Abstract
Alterations in multiple biological functions, such as transcription factor activity, are implicated in the neurobiology of depression based primarily on the characterization of antidepressant efficacy in naïve rodents rather than on models that capture the protracted feelings of anhedonia and helplessness that typify depression. In order to address this issue, the authors developed protocols in rats and mice calling for chronic oral exposure to the stress-associated adrenal hormone, corticosterone (CORT), resulting in anhedonic- and helplessness-like behaviors that are persistent yet reversible by chronic antidepressant treatment. Prior CORT exposure also chronically influences molecular targets hypothesized to contribute to negative mood: One example is phosphorylation of cAMP Response Element Binding protein in the hippocampus and nucleus accumbens. Prior chronic CORT exposure provides an alternative method to chronic mild stress models of depression that is easily replicable and persists well beyond the CORT exposure period, thereby modeling the persistent depressive-like state in man.
Keywords: stress, corticosterone, anhedonia, helplessness, CREB, hippocampus, nucleus accumbens, antidepressant
Depression is a debilitating and chronic illness characterized by feelings of helplessness, anhedonia, and loss of motivation to perform even everyday tasks. Although the relationship between stress and Major Depressive Disorder (MDD) is incompletely understood, stress-induced cortisol release from the adrenal glands and subsequent activation of glucocorticoid receptors in brain (c.f., de Kloet, 2004) likely plays a crucial role, as stressful life events are potent factors that trigger, induce, or exacerbate major depressive episodes. While no stress-induced depression-like syndrome in rodents can fully recapitulate the human condition, a model that produces a persistent, varied behavioral sequela (including helplessness, anhedonia, and loss of sensitivity to reward) sensitive to chronic antidepressant treatment would be a major advance for understanding the neurobiology of depression (c.f., Willner et al., 1987, 2005).
This unit presents a depression model based on oral exposure to the stress hormone corticosterone (CORT) in its hemisuccinate form in rodents (also see Gourley et al., 2008; in press). The use of CORT hemisuccinate for oral administration provides an alternative to dissolving CORT in small amounts of ethanol, which requires treating control animals with ethanol (e.g., Magariños et al., 1998; Nacher et al., 2004). Either of these methods, however, allows for the identification of how CORT contributes to stress-related change in neuronal morphology and behavior (Piazza et al., 1991; Magariños et al., 1998; Nacher et al., 2004).
Dissolution of CORT hemisuccinate in drinking water has been used to administer CORT to nursing pups via the lactating dam (Catalani et al., 2000; Cinque et al., 2003), and to evaluate the reinforcing properties of the hormone (Deroche et al., 2003) and its contribution to vulnerability to drugs of abuse (Piazza et al., 1991). The CORT exposure protocol described here is a chronic exposure method that has been optimized for use in modeling a persistent depressive-like state in rodents and has the great advantage of allowing for multiple behavioral tests after CORT wash-out in the same animals using an etiologically-relevant model of depression that is easily replicable between and within laboratories, and that does not require CORT to be on-board during behavioral testing, which could confound responding [e.g., anxiogenic responses to novelty soon after animals consumed CORT have been previously reported (Ardayfio and Kim, 2006)].
Because the authors' aims were to evaluate long-term effects of CORT, the data presented here were collected from mice and rats 2 weeks or 1 month after the chronic exposure period ended. Optimized dosing protocols for both species are provided, and effects on mobility in the forced swim test, sucrose consumption in a model of anhedonia, and basal endogenous CORT levels are provided. In addition, evidence is provided to demonstrate that the CORT behavioral phenotype is sensitive to reversal by treatment with chronic, but not subchronic, chemical antidepressants in mice. Representative consumption, blood serum CORT, adrenal gland weight, and body weight records are provided and finally, regionally selective changes in biochemical targets relevant to the neurotrophic model of depression and antidepressant efficacy (Duman et al., 1997) are discussed to support the hypothesis that this model can be used to identify biochemical correlates of stress-related insult and to determine whether these alterations are sensitive to reversal by chemical antidepressants at therapeutic doses.
Basic Protocol 1
Oral Corticosterone Exposure in Rats and Mice
CORT hemisuccinate is dissolved in regular tap water in a concentration of 25 μg/ml for mice and 50 μg/ml for rat (free-base) and administered chronically with a weaning period (rather than an abrupt withdrawal from CORT) at the end of the exposure period. Behavioral effects of CORT are expected to be persistent and long-lasting. This model provides a simple, convenient, and easily replicable alternative to stress models of depression in rodents. Animals are maintained on a 12-hour light cycle (0700 on) and are experimentally naïve. The authors' studies have exclusively used group-housed male C57BL/6 mice and pair-housed Sprague-Dawley rats at least 12 weeks of age (e.g., Charles River Laboratories, Kingston, NY).
