Abstract
The study of distal lung morphogenesis and vascular development would be greatly facilitated by an in vitro or ex vivo experimental model. In this study we show that the growth of mouse embryonic day 12.5 lung rudiments implanted underneath the kidney capsules of syngeneic or immunodeficient hosts follows closely lung development in utero. The epithelium develops extensively with both proximal and distal differentiation to the saccular stage. The vasculature also develops extensively. Large blood vessels accompany large airways and capillaries develop within the saccular walls. Interestingly, vessels in the lung grafts develop from endothelial progenitor cells endogenous to the explants and host vessels do not vascularize the grafts independently. This suggests that embryonic lungs possess mechanisms to prevent the inappropriate ingrowth of surrounding vessels. However, vessels in the lung grafts do connect to host vessels, showing that embryonic lungs have the ability to stimulate host angiogenesis and recruit host vessel connections. These data support the hypothesis that the lung vasculature develops by both vasculogenic and angiogenic processes: a vascular network develops in situ in lung mesenchyme, which is then connected to angiogenic processes from central vessels. The lung renal capsule allograft is thus an excellent model to study the development of the pulmonary vasculature and of late fetal lung development that requires a functional blood supply.
Keywords: Lung development, Saccular development, Vascular development, Angiogenesis, Vasculogenesis, Lung allograft
1. Introduction
Normal lung function requires an intimate relationship between airways and blood vessels to form the air-blood interface needed for gas exchange. The elegant design of the lungs achieves this functional coupling by having blood vessels run parallel to the airways and at the end form capillary networks surrounding the alveoli. Despite the importance of the vasculature to lung function, the molecular factors that regulate lung vasularization are not well characterized. Also not known are the regulatory interactions that must be necessary for the coordinated development of airways and blood vessels. Airway development in the mouse starts on embryonic day 9.5 (E9.5) by the budding of the ventral foregut into the surrounding mesenchyme. The epithelium then undergoes a series of repetitive branching, coupled with progressive maturation of epithelial cells, to form the conducting airways and then the respiratory portions of the lungs. Four stages of lung development have been described, based mainly on airway morphology (Boyden, 1977; Burri, 1984). The pseudoglandular stage spans E9.5–16.5 in the mouse and is characterized by growth and branching of the epithelium to form the conducting airways and the beginning of the pulmonary acinus, the respiratory or gas-exchange unit. The pattern of epithelial branching is stereotypical and is determined by epithelial-mesenchymal interactions. At the end of the pseudoglandular period, the airways terminate in a prospective acinus composed of the terminal bronchiole and small clusters of short tubules and buds. The canalicular stage spans E16.5–17.5 in the mouse. During this stage, there is thinning of the intervening mesenchyme and the clusters of tubules and buds develop by lengthening and further peripheral branching and widening of the distal airspaces. The saccular stage spans E17.5–P5 (postnatal day 5) and is characterized by enlargement and further division of the distal airspaces into smooth-walled structures, termed saccules. The wall of these saccules is thick and is composed of epithelial lining on either side of a central core of connective tissue containing two capillary networks. The alveolar stage occurs after birth and is characterized by the remodeling of saccules into alveoli.
During the formation of the epithelium, blood vessels are formed concurrently in the surrounding mesenchyme. DeMello et al. described the presence of vascular spaces surrounding the airways as early as E10 (deMello et al., 1997). This is thought to be a vasculogenic process, the in situ development of a vascular network from endothelial precursor cells derived from lung mesenchyme. Sproutings from central vessels by angiogenesis were also identified by vascular casting following injection of the right heart chambers. Connections between the central and peripheral systems were seen starting on E13–14. The authors suggested that these data support a model of lung vascularization by both angiogenic and vasculogenic processes: the de novo differentiation in the mesenchyme of a primitive vascular plexus (vasculogenesis) that surrounds the growing and branching epithelium. The primitive plexus then remodels into more mature vessels. In addition, angiogenic extensions arise centrally, grow into the lungs and establish connections to the peripheral system. A study by Schachtner et al. using mice with a β-galactosidase gene recombined into the flk-1 locus, thereby marking endothelial cells by their expression of β-galactosidase activity, showed the presence of connections between the aortic sac and the forming primitive vascular network in lung mesenchyme as early as E10.5 (Schachtner et al., 2000). This suggests that central pulmonary vessels are also formed by vasculogenesis. The authors suggested that as more peripheral vessels remodel to form patent tubular structures, they became detectable by intravascular casting, thus giving the impression that the proximal vessels grow by extension. This study also showed that quantitatively, there is a steady increase of vessel formation in the lungs, unlike previous assumption that there is a burst of vessel formation, ‘a vascular phase of lung development’, starting with the canalicular phase.
