Abstract
Background and objectives: While peritoneal dialysis with icodextrin is commonly used in patients with poor peritoneal membrane characteristics, the data on the usefulness of this solution in patients with lower transport characteristics are limited. The study was designed to compare icodextrin to glucose in Chinese prevalent peritoneal dialysis patients of different peritoneal transport characteristics (PET) categories.
Design, setting, participants, & measurements: This was a randomized, double-blind, perspective control study. Stable prevalent continuous ambulatory peritoneal dialysis (CAPD) patients were randomized to either 7.5% icodextrin (ICO) or 2.5% glucose (GLU) solution for 4 wk. Peritoneal membrane function was measured to define PET category in baseline. Creatinine clearance (Ccr), urea nitrogen clearance (CBUN), ultrafiltration (UF) during the long night dwell, dialysate, and metabolic biomarkers were measured at baseline, 2, and 4 wk. UF, Ccr, and CBUN were compared among different PET categories.
Results: A total of 201 CAPD patients were enrolled in the study. There were no baseline differences between the groups. Following 2 and 4 wk of therapy, Ccr, CBUN, and UF were all significantly higher in the ICO versus the GLU group. Additionally, switching to ICO resulted in a significant increase in UF in high, high-average, and low-average transporters as compared with baseline. The extent of increased UF was more obvious in higher transporters. Blood cholesterol level in the ICO group decreased significantly than that in the GLU group.
Conclusion: Compared with glucose-based solution, 7.5% icodextrin significantly improved UF and small solute clearance, even in patients with low-average peritoneal transport.
Control of water and control of solutes are the main goals of current dialysis therapies. In continuous ambulatory peritoneal dialysis (PD), where the peritoneal membrane functions as an endogeneous filter, peritoneal membrane transport characteristics are paramount. Interestingly, clinical experience and scientific studies (1,2) have shown significant differences in these characteristics between Asian and non-Asian patients. Peritoneal membrane function (D/Pcr) in Asian patients is much lower than in non-Asian patients. Indeed, as peritoneal transport is an independent predictor of patient and technique survival (3), the well-known difference in these parameters between Asians and non-Asians on PD may at least partly be due to differences in the peritoneal cavity.
In PD patients, loss of peritoneal membrane integrity gradually occurs with PD therapy and leads to decreased ultrafiltration (UF) (4). This is commonly countered by the introduction of colloid icodextrin solution in the PD regimen (5–9). However, icodextrin was recently introduced in Asia and is not available in mainland China. Thus, there is a paucity of data regarding the performance of this compound in Asian patients, with their unique transport characteristics.
We conducted a randomized, controlled multi-center trial comparing icodextrin and glucose in ethnically Chinese patients, focusing on short-term improvement of ultrafiltration in different peritoneal transport characteristics and metabolic changes.
Materials and Methods
Patients
Prevalent PD patients stable during at least 90 d were included in the study. Inclusion criteria were: (1) age 18 yr or older; (2) CAPD patients, with a minimum of 6L of daily 2.5% Dianeal® (Baxter China Ltd., Shanghai, China) PD-2 or PD-4 dialysate with a night dwell above 8 h; and (3) a night dwell volume of 2L for a minimum of 30 d before inclusion. Exclusion criteria included: (1) documented anaphylaxis with icodextrin; (2) concomittent chronic diseases such as hepatitis, malignancy, severe cardiac diseases, etc.; (3) ongoing infection or known infection within the last 30 d; and (4) planned or ongoing pregnancy. Participation in another clinical or drug trial concurrently was not allowed.
Seven hospitals from different parts of China participated in the study, and the study protocol was reviewed and approved by the Ethics Committee of each one. Informed consent was obtained from all patients before their participation in the trial. The protocol was registered in ClinicalTrials.gov (ClinicalTrials.gov number: NCT00725517). The hospitals were Shanghai Renji Hospital, Peking University First Hospital, The First Affiliated Hospital of Sun Yat-sen University in Guangzhou, Beijing Friendship Hospital, Peking Union Medical College Hospital in Beijing, Shanghai Ruijin Hospital, and Shanghai Changzheng Hospital.