Materials
corticosterone hemisuccinate (4-pregnen-11β 21-DIOL-3 20-DIONE 21-hemisuccinate) (Steraloids, Inc., Newport, RI)
C57bl/6 male mice (group housed: 4-5/cage) or Sprague-Dawley male rats (pair housed), at least 12 weeks of age (e.g., Charles River Laboratories, Jackson Labs)
standard laboratory housing, diet, and light cycle regulation
10 N NaOH and HCl for adjusting pH of solutions
pH meter
disposable Pasteur pipets (for pHing solutions, e.g., Fisher Scientific)
stir plate, magnetic stir bar
standard laboratory rodent water bottles
metric balance
1- or 2-liter clean glass bottles with lids for solution storage
tap water
Weigh out the appropriate amount of CORT hemisuccinate, depending on the species and desired volume, correcting for the added molecular weight of the salt by multiplying the desired dose by a factor of 1.289. In other words, for a desired concentration of 25 μg/ml, dissolve 32.22 mg CORT hemisuccinate into 1 L of tap water. For 50 μg/ml, dissolve 64.45 mg CORT hemisuccinate into 1 L of tap water, etc. The molecular weight of CORT is 346.46; the molecular weight of CORT hemisuccinate is 446.53. Doses in this chapter are expressed as free-base values.
Add CORT to 1 or 2 liters of tap water in a clean glass storage container. Increase the pH to 12-13 with 10 N NaOH using a sterile disposable Pasteur pipette. Stir at room temperature or at 4°C until dissolved (3-7 hours). Stirring at 4°C is preferable because it will slow decay. See Reagents and Solutions for CORT (solid and in aqueous solution) storage conditions and shelf-life.
Following dissolution, neutralize the pH to 7.0-7.4 with 10 N HCl. Add HCl slowly, as the pH will rise and fall dramatically because the solution is not buffered.
Once the CORT solution is prepared, present animals with the CORT in place of normal drinking water for 14 days so CORT provides the only source of hydration throughout the exposure period. To generate an average dose of CORT, weigh bottles daily and divide consumption values by the total body weight of the animals in the cage. In the authors' hands, adult male C57BL/6 mice typically consume 4-6 ml dissolved CORT per day, while adult male Sprague-Dawley rats typically consume 30-60 ml dissolved CORT per day (see Fig. 3). Over the course of this two week period, the average CORT doses the authors have observed range from 5.7-6.9 mg/kg/day for mice consuming a concentration of 25 μg/ml; values are comparable for the rat consuming 50 μg/ml, as rats consume less fluid per gram of body weight than mice.
Wean as follows: 3 days with 50% of the original CORT concentration, and 3 days with 25%, to allow for recovery of endogenous CORT secretion, finally followed by a return to regular drinking water.
Figure 3. Elevated plus maze, blood serum CORT, adrenal gland and body weights, locomotor activity.

a) Oral CORT has been reported to increase anxiety-like behavior when animals are tested immediately after chronic exposure (Ardayfio and Kim, 2006). Because anxiety-like responding to the testing environment, experimenter, etc. could confound several behavioral tests, activity in an elevated plus maze and daytime blood serum CORT was assessed in both species 2 weeks after CORT. No differences in open arm entries and blood serum CORT were found. Mean locomotor activity counts in a novel environment, as measured by photobeam breaks, and post-mortem adrenal gland weights are also reported in the table accompanied by p values from unpaired t-tests. The slight increase in locomotor activity reported would not be expected to confound the results of other tests reported here. (b,d) Representative CORT consumption records for both species are provided. (c,e) Body weights during and after CORT are also shown for both species. CORT-exposed animals are modestly lighter during CORT exposure but regain body weight after exposure such that they do not differ from control animals. Symbols represent means (±SEM) per group.
The entire exposure period is 20 days
Monitor body weights periodically during and after the CORT exposure period to confirm weight loss is not excessive or injurious to the animal. Watch for increased conspecific aggression during CORT exposure and separate animals when appropriate to prevent injury.
Before undertaking further experiments, allow a wash-out period (in which animals are given free access to food and regular drinking water) of at least 2 weeks after the cessation of CORT drinking.
Although most of the data presented in this unit were collected 2 weeks after CORT exposure, anhedonic-like responses to a palatable sucrose solution have been confirmed up to 6 weeks after CORT exposure. A wash-out period is not necessary to detect an effect on the same measure immediately after weaning, but some biochemical effects of prior CORT in the dorsal hippocampus are indeed dependent on the wash-out period (see CRITICAL PERAMETERS; Gourley et al., 2008).
Reagents and solutions
CORT solutions must be changed within 72 hours of dissolution, as CORT will begin to degrade once in solution. Dissolved CORT should be stored at 4°C; stirring at 4°C will also slow degradation. In solid form, CORT hemisuccinate is stable and can be used until the manufacturer's expiration date. Solid CORT can be stored at room temperature or at 4°C.