The study of early epithelial development has been greatly aided by studying branching morphogenesis in lung organ cultures as well as by transgenic and gene targeting studies (Perl and Whitsett, 1999; Warburton et al., 2000; Cardoso, 2001). However, study of epithelial development in the late fetal stages, including saccular formation, has relied primarily on the phenotypes of mice with targeted gene inactivation, since distal lung development does not occur in lung organ cultures. This may be due in part to the lack of a blood supply in the cultured explants. The complex morphogenesis of saccules and alveoli may depend on interactions of the epithelium with a functioning circulatory system to assure the coupling of the two systems. The molecular mechanisms regulating the development of the pulmonary vasculature are not well understood. Thus a model that support vascular formation and late lung development that is accessible to experimental manipulation and free from placental barrier would greatly aid the investigation of the molecular mechanisms regulating these processes. Recently, a model has been described by Schwarz et al. that supports distal lung morphogenesis and vascularization (Schwarz et al., 2000). In this model E14.5 mouse embryonic lung explants are grafted subcutaneously in immunodeficient host mice and allowed to develop for 2 weeks. The authors observed both proximal and distal differentiation of the epithelium as well as extensive neovascularization of the lung grafts. In this paper we describe a lung allograft model that takes advantage of the renal capsule graft model used to study development of other organs. In this model, we observed epithelial development that follows closely its temporal development in utero. We also observed extensive vascular development, and have characterized it in detail, showing that the embryonic lungs must contain endogenous signals that prevent inappropriate neovascularization, while allowing for the development of intrinsic vessels and the recruitment of host vessel connections. This is thus an excellent model for the study of both distal lung morphogenesis and the development of the pulmonary vasculature.
2. Results
2.1. Epithelial development in lung renal capsule allografts
Since renal capsule grafts have been used successfully to study the development of many organs including that of prostate and mammary glands, we reasoned that it might also serve as a model for lung development. We grafted E12.5 embryonic lung rudiments underneath the renal capsules of either syngeneic or immunodeficient mice and studied the course of their development over 8 days. At E12.5, the lung rudiments are relatively simple, containing the main lobar bronchi and a few further branches (Fig. 1A). After 8 days in the renal capsule graft there is extensive development of the lung explants (Fig. 1B). There are no differences in the development of lung rudiments grafted in the kidneys of either syngeneic or immunodeficient mice. The development of the lung explant after 8 days resembles that of E19.5-neonate lungs (Fig. 1C–E). There is tremendous increase in size. The epithelium develops extensively and appears to go through all stages of morphological differentiation. There are large airways lined by columnar ciliated epithelium and surrounded by cartilage, resembling trachea and bronchi (Fig. 1C). There are smaller airways lined by cuboidal epithelium resembling bronchioles (Fig. 1D). There are also structures typical of saccules with walls lined by attenuated epithelial cells (Fig. 1D). These resemble the saccular stage of embryonic lung development (Fig. 1E). To determine more closely the temporal development of the lung grafts, we examined explant development after 4 and 6 days. We found that the development of lung renal capsule graft follows in utero lung development closely. After 4 days the lung grafts resemble E15.5 lungs at the pseudoglandular stage of lung development with many epithelial tubes lined by either high columnar or cuboidal epithelial cells (Fig. 2A,B). After 6 days, there is further development of epithelium with the formation of numerous branched and widened distal airspaces lined by compact cuboidal and flat epithelium with thick intervening mesenchyme (Fig. 2C). This resembles E17.5 lungs at the canalicular stage of epithelial development (Fig. 2D). Thus development of the lung explant in the renal capsule grafts follows closely temporally in utero development, without significant delay. This contrasts with the extent of development achieved in lung organ cultures. A typical in vitro organ culture of E11.5 lung explant shows a modest increase in size combined with several generations of branching after 3 days (Fig. 3A,B). With longer time in culture the explants cease development and start to deteriorate, with dilatation of the terminal buds (Fig. 3C). In vitro cultures of E12.5 lung explant are viable longer and show more branching. However, the epithelium remains in the pseudoglandular stage after 5 days (Fig. 3D). With longer time in culture, the explants also deteriorate and do not continue into canalicular or saccular stages (data not shown).
Fig. 1.
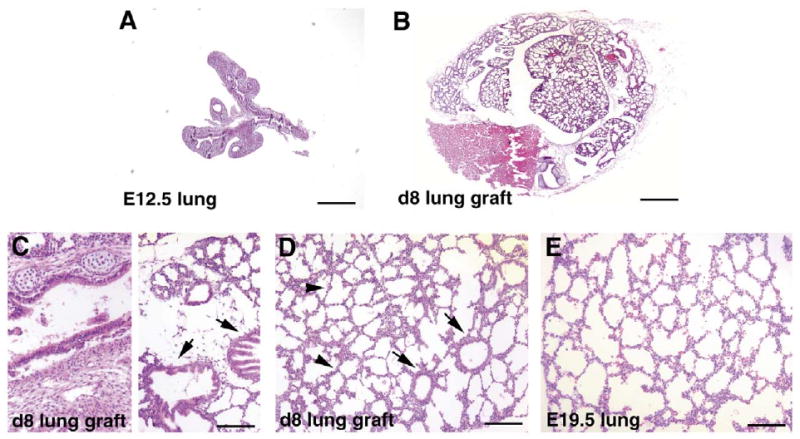
Epithelial development in lung renal capsule grafts. (A) Hematoxylin and eosin (H&E) stained paraffin sections of E12.5 lung rudiment prior to grafting. (B) H&E-stained section of lung rudiment after 8 days of grafting underneath the renal capsule. (C,D) Airway development in day 8 lung renal capsule grafts. Left side of panel (C) shows a large airway lined by ciliated columnar epithelial cells and surrounded by cartilage, right side of panel (C) shows large airways lined by ciliated columnar epithelial cells that are not surrounded by cartilage (arrows); panel D shows smaller airways lined by cuboidal epithelial cells (arrows) and saccular structures lined by attenuated epithelial cells (arrowheads). (E) H&E-stained section of E19.5 embryonic lungs showing the saccular stage of lung development. Bars: A,B, 400 μm; C–E, 100 μm.
