebert SC, Maury WJ, Price JP. Echinacea in infection. Am J Clin Nutr. 2008;87(suppl):488S–92S. doi: 10.1093/ajcn/87.2.488S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombardelli E, Morazzoni P. Hypericum perforatum. Fitoterapia. 1995;66:43–68. [Google Scholar]
- Brenner DM, Widrlechner MP. Amaranthus seed regeneration in plastic tents in greenhouses. FAO/IPGRI Plant Genetic Resources Newsletter. 1998;11:1–4. [Google Scholar]
- Carpenter S, Fehr MJ, Kraus GA, Petrich JW. Chemiluminescent activation of the antiviral activity of hypericin: A molecular flashlight. Proc Natl Acad Sci USA. 1994;91:12273–12277. doi: 10.1073/pnas.91.25.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Witte P, Agostinis P, Van Lint J, Merlevede W, Vandenheede JR. Inhibition of epidermal growth factor receptor tyrosine kinase activity by hypericin. Biochem Pharmacol. 1993;46:1929–1936. doi: 10.1016/0006-2952(93)90633-8. [DOI] [PubMed] [Google Scholar]
- Degar S, Prince AM, Pascual D, Lavie G, Levin B, Mazur Y, Lavie D, Ehrlich LS, Carter C, Meruelo D. Inactivation of the human immunodeficiency virus by hypericin: evidence for photochemical alterations of p24 and a block in uncoating. AIDS Res Hum Retrovir. 1992;8:1929–1936. doi: 10.1089/aid.1992.8.1929. [DOI] [PubMed] [Google Scholar]
- Farnet CM, Wang B, Hansen M, Lipford JR, Zalkow L, Robinson WE, Jr, Siegel J, Bushman F. Human immunodeficiency virus type 1 cDNA integration: New aromatic hydroxylated inhibitors and studies of the inhibition mechanism. Antimicrob Agents Chemother. 1998;42:2245–2253. doi: 10.1128/aac.42.9.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzera M, Zhao J, Khan IA. Hypericum perforatum - Chemical profiling and quantitative results of St. John’s wort products by an improved high-performance liquid chromatography method. J Pharml Sci. 2002;91:623–630. doi: 10.1002/jps.10057. [DOI] [PubMed] [Google Scholar]
- Gaudin M, Simonnet X, Debrunner N. Colletotrichum gloeosporioides as the cause of St. John’s wort (Hypericum perforatum) dieback in Switzerland and breeding for a tolerant variety. In: Ernst E, editor. Hypericum: The Genus Hypericum. Taylor and Francis; London: 2003. pp. 23–42. [Google Scholar]
- Gibbons S, Moser E, Hausmann S, Stavri M, Smith E, Clennett C. An anti-staphylococcal acylphloroglucinol from Hypericum foliosum. Phytochemistry. 2005;66:1472–1475. doi: 10.1016/j.phytochem.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Hammer KDP, Hillwig ML, Solco AKS, Dixon PM, Delate K, Murphy PA, Wurtele ES, Birt DF. Inhibition of PGE2 production by anti-inflammatory Hypericum perforatum extracts and constituents in RAW264.7 mouse macrophage cells. J AgrFood Chem. 2007;55:7323–7331. doi: 10.1021/jf0710074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer KDP, Hillwig ML, Neighbors JD, Sim YJ, Kohut ML, Wiemer DF, Wurtele ES, Birt DF. Pseudohypericin is necessary for the light-activated inhibition of prostaglandin E2 by Hypericum perforatum. Phytochemistry. 2008;69:2354–2362. doi: 10.1016/j.phytochem.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand PD, Jensen KIN. Potential for the biological control of St. John’s-wort (Hypericum perforatum) with an endemic strain of Colletotrichum gloeosporioides. Canad J Pl Pathol. 1991;13:60–70. [Google Scholar]
- Hudson JB, Lopez-Bazzocchi I, Towers GH. Antiviral activities of hypericin. Antiviral Res. 1991;15:101–112. doi: 10.1016/0166-3542(91)90028-p. [DOI] [PubMed] [Google Scholar]
- Ichinari D, Ueki T, Yoshihara K, Kinoshita T. First total synthesis of (±)-hyperolactone. A Chem Commun (Cambridge) 1997;18:1743–1744. [Google Scholar]
- Jacobson JM, Feinman L, Liebes L, Ostrow N, Koslowski V, Tobia A, Cabana BE, Lee D, Spritzler J, Prince AM. Pharmacokinetics, safety, and antiviral effects of hypericin, a derivative of St. John’s Wort plant, in patients with chronic hepatitis C virus infection. Antimicrob Agents Chemother. 2001;45:517–524. doi: 10.1128/AAC.45.2.517-524.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartnig T, Goebel I, Heydel B. Production of hypericin, pseudohypericin and flavonoids in cell cultures of various Hypericum species and their chemotypes. Pl Med. 1996;62:51–3. doi: 10.1055/s-2006-957796. [DOI] [PubMed] [Google Scholar]