Commentary
Background Information
The CORT exposure protocol and data presented in this unit demonstrate that prior exposure to “low-dose” oral CORT (translating to 5-7 mg/kg per day p.o.) induces a persistent depression-like state sensitive to antidepressant treatment in rodents. Prior CORT exposure has several behavioral consequences, including increased immobility in the FST and decreased sucrose consumption in a model of anhedonia (Figs. 1,2). Exploration of an elevated plus maze, baseline daytime endogenous CORT, and adrenal gland weights are unchanged 2 weeks after exposure, thus providing a selectively “depressive-like” behavioral phenotype without interference from increased anxiety-like behavioral or physiological indices (Fig. 3). Although the modest increase in locomotor activity observed after CORT could confound some behavioral tasks, it should be noted that increased ambulatory activity would not be expected to account for decreased mobility in the FST, tail suspension test, or in classic tests of outcome value, as have been previously reported (Gourley et al., 2008; Gourley et al., in press). The authors have also previously confirmed that basic gustatory discrimination processes—as measured by quinine:water discrimination—are intact, indicating the effects of prior CORT on sucrose consumption are unlikely to be due to nonspecific effects on sensory discrimination skills (Gourley et al., in press).
Figure 1. Forced swimming and sucrose consumption after CORT.
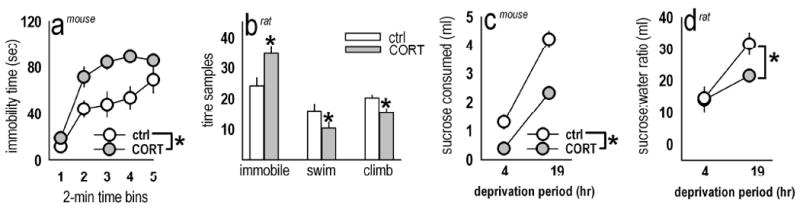
(a) Prior chronic CORT in mice increased immobility time in the FST 2 weeks after exposure [F(1,17)=9.5, p=0.007]. (b) Similarly, in rats previously exposed to 50 μg/ml CORT, analyses revealed a CORT × measure interaction [F(1,2)=12.8, p=0.001], with increased immobility (p=0.001) and decreased climbing and swimming after CORT (ps<0.03). (c) In the sucrose consumption tests, prior CORT at the same doses decreased consumption and preference in mice and rats, respectively. Specifically, CORT-exposed mice of the same body weight consumed significantly less 1% w/v sucrose solution than non-exposed animals after modest deprivation periods [main effect of CORT: F(1,15)=61.1, p<0.001]. (d) In rats, analysis of the ratio of sucrose:water consumed also revealed a main effect of CORT [F(1,10)=6.1, p=0.03]. After 19 hours water deprivation specifically, CORT-exposed rats preferred sucrose to a lesser degree than control counterparts (p=0.006). Bars and symbols represent means (±SEM) per treatment group (*p<0.05). Fig. 1a is reprinted with permission from Gourley et al., in press.
Figure 2. Effects of CORT are sensitive to reversal by chemically distinct antidepressants.

(a) Experimental design: In an effort to reverse the anhedonic response to sucrose with previously well-characterized antidepressants (ADT), mice in a separate cohort were exposed to 25 μg/ml CORT followed by chronic oral amitriptyline (AMI) or fluoxetine (FLX) dissolved in 2% w/v saccharin to mask the taste. Subchronic administration regimens (4 d, p.o.) and ADT-only groups served as controls. (b) One-factor ANOVA with post-hoc tests against the CORT group revealed that both chronic AMI and FLX increased sucrose consumption above CORT-alone levels in mice of equivalent body weight [F(7,77)=7.7, p<0.001, post-hoc ps<0.05], while subchronic AMI and FLX exerted no effects, consistent with clinical observations that ADTs require weeks to exert therapeutic benefits. Animals that had consumed AMI or FLX in the absence of prior CORT also consumed more sucrose. (c) Hippocampal and nucleus accumbens tissue was dissected and immunoblotted for CREB and phosphorylated CREB (ser133) in mice exposed to CORT ± chronic ADTs (i.e., those groups in b indicated by a checkmark). In the dorsal hippocampus, CORT decreased pCREB expression [F(3,21)=3.02, p<0.05; post-hoc p=0.017]. Both antidepressants fully restored pCREB in the dorsal hippocampus. (d) CORT increased pCREB in the nucleus accumbens compared to control mice [F(3,21)=3.08, p=0.05, post-hoc p=0.04], consistent with reports of the “pro-depressive” effects of this substrate here (see Background Information). Neither AMI nor FLX fully restored pCREB to control levels, although they produced a partial recovery, as pCREB levels in both groups differed from control mice at only a trend level of significance (p=0.09 for AMI; p=0.08 for FLX). Bars represent means (±SEM) per treatment group (*p<0.05; †p≤0.09).