Two regiments of dialysate were used in night dwell: 7.5% icodextrin (Extraneal®, Baxter China Ltd.) (ICO group) and 2.5% glucose (Dianeal®, Baxter China Ltd., Shanghai, China) (GLU group). The double-blind status of the study was maintained by labeling the dialysates identically with the words “investigational drug product.” Dialysates were coded only with numbers and stored in a certain place. Randomization was done using a computer program that generated numbers instead of treatment assignments. When a patient was randomized, dialysates with the right number were transported directly to the patient's home. Neither doctors nor patients knew the dialysate regiment. Envelopes that contained the number corresponding to the dialysate regiment were held by personnel not directly involved with the study, and could be opened only in an emergency.
After they agreed to join the study, patients were assessed for peritoneal creatinine clearance, peritoneal transport characteristics (PET), UF volume in night dwell, and blood biochemistry within a week. UF volume was calculated by volume of drain out minus volume before fill in. After the baseline index was assessed, research drugs were transported to a patient's home and were used as night dwell. Adverse events were recorded continuously.
Measurements of Peritoneal Characteristics and Dialysis Efficacy
After an overnight dwell, we assessed drain volume and levels of glucose, creatinine, and urea in the dialysate, as well as vital signs and drained body weight. Blood samples were drawn and immediately sent for analysis. Long dwell UF volume, long dwell peritoneal creatininine clearance, and peritoneal urea nitrogen clearance were calculated (5).
A standard PET was performed at baseline to evaluate a patient's PET. The ratio of creatinine in the dialysate to plasma after a standardized 4-h dwell (D/Pcr) was used to classify transport according to Twardowski (10).
Statistical Analyses
Based upon a power calculation using previously published data (5), and with input from the Chinese State Food and Drug Administration, at least 200 patients were to be enrolled. Patients were randomly assigned to treatments by a computer-generated block randomization scheme, using a 1:1 ratio. Analyses of safety variables were conducted in the intention-to-treat population (ITT, all patients who were randomized to treatment and received at least one exchange of study solution). Efficacy variables were conducted in both the ITT population and per-protocol (PP, finished trial according to protocol) population. A repeated-measures analysis of covariance was used to evaluate differences between treatment groups for the efficacy variables.
Treatment effect was conducted at α = 5%. P values <0.05 were considered significant. For continuous parametric variables, values were reported as mean ± SEM, while for continuous nonparametric data, the median (interquartile range) was used. A paired t test was used to assess differences in parametric variables over time, while Pearson's χ2 test and Fisher's exact test were used to assess categorical data. All analyses were performed using SAS software, version 8.2 (SAS Institute, Cary, NC).
Results
Patients' Characteristics
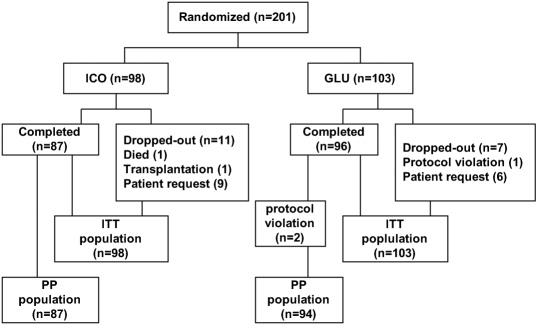
A total of 201 patients were enrolled in the study. There were 96 males and 105 females, with a mean age of 56.1 ± 13.7 yr (range 18 to 81 yr). Eighteen patients (9%) dropped out (Figure 1). During the whole study period, double-blind maintained well, and no events needed to break the blind. Table 1 shows baseline characteristics of the patients. There was no significant difference in either clinical, biochemical, or peritoneal transport characteristics between the groups.
Figure 1.
The flow of study subjects in the study.
Table 1.
