Abstract
Background and objectives: Recently, we showed that soluble TNF-like weak inducer of apoptosis (sTWEAK) plasma levels are diminished in hemodialysis patients and had additive effects with IL-6 on survival. Because sTWEAK plasma level has been associated with the presence of chronic kidney disease (CKD) and cardiovascular disease, we hypothesized that in patients with CKD, sTWEAK levels may relate to the increased prevalence of endothelial dysfunction that usually accompanies the decline of estimated GFR (eGFR).
Design, setting, participants, & measurements: We studied 295 patients with different stages of nondiabetic CKD (52% male; age 47 ± 12 yr), testing the association between sTWEAK plasma levels and CKD stage and the relationship between flow-mediated dilation (FMD) and sTWEAK concentrations. Fifty-five healthy volunteers (51% male; age 47 ± 11 yr) served as matched control subjects.
Results: A gradual decrease in FMD was observed as eGFR decreased. Compared with healthy control subjects, sTWEAK plasma levels were diminished in all stages of CKD and correlated strongly with eGFR. FMD levels were associated with sTWEAK concentrations in univariate analysis. This association persisted after multivariate adjustment for eGFR levels, high-sensitivity C-reactive protein, diastolic BP, and sTWEAK, all of which were found to be significant and independent contributors to FMD.
Conclusions: A decline in eGFR is accompanied by gradual reductions in sTWEAK plasma levels. Because sTWEAK strongly and independently correlated with FMD, our study suggests novel links between sTWEAK and endothelial dysfunction in patients with CKD.
Endothelial dysfunction (ED) is the initial pathophysiologic step in the progression of vascular damage that precedes and leads to clinically manifest cardiovascular diseases (CVD) (1–3). ED is highly prevalent in patients with moderate to advanced chronic kidney disease (CKD) (4,5) and is linked to the elevated cardiovascular risk of this patient population (6,7).
The cause of ED is complex and involves dysregulation of multiple pathways. One of those could be mediated by the TNF-like weak inducer of apoptosis (TWEAK, TNFSF12), a member of the TNF superfamily of cytokines (8). TWEAK is a type II transmembrane glycoprotein (30 kD) that circulates in plasma as a soluble form (sTWEAK) with a molecular weight of 18 kD (9,10). TWEAK is widely expressed and can be found in pancreas, intestine, heart, brain, lung, ovary, the vasculature, skeletal muscle, liver, and kidney (8,11). Binding of TWEAK to its receptor, Fn14 (10), mediates multiple biologic effects such as cellular growth, proliferation and migration, osteoclastogenesis, angiogenesis, and apoptosis (12–15). In addition, TWEAK induces the expression of different cell adhesion molecules and proinflammatory cytokines (16–18) through NF-κB activation.
In previous studies, we observed that plasma sTWEAK concentrations are diminished in patients who undergo hemodialysis (HD) or have carotid atherosclerosis (19,20). We thus suggested that sTWEAK could be a novel biomarker of CVD; however, the implications of sTWEAK levels in earlier CKD stages as well as its relation with ED are unknown. In this article, we investigated sTWEAK plasma concentrations in 295 patients who had various stages of nondiabetic CKD, and in whom measurements of flow-mediated dilation (FMD) were also performed.
Materials and Methods
Patients
The patients were referrals to the Renal Unit of the Gülhane School of Medicine Medical Center (Ankara, Turkey) because of suspected or manifest renal failure. They all received for the first time a diagnosis of CKD according to their estimated GFR (eGFR) and the presence of kidney injury as defined by National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K/DQOI) Guidelines (21). To diminish any confounders that may influence patients with ED, patients with previous clinical history of CVD and nephrotic syndrome were not included. At the same time, smoking. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, statins, and supplemental vitamins were exclusion criteria. In addition, patients with a previous diagnosis of diabetes, current use of oral antidiabetic medication or insulin, or a fasting glucose level >126 mg/dl were excluded. Finally, other exclusion criteria than unwillingness to participate in the study were applied.
Following this criteria, 295 patients with CKD and a mean age of 47 ± 11 yr were included in the study. The clinical characteristics of the study groups are given in Table 1. Hypertension was defined as systolic BP (SBP) ≥140 mmHg or diastolic BP (DBP) ≥90 mmHg on repeated measurements or the use of antihypertensive drugs. Sixty-four of the patients were on antihypertensive therapy (40 patients were treated with calcium channel antagonists, 10 with β-blocker agents, and seven with loop diuretics). Patients with stage 5 CKD were required to be under regular HD for at least 6 mo before inclusion in the study.
Table 1.