Oral CORT as an alternative or complement to stress depression models
Stressful life events play a major role in the etiology and/or exacerbation of MDD (Kendler et al., 1999), but environmental stress manipulations in rodents, such as learned helplessness, chronic mild stress (CMS), and repeated social defeat, are hampered by protocol variability and reported difficulties in replication (Matthews et al., 1995; Forbes et al., 1996, but see Willner, 2005; Vollmayr and Henn, 2001; Nestler et al., 2002), highlighting the need for a depression model easily replicable between laboratories. The CORT protocol presents a strong candidate since it carries a high degree of face, predictive, and construct validity. For example, in line with CMS models and several cases of human depression, circulating CORT levels do not differ in prior CORT-exposed and control animals at test, suggesting stress-related insult persists long after HPA axis disruption (Fig. 3; Gourley et al., in press). CORT exposure also induces antidepressant-sensitive anhedonia (Fig. 2) and decreased sensitivity to reward (Gourley et al., 2008)—hallmarks of MDD—as well as immobility in the FST. The authors acknowledge using the FST as an assay of depressive-like behavior is controversial because, like novelty-suppressed feeding and tail suspension, the measure has been validated by sensitivity to antidepressant treatments, not etiological relevance; nonetheless, the FST is a widely used behavioral assay, and consistent with this practice, prior CORT robustly increased immobility in both species (Fig. 1).
Although a MDD diagnosis requires multiple symptoms, few established depression models or assays incorporate several behavioral features of depression, such as symptom chronicity, sensitivity to chronic but not acute antidepressants, or robust motivational dysfunction, in a single model or test. For example, both acute and chronic antidepressants decrease “helplessness” in naïve animals in the FST, effects highly inconsistent with the typical antidepressant efficacy in humans, whereas CORT-exposed mice show increased hedonic response to sucrose—which can be measured multiple times in the same animals—only after chronic AMI or FLX (Fig. 2). Non-stress paradigms produce conflicting results in models of hedonic/motivational loss in MDD (Shalev and Nafkafi, 2002; Rüedi-Bettschen et al., 2005; Barr and Phillips, 1999; Russig et al., 2003). The efficacy with which CMS decreases motivated behaviors has also been questioned (Barr and Phillips, 1998), but the authors have found marked consistency in suppressed primary motivation to acquire food reward after prior CORT exposure (Gourley et al., 2008; in press). Although Kleen and colleagues (2006) reported decreased responding in a similar task after CMS, the primary and incentive motivational deficits reported could not be dissociated.
CORT is sufficient to alter molecular targets implicated in depression and antidepressant efficacy
The neurotrophic hypothesis of depression and antidepressant efficacy was based in part on observations that stress decreases activity of hippocampal CREB, a transcriptional regulator of the growth factor, Brain-derived Neurotrophic Factor (BDNF), or BDNF itself, while antidepressants produce opposite effects (Smith et al., 1995; Schaaf et al., 1997, 1998; Nibuya et al., 1996; Duman et al., 1997; Prickaerts et al., 2006). In one of the few reports addressing the long-lasting consequences of stress exposure on neurotrophin-regulated signaling targets, Laifenfeld and colleagues (2005) reported decreased hippocampal CREB phosphorylation 4 months after chronic environmental stress. Here we demonstrate that CORT, a major stress substrate, is itself sufficient to persistently decrease hippocampal CREB phosphorylation up to one month after CORT exposure, and subsequent treatment with chronic AMI or FLX can also normalize expression (Fig. 2).
In the nucleus accumbens, prior CORT exposure increases pCREB (Fig. 2), also consistent with previous work on this target using other models of depression (Pliakas et al., 2001; Barrot et al., 2002; Newton et al., 2002; Green et al., 2006). Importantly, a partial rescue of pCREB was achieved with chemically distinct chronic antidepressants, thus suggesting the “prior CORT exposure” model may be a powerful method with which to understand the manner in which antidepressants act in multiple brain regions of rodents displaying depressive-like behavior, as opposed to naïve rodents. Much of what is believed to be known about neurobiological factors of MDD, including the potential importance of CREB-regulated signaling, is based upon our understanding of (often rapid) actions of standard antidepressants in otherwise naïve rodents. For example, although enhanced hippocampal CREB activity may mediate antidepressant efficacy in naïve rodents (Nibuya et al., 1996; Thome et al., 2000, Tiraboschi et al., 2004; but see Conti et al., 2002), whether identical mechanisms are responsible for reversal of a depression-like phenotype is not known; the CORT model is ideally suited to addressing these hypotheses.
Critical Parameters
CORT dose sensitivity
Despite evidence for morphological and neurochemical alterations in the hippocampus after a high dose of oral CORT in rats (400 μg/ml, Magariños et al., 1998; Nacher et al., 2004), the highest CORT doses used in our initial unpublished behavioral studies (300 and 400 μg/ml) were ineffective in producing a persistent depressive-like phenotype in mice, as measured by sucrose consumption, mobility in the FST, and instrumental assays of sensitivity to reward. We did not pursue these negative findings because the health of the mice also suffered, sometimes lethally, with these CORT concentrations. By contrast, behavioral and biochemical findings after “low-dose” CORT (25-100 μg/ml) are robust and replicable without excessive threat to animal health, particularly at the lower 25 μg/ml concentration, which is now used exclusively in our laboratory. In Sprague-Dawley rats, we find that 50 and 100 μg/ml exert comparable, if not indistinguishable, effects on behavioral responding; concentrations >100 μg/ml have not been tested.
