Abstract
Background and objectives: The feasibility and additional value of combining bioimpedance analysis (BIA) with near-subject absolute measurement of total body water using deuterium dilution (TBWD) in determining longitudinal fluid status was investigated.
Design, setting, participants, & measurements: Fifty-nine hemodialysis patients (17 female; age 58.4 ± 16.1 yr; body mass index 27.0 ± 5.4) were enrolled into a 12-mo, two-center, prospective cohort study. Deuterium concentration was measured in breath by flowing-afterglow mass spectrometry using a validated protocol ensuring full equilibration with the TBW; BIA was measured using a multifrequency, multisegmental device. Comorbidity was quantified by the Stoke score. Clinicians were blinded to body composition data.
Results: At baseline and 12 mo, there was an incremental discrepancy between TBWBIA and TBWD volumes such that greater comorbidity was associated with increasing overhydration. Forty-three patients who completed the study had no longitudinal differences in the prescribed or achieved postdialysis weights. In contrast, TBWD increased without a change in TBWBIA (mean difference −0.10 L). Changes in TBW and lean body mass differed according to baseline comorbidity; without comorbidity, BIA also identified an increase in TBW and lean body mass, whereas with increasing comorbid burden, BIA failed to demonstrate increases in tissue hydration identified by TBWD.
Conclusions: Combined near-patient measurements of absolute and BIA-estimated TBW are achievable in a dialysis facility by identifying changes in body composition not fully appreciated by routine assessment. BIA underestimates tissue overhydration that is associated with comorbidity, resulting in reduced sensitivity to longitudinal increases during a 12-mo period.
The management of fluid status in patients with advanced renal failure (stage 5 chronic kidney disease) is both difficult and critical because high mortality is driven by cardiovascular disease in a setting of chronic fluid overload and suboptimal nutrition. The difficulty has two major components: (1) The practical problems that interfere with achieving good volume status, such as excess interdialytic weight gain (1), intradialytic hypotension, cardiac stunning (2), and impaired baroreceptor reflexes (3), all associated with increased mortality, and (2) the problem of determining accurate fluid status in hemodialysis (HD) patients. The latter is especially confounded by longitudinal changes in body composition, frequently abnormal at the start of dialysis but progressively changing and often deteriorating, especially in patients with additional comorbidities (4).
Bioimpedance analysis (BIA) is a promising tool to aid clinicians with this problem. In measuring resistance, inversely related to tissue water content and reactance, proportional to the cell membranes and thus cell mass, it provides the clinician with an index of how these two components of body composition are in proportion to each other and how they change longitudinally with time (4,5). The challenge arises in converting these measures into liters of body fluid (and so directly influencing dialysis prescription), which requires use of algorithms that all require some degree of assumption when replacing actual measurement (5). They usually describe healthy populations well but have wide confidence limits when compared with gold standard methods such as stable isotope dilution. These problems are magnified in unhealthy individuals, in whom body composition is usually abnormal and in particular when tissue hydration is disturbed. To resolve this problem, we developed a near-patient rapid breath test method that is designed to enable absolute measurements of total body water (TBW) using the gold standard deuterium (D) dilution method in the HD clinic (6,7). We present a proof-of-principle study to demonstrate both that it is feasible to do this in a busy dialysis facility and that there is added value in combining the absolute TBW measurement by breath D dilution with the relative fluid status and body composition technique of a multifrequency, multisegmental BIA.
Materials and Methods
Study Design
This was a prospective, longitudinal, observational study of prevalent HD patients who were treated in two university hospital dialysis facilities. Patients underwent fluid status and body composition assessment using BIA and near-patient D dilution at baseline and at 12 mo. The clinical dry weight of patients was assessed and regularly reviewed as part of their routine care by clinicians who were blinded to the body composition data. The patients' comorbidities were recorded using the Stoke comorbidity score (8), a validated tool that predicts survival in HD patients (9). The score is an arithmetic sum of up to seven comorbidities: Ischemic heart disease, peripheral vascular disease (includes cerebrovascular), left ventricular dysfunction, diabetes, connective tissue disorder, active noncutaneous malignancy, and another life threatening illness. Subjective global assessment was performed at baseline and 12 mo to assess nutritional status. TBW by anthropometric equations (Watson, Hume-Weyer, Chumlea, and Chertow equations; see appendix) were calculated on the basis of their postdialysis weight (10,11). The study protocol was approved by the local ethics committees, and all patients gave their written informed consent.
