Abstract
Background and objectives: Serum alkaline phosphatase has been associated with increased mortality in hemodialysis patients but its associations with mortality in chronic kidney disease (CKD) stages III and IV are unknown.
Design, settings, participants & measurements: In 1094 participants in the African-American Study of Kidney Disease and Hypertension (AASK) database, the associations of serum alkaline phosphatase with mortality and cardiovascular events were examined in Cox models.
Results: The mean (±SD) age was 54 ± 11 yr, and 61% were men. The median alkaline phosphatase was 80 IU/L, and interquartile range was 66 to 97 IU/L. The mean follow-up was 4.6 yr. There were 105 (9.6%) all-cause deaths and 149 (13.6%) cardiovascular events. Each doubling of serum alkaline phosphatase was significantly associated with increased hazard [hazard ratio (HR) 1.60, 95% confidence interval (CI) 1.08 −2.36] of all-cause mortality adjusted for demographics, drug and blood pressure groups, and comorbidity. With further adjustment for liver function tests as well as serum calcium and phosphorus, each doubling of serum alkaline phosphatase remained significantly associated with increased mortality (HR 1.55, 95% CI 1.03 to 2.33). Serum alkaline phosphatase was not significantly associated with increased risk of cardiovascular events.
Conclusions: Independent of liver function tests and serum calcium and phosphorus, higher levels of serum alkaline phosphatase are associated with increased mortality in the CKD population. Further studies are warranted to identify the potential mechanisms for this association.
Experimental studies suggest alkaline phosphatase might promote vascular calcification (1,2). Indeed, higher levels of serum alkaline phosphatase were independently associated with progressive arterial calcification in a longitudinal study of stage IV and V chronic kidney disease (CKD) patients (3). Furthermore, serum alkaline phosphatase was independently associated with increased mortality in hemodialysis patients (4–6). However, there is a paucity of data on whether serum alkaline phosphatase is an independent predictor of mortality in CKD stages III and IV. Therefore, we examined the associations of serum alkaline phosphatase with cardiovascular events and mortality in the African-American Study of Kidney Disease and Hypertension (AASK) database.
Materials and Methods
The details of the AASK design have been published earlier (7,8). In brief, the AASK study was a multicenter, prospective, controlled, blinded, randomized trial sponsored by the National Institutes of Health. This trial examined (1) the effect of strict blood pressure (BP) control [mean arterial pressure (MAP) <92 mmHg or <125/75 mmHg] versus usual BP control (MAP, 102 to107 mmHg or 140/90 mmHg), and (2) the effect of three classes of antihypertensive agents (calcium channel blocker, angiotensinogen converting enzyme inhibitor, and β-blocker therapy) on the rate of change in GFR in African Americans. Patients were randomly assigned to receive amlodipine, ramipril, or metoprolol, and other drugs were added stepwise to achieve the BP goals.
AASK participants were 1094 African-American men and women, age 18 to 70 yr, nondiabetic, hypertensive (diastolic BP ≥95 mmHg) and had reduced GFR between 20 and 65 ml/min/1.73 m2.
Recruitment into the full-scale trial began in February 1995, with planned follow-up through September 2001. Using standardized forms, trained personnel obtained data on baseline demographic, clinical, and laboratory data. At a seated position, 10 to 20 ml of blood was collected in serum separator tubes, allowed to clot at room temperature for at least 30 min, and then centrifuged. Serum samples were mailed overnight in a frozen pack to the Central Biochemistry Laboratory at the Cleveland Clinic for standardized measurements. Chemistry 18 panels [electrolytes, BUN, serum creatinine, alkaline phosphatase, albumin, bilirubin, lactate dehydrogenase (LDH), aspartate aminotransferase (AST), γ-glutamyltransferease (GGT), calcium, and phosphorus] were performed on a Roche Hitachi 747 or Beckman CX3 Delta analyzer. GFR was assessed by using radioiodine-iothalamate clearance twice during baseline, then at 3, 6, and every 6 mo thereafter.