Fig. 2.
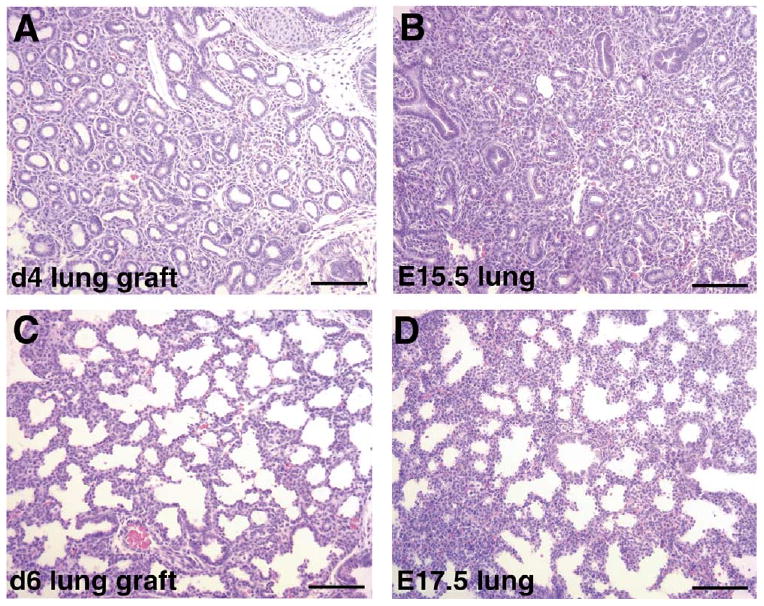
Epithelial development in lung renal capsule grafts at 4 and 6 days. (A,B) H&E-stained sections of lung rudiment after 4 days of grafting (A) and of embryonic lung at E15.5 (B), showing the pseudoglandular stage of embryonic lung development with branching epithelial tubes surrounded by thick mesenchyme. (C,D): H&E-stained sections of lung rudiment after 6 days of grafting (C) and of embryonic lung at E17.5 (D), showing the canalicular stage of embryonic lung development with branched and widened distal airspaces surrounded by thick mesenchyme. Bar: 100 μm.
Fig. 3.
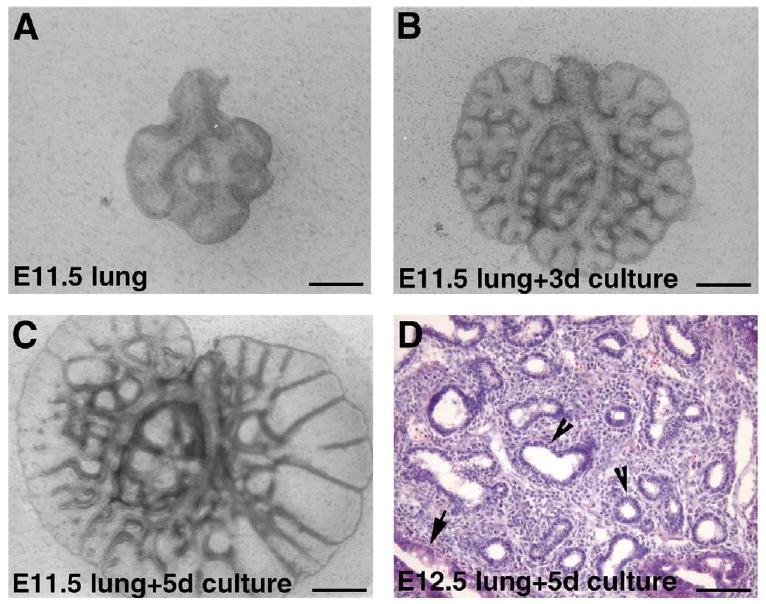
Epithelial development in lung organ cultures. (A) E11.5 lung on the day of dissection. (B) E11.5 lung after 3 days in organ culture. (C) E11.5 lung after 5 days in organ culture. (D) H&E-stained sections of E12.5 lung after 5 days in organ culture. Epithelial tubes are lined by columnar (arrow) or cuboidal (arrowhead) epithelial cells, consistent with the pseudoglandular stage. Bars: A–C, 400 μm; D, 100 μm.
To determine if epithelial differentiation occurs normally in the lung grafts, we assayed for the expression of proximal and distal epithelial cell markers. Clara cell specific protein (CCSP) is expressed by nonciliated bronchiolar epithelial (Clara) cells and indicates proximal differentiation (Hackett et al., 1992; Strum et al., 1992). Surfactant protein C (proSP-C) is a marker for type II epithelial cells, indicative of distal differentiation (Kalina et al., 1992). In the late fetal stage, SP-C is expressed by cells located in the distal acinar tubules and buds and in cells of the saccular walls. Immunostaining for CCSP and proSP-C showed expression of these two markers in the appropriate structures in the lung grafts and at the appropriate times of development. CCSP is expressed by bronchiolar epithelial cells (Fig. 4A,B). ProSP-C is expressed by attenuated epithelial cells in the distal acinar tubules and buds in day 6 lung grafts (canalicular stage, Fig. 4C), and in the saccular walls in day 8 lung grafts (saccular stage, Fig. 4D).