TBWD Measurement Using Flowing-Afterglow Mass Spectrometry
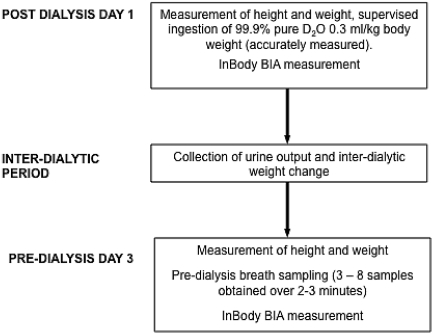
Flowing-afterglow mass spectrometry (FA-MS) is a prototype device developed by Trans Spectra Ltd, UK, which enables real-time quantification of deuterated water vapor in exhaled breath and hence near-subject noninvasive measurement of absolute TBWD using the dilution principle validated in healthy individuals (6,7). The FA-MS method has been detailed in previous publications, in which it has been shown to measure the D content of water vapor in breath or the head space of liquids to within 1% (12). The D abundance was obtained using the FA-MS data acquisition program (SCILIB library 4.96, Patrik Spanel, Trans Spectra Ltd., http://www.transpectra.com). The breath-sampling protocol used to measure TBWD was published recently (13) and is represented in Figure 1. Briefly, patients were administered after dialysis a measured dose of D2O, in the range of 20 to 30 ml according to postdialysis body weight. TBWD was measured on the next dialysis day. Interdialytic urine outputs were measured and their interdialytic weight changes were recorded to calculate the TBWD on the day of ingestion of D2O (see Figure 1). Using this protocol, repeated independent measures of TBW were highly correlated (R = 0.99, P < 0.001) with a coefficient of variation of 2.6%.
Figure 1.
Protocol for determining TBWBIA and TBWD.
Measurement of Body Composition and Fluid Status Using BIA
BIA measurements of resistance and reactance were taken in the recumbent position after 5 min of rest immediately before dialysis and at least 15 min after dialysis using a multifrequency (1, 5, 50, 250, 500, and 1000 kHz), multisegmental, eight-point contact tetrapolar BIA device (InBody S20; Biospace, Seoul, Korea). This device has previously been validated in normal subjects using both D dilution and dual-energy x-ray absorptiometry (14,15). TBWBIA, extracellular water (ECW), lean body mass (LBM), skeletal mass, and body fat mass were derived on the basis of the manufacturer's impedance algorithm, which sums estimates for each of the limbs and the trunk.
Statistical Analysis
Statistical analyses were performed using SPSS 16.0.1 (SPSS, Chicago, IL). Correlations between TBWD, TBWBIA, and those estimated by anthropometric equations were assessed using Pearson correlation coefficient. The paired t test was used to compare longitudinal mean values. ANOVA was performed to compare multiple mean values and effects of the variables, with a Bonferroni correction as the post hoc test.
Results
Patients
Fifty-nine HD patients (demographics are summarized in Table 1) were recruited. They underwent thrice-weekly bicarbonate-based in-center HD. Dialysis time ranged from 240 to 300 min. The mean vintage of dialysis was 3.3 ± 3.9 yr, and mean single-pool Kt/V was 1.43 ± 0.46. Of the patients who did not complete the study (n = 16), there were seven deaths, three transplants, and six withdrawals (one because of hospital admission). Comparison of body composition at baseline of patients who did not with those who did complete the study demonstrated lower LBM (44.7 versus 50.8 kg; P = 0.056), skeletal muscle mass (25.5 versus 29.5 kg; P = 0.048), TBWD (36.5 versus 41.4 L; P = 0.043), and TBWBIA (35.0 versus 39.8 L; P = 0.058).
Table 1.
Baseline demographics of all patients and those who completed the study
| Demographic | All Patients(n = 59) | Patients Who Completed the Study(n = 43) |
|---|---|---|
| Age (yr; mean ± SD) | 58.4 ± 16.1 | 58.4 ± 15.6 |
| Gender (F:M) | 17:42 | 10:32 |
| Ethnicity (n [%]) | ||
| white | 53 (89.8) | 37 (88.1) |
| Afro-Caribbean | 5 (8.5) | 4 (9.5) |
| Asian | 1 (1.7) | 1 (2.4) |
| Diabetes (n [%]) | 19 (32.2) | 13 (31.0) |
| Stoke comorbidity score | ||
| low | 16 (27.1) | 13 (31.0) |
| medium | 29 (49.2) | 22 (52.4) |
| high | 14 (23.7) | 7 (16.7) |
| SGA (n [%]) | ||
| A | 50 (84.7) | 36 (85.7) |
| B | 9 (15.3) | 6 (14.3) |
| Height (m; mean ± SD) | 1.71 ± 0.09 | 1.72 ± 0.09 |
| Weight post-HD (kg; mean ± SD) | 78.5 ± 18.5 | 79.1 ± 18.9 |
| Clinical dry weight (kg; mean ± SD) | 78.4 ± 18.5 | 78.9 ± 18.9 |
| BMI (mean ± SD) | 26.7 ± 5.4 | 26.5 ± 5.7 |
SGA, subjective global assessment.