Hospitalizations and deaths were tracked by study personnel. The AASK Clinical Outcome committee, which made the final determination of the event status, reviewed hospitalizations and deaths classified by the clinical sites as potential cardiovascular events. A cardiovascular event was defined as a cardiovascular death or hospitalization because of myocardial infarction, congestive heart failure, cerebrovascular event, or coronary revascularization. A composite kidney end point was defined as the occurrence of GFR event (defined as a confirmed reduction in GFR by 50% or by 25 ml/min per 1.73 m2 from the mean of the 2 baseline GFR values), renal replacement therapy, or death.
Statistical Methods
Baseline characteristics were summarized and compared between the low and high alkaline phosphatase groups (defined by median value of 80 IU/L of alkaline phosphatase) using independent two-sample t tests or Wilcoxon rank-sums tests as appropriate for continuous variables and Fisher's exact test for categorical variables.
The cross-sectional associations of baseline factors with the occurrence of a high baseline serum alkaline phosphatase level (≥80 IU/L) were examined in a multivariate logistic regression model.
The associations of serum alkaline phosphatase with time to all-cause death; time to first cardiovascular event; and time to the composite of death, GFR event, or dialysis were examined using separate Cox regression analyses. Analyses of all-cause mortality were censored at the administrative end date for the trial. Deaths that occurred after the onset of ESRD but before the administrative censoring date were included in the analyses. Analyses of the cardiovascular end point were censored at the administrative end date for the trial, noncardiovascular death, or the occurrence of ESRD because the Clinical Outcome committee did not adjudicate on events that occurred after the onset of ESRD.
Because serum alkaline phosphatase levels had a positively skewed distribution, the values were transformed logarithmically. The results of these models are reported as the hazard ratios associated with each 2-fold increase in the baseline serum alkaline phosphatase level. For each outcome, Cox regression models were first fit with log-transformed serum alkaline phosphatase as the predictor variable, adjusted for demographics (age and gender), AASK BP and drug groups, baseline GFR, urine protein-to-creatinine ratio, MAP, atherosclerotic conditions (coronary artery disease, cerebrovascular disease, and peripheral vascular disease), congestive heart failure, body mass index (BMI), and hematocrit. In these models, clinical center was included as a stratification variable. Next, liver function tests [serum albumin, AST, alanine aminotransferase (ALT), total bilirubin and LDH] were added into the Cox models. Finally, serum calcium and phosphorus were added to examine whether the associations of alkaline phosphatase with outcomes were independent of these parameters. Separate Cox regression models, with the same sets of covariates, were used to relate all-cause mortality, the first cardiovascular event, and the composite of death, dialysis, or GFR event to a dichotomous variable defined by alkaline phosphatase levels of 80 IU/L or more or less than 80 IU/L, the approximate median baseline alkaline phosphatase value.
Key assumptions of the Cox regression models, including linear effects of baseline factors and proportional hazards over time, were evaluated by preliminary diagnostic analyses. First, quadratic terms were tested for each continuous variable to test for the presence of nonlinear effects of continuous covariates. Only BMI and serum albumin had nonlinear associations with time to death. Therefore, both linear and quadratic terms were included for these variables in each of the Cox regressions described above.
Second, interactions of each predictor with follow-up time were tested to evaluate the assumption of proportional hazards. Only serum alkaline phosphatase had evidence of nonproportionality for all-cause death. Therefore, the associations of alkaline phosphatase with mortality in the first 2 yr and later years were separately examined.
Results
All 1094 AASK participants that were randomized had baseline serum alkaline phosphatase measured. The mean (±SD) age was 54 ± 11 yr, 61% were men, and all were self-identified African Americans. Because diabetes was an exclusion criterion, none had known diabetes. Congestive heart failure (3%) and atherosclerotic conditions (14%) were present. The mean (±SD) baseline MAP was 114 ± 16 mmHg and BMI was 30.5 ± 6.6 kg/m2.
The distribution of serum alkaline phosphatase was positively skewed. The median alkaline phosphatase was 80 IU/L and the interquartile range was 66 to 97 IU/L. There were 554 and 540 patients randomized to the standard MAP and low MAP arms, respectively, and 436, 441, and 217 patients randomized to ramipril, metoprolol, and amlodipine arms, respectively. There were 149 (13.6%) cardiovascular events over a mean of 4.1 yr of follow-up and 105 (9.6%) all-cause deaths over a mean of 4.6 yr of follow-up.