Clinical characteristics of study groups
| Characteristic | Control(n = 55) | CKD |
||||
|---|---|---|---|---|---|---|
| Stage 1 (≥90; n = 57) | Stage 2 (60 to 89; n = 61) | Stage 3 (30 to 59; n = 60) | Stage 4 (15 to 29; n = 60) | Stage 5 (<15; HD; n = 57) | ||
| Age (yr; mean ± SD) | 47 ± 11 | 46 ± 11 | 49 ± 10 | 45 ± 13 | 47 ± 12 | 46 ± 12 |
| Male gender (n) | 28 | 31 | 34 | 29 | 32 | 27 |
| BMI (kg/m2; mean ± SD) | 26.1 ± 2.0 | 26.4 ± 2.2 | 26.3 ± 3.1 | 25.8 ± 2.6 | 25.9 ± 2.8 | 25.2 ± 2.7 |
| Cause of CKD (n) | ||||||
| glomerulonephritis | 11 | 13 | 10 | 12 | 9 | |
| hypertension | 10 | 9 | 14 | 13 | 11 | |
| ADPKD | None | 4 | 5 | 3 | 4 | 4 |
| reflux nephropathy | 2 | 1 | 2 | 1 | 3 | |
| unknown | 30 | 35 | 31 | 30 | 30 | |
| Antihypertensive drugs (n) | ||||||
| calcium-channel blockers | 6 | 7 | 9 | 10 | 8 | |
| β blockers | None | 2 | 1 | 2 | 2 | 3 |
| loop diuretics | 2 | 1 | 3 | 1 | 0 | |
ADPKD, autosomal dominant polycystic kidney disease; BMI, body mass index.
The patients were allocated to eGFR levels from stages 1 to 5 as determined by K/DOQI (Table 1). The calculation was made according to the simplified version of the Modification of Diet in Renal Disease (MDRD) formula as defined by Levey et al. (22): [GFR = 186 × protein catabolic rate−1.154 × age−0.203 × 1.212 (if black) × 0.742 (if female)].
Fifty-five age-matched (47 ± 11 yr) and gender-matched (50% male) nonsmoker, healthy individuals were enrolled as control subjects. These control subjects had no history of metabolic or other serious concomitant diseases, renal dysfunction, or disorders of lipid metabolism. All procedures in this study were carried out in accordance with institutional and national ethical guidelines for human studies. The ethical committee of Gülhane School of Medicine approved the study. Informed consent was obtained from each participant.
Laboratory Measurements
The arterial BPs were measured by a physician three times after a 15-min resting period in the morning, and mean values were calculated for SBP and DBP for all participants. After an overnight fasting (nondialysis day for HD patients), the venous blood samples from patients and control subjects were obtained to calculate the fasting plasma glucose, serum albumin, total serum cholesterol, triglycerides, and HDL and LDL cholesterol. Total plasma cholesterol, triglycerides, and HDL cholesterol were measured by enzymatic colorimetric method with Olympus AU 600 autoanalyzer using reagents from Olympus Diagnostics, GmbH (Hamburg, Germany). LDL cholesterol was calculated by Friedewald's formula (23). Plasma concentrations of sTWEAK were determined in duplicate with commercially available ELISA kits (Bender MedSystems, Vienna, Austria). The minimum detectable level of sTWEAK was 10 pg/ml. Intra- and interassay coefficients of variation were 7.9 and 9.2%, respectively. Basal insulin level was measured by the coated tube method (DPC-USA). An insulin resistance score Homeostasis Model Assessment-Insulin resistance (HOMA-IR) was computed by the following formula (24): HOMA-IR = fasting plasma glucose (mg/dl) × immunoreactive insulin (μIU/ml)/405. For measurement of high-sensitivity C-reactive protein (hsCRP), serum samples were diluted at a ratio of 1:101 with the diluents solution. Calibrators, kit controls, and serum samples all were added on each microwell with an incubation period of 30 min. After three washing intervals, 100 μl of enzyme conjugate (peroxidase-labeled anti-CRP) was added on each microwell for additional 15-min incubation period at room temperature in the dark. The reaction was stopped with a stop solution, and photometric measurement was performed at the 450-nm wavelength. The amount of serum samples was calculated as mg/L with a graphic that was made by noting the absorbance levels of the calibrators.
Vascular Assessment
According to the method of Celermajer et al. (25), the endothelium-dependent vasodilation (FMD) and endothelium-independent vasodilation (nitroglycerine-mediated dilation [NMD]) of the brachial artery were assessed by using high-resolution ultrasound. Measurements were made by a single observer using an ATL 5000 ultrasound system (Advanced Technology Laboratories, Bothell, WA) with a 12-MHz probe. The nonfistula arm was used in the dialysis group. All vasoactive medications were withheld for 24 h before the procedure. The participants remained at rest in the supine position for at least 15 min before the examination started. The participant's arm was comfortably immobilized in the extended position to allow consistent recording of the brachial artery 2 to 4 cm above the antecubital fossa. Three adjacent measurements of end-diastolic brachial artery diameter were made from single two-dimensional frames. All ultrasound images were recorded on S-VHS videotape for subsequent blinded analysis. A pneumatic tourniquet was inflated to 200 mmHg with obliteration of the radial pulse. After 5 min, the cuff was deflated. Flow measurements were made 60 s after deflation. After an additional 15 min, measurements were repeated and again 3 min after administration of sublingual glyceryl trinitrate 400 μg orally. The maximum FMD and NMD dilation diameters were calculated as the average of the three consecutive maximum diameter measurements. The FMD and NMD were then calculated as the percentage change in diameter compared with baseline resting diameters.