TBWD Compared with TBW Estimated by Anthropometric Equations and BIA at Baseline and 12 Mo
TBWD at baseline and at 12 mo were highly correlated with TBW estimated by anthropometric equations and BIA (Table 2). There was also a high level of correlation between the resistance indices at all frequencies measured by the InBody BIA device (R = 0.841 to 0.898, P < 0.001) with the absolute TBWD.
Table 2.
Mean differences and Pearson correlation between TBWD and TBW estimated by anthropometric equations or BIA at baseline (for all patients and those who completed the study) and at 12 mo
| Parameter | Baseline |
12 Mo(n = 43) |
||||
|---|---|---|---|---|---|---|
| All Patients at Baseline(n = 59) |
Patients Who Completed the Study(n = 43) |
|||||
| Mean Difference (L) | Correlationsa | Mean Difference (L) | Correlationsa | Mean Difference (L) | Correlationsa | |
| TBWD − TBWWatson | −0.29 ± 3.80 | 0.90 | −0.08 ± 4.00 | 0.88 | 2.86 ± 3.83 | 0.90 |
| TBWD − TBWHume | −1.30 ± 3.96b | 0.89 | −1.06 ± 4.10 | 0.88 | 1.74 ± 4.13 | 0.88 |
| TBWD − TBWChertow | −4.40 ± 3.96 | 0.90 | −4.30 ± 4.20 | 0.89 | −1.40 ± 3.87 | 0.90 |
| TBWD − TBWChumlea | −1.95 ± 4.28 | 0.87 | −2.06 ± 4.30 | 0.87 | 0.82 ± 4.05c | 0.89 |
| TBWD − TBW58 | −5.54 ± 5.81 | 0.84 | −5.30 ± 4.20 | 0.84 | −2.36 ± 6.32 | 0.81 |
| TBWD − TBWBIA | 1.62 ± 3.11 | 0.94 | 1.29 ± 3.30 | 0.93 | 3.88 ± 3.91 | 0.90 |
All P < 0.001.
The best agreement of TBWD was with TBWWatson at baseline.
The best agreement of TBWD was with TBWChumlea at 12 mo.
Relationship among TBWD, TBWBIA, and Comorbidity at Baseline and 12 Mo
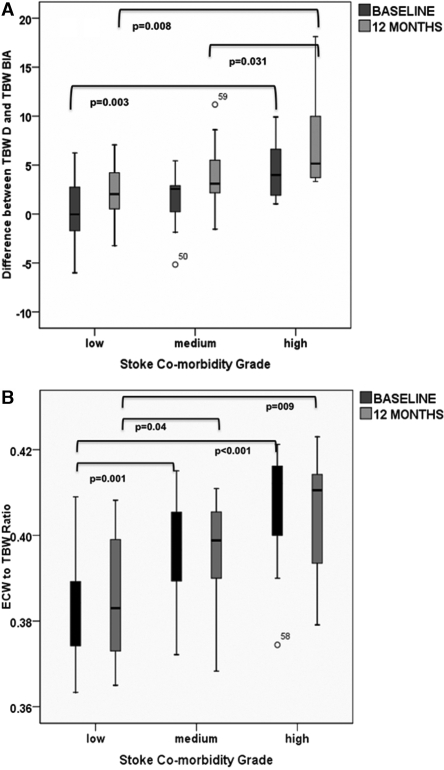
Despite this high level of correlation between measured and estimated TBW for the whole population, there were significant differences between absolute TBWD and the TBWBIA both at baseline and at 12 mo, such that BIA underestimated the volume by 1.62 ± 3.11 and 3.88 ± 3.91 L, respectively (both P < 0.001). At both time points, this difference was very significantly associated with comorbidity burden (Figure 2). At baseline, this discrepancy was incrementally greater the higher the comorbidity score [ANOVA F (2,56) = 6.20; P < 0.005, ω = 0.39]. This increasing discrepancy demonstrated an incremental linear effect [ANOVA F (1,56) = 12.19; P = 0.001, ω = 0.41; Figure 2A], which was also observed at 12 mo, with patients in the higher comorbidity group having the greatest difference between the TBWD and TBWBIA measurements [ANOVA F (2,39) = 5.33 (P < 0.01, ω = 0.41) versus F (1,39) = 10.28 (P = 0.003, ω = 0.44)]. In fact, the discrepancy was more marked at 12 mo and was associated with increased obesity as determined by BMI (R = 0.47, P = 0.002).
Figure 2.
(A) Difference between measured TBWD and estimated TBWBIA at baseline and 12 mo according to grade of comorbidity; at both time points, BIA underestimates the TBW with increasing comorbidity. (B) Ratio of ECW:TBW derived from BIA at baseline and 12 mo according to grade of comorbidity; comorbidity is associated with an increased ratio. Data are mean, interquartile range (box), and complete range. In both cases, P values refer to unpaired data, ANOVA (for paired data analysis, see Table 3). For statistical tests of the incremental effect of comorbidity, see text.