Fig. 4.
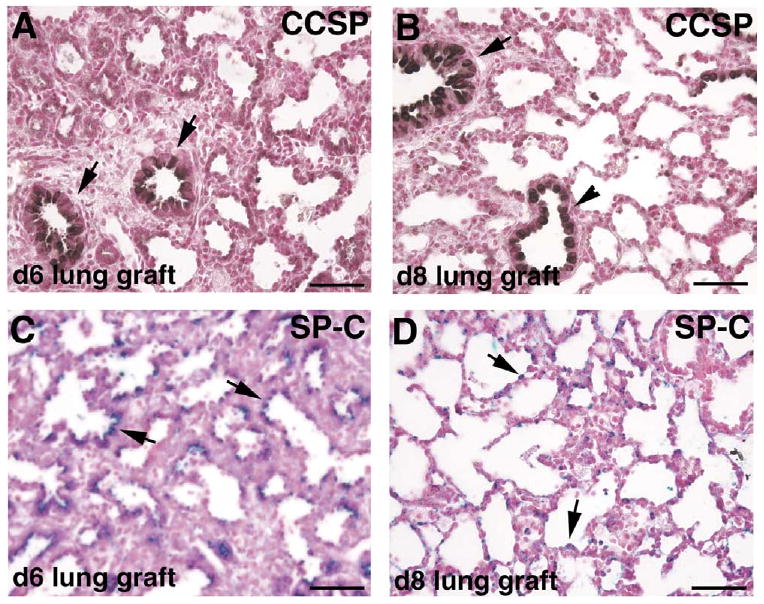
Epithelial differentiation in lung renal capsule grafts. (A,B) Immunostaining for Clara cell specific protein (CCSP) of day 6 (A) and day 8 (B) lung grafts; positively stained (brown) cells are found in airways lined by columnar epithelial cells (arrows) and in those lined by cuboidal epithelial cells (arrowhead). (C,D) Immunostaining for pro surfactant protein C (proSP-C) of day 6 (C) and day 8 (D) lung grafts; positively stained (blue) cells are found in the distal acinar tubules and buds (C, arrows) and in the saccular walls (D, arrows). Bar: 50 μm.
2.2. Blood vessel development in lung renal capsule allografts
In the E12.5 lung there is a network of blood vessels in the mesenchyme surrounding the distal epithelial tubes as evidenced by immunostaining for the endothelial cell marker CD31 (PECAM-1). There are capillary-like vessels surrounding the budding epithelial tubes as well as vessels that have larger lumen (Fig. 5A). After 8 days of grafting under the kidney capsules, there is extensive development of blood vessels in the lung explants. On hematoxylin and eosin staining of day 8 lung grafts, blood vessels of different sizes are apparent as tubular structures with red blood cells in them. There are large vessels that are found along side large airways (Fig. 5B). There are also apparent capillaries in the saccular walls as evident by the presence of red blood cells in these walls (Fig. 5C). Immunostaining for CD31 confirms that there is an extensive network of blood vessels in the lung grafts, including capillaries in the saccular walls (Fig. 5D). The vessels are found on day 4 as capillary networks surrounding epithelial tubes (data not shown), and by day 6 are evident as both large vessels and capillary networks in the mesenchyme surrounding airspaces (Fig. 5E). This contrasts with the amount of vascular development achieved in organ cultures. E11.5 lung explants cultured in vitro for 3 days show development of a plexus of capillary-like network in the mesenchyme surrounding the epithelial tubes (Fig. 5F). This has also been observed previously by other investigators (Healy et al., 2000). There is no further development of the vasculature in vitro; specifically, there is no remodeling of the capillary plexus into larger-sized vessels (data not shown).
Fig. 5.
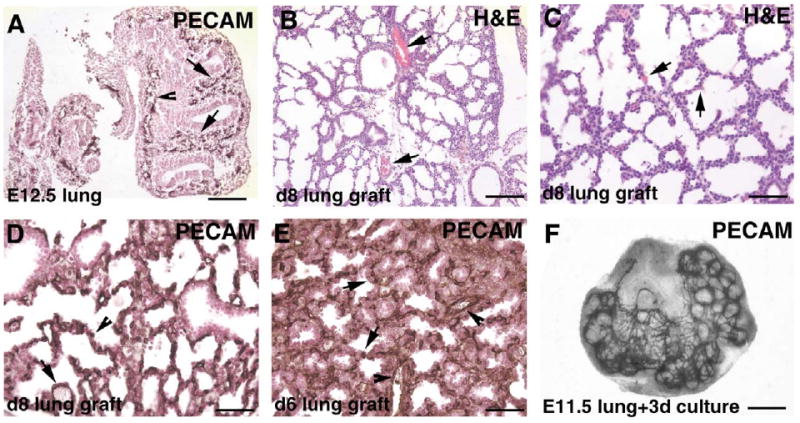
Blood vessel development in lung renal capsule grafts. (A) Blood vessels in the E12.5 lung shown by immunostaining for the endothelial cell marker CD31 (PECAM-1). At this stage, there is a network of capillaries in the mesenchyme surrounding the epithelial tubes (arrows), as well as some vessels with larger lumen (arrowhead). (B,C) H&E-stained paraffin sections of day 8 lung graft, showing large vessels with red blood cells (B, arrows) and capillaries with red blood cells in the saccular walls (C, arrows). (D,E) Immunostaining for the endothelial cell marker CD31 show both large vessels (arrows) and extensive networks of capillaries (arrowheads) in day 8 (D) and day 6 (E) lung grafts. (F) Blood vessels in the E11.5 lung after 3 days in culture shown by whole-mount immunostaining for the endothelial cell marker CD31. There is a plexus of capillaries surrounding the epithelial tubes. Bar: A,B, 100 μm; C–E, 50 μm; F, 400 μm.