Statistical Analysis
Non-normally distributed variables were expressed as median (range), and normally distributed variables were expressed as mean ± SD as appropriate. P < 0.05 was considered to be statistically significant. Between-group comparisons were assessed for nominal variables with the χ2 test. Difference among the groups was analyzed by one-way ANOVA test followed by post hoc Tukey Kramer test for multiple comparisons. Spearman rank correlation was used to determine correlations between two variables. Stepwise multivariate regression analysis was used to assess the predictors for FMD levels. All of the statistical analyses were performed by using SPSS 11.0 (SPSS, Chicago, IL) statistical package.
Results
The characteristics of the study participants are presented in Tables 1 and 2. No significant differences between patients and control subjects were found with regard to age, body mass index, SBP, DBP, serum albumin, total cholesterol, LDL cholesterol, HDL cholesterol, or triglyceride levels. All of these parameters were similar within the various CKD groups.
Table 2.
Laboratory and vascular assessments according to groups
| Parameter | Control(n = 55) | CKD |
Pa | ||||
|---|---|---|---|---|---|---|---|
| Stage 1 (≥90; n = 57) | Stage 2 (60 to 89; n = 61) | Stage 3 (30 to 59; n = 60) | Stage 4 (15 to 29; n = 60) | Stage 5 (<15; HD; n = 57) | |||
| eGFR (ml/min per 1.73 m2; median [range]) | 118 (98 to 129) | 97 (88 to 108)b | 69 (60 to 93)b | 38 (17 to 58)b | 21 (15 to 37)b | 9 (2 to 14)b | <0.001 |
| Serum albumin (g/dl; median [range]) | 4.2 ± 0.4 | 4.1 ± 0.3 | 4.2 ± 0.3 | 4.2 ± 0.4 | 4.1 ± 0.3 | 4.0 ± 0.3 | 0.134 |
| SBP (mmHg; median [range]) | 131 (119 to 139) | 132 (115 to 157) | 133 (115 to 145) | 133 (110 to 142) | 131 (111 to 151) | 133 (113 to 141) | 0.803 |
| DBP (mmHg; median [range]) | 83.0 (76.0 to 89.0) | 82.0 (71.0 to 94.0) | 83.0 (73.0 to 90.0) | 84.5 (80.0 to 92.0) | 83.5 (71.0 to 93.0) | 83.0 (71.0 to 92.0) | 0.041 |
| Plasma insulin (μIU/ml; median [range]) | 6.8 (4.5 to 9.8) | 6.5 (5.0 to 9.7) | 6.7 (4.9 to 9.8) | 7.0 (4.9 to 13.0) | 6.7 (5.1 to 15.2) | 7.0 (5.5 to 13.5) | 0.107 |
| Plasma glucose (mg/dl; median [range]) | 85 (66 to 104) | 82 (70 to 108) | 88 (67 to 104) | 88 (68 to 105) | 90 (70 to 106) | 86 (68 to 109) | 0.057 |
| HOMA-IR (median [range]) | 1.3 (0.9 to 2.1) | 1.4 (1.0 to 2.4) | 1.4 (0.9 to 3.0) | 1.4 (1.0 to 3.1) | 1.4 (1.1 to 3.4) | 1.5 (1.1 to 3.0) | 0.070 |
| Total cholesterol (mg/dl; median [range]) | 192 (159 to 235) | 193 (160 to 231) | 194 (170 to 235) | 193 (171 to 237) | 192 (159 to 236) | 192 (149 to 235) | 0.092 |
| Triglycerides (mg/dl; median [range]) | 138 (115 to 167) | 137 (103 to 179) | 142 (106 to 159) | 138 (107 to 168) | 136 (124 to 162) | 137 (124 to 166) | 0.384 |
| LDL cholesterol (mg/dl; mean ± SD) | 122 ± 12 | 125 ± 16 | 130 ± 15 | 125 ± 16 | 126 ± 16 | 120 ± 20 | 0.031 |
| HDL cholesterol (mg/dl; median [range]) | 44 (27 to 53) | 46 (31 to 51) | 46 (26 to 54) | 43 (26 to 50) | 43 (28 to 63) | 43 (26 to 59) | 0.118 |
| hsCRP (mg/L; median [range]) | 2.0 (1.0 to 4.0) | 6.0 (3.2 to 10.0)b | 10.0 (5.0 to 15.0)b | 16.0 (6.5 to 22.0)b | 22.0 (8.0 to 28.0)b | 26.0 (11.0 to 37.0)b | <0.001 |
| NMD (%; median [range]) | 13.0 (11.9 to 13.9) | 13.0 (11.8 to 13.8) | 13.0 (12.4 to 13.8) | 12.9 (12.0 to 13.8) | 13.0 (11.6 to 13.8) | 12.0 (10.0 to 13.3)b | <0.001 |
| FMD (%; median [range]) | 9.0 (7.5 to 12.4) | 8.4 (7.2 to 9.7)b | 7.3 (6.7 to 8.3)b | 6.9 (6.2 to 8.2)b | 6.2 (5.2 to 8.2)b | 5.2 (4.0 to 7.2)b | <0.001 |
| sTWEAK (pg/ml; median [range]) | 445 (326 to 634) | 378 (146 to 678)b | 322 (145 to 479)b | 263 (142 to 381)b | 234 (139 to 357)b | 174 (114 to 313)b | <0.001 |
One-way ANOVA test.