Relationship between Comorbidity Grades with ECW/TBWBIA and TBWD
The ECW/TBWBIA was greatest in patients with higher comorbidity grade at baseline and at 12 mo (Figure 2B); however, higher ECW/TBWBIA was also associated with lower LBM at baseline (R = 0.28, P = 0.03) and at 12 mo (R = 0.40, P = 0.008), and there were no differences in ECW normalized for height according to degree of comorbidity, indicating that the abnormal ECW/TBWBIA was mainly due to the reduced muscle mass (TBW) component of the ratio. The difference between TBWD and TBWBIA was greater in patients with higher ECW/TBWBIA at baseline and at 12 mo (R = 0.40, P = 0.001; and R = 0.40, P = 0.008 respectively), again suggesting that the patients were more overhydrated than the BIA indicated.
Longitudinal Paired Analysis of Fluid Status and Body Composition of Patients According to Comorbidity
For the whole population, BIA did not identify significant longitudinal changes in body composition, including TBW (0.08 L; 95% confidence interval [CI] −0.79 to 0.96), body fat mass (−0.52 L; 95% CI −1.7 to 0.66), ECW (0.07 L; 95% CI −0.37 to 0.50), ECW/TBW, or LBM (0.1 kg; 95% CI −0.10 to 0.15) in the 43 patients who completed the study. Equally, there were no overall changes in the clinician-prescribed dry weights (−0.43 kg; 95% CI −1.7 to 0.83) or in the achieved postdialysis weights (−0.46 kg; 95% CI −1.63 to 0.71). In contrast, there was a significant increase in the TBWD (2.3 L; 95% CI 1.51 to 3.08; P < 0.001). These overall mean differences mask significantly dif-ferent patterns of changing body composition at the comorbidity subgroup (Tables 3 and 4, Figure 3) and individual levels (Figure 4). In patients without comorbid disease, BIA did identify a significant increase in LBM and TBW, indicating that the increase in TBWBIA and TBWD was largely attributable to a gain in muscle mass, although there was also an increase in the estimated ECWBIA. In patients with intermediate comorbidity, BIA did not identify significant changes, but TBWD indicated an overall increase in tissue hydration. In the patients with more than two comorbidities, only seven (50%) remained in the study at follow-up. They had NS reduction in weight and LBM combined with an increase in TBWD, resulting in a significant increase in their overall hydration (TBWD normalized for weight).
Table 3.
Paired measures of body composition at baseline and 12 mo according to grade of comorbidity in the 43 patients who completed the study
| Body Composition Measure | Comorbidity 0 (n = 14) |
Comorbidity 1 to 2 (n = 22) |
Comorbidity >2 (n = 7) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Mo | P (95% CI) | Baseline | 12 Mo | P (95% CI) | Baseline | 12 Mo | P (95% CI) | |
| Actual weight (kg) | 82.9 ± 14.8 | 82.8 ± 15.3 | 0.947 (−3.00 to 2.90) | 78.7 ± 20.0 | 78.4 ± 19.3 | 0.599 (−1.40 to 0.80) | 78.4 ± 24.0 | 76.7 ± 25.0 | 0.342 (−5.70 to 2.30) |
| Dry weight (kg) | 82.7 ± 14.9 | 82.7 ± 15.2 | 1.000 (−3.60 to 3.60) | 78.5 ± 17.8 | 78.2 ± 18 | 0.604 (−1.40 to 0.80) | 78.4 ± 23.0 | 76.6 ± 23.0 | 0.344 (−5.70 to 2.30) |
| BFM (kg) | 21.7 ± 9.7 | 20.5 ± 11.8 | 0.348 (−4.10 to 1.50) | 27.0 ± 12.1 | 26.6 ± 13.1 | 0.564 (−1.70 to 0.90) | 28.3 ± 16.7 | 28.9 ± 16.9 | 0.762 (−3.40 to 4.50) |
| LBM (kg) | 53.9 ± 11.1 | 54.2 ± 10.5 | 0.048 (0.01 to 2.5) | 48.7 ± 11.6 | 48.8 ± 10.3 | 0.934 (−1.40 to 1.50) | 47.2 ± 11.0 | 45.2 ± 13.0 | 0.371 (−7.30 to 3.20) |
| ECF (kg) | 16.3 ± 3.3 | 16.8 ± 3.3 | 0.023 (0.08 to 0.90) | 15.1 ± 3.8 | 15.1 ± 3.2 | 0.921 (−0.60 to 0.70) | 14.9 ± 3.1 | 14.3 ± 3.8 | 0.411 (−2.60 to 1.20) |
| ECF/ht (kg/m) | 9.26 ± 1.4 | 9.5 ± 1.4 | 0.024 (0.04 to 0.52) | 8.8 ± 1.8 | 8.8 ± 1.5 | 0.875 (−0.30 to 0.40) | 8.9 ± 1.7 | 8.5 ± 2.1 | 0.401 (−1.60 to 0.80) |
| TBWBIA (kg) | 42.8 ± 8.7 | 43.8 ± 8.5 | 0.044 (0.03 to 1.95) | 38.1 ± 9.1 | 38.2 ± 8.0 | 0.931 (−1.10 to 1.20) | 37.1 ± 8.4 | 35.5 ± 10.0 | 0.384 (−5.80 to 2.60) |
| TBWD (kg) | 44.2 ± 6.8 | 47.3 ± 7.1 | 0.001 (1.60 to 4.60) | 39.7 ± 8.6 | 41.8 ± 9.1 | 0.001 (0.90 to 3.20) | 41.6 ± 9.0 | 43.2 ± 9.6 | 0.157 (−0.80 to 3.80) |
| TBWD/wt (%) | 53.5 ± 5.4 | 57.6 ± 7.3 | 0.006 (1.40 to 6.80) | 51.6 ± 7.6 | 54.2 ± 6.9 | 0.004 (0.00 to 4.30) | 54.1 ± 4.8 | 58.0 ± 7.6 | 0.05 (0.00 to 7.50) |
Table 4.