The use of a wash-out period after CORT
The hypotheses the authors have been interested in pursuing relate to the long-lasting and persistent effects of prior chronic CORT on several behavioral and biochemical measures. With few exceptions, it is not known if results from animals tested immediately after CORT exposure will precisely resemble those represented here. Certainly, some immediate and long-term biochemical consequences are similar: For example, expression of trkB, the receptor for BDNF, is decreased in the dentate gyrus of the hippocampus immediately after CORT exposure, while phosphorylation of the receptor is attenuated 2 weeks and 1 month after exposure (Gourley et al., 2008; unpublished data). Both decreased receptor expression and phosphorylation would be indicative of decreased activity of intracellular cascades associated with receptor binding.
On the other hand, we have identified heat-shock proteins associated with depression and bipolar disorder in humans to be increased in the mouse hippocampus only after a 2-week or 1-month CORT wash-out period, and not immediately after the cessation of CORT drinking (Gourley et al., 2008). In tests of attentional function in rats, oral CORT (100 μg/ml) impairs behavioral performance after wash-out, but not during CORT exposure (A. Betz, P. Olausson, and J.R. Taylor, unpublished data). Together, these data indicate that at least some complex behavioral and biochemical consequences of prior CORT exposure are temporally dynamic. Further elucidating the regionally specific mechanisms by which chronic stress hormone exposure and wash-out influences the development, expression, and progression of depressive-like symptomology in rodents may provide additional insight into stress-associated psychopathologies in humans and new avenues for antidepressant drug development.
Troubleshooting
At higher concentrations, CORT may not dissolve within the recommended time window. Stirring longer than the recommended 3-7 hrs (e.g., overnight at 4°C) does not harm the compound; all doses discussed here would be expected to dissolve within 24 hrs. After dissolving CORT, the authors have not experienced it coming out of solution again even if the pH drops below the desired range. If CORT “falls out,” the authors recommend discarding the solution and starting over.
Increased conspecific aggression may be observed while animals, particularly C57BL/6 mice, are consuming CORT. In this instance, animals should be re-housed separately, as increased fighting may result in death. Over-grooming may also occur, such that the animals remove patches of fur. As long as the skin is not broken, this behavior is not considered harmful, and fur should re-grow when the CORT consumption period ends. If skin is broken, the animal should be removed from group housing, and topical antibiotics can be applied to prevent infection during the remaining CORT administration period. Nestlets can be added to the animals' pens to aid in temperature regulation.
The authors have not experienced a situation in which the animals refuse to drink CORT, but it is possible that other strains of mice or rats might find the compound aversive. Adding 2% w/v saccharin increases the palatability of other compounds, such as chemical antidepressants (see Fig. 1; Caldarone et al., 2003; Gourley et al., 2008); adding saccharin to CORT would not be expected to alter its pharmacological effects.
Anticipated Results
In this section, the authors provide representative data illustrating the multiple effects of oral CORT exposure using the protocol described above. Methodological details and testing parameters were as follows:
Forced swim test (FST; UNIT 8.10A)
FSTs in mice were conducted in single 10-min sessions in glass cylinders (24 cm × 15.5 cm diameter) filled to 10 cm with 27°C water (see Gourley et al., in press, Supplementary Methods) that was changed between animals. Total time spent immobile, defined as only those movements necessary to keep the head above water, was scored in 2-min bins by a single, blinded rater.
In rats, immobility and escape behaviors were assessed in the traditional 2-day FST (UNIT 8.10A) conducted under red light. Forced swim tanks were 46 cm tall × 20 cm in diameter; water depth was 30 cm. Water was changed between each animal and maintained at 27°C. A single blinded experimenter rated behavior every 5 sec on day 2 as follows: Immobility consisted of only those movements necessary to keep the head above water. A swimming rating required active swimming motions such as moving around the perimeter or diving into the cylinder. Climbing was scored when the rat made vigorous movements against the wall of the cylinder with its paws extended fully out of the water. In both species, two-factor repeated measures (RM)-analysis of variance (ANOVA) was used to analyze differences between groups.