Table 1 summarizes the clinical characteristics of the study participants by low and high alkaline phosphatase groups defined by the median value (80 IU/L). The high alkaline phosphatase group had higher proportion of women, lower GFR, and greater proteinuria. There were no significant differences across the alkaline phosphatase groups regarding serum calcium and phosphorus as well as serum AST, ALT, and bilirubin. However, the high alkaline phosphatase group had higher GGT levels. In a multivariate logistic regression model, male gender [odds ratio (OR) 0.74, 95% confidence interval (CI) 0.56 to 0.97], GFR (OR 0.87, 95% CI 0.78 to 0.97 for each 10-ml/min/1.73 m2 increase), and GGT (OR 1.08, 95% CI 1.04 to 1.13 for each 10-IU/L increase) were significantly associated with the high alkaline phosphatase group.
Table 1.
Baseline characteristics of AASK cohort by serum alkaline phosphatase groups
| Baseline Characteristic | Alkaline Phosphatase <80 (mean 63 ± 10) IU/L (n = 527) | Alkaline Phosphatase ≥80 (mean 106 ± 37) IU/L (n = 567) | P Valuea |
|---|---|---|---|
| Demographics and trial characteristics | |||
| age (yr) | 54.0 ± 10.8 | 54.9 ± 10.6 | 0.18 |
| male gender (%) | 342 (65) | 327 (58) | 0.02 |
| Randomized drug group | |||
| ramipril (%) | 201 (38) | 235 (41) | 0.27 |
| metoprolol (%) | 214 (41) | 227 (40) | 0.85 |
| amlodipine (%) | 112 (21) | 105 (19) | 0.29 |
| Randomized BP group | |||
| strict control (%) | 253 (48) | 287 (51) | 0.40 |
| usual control (%) | 274 (52) | 280 (49) | 0.40 |
| Clinical characteristics | |||
| atherosclerotic conditions (%) | 73 (14) | 82 (14) | 0.80 |
| congestive heart failure (%) | 12 (2) | 16 (3) | 0.70 |
| current or past smoking (%) | 290 (55) | 343 (60) | 0.08 |
| BMI (kg/m2) | 30.7 ± 6.4 | 30.4 ± 6.8 | 0.50 |
| MAP (mmHg) | 114.5 ± 16.0 | 113.6 ± 16.1 | 0.36 |
| GFR (ml/min/1.73 m2) | 47.8 ± 13.4 | 45.1 ± 13.7 | 0.001 |
| Laboratory data | |||
| serum AST (IU/L) | 23.0 ± 13.1 | 23.3 ± 12.1 | 0.71 |
| serum LDH (IU/L) | 177.7 ± 41.4 | 179.8 ± 44.2 | 0.41 |
| serum total bilirubin (mg/dl) | 0.5 ± 0.2 | 0.5 ± 0.3 | 0.09 |
| serum GGT (IU/L) | 38.5 ± 64.3 | 54.8 ± 70.5 | <0.001 |
| serum albumin (g/dl) | 4.3 ± 0.3 | 4.3 ± 0.4 | 0.88 |
| urine protein-to-creatinine ratiob (mg/g) | 70 (30 – 310) | 90 (30 – 410) | 0.03 |
| serum calcium (mg/dl) | 9.2 ± 0.5 | 9.2 ± 0.5 | 0.75 |
| serum phosphorus (mg/dl) | 3.5 ± 0.7 | 3.5 ± 0.7 | 0.89 |
| serum calcium phosphorus product (mg/dl)b | 32.2 ± 7.0 | 32.3 ± 6.3 | 0.95 |
P values calculated by ANOVA for continuous variables and Fisher's exact tests for categorical variables.
Geometric mean (interquartile range) presented.
As shown in Table 2, each doubling of serum alkaline phosphatase was significantly associated with a 60% higher hazard of all-cause mortality adjusted for demographics (age, gender, and race), drug and BP groups, atherosclerosis, congestive heart failure, MAP, BMI, smoking, GFR, and proteinuria. With further adjustment for liver function tests as well as serum calcium and phosphorus, each doubling of serum alkaline phosphatase remained significantly associated with increased mortality (Table 2).
Table 2.