Number and type of actual comorbidities present by comorbidity group
| Spectrum of Comorbiditities | Comorbidity 0 (n = 14) | Comorbidity 1 to 2 (n = 22; n[%]) | Comorbidity >2 (n = 7; n[%]) |
|---|---|---|---|
| Ischemic heart disease | 0 | 8 (36) | 7 (100) |
| Peripheral vascular disease | 0 | 5 (23) | 4 (57) |
| Left ventricular dysfunction | 0 | 3 (14) | 4 (57) |
| Diabetes | 0 | 7 (32) | 5 (71) |
| Connective tissue disease | 0 | 0 (0) | 0 (0) |
| Malignancy | 0 | 3 (14) | 1 (14) |
| Significant other life-threatening condition | 0 | 8 (36) | 6 (86) |
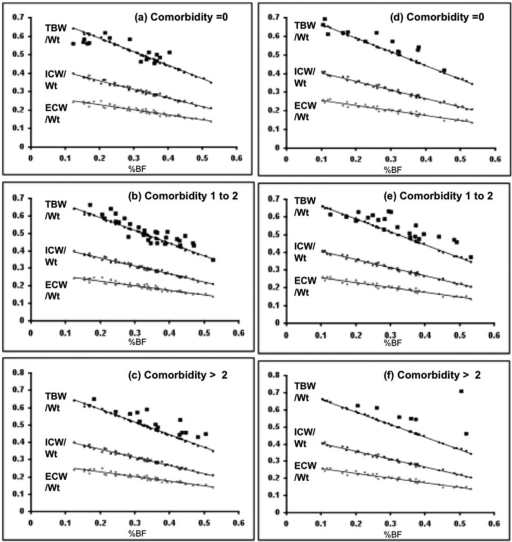
Figure 3.
Each panel shows the relationship between body water spaces normalized to body weight, TBW/wt (●), Intra-cellular water/wt (○), and ECW/wt (●) and percentage of body fat (x axis) as determined by BIA. The linear regression lines indicate the BIA algorithm-derived fluid volumes (all points very close to the line) for these fluid volumes; for TBW especially, this can be seen to assume the same hydration fraction (0.73 for lean tissue) for all patients. Superimposed is the measured TBWD/wt (■). As comorbidity increases (baseline [A through C]) and longitudinally (12 mo [D through F]), it can be seen that although the TBWD/wt runs parallel to the BIA predictions, the patients are relatively more overhydrated.
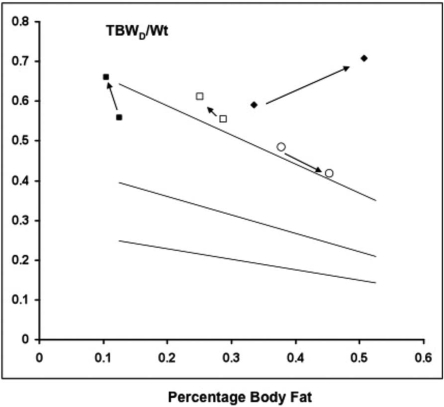
Figure 4.