Tukey-Kramer test, statistically (P < 0.05) compared with control group.
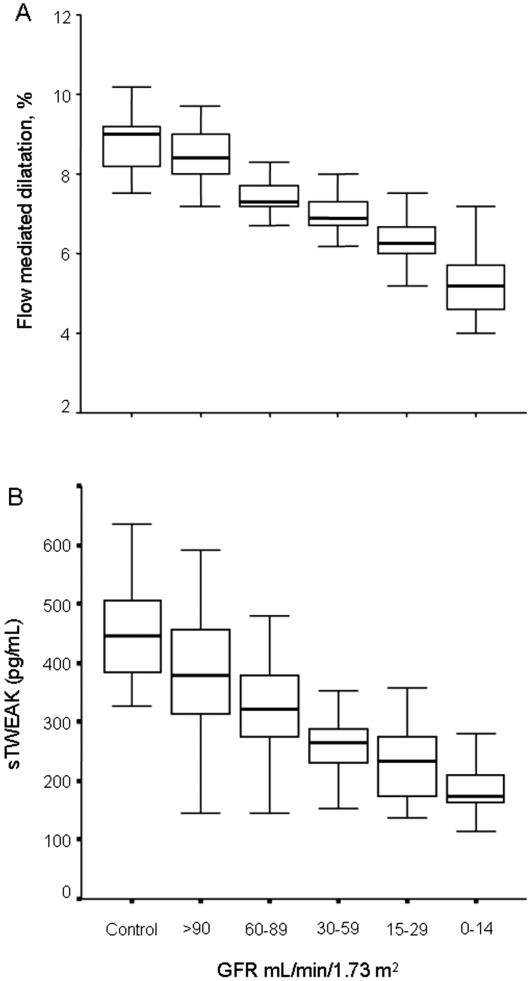
As expected, groups with stages 1 through 5 CKD had gradually diminished FMD values (P < 0.001; Figure 1A) and increased hsCRP values as compared with control subjects. NMD levels were similar between patients within stages 1 through 4 CKD and control subjects (Table 2), whereas NMD was lower in the stage 5 CKD group (P < 0.001). At the same time, across increasing CKD stages, gradual decreases in sTWEAK plasma levels were observed as compared with control subjects (P < 0.001; Table 2, Figure 1B). The lowest sTWEAK concentration was found in the stage 5 CKD group.
Figure 1.
(A and B) Box plots showing the decrease in FMD (A) or in sTWEAK (B) in parallel with the reduction in eGFR.
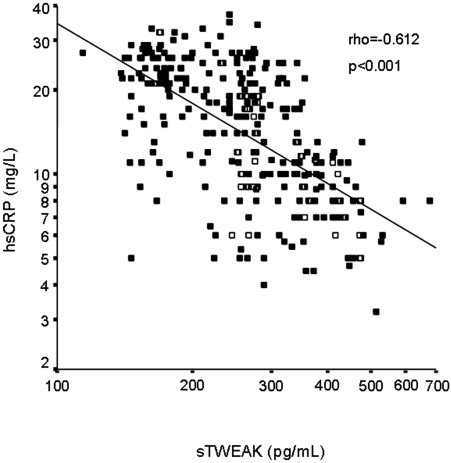
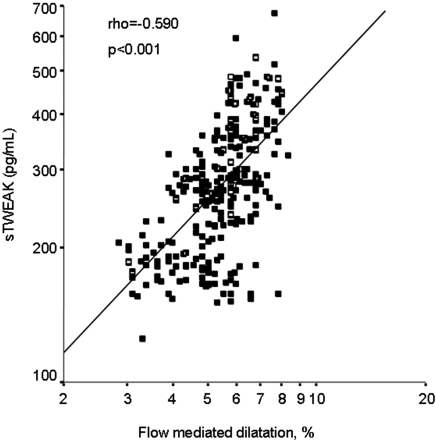
In univariate analysis, sTWEAK plasma levels negatively correlated with hsCRP (ρ = −0.612; P < 0.001; Figure 2), DBP (ρ = −0.131; P = 0.02), and HOMA (ρ = −0.120; P = 0.04; Table 3), whereas it was positively associated with eGFR (ρ = 0.704; P < 0.001), FMD (ρ = 0.590; P < 0.001; Figure 3), and NMD (ρ = 0.232; P < 0.001).