Associations of serum alkaline phosphatase with all-cause mortality
| Model/Association | Hazard Ratio | 95% CI |
|---|---|---|
| Continuous variable models for each 2-fold increase in baseline serum alkaline phosphatase | ||
| model 1a | 1.60 | 1.08 to 2.36 |
| model 2b | 1.55 | 1.03 to 2.33 |
| model 3c | 1.55 | 1.03 to 2.33 |
| Categorical variable models for high versus low alkaline phosphatase group | ||
| model 1a | 1.36 | 0.90 to 2.04 |
| model 2b | 1.30 | 0.86 to 1.96 |
| model 3c | 1.30 | 0.86 to 1.96 |
Model 1 adjusted for demographics (age and gender), drug and BP groups, atherosclerosis, congestive heart failure, MAP, BMI, smoking, GFR, and proteinuria.
Model 2 further adjusted for liver function tests (serum AST, albumin, LDH, total bilirubin, and GGT).
Model 3 further adjusted for serum calcium and phosphorus.
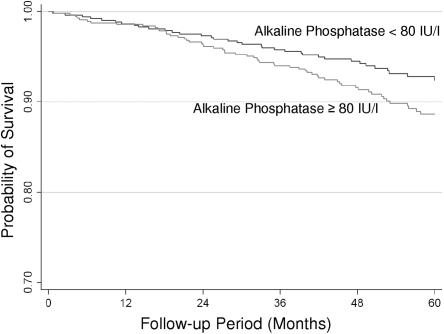
The unadjusted association of serum alkaline phosphatase groups with mortality is shown in Figure 1. When expressed as a dichotomous factor, the high alkaline phosphatase group was associated with increased mortality when compared with the low alkaline phosphatase group, but this was NS (Table 2).
Figure 1.
Associations of serum alkaline phosphatase groups with survival in the AASK cohort.
In sensitivity analyses, only BMI and serum albumin had evidence of nonlinearity in their associations with mortality. Although BMI and serum albumin were not significantly associated with serum alkaline phosphatase (Table 1), addition of quadratic terms for BMI and serum albumin slightly attenuated the associations of serum alkaline phosphatase with mortality (for each doubling of serum alkaline phosphatase HR 1.47, 95% CI 0.97 to 2.24).
Serum alkaline phosphatase had evidence of nonproportionality in its associations with mortality. In a time-dependent multivariable Cox model adjusted for demographics (age, gender and race), drug and BP groups, atherosclerosis, congestive heart failure, MAP, BMI, smoking, GFR, proteinuria, liver function tests (serum AST, albumin, LDH, total bilirubin, and GGT), and serum calcium and phosphorus, each doubling of serum alkaline phosphatase was not associated with increased mortality in the first 2 yr of follow-up (HR 0.91, 95% CI 0.43 to 1.92), but was associated with increased mortality after 2 yr of follow-up (HR 1.95, 95% CI 1.23 to 3.08).
Serum alkaline phosphatase was not associated with cardiovascular events or the composite of death, dialysis, or GFR event (Tables 3 and 4).
Table 3.
Associations of serum alkaline phosphatase with the cardiovascular composite end point
| Model/Association | Hazard Ratio | 95% CI |
|---|---|---|
| Continuous variable models for each 2-fold increase in baseline serum alkaline phosphatase | ||
| model 1a | 1.04 | 0.72 to 1.50 |
| model 2b | 1.09 | 0.74 to 1.58 |
| model 3c | 1.08 | 0.74 to 1.58 |
| Categorical variable models for high versus low alkaline phosphatase group | ||
| model 1a | 1.16 | 0.83 to 1.63 |
| model 2b | 1.18 | 0.83 to 1.66 |
| model 3c | 1.18 | 0.83 to 1.66 |
Model 1 adjusted for demographics (age and gender), drug and BP groups, atherosclerosis, congestive heart failure, MAP, BMI, smoking, GFR, and proteinuria.
Model 2 further adjusted for liver function tests (serum AST, albumin, LDH, total bilirubin, and GGT).
Model 3 further adjusted for serum calcium and phosphorus.
Table 4.