Four examples of longitudinal body composition change in individual patients. The linear regression lines and layout are explained in the legend to Figure 3. Patient 1 (■), a 26-yr-old man with no comorbidity, lost 1.5 kg of actual weight during the study; despite this, he increased LBM and TBWD without becoming overhydrated. Patient 2 (○), a 43-yr-old woman without comorbidity, gained 16 kg during the year, increasing her body mass index from 31.4 to 37.5. BIA changes confirm the increase in obesity, TBWD confirms that her hydration status remained normal. Patient 3 (□) is a 50-yr-old man who had difficulty in achieving clinical dry weight as a result of intradialytic hypotension. He lost 3.5 kg during the year of study, but this is due in part to having undergone a nephrectomy. BIA suggests that weight loss is fat, but the disproportionate increase in TBWD indicates that he has become relatively overhydrated (not identified by BIA). Patient 4 (♦) is a 78-yr-old woman with severe comorbidity, including coronary heart disease, diabetes, and heart failure. She lost 9 kg in body weight. The BIA indicates an increase in percentage of body fat, but this is due to excessive loss in lean tissues. TBWD/wt is increased at baseline, indicating overhydration, but becomes much more so during the study, again not identified by the BIA.
Discussion
This is the first study to describe the combined use of relative and absolute point-of-care methods to monitor longitudinal fluid status and body composition in a cohort of HD patients. We demonstrated that these near-subject methods were well tolerated by patients and feasible in the busy environment of a dialysis facility. Using these techniques, we were able to identify and quantify overhydration that was proportional to comorbid burden during the 12 mo of the study. We also identified changes in body composition and hydration status that also varied by comorbidity, which seemingly may not have been apparent on clinical assessment. Specific case examples were identified that demonstrate the value of combining these measures in tracking individual changes in body composition (Figure 4).
Although many studies describe the use of BIA to assess body composition in HD patients, these are generally cross-sectional in nature and only occasionally validated by contemporary gold standard methods such as stable isotope dilution (16–18). As with other studies, we found a strong correlation between all of our measures and estimates of TBW, and the excellent correlation and agreement between TBWD and TBWBIA (truly independent measures), is similar to that reported in the literature for both normal (7) and dialysis populations. BIA was also shown previously to predict survival in dialysis patients: Using the relationship between measured reactance and resistance, expressed as either the phase angle or the ratio of algorithm-derived fluid compartments (e.g., ECW:TBW), this is a strong predictor independent of age and other factors (19). We also found that this BIA measure (ECW:TBW) was very significantly related to the Stoke comorbidity score but that it was the reduced TBW component of the ratio that explained almost all of this relationship, reflecting the muscle wasting associated with disease burden, age, and inflammation as mortality risk increases.
One of the novel observations in this study is the increasing discrepancy between BIA-derived and isotope-measured TBW as comorbid burden increased. There are two possible explanations for this: Either BIA is systematically overestimating the intracellular component of TBW in all patients but disproportionately more so in those with less comorbidity, or it is underestimating TBW in more comorbid patients as a result of the ECW component. For several reasons, the latter is more likely, not least because the InBody algorithm has been validated in normal European individuals, for whom it does not overestimate TBW (14), and the absolute measured TBW was higher, not lower, than estimated by BIA. There are several explanations as to why the BIA might underestimate the TBW, in particular the ECW component, in sicker patients. First, BIA is relatively insensitive to truncal fluid and fluid in third spaces such as the pleural cavity, where fluid may accumulate in patients with more severe comorbidity. Second, related to this, BIA is less accurate when body composition, especially body shape, is more abnormal, for example when patients are more obese. In this study, the discrepancy between TBWD and TBWBIA did increase with increasing body mass index, although this might well be a secondary association with comorbidity. Third and likely of greatest importance, however, is the in-built assumptions of the algorithms used by all BIA devices to compute absolute volumes, which assume a fixed value (usually 0.73) for the hydration of lean tissues (demonstrated in Figure 3). That tissues are significantly overhydrated, including fat and lean body structures, in the presence of increased comorbid burden is highly plausible in HD patients, and the data presented here suggest that this typically ranges between 1.5 and 5.0 L. That this was found to be even more marked at 12 mo than at baseline and in both study centers underpins the reproducibility and thus significance of this observation.
Although this excess hydration associated with comorbidity in HD patients, as distinct from an abnormal ECW-TBW ratio, has not been reported previously, it is worth noting that in a recent study that evaluated a new algorithm for determining fluid volumes from multifrequency BIA, TBW and extracellular fluid (ECF) volume, measured by D and sodium bromide dilution, respectively, were underestimated by BIA in the study center in which all of the patients were on dialysis when compared with centers that investigated healthy subjects (18). Chamney et al. (20) proposed a new approach to modeling fluid volumes in individuals who are likely to have excess ECF: The measured intracellular water minus body weight is subtracted from the measured ECW volume, using derived constants to correct each component for normal hydration so that the net difference reflects the quantity of excess fluid. Conceptually, this is strongly supported by the observations in this study; however, when using this approach, which still relies on the strict use of algorithms, we were not able to observe a relationship between excess fluid and comorbidity using the BIA-derived volumes alone, only when the measured TBWD volumes were substituted, indicating the additional value of obtaining an absolute individual value for TBW when estimating fluid excess.