The large difference between lung development in the organ culture system and in the renal capsule allograft model raises the question as to whether vascular and distal lung development requires blood vessel development from precursors arriving from outside the lungs. Alternatively, the lack of perfusion in organ culture may prevent more than rudimentary development. To determine if blood vessels in the grafts develop from neovascularization of the grafts by host vessels or from endothelial cells endogenous to the lung explants, we grafted wild-type embryonic lung rudiments under the renal capsules of host mice that carry a β-galactosidase transgene recombined into the flk-1 locus (Shalaby et al., 1995). Flk-1 (VEGFR2) is a receptor for vascular endothelial growth factor (VEGF) and is expressed by endothelial cells. Thus, host endothelial cells can be identified by the expression of β-galactosidase activity. Substrate staining of frozen sections of the day 8 lung grafts for β-galactosidase activity showed that there are no endothelial cells in the grafts expressing this enzyme, indicating that host endothelial cells have not vascularized the grafts (Fig. 6A). Staining of sections of the kidney harboring the grafts showed that host endothelial cells do indeed express this enzyme, as shown by blue staining of endothelial cells of the glomerular tufts, and of vessels adjacent to the tubules (Fig. 6B). Thus vessels that develop in the grafts come from endothelial cells endogenous to the graft itself.
Fig. 6.
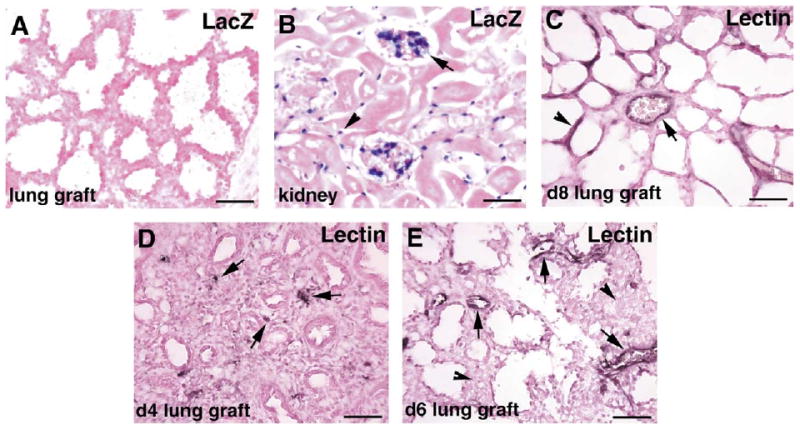
Lung graft vessels are endogenous to the grafts but are connected to host vessels. (A,B) β-Galactosidase staining of lung graft (A) and kidney (B) sections from wild-type lung rudiments that were grafted into KDR.LacZ mice for 8 days. Host endothelial cells in the glomerular tufts (arrow) and between the tubules (arrowhead) in the host kidney stain positive (blue) for β-galactosidase activity (B), but there are no host endothelial cells in the graft (A). (C) Intravenous perfusion of biotinylated lectin into the host shows that vessels in the 8-day lung grafts are connected to the host circulation, as evidenced by lectin staining of both large vessel (arrow) and capillaries (arrowhead) in the grafts. (D,E) Intravenous perfusion of biotinylated lectin into the host after 4 days (D) and 6 days (E) of grafting shows dot-like areas of lectin staining (arrows) within the mesenchyme of the lung grafts at 4 days, and lectin staining of larger caliber vessels (arrows), but no staining of capillaries in the mesenchyme (arrowheads) at 6 days. Bar: 50 μm.