Figure 2.
Scatter plot showing the significant negative relationship between sTWEAK plasma levels and hsCRP concentrations.
Table 3.
Spearman rank correlations between sTWEAK levels and selected parameters in patients with CKD
| Parameter | ρ | P |
|---|---|---|
| Age | 0.002 | 0.975 |
| BMI | 0.197 | 0.001 |
| eGFR (ml/min per 1.73 m2) | 0.704 | <0.001 |
| Serum albumin (g/dl) | 0.114 | 0.061 |
| SBP (mmHg) | 0.032 | 0.583 |
| DBP (mmHg) | −0.131 | 0.025 |
| Plasma insulin (μIU/ml) | −0.100 | 0.086 |
| Plasma glucose (mg/dl) | −0.085 | 0.147 |
| HOMA-IR | −0.120 | 0.039 |
| Total cholesterol (mg/dl) | 0.074 | 0.206 |
| Triglycerides (mg/dl) | 0.018 | 0.758 |
| LDL cholesterol (mg/dl) | 0.090 | 0.121 |
| HDL cholesterol (mg/dl) | 0.045 | 0.443 |
| hsCRP (mg/L) | −0.612 | <0.001 |
| NMD (%) | 0.232 | <0.001 |
| FMD (%) | 0.590 | <0.001 |
Figure 3.
Scatter plot showing the significant positive relationship between sTWEAK plasma levels and FMD.
To clarify whether sTWEAK is an independent predictor of ED in these patients, we performed a multiple regression model. Variables that were expected to influence FMD (gender, age, total cholesterol, DBP, eGFR, HOMA, and hsCRP) as well as sTWEAK were included in the multivariate analysis. In such model, eGFR levels, hsCRP, DBP, and sTWEAK were significantly associated with FMD levels (Table 4).
Table 4.
Multivariable regression analysis of predictors of FMD in patients with CKD
| Parameter | Estimate | SE | β | P |
|---|---|---|---|---|
| Intercept | 3.79 | 0.966 | <0.001 | |
| eGFR (ml/min) | 1.59E-02 | 0.003 | 0.435 | <0.001 |
| hsCRP (mg/L) | −4.4E-02 | 0.011 | −0.281 | <0.001 |
| DBP (mmHg) | 3.07E-02 | 0.010 | 0.109 | 0.003 |
| sTWEAK (pg/ml) | 1.68E-03 | 0.001 | 0.134 | 0.010 |
Variables that are known to influence FMD levels (age, gender, total cholesterol, eGFR, hsCRP, and DBP) as well as sTWEAK were included in the models. Whole-model adjusted r2 = 0.62.
Discussion
In this study, we investigated sTWEAK plasma levels across various CKD stages. We could observe a gradual decrease in sTWEAK values concomitant with eGFR decline. At the same time, we showed for the first time an independent relationship between sTWEAK levels and ED in 295 patients with CKD. These results therefore agree with previous reports on diminution in sTWEAK plasma levels in patients with CKD (26) and in HD patients (19).
In accordance with previous reports (19,20), sTWEAK was inversely associated with the inflammatory biomarker CRP; however, sTWEAK did not relate to lipid profile or glucose/insulin concentrations, which seems to be in contradiction to previous studies (20–26). Nonetheless, those studies cannot be extrapolated directly to our data, because we included young patients with CKD, who were free from both diabetes and cardiovascular history. The observed significant inverse association between HOMA-IR and sTWEAK may indicate, however, some indirect relation to insulin resistance.
The main finding in our study is the independent association between sTWEAK and ED. The process by which ED is associated with sTWEAK plasma levels is still poorly characterized but could be related with the expression of TWEAK or its receptor, Fn14, by endothelial cells (27,28). In this context, expression and release of other members of the TNF superfamily are abnormal in patients with ED. Soluble Fas ligand plasma levels are diminished in patients with carotid atherosclerosis (29) and in individuals at high cardiovascular risk (30). Furthermore, forearm vasodilator responses to reactive hyperemia are associated with soluble Fas ligand concentration in patients with coronary artery disease (31). Altogether, these data may therefore indicate that members of the TNF superfamily could be directly related with ED.