Previous longitudinal studies using BIA to detect changes in body composition usually focused on within-dialysis treatment changes, examining vector length, total body, or segmental changes associated with acute fluid removal (21–23) or changes in derived ECF volume in the short term, in an attempt to define the appropriate dry weight for a patient (24). Generally, these studies have been able to identify relative changes in resistance and impedance in keeping with short-term fluid shifts, although they have not usually been validated with paired changes using isotopic dilution methods. Another new finding of this study was that longitudinal changes during a 12-mo period were influenced by comorbidity, not unexpected, but also that BIA was less sensitive to change in TBW than D dilution. This is in contrast to the stable body composition and good agreement between BIA and TBWD measured using the FA-MS method that we observed during a 12-mo period in a cohort of healthy subjects (25). It is notable that in the low comorbidity group, in which BIA detected an increase in TBW, this was associated with an increase in LBM, whereas BIA was less sensitive to increases in TBW in the more comorbid patients, in whom these changes will reflect increasing ECW. This again suggests that the BIA algorithm is better at identifying differences in TBW when associated with changing normally hydrated LBM rather than that associated with overhydration of tissues.
The prescribed “dry weights” for these groups of patients were overall static, which seems to suggest that clinicians were relatively unaware of these differences in body composition. The potential for additional information that might be of clinical value in these point-of-care measures of body composition is well illustrated in the example cases shown. For example, it is reassuring to the clinician that a change in body composition has been favorable (e.g., TBW increase associated with an increase in muscle mass rather than overhydration or when a dramatic increase in weight can be attributed to fat rather than fluid). It is also clear that in some individuals, severe overhydration can occur; this is especially worrying when absolute TBW is increasing despite a BIA predicted fall in TBW as a proportion of body weight as a result of accelerated loss in muscle mass that might, in error, be seen as increasing body fat.
Conclusions
There is a case for combining both absolute and relative measures of body composition to monitor longitudinal body composition in dialysis patients. This is also now a feasible option. Further research is required to determine the actual value of these measurements in terms of clinical benefit to patients.
Disclosures
D.S. and P.S. are directors of a device development company, Trans Spectra Ltd, UK, which built the FA-MS device prototype.
Acknowledgments
C.C. was supported by grants from Kidney Research UK (RP17/1/2004) and the Baxter Extramural Grant Programme, 2006. FA-MS instrument development was supported by the Wellcome Trust (GR067 160MA).
We a very grateful to the patients and staff of the North Staffordshire and Derby Renal Units for support of this research.
Appendix: Equations Used to Derive Estimates of TBW
Watson
Male = 2.447 − (0.09156 × age) + (0.1074 × height) + (0.3362 × weight)
Female = −2.097 + (0.1069 × height) + (0.2466 × weight)
Hume Male = (0.194786 × height) + (0.296785 × weight) − 14.012934
Female = (0.34454 × height) + (0.183809 × weight) − 35.270121
Chertow
Male = (−0.000672 × weight2) + (0.0725 × weight) + (0.1270 × height) + (0.001861 × weight × height) + (0.001041 × weight × age) + (−0.1098 × age)
Female = (−0.000672 × weight2) + (−0.0401 × weight) + (0.1270 × height) + (0.001861 × weight × height) + (0.001041 × weight × age) + (−0.0749 × age)
For patients with diabetes, add 0.58 L to the above results.