To determine if vessels in the grafts are connected to and perfused by the host circulation, we injected the host mice intravenously via the tail vein with a solution of biotinylated Lycopersicon esculentum (tomato) lectin prior to sacrifice and harvest of the grafts. The injected lectin circulates in the host vasculature and binds to endothelial cells. Subsequent staining of tissue sections with horseradish peroxidase-conjugated streptavidin to expose the presence of biotin reveals vessels that are connected to the host circulation and therefore exposed to the injected biotinylated lectin. The results show that the endothelial lining of vessels in the day 8 lung grafts are labeled with the injected lectin (Fig. 5C). Thus vessels in the grafts are connected to and perfused by host vessels. To determine the time course of host vessel connections to lung graft vessels, we injected biotinylated lectin into host mice harboring lung explants after 4 and 6 days of grafting. Subsequent staining of tissue sections for biotinylated lectin show that after 4 days there are scattered spots of lectin staining within the mesenchyme of the lung grafts (Fig. 6D). These may be capillary sprouts from the host that have grown into the lung explants. After 6 days, some of the larger vessels in the grafts are stained with the lectin, indicating their connections to host vessels (Fig. 6E). However, the capillary networks in the mesenchyme normally present by this time (see Fig. 5E) are not yet connected, as evidenced by their lack of lectin staining.
3. Discussion
We have established the renal capsule allografts of embryonic lung explants as an excellent model to study lung development. As renal capsule grafts, development of the lung explants occurs extensively and follows closely in utero development. This model offers great advantage over lung explant cultures. In contrast to the routine cultures of E11.5 lung explants, in which only several generations of growth and branching morphogenesis of the epithelium are achieved, we observed in the lung renal capsule allografts all the morphological stages of embryonic lung epithelial differentiation. The epithelium goes through the pseudoglandular, canalicular, and saccular stages at a pace similar to in utero development. Study of the expression of both proximal (CCSP) and distal (proSP-C) epithelial cell markers supports the normal differentiation of epithelial cells. Previous study by Schwarz et al. (2000) using subcutaneous lung allografts also showed distal lung morphogenesis. However, there are several differences between this model and ours. First, we implant lung rudiments at an earlier stage, E12.5 instead of E14.5, thus allowing for the study of an earlier period of lung development. Secondly, development in our model occurs in a similar time frame to development in utero with only a slight delay. E12.5 + 4 days lung grafts are similar in developmental stage to E15.5 lungs. E12.5 + 6 days lung grafts are similar in developmental stage to E17.5 lungs, and E12.5 + 8 days lung grafts are similar to E19.5-neonate lungs. In the subcutaneous model, it takes up to two weeks for the E14.5 lung rudiments to develop to the saccular stage.
A novel aspect of the current model is the development of the vascular system, which we have characterized in some details. We showed that E12.5 lung explants contain intrinsic endothelial cells and/or endothelial cell precursors that can form the lung vasculature, including both large vessels and capillaries in the saccular walls. We also showed that there are no vessels of host origin in the grafts. This is consistent with a previous study using quail-chick chimeras. Intracoelomic grafts of quail organ rudiments into chick hosts and vice versa showed that the vasculature in the lung grafts is derived from endothelial cell precursors intrinsic to the grafts and that host endothelial cells do not invade the grafts (Pardanaud et al., 1989). The subcutaneous lung allograft model by Schwarz et al. (2000) also showed development of blood vessels endogenous to the grafts. Interestingly, in our study, even though the lung explants are not vascularized by host blood vessels, the intrinsically developed graft vessels are connected to the host circulation, suggesting that the lung explants are able to stimulate host neoangiogenesis. However, these host vessels do not vascularize the grafts and grow independently of intrinsically developed graft vessels. Instead, they find and connect to the graft vessels. In explants that have been grafted into KDR.LacZ hosts, one can potentially demonstrate the anastomotic connections between host and graft vessels by double staining for lacZ and CD31. However, we have not detected lacZ staining in the grafts, even after analyzing many serial sections. These anastomotic connections may be either very rare or very peripheral and therefore may not be easily found.
This is the first example of a tissue that can develop its own intrinsic vessels, and yet recruit connections from surrounding host vessels. This may recapitulate how the vascularization of the lungs, and perhaps also of other organs, occurs. The fact that host vessels do not vascularize the lung grafts and grow independently of graft vessels, but instead find and connect to intrinsically developed graft vessels is of great interest. It shows that the developing lungs may contain strict regulatory mechanisms to direct the growth of blood vessels. This may include angiogenic inhibitors to prevent extrapulmonary vessels to inappropriately vascularize the lungs. Since the lungs are positioned next to the heart and the great vessels, such a mechanism may be necessary. The connection between host and graft vessels is also of great interest. How blood vessels are attracted to each other and form connections is a fundamental process during tissue vascularization. Yet little is known about the molecular factors involved. The ephrins and their ligands have been implicated in this process due to their complementary expression on arteries and veins (Wang et al., 1998). The lung renal capsule grafts may be useful in studying the function of these genes as well as other genes in this process.
Saccular development is currently not well understood. This is partly due to the lack of an accessible experimental model. Study of gene function in distal lung development has relied principally on transgenic or gene targeting studies. These studies are labor intensive and time consuming. The observation that saccular development occurs in our model makes it useful for the study of the regulatory mechanisms of saccular formation. With the renal capsule graft model, it will now be feasible to do gain-and loss-of-function studies more readily by the administration of reagents that either augment or inhibit gene function to the host mice. There is no placental barrier to overcome. The renal capsule graft model can also complement transgenic or gene targeting studies. Study of the function in lung development of genes whose deficiency results in early embryonic lethality can be carried out if the mutant embryos die after lung formation has initiated. We grafted E12.5 lung rudiments for ease of manipulation, but lungs of earlier gestational stage may also be grafted, depending on technical skill of the investigator. Thus lung rudiments from mutant embryos can be dissected out and grafted and their development studied. Lung grafts can also be used to distinguish between a direct effect on the lungs and an indirect effect due to a global and systemic effect of gene deficiency on the embryos. In this case, grafting the lung rudiments and observe their development isolated from the rest of the embryos will distinguish between these two effects.