The mechanisms that lead to lower sTWEAK levels are poorly understood. A pathologic role of TWEAK has been demonstrated in animal models of kidney injury (32,33). In this regard, TWEAK is expressed in several tissues, but the expression of its receptor, Fn14, is relatively low in the kidney (33) and undetectable in the arterial wall (34). Under pathologic conditions, Fn14 expression is increased, and cells, including vascular cells, could be sensitized to TWEAK, leading to injurious actions such as apoptosis and production of proinflammatory mediators (33,34). In such scenario, unknown mechanisms may lead to low TWEAK concentrations to try unsuccessfully to limit tissue injury: Low levels of sTWEAK in patients with CKD may be a compensatory mechanism to protect from the consequences of Fn14 activation (9). In this sense, it was recently reported that both CD163 (a TWEAK scavenger receptor) and sTWEAK are expressed in an opposite trend in human carotid atherosclerotic plaques (35). Moreover, CD163-expressing macrophages can bind and internalize sTWEAK in vitro (35). The reduction of sTWEAK in patients with CKD could be related with the presence of its scavenger receptor CD163, which is upregulated in patients with stage 5 CKD (36). This increment could facilitate TWEAK degradation by inflammatory macrophages, leading to low TWEAK levels (37). In this context, low TWEAK levels may relate to the degree of macrophage activation.
A second possibility is that a defective shedding of TWEAK is responsible for the diminution of sTWEAK plasma levels observed in our study. As an example, previous studies demonstrated that LEu554Phe polymorphisms in the E-selectin gene display a more severe degree of atherosclerosis (38). An inverse relationship between carotid intima-media thickness, plasma E selectin, and cardiovascular mortality in patients with ESRD has also been described (39,40). Such an association is in keeping with the functional effect of this polymorphism, which favors E-selectin anchoring to the endothelium surface, thereby limiting the shedding of E-selectin into the bloodstream (38).
Finally, different effects of TWEAK in cardiovascular diseases have been suggested (41). Augmented circulating TWEAK levels in mice through genetic approaches resulted in the development of dilated cardiomyopathy, with subsequent cardiac dysfunction and early mortality, a process dependent on the Fn14 cellular receptor (42). The interaction between TWEAK and Fn14 also has several potential proatherogenic effects in cultured cells, which may be important in the pathogenesis of atherosclerosis. TWEAK through Fn14 can induce intercellular adhesion molecule 1 and E-selectin expression in human umbilical vein endothelial cells in culture (27). Stimulation of human umbilical vein endothelial cells with TWEAK also induces the secretion of proinflammatory cytokines and chemokines such as IL-8 and monocyte chemoattractant protein 1 (27), increasing the inflammatory response. In addition, TWEAK augments vascular smooth muscle cell proliferation (13) and induces metalloproteinase activity (16), favoring atherosclerotic plaque destabilization. Finally, TWEAK and Fn14 are expressed in human carotid atherosclerotic plaques (43).
Some limitations of this study should be considered. First, our observation was cross-sectional in nature. Second, because patients with diabetes were excluded, this may limit the value of the observed results, which may not be able to be extrapolated to patients with diabetes. Third, we found surprisingly strong associations between the investigated factors, possibly as a result of the strict inclusion criteria of our patient material and meaning that the results may be hard to transfer to more heterogeneous populations; however, our data are in agreement with the present understanding of the sTWEAK pathophysiology in CKD (19,20). Finally, by assessing GFR indirectly, we may have limited the power of the study to show the true extent of progression to stage 5 CKD.
Conclusions
This study demonstrates that sTWEAK plasma concentrations are negatively associated with GFR decline. Finally, we report an association between sTWEAK and functional changes in FMD in patients with CKD, independent of inflammation and BP, suggesting that this protein may play a previously unrecognized role in the ED of uremia.
Disclosures
None.