Associations of serum alkaline phosphatase with the composite of death, dialysis, or GFR event
| Model/Association | Hazard Ratio | 95% CI |
|---|---|---|
| Continuous variable models for each 2-fold increase in baseline serum alkaline phosphatase | ||
| model 1a | 1.07 | 0.76 to 1.33 |
| model 2b | 1.03 | 0.82 to 1.30 |
| model 3c | 1.03 | 0.82 to 1.30 |
| Categorical variable models for high versus low alkaline phosphatase group | ||
| model 1a | 0.98 | 0.79 to 1.21 |
| model 2b | 0.96 | 0.77 to 1.19 |
| model 3c | 0.97 | 0.78 to 1.20 |
Model 1 adjusted for demographics (age and gender), drug and BP groups, atherosclerosis, congestive heart failure, MAP, BMI, smoking, GFR, and proteinuria.
Model 2 further adjusted for liver function tests (serum AST, albumin, LDH, total bilirubin, and GGT).
Model 3 further adjusted for serum calcium and phosphorus.
Discussion
In hemodialysis patients, high serum alkaline phosphatase was associated with increased mortality (4,6). An analysis of the Dialysis Outcomes and Practice Patterns Study database found that elevated serum alkaline phosphatase levels in hemodialysis patients were associated with higher risk of hospitalization and death (5). To our knowledge, there are no published studies on the associations of serum alkaline phosphatase levels with mortality in CKD stages III and IV.
The results of the study presented here suggest that independent of liver function tests and serum calcium and phosphorus, serum alkaline phosphatase might be a risk factor for death in African-American patients in CKD stages III and IV. The potential mechanisms for this observation remain unclear. However, there is mounting evidence that alkaline phosphatase can promote vascular calcification by hydrolyzing pyrophosphate in the arterial wall (1,9). For instance, in calcified diabetic arteries, alkaline phosphatase is upregulated (9). In the aorta of uremic rats, the hydrolysis rate of pyrophosphate was increased compared with controls (1). This increase was reduced by levamisole, a nonspecific inhibitor of alkaline phosphatase. These experimental data suggest a role for alkaline phosphatase in the development of uremic calcification. Indeed, in a longitudinal study of 134 stage IV and V CKD patients, higher levels of serum alkaline phosphatase were associated with progressive arterial calcification (3). This association was independent of serum calcium, phosphorus, parathyroid hormone (PTH), fetuin A, and C-reactive protein. In another study of 135 stages I through V CKD patients with limited adjustment for gender, CKD stage, serum PTH, and tartrate-resistant acid phosphatase 5b, the highest tertile of alkaline phosphatase was associated with increased risk of cardiovascular events (10).
Apart from vascular calcification, inflammation, and insulin resistance are other potential mechanisms for the association between higher serum alkaline phosphatase levels and increased mortality. Serum alkaline phosphatase has been associated with elevated C-reactive protein levels (11,12). In the Insulin Resistance Atherosclerosis Study, compared with the lowest quartile of serum alkaline phosphatase, those in the highest quartile had 2.3-fold higher odds of developing incident metabolic syndrome over a mean follow-up of 5.2 yr (13).
Because hypovitaminosis D is known to be associated with elevated serum alkaline phosphatase levels (14), another potential explanation for increased mortality associated with serum alkaline phosphatase is decreased vitamin D levels. Because serum vitamin D levels were not measured in the AASK cohort, we are unable to examine this hypothesis. Thus, there are multiple potential mechanisms for the associations of elevated serum alkaline phosphatase with increased mortality.
The cross-sectional association of serum alkaline phosphatase with greater proteinuria at baseline (Table 1) is intriguing. One might speculate that alkaline phosphatase might play a role in kidney injury. However, higher levels of serum alkaline phosphatase are not associated with the kidney composite.
The strengths of the study presented here include careful collection of data in the AASK study and centralized measurement of serum alkaline phosphatase. The limitations of the study include that of retrospective analyses of an existing database and unavailability of serum PTH and vitamin D levels in the AASK database. Information on bone-specific alkaline phosphatase is also unavailable. Nonetheless, data on serum GGT (which is clinically used to differentiate between bone versus liver source of alkaline phosphatase) were available. Even when adjusted for liver enzymes, elevated serum alkaline phosphatase remained associated with increased mortality. Finally, the AASK cohort consisted only of African Americans and further studies in other races are needed.
In summary, serum alkaline phosphatase is associated with increased mortality in African Americans with stage III and IV CKD independent of serum calcium and phosphorus and liver function tests. Further studies are warranted to determine the mechanisms of the increased mortality associated with higher levels of serum alkaline phosphatase.