4. Experimental procedures
4.1. Transplantation of embryonic lung rudiments underneath the renal capsule
E12.5 lungs were dissected from pregnant CD-1, C57Bl6, or C3H mothers (the day of the vaginal plug is considered E0.5) and placed in PBS on ice. Adult host mice were anesthetized, and the kidney exposed through a dorsal incision. An incision was made in the thin membranous capsule, and individual E12.5 lung was placed as a whole underneath the capsule. Two lungs were placed per kidney, and either one or both kidneys are used. After grafting, the kidney was placed back into its position, the muscle layer was closed with silk sutures, and the skin was stapled. CD-1 lungs were grafted into immunodeficient mice; C57Bl6 and C3H lungs were grafted into C57Bl6 or C3H hosts, respectively. After 4, 6, or 8 days (the day of grafting is day 0), the host mice were killed and the grafts removed for analysis.
4.2. Lung organ cultures
E12.5 lungs were dissected from pregnant CD1 mice and cultured on top of a Corning Nuclepore polycarbonate membrane of 13-mm diameter and 8 μm pore size (VWR, Brisbane, CA) at 37 °C in an atmosphere of ambient air + 5% CO2. The membrane is floated on top of 1 ml of culture medium in a well of a 24-well tissue culture plate. The culture medium is BGJb medium containing 1 μg/ml BSA, 10 μg/ml transferrin, and 50 μg/ml gentamycin.
4.3. Histological analysis and Immunohistochemistry
After removal, the lung grafts were fixed in 4% paraformaldehyde at 4 °C overnight and then processed for paraffin sections. For histological analysis, the sections were stained with hematoxylin and eosin. For immunohistochemistry with CD31 (PECAM-1) antibody, the sections were deparaffinized, washed in PBS, quenched in PBS + 3% H2O2 for 5 min, washed in PBS, then briefly treated with 0.1% Trypsin made in PBS for a few minutes at 37 °C. The slides are then washed in PBS and blocked in PBS + 2% normal serum for 2 h. The sections were then incubated with rat monoclonal anti mouse CD31 antibody (clone MEC 13.3, Pharmingen, San Diego, CA) diluted 1:100 in PBS + 1 mg/ml BSA at 4 °C overnight. Following primary antibody incubation, the slides were washed in PBS, incubated with biotinylated secondary antibody for 1 h, washed in PBS, then incubated with Vector Elite ABC reagent (Vector Laboratory, Burlingame, CA) for 1 h, washed in PBS, and developed with DAB substrate. For immunohistochemistry with proSP-C and CCSP antibodies, the sections were deparaffinized, washed in PBS, quenched in methanol + 0.5% H2O2 for 5 min, washed in PBS pH 7.2, then blocked in 0.1 M phosphate buffer pH 7.4 + 0.2% Triton X-100 and 2% normal serum for 2 h. The sections were then incubated with primary antibody diluted in 0.1 M phosphate buffer pH 7.4 + 0.2% Triton X-100 + 1 mg/ml BSA at 4 °C overnight. Following primary antibody incubation, the slides were washed in 0.1 M phosphate buffer pH 7.4 + 0.2% Triton X-100, incubated with biotinylated secondary antibody for 1 h, washed in 0.1 M phosphate buffer pH 7.4 + 0.2% Triton X-100, then incubated with Vector Elite ABC reagent for 1 h. The slides were then washed in 0.1 M phosphate buffer pH 7.5 + 0.2% Triton X-100, and developed with DAB substrate. Rabbit anti human proSP-C antibody is from Chemicon (Temecula, CA) and was used at dilution 1:500. Rabbit anti rat CCSP antibody is a gift from Dr. Gurmukh Singh (Department of Veteran Affairs, Pittsburg Health Care Systems, Pittsburgh, PA) and was used at dilution 1:5000.
4.4. Whole-mount immunostaining
Tissues were fixed in 4% paraformaldehyde overnight at 4 °C, washed in PBS, dehydrated in a graded series of methanol, then quenched in 5% H2O2 in methanol for 30 min. The tissues were then rehydrated in a graded series of methanol to PBS, blocked in PBS-BT (PBS + 1% BSA and 0.1% Triton X-100) at room temperature for 30 min, incubated with rat anti mouse CD31 antibody diluted 1:100 in PBS-BT overnight at 4 °C. The tissues were then washed in PBS-BT, incubated with HRP-conjugated goat anti rat IgG antibody (Jackson Laboratories, Westgrove, PA) diluted in PBS-BT for 1 h at room temperature, washed in PBS-BT, and developed with DAB substrate.