Acknowledgments
This work was supported by grants from Ministerio de Educación y Ciencia (SAF 2007-60896 and SAF 2007-63648), Ministerio de Sanidad y Consumo, Instituto Carlos III (RD06/0014/0035), Loo and Hans Osterman Foundation, Fondo de Investigaciones Sanitarias (CP04/00060 and 06/0046, Programa Intensificación Actividad Investigadora ISCIII/Agencia Laín-Entralgo to A.O.), and Comunidad de Madrid (FRACM/S-BIO0283/2006 and S2006/GEN-0247).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Libby P, Ridker PM, Maseri A: Inflammation and atherosclerosis. Circulation 105: 1135– 1143, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA: The clinical implications of endothelial dysfunction. J Am Coll Cardiol 42: 1149– 1160, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Vita JA, Keaney JF, Jr: Endothelial function: A barometer for cardiovascular risk? Circulation 106: 640– 642, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Endemann DH, Schiffrin EL: Endothelial dysfunction. J Am Soc Nephrol 15: 1983– 1992, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A: Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 101: 948– 954, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32: S112– S119, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Tonelli M, Pfeffer MA: Kidney disease and cardiovascular risk. Annu Rev Med 58: 123– 139, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, Garcia I, Browning JL: TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem 272: 32401– 32410, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Winkles JA: The TWEAK-Fn14 cytokine-receptor axis: Discovery, biology and therapeutic targeting. Nat Rev Drug Discov 7: 411– 425, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiley SR, Winkles JA: TWEAK, a member of the TNF superfamily, is a multifunctional cytokine that binds the TweakR/Fn14 receptor. Cytokine Growth Factor Rev 14: 241– 249, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Marsters SA, Sheridan JP, Pitti RM, Brush J, Goddard A, Ashekenazi A: Identification of a ligand for the death-domain containing receptor Apo3. Curr Biol 8: 525– 528, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Desplat-Jego S, Varriale S, Creidy R, Terra R, Bernard D, Khrestchatisky M, Izui S, Chicheportiche Y, Boucraut J: TWEAK is expressed by glial cells, induces astrocyte proliferation and increases EAE severity. J Neuroimmunol 133: 116– 123, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Lynch CN, Wang YC, Lund JK, Chen YW, Leal JA, Wiley SR: TWEAK induces angiogenesis and proliferation of endothelial cells. J Biol Chem 274: 8455– 8459, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Polek TC, Talpaz M, Darnay BG, Spivak-Kroizman T: TWEAK mediates signal transduction and differentiation of RAW264.7 cells in the absence of Fn14/TweakR: Evidence for a second TWEAK receptor. J Biol Chem 278: 32317– 32323, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Ho DH, Vu H, Brown SA, Donohue PJ, Hanscom HN, Winkles JA: Soluble tumor necrosis factor-like weak inducer of apoptosis overexpression in HEK293 cells promotes tumor growth and angiogenesis in athymic nude mice. Cancer Res 64: 8968– 8972, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Kang YJ, Kim WJ, Woo DK, Lee Y, Kim DI, Park YB, Kwon BS, Park JE, Lee WH: TWEAK can induce pro-inflammatory cytokines and matrix metalloproteinase-9 in macrophages. Circ J 68: 396– 399, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Saas P, Boucraut J, Walker PR, Quiquerez AL, Billot M, Desplat-Jego S, Chicheportiche Y, Dietrich PY: TWEAK stimulation of astrocytes and the proinflammatory consequences. Glia 32: 102– 107, 2000 [PubMed] [Google Scholar]
- 18.Saitoh T, Nakayama M, Nakano H, Yagita H, Yamamoto N, Yamaoka S: TWEAK induces NF-kappaB2 p100 processing and long lasting NF-kappaB activation. J Biol Chem 278: 36005– 36012, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Carrero JJ, Ortiz A, Qureshi AR, Martín-Ventura JL, Bárány P, Heimbürger O, Marrón B, Metry G, Snaedal S, Lindholm B, Egido J, Stenvinkel P, Blanco-Colio LM: Additive effects of soluble TWEAK and inflammation on mortality in hemodialysis patients. Clin J Am Soc Nephrol 4: 110– 118, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanco-Colio LM, Martin-Ventura JL, Munoz-Garcia B, Orbe J, Paramo JA, Michel JB, Ortiz A, Meilhac O, Egido J: Identification of soluble tumor necrosis factor-like weak inducer of apoptosis (sTWEAK) as a possible biomarker of subclinical atherosclerosis. Arterioscler Thromb Vasc Biol 27: 916– 922, 2007 [DOI] [PubMed] [Google Scholar]
- 21.National Kidney Foundation: K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[ Suppl 3]: S1– S202, 2004 [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461– 470, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem, 18: 499– 502, 1972 [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412– 419, 1985 [DOI] [PubMed] [Google Scholar]
- 25.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID: Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111– 1115, 1992 [DOI] [PubMed] [Google Scholar]