Sucrose consumption
Sucrose consumption was also evaluated in a model of anhedonia, a hallmark symptom of depression. In both species, the protocol first required 48 hours habituation to a 1% (for mice) or 5% (for rats) w/v sucrose solution (Sigma), during which the solution replaced regular drinking water. Animals were then habituated to modest water deprivation with 4, 14, and then 19 hrs of water deprivation over consecutive days, followed by 1-hr access to the sucrose solution. In mice, only the sucrose solution was provided during this 1-hr period, but both sucrose and water were provided to the rats, in which the larger consumption volume better allows for preference measures. The location of water and sucrose were counter-balanced between and within groups and alternated daily. Finally, each animal was allowed 1 hr access to the sucrose solution in its home cage while its cagemates were temporarily housed in a clean cage in the colony room. Each successive individual animal had 1 hr of access to the solution in such a way that the average water deprivation period per cage was 4 and then 19 hours. Data were analyzed by two-factor (deprivation period × CORT) RM-ANOVA.
To confirm the depressive-like phenotype induced by CORT was sensitive to reversal by chronic antidepressant treatment, a separate group of mice was exposed to CORT, then provided amitriptyline hydrochloride (AMI) (200 μg/ml in accordance with Caldarone et al., 2003; Sigma) or fluoxetine hydrochloride (FLX) (160 μg/ml; a generous donation from Dr. Ronald Duman, can be purchased from Sigma) in the drinking water for 2 or 3 weeks, respectively. Four-day treatment groups served as additional comparison groups, and all compounds were dissolved in 2% w/v saccharin and tap water. All mice consumed saccharin for 3 weeks to match the chronic fluoxetine-treated mice. See the timeline in Figure 2. Sucrose consumption testing proceeded as described above; the 19-hour deprivation data are shown. The data were analyzed by ANOVA with post-hoc tests against the CORT-only group.
Post-mortem measures
Several groups from the antidepressant sucrose consumption experiment were selected for post-mortem analyses by immunoblot to evaluate whether biochemical targets known to be affected by antidepressant treatments were similarly affected in this model. These mice were sacrificed by rapid decapitation 1 month after CORT exposure to allow for antidepressant treatments described and outlined in figure 2. Immediately after decapitation, brains were rapidly frozen on dry ice and cut into 1-mm slices using a chilled mouse brain matrix (Plastics One, Roanoke, VA) to dissect tissue for Western blots. Bilateral punches (1.2-mm diameter; Fine Science Tools, Foster City, CA) were taken from the nucleus accumbens and dorsal hippocampus, including the surrounding CA1/CA3 area (in accordance with Paxinos and Franklin, 2002). Tissue was processed using standard immunoblotting techniques. The phosphorylation state of cAMP Response Element Binding (CREB) protein was determined using anti-CREB (Ms, 1:500) and anti-ser 133-CREB (Rb; 1:500) (Cell Signaling, Beverly, MA). Membranes were incubated with primary antibodies at 4°C overnight and then incubated with IRDye 700 Dx Anti-Rb IgG and IRDye 800 Dx Anti-Ms IgG (both 1:5,000; Rockland Immunochemicals, Gilbertsville, PA).
Bands were quantified using fluorescent densitometry analysis (Odyssey Infrared Imaging System). Membranes were re-probed with anti-GAPDH (Ms; 1:40K; Advanced Immunochemical Inc., Long Beach, CA) to determine total protein levels. pCREB was normalized to total CREB, which was not changed. Data were converted to a percent of control samples from the same membrane to control for variance between gels and square-root transformed to preserve required homogeneity of variance. Group means were analyzed by ANOVA with post-hoc comparisons against control (water drinking) mice.
Elevated plus maze (UNIT 8.3), ambulation
To evaluate the behavioral specificity of the CORT-induced depressive-like phenotype, additional measures were collected from rats and mice. Two weeks after CORT, animals were allowed to explore an elevated plus maze (UNIT 8.3) in a conventional assay of anxiety. Tests were conducted in a single session under red light in clean mazes with four arms (mouse=15, rat=50 cm long), two of which were enclosed (mouse=30, rat=40 cm high walls). Mazes were raised off the table (mouse=30, rat=50 cm), and animals were initially placed on the center platform (mouse=5×5, rat=10×10 cm) and videotaped. Entries into open and closed arms (mouse=5, rat=7 min) were scored by a single blinded rater and analyzed by t-test.
To evaluate whether behavioral changes could be attributed to gross locomotor differences between groups, the ambulatory activity of mice and rats (UNIT 8.1) was monitored in clean, novel cages 2 weeks after CORT using the automated Omnitech (Columbus, OH) Digiscan Micromonitor system equipped with 16 photocells. Data were represented as photobeams broken in 1 hr and were analyzed by t-test.
To determine whether circulating endogenous CORT levels were changed after weaning, a separate group of experimentally naïve mice and rats were sacrificed between 0800-0900 hrs (i.e., within 2 hrs of lights on) 2 weeks after CORT exposure. Adrenal glands were carefully excised from the surrounding adipose tissue and weighed as an approximate reflection of recent hypothalamic-pituitary-adrenal axis activity. Trunk blood was collected in chilled microcentrifuge (1.7-ml, mouse) or Falcon (15-ml, rat) tubes and centrifuged for 1 hr at 5°C. Serum was extracted, and CORT content was assayed in duplicate using a corticosterone enzyme immunoassay kit in accordance with the manufacturer's instructions (Assay Designs, Inc., Ann Arbor, MI). Samples were diluted 10-fold with assay buffer to fall within the detection limits of the assay. Fluorescence values were analyzed by t-test, and means and SEMs are recorded in Figure 3. Representative body weights during and after exposure are also presented and were analyzed by 2-factor (group × time point) RM-ANOVA.