4.5. β-Galactosidase activity staining
E12.5 lungs from C57Bl6 pregnant mothers were dissected and grafted underneath the kidney capsules of C57Bl6/KDR.LacZ host mice (Jackson Labs, Bar Harbor, ME). After 8 days, the host mice were killed and the lung grafts removed. Pieces of the kidney harboring the grafts were also removed for positive control. The lung grafts or kidney fragments were then fixed in 0.2% paraformaldehyde in PIPES buffer, pH 6.9, at 4 °C overnight, equilibrated in 30% sucrose in PBS for several hours and embedded in OCT. Frozen sections (10 mm) were stained for β-galactosidase activity as follows. The slides were washed in PBS + 2 mM MgCl2 for 5 min and incubated in Xgal staining solution (5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 1 mg/ml Xgal in PBS + 2 mM MgCl2) at 37 °C until color developed. The slides were then washed in PBS + 2 mM MgCl2, rinsed in ddH2O, counterstained with contrast red (KPL laboratory, Gaithersburg, MD), dehydrated in a series of methanol, cleared in xylene, and mounted.
4.6. Labeling of blood vessels by intravenous injection of biotinylated lectin
Before sacrifice the host mice were injected intravenously via the tail vein with 200 μl of a solution of 0.5 mg/ml biotinylated Lycopersicon esculentum (tomato) lectin (Vector Laboratory) in PBS. After 20 min, the mice were killed, and the lung grafts as well as pieces of the kidney harboring the grafts were removed and washed briefly in PBS. The lung grafts or kidney fragments were then fixed in 4% paraformaldehyde at 4 °C overnight, equilibrated in 30% sucrose in PBS for several hours and embedded in OCT. Frozen sections (10 μm) were washed in PBS, quenched in PBS + 3% H2O2 for 5 min, washed again in PBS, and incubated with either PBS or Vectastain elite ABC reagent for 1 h at room temperature, then washed in PBS and developed with DAB substrate. After staining, the sections were counterstained with contrast red, air dried, and mounted.
Acknowledgments
This study was supported by grants from the National Institutes of Health (HL03880 and HL69925) to T.H.V., and by grants from the Human Frontier Science Program (RG005/199M), and the National Institutes of Health (AI053194) to Z.W.
References
- Boyden EA. Development and growth of the airways. In: Hodson WA, editor. Development of the Lung, Lung Biology in Health and Disease. Marcel Dekker; New York: 1977. pp. 3–35. [Google Scholar]
- Burri PH. Fetal and postnatal development of the lung. Annu Rev Physiol. 1984;46:617–628. doi: 10.1146/annurev.ph.46.030184.003153. [DOI] [PubMed] [Google Scholar]
- Cardoso WV. Molecular regulation of lung development. Annu Rev Physiol. 2001;63:471–494. doi: 10.1146/annurev.physiol.63.1.471. [DOI] [PubMed] [Google Scholar]
- deMello DE, Sawyer D, Galvin N, Reid LM. Early fetal development of lung vasculature. Am J Respir Cell Mol Biol. 1997;16:568–581. doi: 10.1165/ajrcmb.16.5.9160839. [DOI] [PubMed] [Google Scholar]
- Hackett BP, Shimizu N, Gitlin JD. Clara cell secretory protein gene expression in bronchiolar epithelium. Am J Physiol. 1992;262:L399–L404. doi: 10.1152/ajplung.1992.262.4.L399. [DOI] [PubMed] [Google Scholar]
- Healy AM, Morgenthau L, Zhu X, Farber HW, Cardoso WV. VEGF is deposited in the subepithelial matrix at the leading edge of branching airways and stimulates neovascularization in the murine embryonic lung. Dev Dyn. 2000;219:341–352. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1061>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Kalina M, Mason RJ, Shannon JM. Surfactant protein C is expressed in alveolar type II cells but not in Clara cells of rat lung. Am J Respir Cell Mol Biol. 1992;6:594–600. doi: 10.1165/ajrcmb/6.6.594. [DOI] [PubMed] [Google Scholar]
- Pardanaud L, Yassine F, Dieterlen-Lievre F. Relationship between vasculogenesis, angiogenesis and haemopoiesis during avian ontogeny. Development. 1989;105:473–485. doi: 10.1242/dev.105.3.473. [DOI] [PubMed] [Google Scholar]
- Perl AK, Whitsett JA. Molecular mechanisms controlling lung morphogenesis. Clin Genet. 1999;56:14–27. doi: 10.1034/j.1399-0004.1999.560103.x. [DOI] [PubMed] [Google Scholar]
- Schachtner SK, Wang Y, Scott Baldwin H. Qualitative and quantitative analysis of embryonic pulmonary vessel formation. Am J Respir Cell Mol Biol. 2000;22:157–165. doi: 10.1165/ajrcmb.22.2.3766. [DOI] [PubMed] [Google Scholar]
- Schwarz MA, Zhang F, Lane JE, Schachtner S, Jin Y, Deutsch G, Starnes V, Pitt BR. Angiogenesis and morphogenesis of murine fetal distal lung in an allograft model. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1000–L1007. doi: 10.1152/ajplung.2000.278.5.L1000. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Strum JM, Compton RS, Katyal SL, Singh G. The regulated expression of mRNA for Clara cell protein in the developing airways of the rat, as revealed by tissue in situ hybridization. Tissue Cell. 1992;24:461–471. doi: 10.1016/0040-8166(92)90062-c. [DOI] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev. 2000;92:55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]