- 26.Kralisch S, Ziegelmeier M, Bachmann A, Seeger J, Lossner U, Bluher M, Stumvoll M, Fasshauer M: Serum levels of the atherosclerosis biomarker sTWEAK are decreased in type 2 diabetes and end-stage renal disease. Atherosclerosis 199: 440– 444, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Harada N, Nakayama M, Nakano H, Fukuchi Y, Yagita H, Okumura K: Pro-inflammatory effect of TWEAK/Fn14 interaction on human umbilical vein endothelial cells. Biochem Biophys Res Commun 299: 488– 493, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Donohue PJ, Richards CM, Brown SA, Hanscom HN, Buschman J, Thangada S, Hla T, Williams MS, Winkles JA: TWEAK is an endothelial cell growth and chemotactic factor that also potentiates FGF-2 and VEGF-A mitogenic activity. Arterioscler Thromb Vasc Biol 23: 594– 600, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Blanco-Colio LM, Martín-Ventura JL, Sol JM, Díaz C, Hernández G, Egido J: Decreased circulating Fas ligand in patients with familial combined hyperlipidemia or carotid atherosclerosis: Normalization by atorvastatin. J Am Coll Cardiol 43: 1188– 1194, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Blanco-Colio LM, Martín-Ventura JL, de Teresa E, Farsang C, Gaw A, Gensini G, Leiter LA, Langer A, Martineau P, Hérnandez G, Egido J: ACTFAST investigators: Increased soluble Fas plasma levels in subjects at high cardiovascular risk—Atorvastatin on Inflammatory Markers (AIM) study, a substudy of ACTFAST. Arterioscler Thromb Vasc Biol 27: 168– 174, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Blanco-Colio LM, Martín-Ventura JL, Tuñón J, García-Camarero T, Berrazueta JR, Egido J: Soluble Fas ligand plasma levels are associated with forearm reactive hyperemia in subjects with coronary artery disease: A novel biomarker of endothelial function? Atherosclerosis 201: 407– 412, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Zhao Z, Burkly LC, Campbell S, Schwartz N, Molano A, Choudhury A, Eisenberg RA, Michaelson JS, Putterman C: TWEAK/Fn14 interactions are instrumental in the pathogenesis of nephritis in the chronic graft-versus-host model of systemic lupus erythematosus. J Immunol 179: 7949– 7958, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Sanz AB, Justo P, Sanchez-Nino MD, Blanco-Colio LM, Winkles JA, Kreztler M, Jakubowski A, Blanco J, Egido J, Ruiz-Ortega M, Ortiz A: The cytokine TWEAK modulates renal tubulointerstitial inflammation. J Am Soc Nephrol 19: 695– 703, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munoz-Garcia B, Martin-Ventura JL, Martinez E, Sanchez S, Hernandez G, Ortega L, Ortiz A, Egido J, Blanco-Colio LM: Fn14 is upregulated in cytokine-stimulated vascular smooth muscle cells and is expressed in human carotid atherosclerotic plaques: Modulation by atorvastatin. Stroke 37: 2044– 2053, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Moreno JA, Muñoz-García B, Martín-Ventura JL, Madrigal-Matute J, Orbe J, Páramo JA, Ortega L, Egido J, Blanco-Colio LM: The CD163-expressing macrophages recognize and internalize TWEAK potential consequences in atherosclerosis. Atherosclerosis May3, 2009. [ epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Axelsson J, Møller HJ, Witasp A, Qureshi AR, Carrero JJ, Heimbürger O, Bárány P, Alvestrand A, Lindholm B, Moestrup SK, Stenvinkel P: Changes in fat mass correlate with changes in soluble sCD163, a marker of mature macrophages, in patients with CKD. Am J Kidney Dis 48: 916– 925, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Bover LC, Cardo-Vila M, Kuniyasu A, Sun J, Rangel R, Takeya M, Aggarwal BB, Arap W, Pasqualini R: A previously unrecognized protein-protein interaction between TWEAK and CD163: Potential biological implications. J Immunol 178: 8183– 8194, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Wenzel K, Ernst M, Rohde K, Baumann G, Speer A: DNA polymorphisms in adhesion molecule genes: A new risk factor for early atherosclerosis. Hum Genet 97: 15– 20, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Testa A, Benedetto F, Spoto B, Pisano A, Tripepi G, Mallamaci F, Malatino LS, Zoccali C: The E-selectin gene polymorphisms and carotid atherosclerosis in end-stage renal disease. Nephrol Dial Transplant 21: 1921– 1926, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Malatino LS, Stancanelli B, Cataliotti A, Bellanuova I, Fatuzzo P, Rapisarda F, Leonardis D, Tripepi G, Mallamaci F, Zoccali C: Circulating E-selectin as a risk marker in patients with end-stage renal disease. J Intern Med 262: 479– 487, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Ortiz A, Sanz AB, García BM, Moreno JA, Niño MD, Martín-Ventura JL, Egido J, Blanco-Colio LM: Considering TWEAK as a target for therapy in renal and vascular injury. Cytokine Growth Factor Rev 20: 251– 258, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Jain M, Jakubowski A, Cui L, Shi J, Su L, Bauer M, Guan J, Lim CC, Naito Y, Thompson JS, Sam F, Ambrose C, Parr M, Crowell T, Lincecum JM, Wang MZ, Hsu YM, Zheng TS, Michaelson JS, Liao R, Burkly LC: A novel role for tumor necrosis factor-like weak inducer of apoptosis (TWEAK) in the development of cardiac dysfunction and failure. Circulation 119: 2058– 2068, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blanco-Colio LM, Martín-Ventura JL, Muñoz-Garcia B, Moreno JA, Meilhac O, Ortiz A, Egido J: TWEAK and Fn14: New players in the pathogenesis of atherosclerosis. Front Biosci 12: 3648– 3655, 2007 [DOI] [PubMed] [Google Scholar]