Time Considerations
Experimenters should allow up to 24 hours for CORT dissolution, and because the CORT solution needs to be changed every 72 hours, experimenters must be able to prepare CORT every 3 days. The exposure period is substantial—20 days—and a wash-out period after CORT exposure is recommended to model the chronic depressive-like state (see CRITICAL PERAMETERS), potentially resulting in the addition of several weeks to the experiment duration. Pilot experiments will be required to determine an appropriate timeline for each experiment, depending on the experimental aims. One additional factor to consider in behavioral testing is the possibility of testing animals before and after CORT exposure, allowing for within-subjects comparisons, which may be statistically advantageous and potentially minimize the number of animals used for a given project. For example, the sucrose consumption protocol outlined here could easily be used to test animals before and after CORT to investigate changing responses to sucrose as a function of CORT exposure. This approach would add several days to the experimental timeline.
Acknowledgments
The authors gratefully acknowledge assistance from Peter Olausson and Drew Kiraly in the genesis and optimization of the methods discussed. D. Kiraly also conducted the Western blot analyses shown here. Finally, we also thank Paul Hitchcott for intellectual contributions. Supported by NIH MH 25642 (JRT), AA 17537 (JRT), and MH 79680 (SLG), the Beinecke Brothers Foundation (SLG), and the Connecticut State Department of Mental Health and Addiction Services (JRT).
Footnotes
NOTE: All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) or conform to governmental regulations regarding the care and use of laboratory animals.
The authors declare they have no competing financial interests.
Works cited
- Ardayfio P, Kim KS. Anxiogenic-like effects of chronic corticosterone in the light-dark emergence task in mice. Behav Neurosci. 2006;120:249–256. doi: 10.1037/0735-7044.120.2.249. [DOI] [PubMed] [Google Scholar]
- Barr AM, Phillips AG. Chronic mild stress has no effect on responding by rats for sucrose under a progressive ratio schedule. Physiol Behav. 1998;64:591–597. doi: 10.1016/s0031-9384(98)00060-2. [DOI] [PubMed] [Google Scholar]
- Barr AM, Phillips AG. Withdrawal following repeated exposure to d-amphetamine decreases responding for a sucrose solution as measured by a progressive ratio schedule of reinforcement. Psychopharmacology. 1999;141:99–106. doi: 10.1007/s002130050812. [DOI] [PubMed] [Google Scholar]
- Barrot M, Olivier JDA, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldarone BJ, Karthigeyan K, Harrist A, Hunsberger JG, Witmack E, King SL, Jatlow P, Picciotto MR. Sex differences in response to oral amitriptyline in three animal models of depression in C57BL/6J mice. Psychopharmacology. 2003;170:94–101. doi: 10.1007/s00213-003-1518-7. [DOI] [PubMed] [Google Scholar]
- Catalani A, Casolini P, Scaccianoce S, Patacchioli FR, Spinozzi P, Angelucci L. Maternal corticosterone during lactation permanently affects brain corticosteroid receptors, stress response and behaviour in rat progeny. Neuroscience. 2000;100:319–325. doi: 10.1016/s0306-4522(00)00277-3. [DOI] [PubMed] [Google Scholar]
- Cinque C, Zuena AR, Casolini P, Ngomba RT, Melchiorri D, Maccari S, Nicoletti F, Gerevini D, Catalina A. Reduced activity of hippocampal group 1 metabotropic glutamate receptors in learning-prone rats. Neuroscience. 2003;122:277–284. doi: 10.1016/s0306-4522(03)00442-1. [DOI] [PubMed] [Google Scholar]
- Conti AC, Cryan JF, Dalvi A, Lucki I, Blendy JA. cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J Neurosci. 2002;22:3262–3268. doi: 10.1523/JNEUROSCI.22-08-03262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet RE. Hormones and the stressed brain. Ann N Y Acad Sci. 2004;1018:1–15. doi: 10.1196/annals.1296.001. [DOI] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Deminiere JM, Le Moal M, Simon H. Rats orally self-administer corticosterone. Brain Research. 1993;622:315–320. doi: 10.1016/0006-8993(93)90837-d. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Forbes NF, Stewart CA, Matthews K, Reid I. Chronic mild stress and sucrose consumption: Validity as a model of depression. Physiol Behav. 1996;60:1481–1484. doi: 10.1016/s0031-9384(96)00305-8. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Kiraly DD, Howell JL, Olausson P, Taylor JR. Acute hippocampal BDNF restores motivational drive and forced swimming after corticosterone. Biol Psychiatry. doi: 10.1016/j.biopsych.2008.06.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS, Taylor JR. Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol Psychiatry. 