- Kraus GA, Jeon I. Use of allylic strain to enforce stereochemistry. A direct synthesis of calamenenes from Hypericum elodeoides. Organic Lett. 2006;8:5315–6316. doi: 10.1021/ol0621194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus GA, Pratt D, Tossberg J, Carpenter S. Antiretroviral activity of synthetic hypericin and related analogs. Biochem Biophys Res Commun. 1990;172:149–153. doi: 10.1016/s0006-291x(05)80185-8. [DOI] [PubMed] [Google Scholar]
- Kraus GA, Wei J. A direct synthesis of Hyperolactone C. J Nat Prod. 2004;67:1039. doi: 10.1021/np0498962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn D. Hypericin: an answer for safer blood? Common Factor. 1995;10:36. [PubMed] [Google Scholar]
- Laurent D, Baumann F, Benoit AG, Mortelecqe A, Nitatpattana N, Desvignes I, Debitus C, Laille M, Gonzalez JP, Chungue E. Structure-activity relationships of dengue antiviral polycyclic quinines. Southeast Asian J Trop Med Public Health. 2005;36:901–905. [PubMed] [Google Scholar]
- Lavie G, Mazur Y, Lavie D, Prince AM, Pascual D, Liebes L, Levin B, Meruelo D. Hypericin as an inactivator of infectious viruses in blood components. Transfusion. 1995;35:392–400. doi: 10.1046/j.1537-2995.1995.35595259149.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Bazzocchi I, Hudson JB, Towers GH. Antiviral activity of the photoactive plant pigment hypericin. Photochem Photobiol. 1991;54:95–98. doi: 10.1111/j.1751-1097.1991.tb01990.x. [DOI] [PubMed] [Google Scholar]
- Marais JPJ, Ferreira D, Slade D. Stereoselective synthesis of monomeric flavonoids. Phytochemistry. 2005;66:2145–2176. doi: 10.1016/j.phytochem.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Mathela DK, Mathela CS, Dev V. Volatile constituents of Hypericum elodeoides Chois. J Indian Chem Soc. 1984;61:792–793. [Google Scholar]
- McCoy J. Utilizing the national plant germplasm system for medicinal plant research. In: Janick J, Whipkey A, editors. Issues in New Crops and New Uses. 2007. In press. [Google Scholar]
- Meruelo D, Lavie G, Lavie D. Therapeutic agents with dramatic antiretroviral activity and little toxicity at effective doses: aromatic polycyclic diones hypericin and pseudohypericin. Proc Natl Acad Sci USA. 1988;85:5230–5234. doi: 10.1073/pnas.85.14.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- Naesens L, Bonnafous P, Agut H, De Clercq E. Antiviral activity of diverse classes of broad-acting agents and natural compounds in HHV-6-infected lymphoblasts. J Clin Virol. 2006;37(Suppl 1):S69–75. doi: 10.1016/S1386-6532(06)70015-4. [DOI] [PubMed] [Google Scholar]
- Park J, English DS, Wannemuehler Y, Carpenter S, Petrich JW. The role of oxygen in the antiviral activity of hypericin and hypocrellin. Photochem Photobiol. 1998;68:593–597. [PubMed] [Google Scholar]
- Percifield RJ, Hawkins JS, McCoy J, Widrlechner MP, Wendel JF. Genetic diversity in Hypericum and AFLP markers for species-specific identification of H. perforatum L. Pl Med. 2007 doi: 10.1055/s-2007-993749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed-Inderbitzin E, Maury W. Cellular specificity of HIV-1 replication can be controlled by LTR sequences. Virology. 2003;314:680–695. doi: 10.1016/s0042-6822(03)00508-7. [DOI] [PubMed] [Google Scholar]
- Robson NKB. Hypericum botany. In: Ernst E, editor. Hypericum: The Genus Hypericum. Taylor and Francis; London: 2003. pp. 1–22. [Google Scholar]
- US Department of Agriculture. Germplasm Resources Information Network Database. 2007 Accessed on the Internet at http://www.ars-grin.gov/npgs on 27 August 2007.
- Viola H, Wasowski C, Levi de Stein M, Wolfman C, Silveira R, Dajas F, Medina JH, Paladini A C. Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects. Pl Med. 1995;61:213–216. doi: 10.1055/s-2006-958058. [DOI] [PubMed] [Google Scholar]
- Vyas GN. Inactivation and removal of blood-borne viruses. Transfusion. 1995;35:367–370. doi: 10.1046/j.1537-2995.1995.35595259143.x. [DOI] [PubMed] [Google Scholar]
- Widrlechner MP, Abel CA, Wilson RL. Ornamental seed production in field cages with insect pollination. Comb Proc IPPS. 1997;46:512–516. [Google Scholar]
- Widrlechner MP, McKeown KA. Assembling and characterizing a comprehensive Echinacea germplasm collection. In: Janick J, Whipkey A, editors. Trends in New Crops and New Uses. ASHS Press; Alexandria, VA.: 2002. pp. 506–508. [Google Scholar]