Disclosures
None.
Acknowledgments
The AASK study was conducted by the AASK investigators and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). This manuscript was not prepared in collaboration with investigators of the AASK study and does not necessarily reflect the opinions or views of the AASK study or the NIDDK.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Lomashvili KA, Garg P, Narisawa S, Millan JL, O'Neill WC: Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: Potential mechanism for uremic vascular calcification. Kidney Int 73: 1024– 1030, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narisawa S, Harmey D, Yadav MC, O'Neill WC, Hoylaerts MF, Millan JL: Novel inhibitors of alkaline phosphatase suppress vascular smooth muscle cell calcification. J Bone Miner Res 22: 1700– 1710, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Sigrist MK, Taal MW, Bungay P, McIntyre CW: Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clin J Am Soc Nephrol 2: 1241– 1248, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Lee GH, Benner D, Regidor DL, Kalantar-Zadeh K: Impact of kidney bone disease and its management on survival of patients on dialysis. J Ren Nutr 17: 38– 44, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Blayney MJ, Pisoni RL, Bragg-Gresham JL, Bommer J, Piera L, Saito A, Akiba T, Keen ML, Young EW, Port FK: High alkaline phosphatase levels in hemodialysis patients are associated with higher risk of hospitalization and death. Kidney Int 75: 1114, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD: Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771– 780, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, Briggs JP, Charleston J, Cheek D, Cleveland W, Douglas JG, Douglas M, Dowie D, Faulkner M, Gabriel A, Gassman J, Greene T, Hall Y, Hebert L, Hiremath L, Jamerson K, Johnson CJ, Kopple J, Kusek J, Lash J, Lea J, Lewis JB, Lipkowitz M, Massry S, Middleton J, Miller ER, III, Norris K, O'Connor D, Ojo A, Phillips RA, Poque V, Rahman M, Randall OS, Rostrand S, Schulman G, Smith W, Thornely-Brown D, Tisher CC, Toto RD, Wright JT, Jr, Xu S.African American Study of Kidney Disease and Hypertension (AASK) Study Group: Effect of ramipril vs. amlodipine on renal outcomes in hypertensive nephrosclerosis: A randomized controlled trial. JAMA 285: 2719– 2728, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Wright JT, Jr., Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Herbert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostrand SG; African American Study of Kidney Disease and Hypertension Study: Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 288: 2421– 2431, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Fadini GP, Pauletto P, Avogaro A, Rattazzi M: The good and the bad in the link between insulin resistance and vascular calcification. Atherosclerosis 193: 241– 244, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Fahrleitner-Pammer A, Herberth J, Browning SR, Obermayer-Pietsch B, Wirnsberger G, Holzer H, Dobnig H, Malluche HH: Bone markers predict cardiovascular events in chronic kidney disease. J Bone Miner Res 23: 1850– 1858, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Cheung BM, Ong KL, Cheung RV, Wong Ly, Wat NM, Tam S, Leung GM, Cheng CH, Woo J, Janus ED, Lau CP, Lam TH, Lam KS: Association between plasma alkaline phosphatase and C-reactive protein in Hong Kong Chinese. Clin Chem Lab Med 46: 523– 527, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Kerner A, Avizohar O, Sella R, Bartha P, Zinder O, Markiewicz W, Levy Y, Brook GJ, Anoson D: Association between elevated liver enzymes and C-reactive protein: possible hepatic contribution to systemic inflammation in the metabolic syndrome. Arterioscler Thromb Vasc Biol 25: 193– 197, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Hanley AJ, Williams K, Festa A, Wagenknecht LE, D'Agostino RB, Jr., Haffner SM: Liver markers and development of the metabolic syndrome: The insulin resistance atherosclerosis study. Diabetes 54: 3140– 3147, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Jean G, Terrat JC, Vanel T, Hurot JM, Lorriauz C, Mayor B, Chazot C: Daily oral 25-hydroxycholecalciferol supplementation for vitamin D deficiency in haemodialysis patients: Effects on mineral metabolism and bone markers. Nephrol Dial Transplant 23: 3670– 3676, 2008 [DOI] [PubMed] [Google Scholar]