2008;63:353–359. doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Hommel JD, DiLeone RJ, Kumar A, Theobald DE, Neve RL, Nestler EJ. Induction of inducible cAMP early repressor expression in nucleus accumbens by stress or amphetamine increases behavioral responses to emotional stimuli. J Neurosci. 2006;26:8235–8242. doi: 10.1523/JNEUROSCI.0880-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kleen JK, Sitomer MT, Killeen PR, Conrad CD. Chronic stress impairs spatial memory and motivation for reward without disrupting motor ability and motivation to explore. Behav Neurosci. 2006;120:842–851. doi: 10.1037/0735-7044.120.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laifenfeld D, Kerry R, Grauer E, Klein E, Ben-Shacher D. Antidepressant and prolonged stress in rats modulate CAM-L1, laminin, and pCREB, implicated in neuronal plasticity. Neurobiol Dis. 2005;20:432–441. doi: 10.1016/j.nbd.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Magariños AM, Orchinik M, McEwen BS. Morphological changes in hippocampal CA3 regions induced by non-invasive glucocorticoid administration: a paradox. Brain Res. 1998;809:314–318. doi: 10.1016/s0006-8993(98)00882-8. [DOI] [PubMed] [Google Scholar]
- Matthews K, Forbes N, Reid IC. Sucrose consumption as an hedonic measure following chronic unpredictable mild stress. Physiol Behav. 1995;57:241–248. doi: 10.1016/0031-9384(94)00286-e. [DOI] [PubMed] [Google Scholar]
- Nacher J, Pham K, Gil-Fernandex V, McEwen BS. Chronic restraint stress and chronic corticosterone treatment modulate differentially the expression of molecules related to structural plasticity in the adult rat piriform cortex. Neuroscience. 2004;126:503–509. doi: 10.1016/j.neuroscience.2004.03.038. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Newton SS, Thome J, Wallace TL, Shirayama Y, Schlessinger L, Sakai N, Chen J, Neve R, Nestler EJ, Duman RS. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nester EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Academic Press; San Diego, CA: 2002. [Google Scholar]
- Piazza PV, Maccari S, Deminiere JM, le Moal M, Mormede P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci U S A. 1991;88:2088–2092. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA. Altered responsiveness to cocaine and increase immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in the nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prickaerts J, van den Hove DLA, Fieren FLP, Kia HK, Lenaerts I, Steckler T. Chronic corticosterone manipulations in mice affect brain cell proliferation rates, but only partly affect BDNF protein levels. Neurosci Lett. 2006;396:12–16. doi: 10.1016/j.neulet.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Rüedi-Bettschen D, Pedersen EM, Feldon J, Pryce CR. Early deprivation under specific conditions leads to reduced interest in reward in adulthood in Wistar rats. Behav Brain Res. 2005;156:297–310. doi: 10.1016/j.bbr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Russig H, Pezze MA, Nanz-Bahr NI, Pryce CR, Feldon J, Murphy CA. Amphetamine withdrawal does not produce a depressive-like state in rats as measured by three behavioral tests. Behav Pharmacol. 2003;14:1–18. doi: 10.1097/00008877-200302000-00001. [DOI] [PubMed] [Google Scholar]
- Schaaf MJM, Hoetelmans E, de Kloet R, Vreugdenhil E. Corticosterone regulates expression of BDNF and trkB but not NT-3 and trkC mRNA in the rat hippocampus. J Neurosci Res. 1997;48:334–341. [PubMed] [Google Scholar]
- Schaaf MJM, de Jong J, de Kloet ER, Vreugdenhil E. Down-regulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 1998;813:112–120. doi: 10.1016/s0006-8993(98)01010-5. [DOI] [PubMed] [Google Scholar]
- Shalev U, Kafkafi N. Repeated maternal separation does not alter sucrose-reinforced and open-field behaviors. Pharmacol Biochem Behav. 2002;73:115–122. doi: 10.1016/s0091-3057(02)00756-6. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1767–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome J, Sakai N, Shin K, Steffen C, Zhang YJ, Impey S, Storm D, Duman RS. cAMP response element-mediated gene transcription is upregulated by chronic antidepressant treatment. J Neurosci. 2000;20:4030–4036. doi: 10.1523/JNEUROSCI.20-11-04030.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraboschi E, Tardito D, Kasahara J, Moraschi S, Pruneri P, Gennarelli M, Racagni G, Popoli M. Selective phosphorylation of nuclear CREB by fluoxetine is linked to activiation of CaM kinase IV and MAP kinase cascades. Neuropsychopharmacology. 2004;29:1831–1840. doi: 10.1038/sj.npp.1300488. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Henn FA. Learned helplessness in the rat: Improvements in validity and reliability. Brain Res Protoc. 2001;8:1–7. doi: 10.1016/s1385-299x(01)00067-8. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of a sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;95:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]