Chumlea
Male = 23.04 − (0.03 × age) + (0.50 × weight) − (0.62 × body mass index)
Male (black) = −18.39 − (0.09 × age) + (0.34 × weight) + (0.25 × height)
Female = −10.50 − (0.01 × age) + (0.20 × weight) + (0.18 × height)
Female (black) = −16.71 − (0.05 × age) + (0.22 × weight) + (0.24 × height)
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, Fonarow GC: Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation 119: 671– 679, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McIntyre CW, Burton JO, Selby NM, Leccisotti L, Korsheed S, Baker CS, Camici PG: Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol 3: 19– 26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chesterton LJ, Sigrist MK, Bennett T, Taal MW, McIntyre CW: Reduced baroreflex sensitivity is associated with increased vascular calcification and arterial stiffness. Nephrol Dial Transplant 20: 1140– 1147, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Johansen KL, Kaysen GA, Young BS, Hung AM, da Silva M, Chertow GM: Longitudinal study of nutritional status, body composition, and physical function in hemodialysis patients. Am J Clin Nutr 77: 842– 846, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Jaffrin MY, Morel H: Body fluid volumes measurements by impedance: A review of bioimpedance spectroscopy (BIS) and bioimpedance analysis (BIA) methods. Med Eng Phys 30: 1257– 1269, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Davies S, Spanel P, Smith D: Rapid measurement of deuterium content of breath following oral ingestion to determine body water. Physiol Meas 22: 651– 659, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Smith D, Engel B, Diskin AM, Spanel P, Davies SJ: Comparative measurements of total body water in healthy volunteers by online breath deuterium measurement and other near-subject methods. Am J Clin Nutr 76: 1295– 1301, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies SJ, Phillips L, Naish PF, Russell G: Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant 17: 1085– 1092, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Van Manen JG, Korevaar JC, Dekker FW, Boeschoten EW, Bossuyt PM, Krediet RT: Adjustment for comorbidity in studies on health status in ESRD patients: Which comorbidity index to use? J Am Soc Nephrol 14: 478– 485, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Chumlea WC, Guo SS, Zeller CM, Reo NV, Baumgartner RN, Garry PJ, Wang J, Pierson RN, Jr, Heymsfield SB, Siervogel RM: Total body water reference values and prediction equations for adults. Kidney Int 59: 2250– 2258, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Chertow GM, Lazarus JM, Lew NL, Ma L, Lowrie EG: Development of a population-specific regression equation to estimate total body water in hemodialysis patients. Kidney Int 51: 1578– 1582, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Spanel P, Smith D: Accuracy and precision of flowing afterglow mass spectrometry for the determination of the deuterium abundance in the headspace of aqueous liquids and exhaled breath water. Rapid Commun Mass Spectrom 15: 867– 872, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Chan C, Smith D, Spanel P, McIntyre CW, Davies SJ: A non-invasive, on-line deuterium dilution technique for the measurement of total body water in haemodialysis patients. Nephrol Dial Transplant 23: 2064– 2070, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bedogni G, Malavolti M, Severi S, Poli M, Mussi C, Fantuzzi AL, Battistini N: Accuracy of an eight-point tactile-electrode impedance method in the assessment of total body water. Eur J Clin Nutr 56: 1143– 1148, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Volgyi E, Tylavsky FA, Lyytikainen A, Suominen H, Alen M, Cheng S: Assessing body composition with DXA and bioimpedance: Effects of obesity, physical activity, and age. Obesity (Silver Spring) 16: 700– 705, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Chertow GM, Lowrie EG, Wilmore DW, Gonzalez J, Lew NL, Ling J, Leboff MS, Gottlieb MN, Huang W, Zebrowski B, et al. : Nutritional assessment with bioelectrical impedance analysis in maintenance hemodialysis patients. J Am Soc Nephrol 6: 75– 81, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Chertow GM, Lazarus JM, Lew NL, Ma L, Lowrie EG: Bioimpedance norms for the hemodialysis population. Kidney Int 52: 1617– 1621, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Moissl UM, Wabel P, Chamney PW, Bosaeus I, Levin NW, Bosy-Westphal A, Korth O, Muller MJ, Ellegard L, Malmros V, Kaitwatcharachai C, Kuhlmann MK, Zhu F, Fuller NJ: Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas 27: 921– 933, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Chertow GM, Jacobs DO, Lazarus JM: Phase angle predicts survival in hemodialysis patients. J Ren Nutr 7: 204– 207, 1997 [Google Scholar]
- 20.Chamney PW, Wabel P, Moissl UM, Muller MJ, Bosy-Westphal A, Korth O, Fuller NJ: A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am J Clin Nutr 85: 80– 89, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Piccoli A: Identification of operational clues to dry weight prescription in hemodialysis using bioimpedance vector analysis. The Italian Hemodialysis-Bioelectrical Impedance Analysis (HD-BIA) Study Group. Kidney Int 53: 1036– 1043, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Pillon L, Piccoli A, Lowrie EG, Lazarus JM, Chertow GM: Vector length as a proxy for the adequacy of ultrafiltration in hemodialysis. Kidney Int 66: 1266– 1271, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Zhu F, Kuhlmann MK, Kaysen GA, Sarkar S, Kaitwatcharachai C, Khilnani R, Stevens L, Leonard EF, Wang J, Heymsfield S, Levin NW: Segment-specific resistivity improves body fluid volume estimates from bioimpedance spectroscopy in hemodialysis patients. J Appl Physiol 100: 717– 724, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Chamney PW, Kramer M, Rode C, Kleinekofort W, Wizemann V: A new technique for establishing dry weight in hemodialysis patients via whole body bioimpedance. Kidney Int 61: 2250– 2258, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Engel B, Spanel P, Smith D, Diskin AM, Davies SJ: Longitudinal measurements of total body water and body composition in healthy volunteers by online breath deuterium measurement and other near-subject methods. Int J Body Compos Res 2: 99– 106, 2004 [PMC free article] [PubMed] [Google Scholar]