Baseline characteristics of enrolled patients, grouped according to therapya (n = 201)
| 7.5% Icodextrin Group (ICO) | 2.5% Glucose Group (GLU) | P Value | |
|---|---|---|---|
| N | 98 | 103 | |
| Age (yr old) | 56.8 ± 13.5 | 55.4 ± 14.0 | 0.42 |
| Male/female | 51/47 | 45/58 | 0.24 |
| CAPD duration (m) | 28.2 ± 24.9 | 29.8 ± 26.0 | 0.72 |
| eGFR (ml/min) | 2.38 ± 2.05 | 2.26 ± 2.26 | 0.66 |
| Blood Cr concentration (umol/L) | 997.7 ± 313.6 | 989.10 ± 297.16 | 0.89 |
| Blood BUN concentration (mmol/L) | 20.11 ± 6.82 | 20.47 ± 5.54 | 0.57 |
| Net ultrafiltration volume in long dwell (ml) | 235.92 ± 246.48 | 267.96 ± 239.27 | 0.38 |
| Long-term Peritoneal Ccr (ml/min) | 2.97 ± 0.72 | 3.16 ± 0.86 | 0.06 |
| Peritoneal transport character (PET) | 0.35 | ||
| high, n (%) | 9 (9.2) | 15 (14.6) | |
| high-average, n (%) | 39 (39.8) | 33 (32.0) | |
| low-average, n (%) | 35 (35.7) | 46 (44.7) | |
| low, n (%) | 14 (14.3) | 7 (6.8) | |
| Primary ethiology of kidney disease | 0.16 | ||
| chronic glomerulonephritis, n (%) | 41 (41.8) | 37 (35.9) | |
| diabetic nephropathy, n (%) | 24 (24.5) | 21 (20.4) | |
| hypertension, n (%) | 14 (14.3) | 13 (12.6) | |
| others, n (%) | 19 (19.3) | 32 (31.1) |
All the patients received 2.5% Dianeal® PD-2 or PD-4 dialysate in long dwell time in baseline.
Values are expressed as mean ± SEM; CAPD, continuous ambulatory peritoneal dialysis; eGFR, estimated glomerular filtration rate; PET, peritoneal equilibration test; ESRF, end stage renal failure.
Adverse Events
One patient died due to a traffic accident. There were two episodes of bacterial peritonitis in the ICO group, which resolved following antibiotic treatment but resulted in patient censoring. A total of ten episodes were defined as related to drugs. Table 2 shows events related to drugs There were no statistically significant differences between the two groups in the incidence of adverse events.
Table 2.
Ten adverse events defined as related to drugs during study in ITT population
| ICO Group |
GLU Group |
||
|---|---|---|---|
| AE | n (%) | AE | n (%) |
| dizzy | 1 (1%) | hypotention | 1 (1%) |
| thirsty | 2 (2%) | Rash in skin | 1 (1%) |
| fatigue | 2 (2%) | vomit | 1 (1%) |
| edema | 1 (1%) | uncomfortable in abdomen | 1 (1%) |
Changes of Peritoneal Creatinine Clearance
Although peritoneal creatinine clearance (CCr) had a tendency of lower numerical value in the ICO group at baseline, it was significantly higher in the ICO versus the GLU group (3.53 ± 0.77 ml/min versus 3.02 ± 0.80 ml/min, P < 0.05) after 4 wk. Also, compared with baseline, the increase of net peritoneal CCr was higher in patients with peritoneal higher transport than in those with lower transport. Changes in peritoneal CCr during the study grouped according to initial PET are shown in Table 3. The results in the PP population were similar to those in the ITT population.
Table 3.
ΔCcr and ΔUF (baseline compared with 2 and 4 wk) grouped according to baseline PET transport characteristic group in ITT population
| PET Category | Time | ΔCcr (ml/min) |
ΔUF (ml) |
||||
|---|---|---|---|---|---|---|---|
| GLU | ICO | P1 | GLU | ICO | P2 | ||
| High trspt | 2W | 0.16 ± 0.14 | 0.98 ± 0.18 | 0.0003 | −67.6 ± 59.2 | 495.6 ± 77.2 | <0.0001 |
| (n = 24) | 4W | −0.17 ± 0.18 | 0.75 ± 0.23 | 0.0014 | 17.7 ± 61. 4 | 456.3 ± 79.0 | <0.0001 |
| HA trspt | 2W | 0.19 ± 0.09 | 0.82 ± 0.09 | <0.0001 | 37.5 ± 37.1 | 428.3 ± 36.4 | <0.0001 |
| (n = 72) | 4W | −0.02 ± 0.12 | 0.78 ± 0.11 | <0.0001 | 13.0 ± 39.0 | 439.3 ± 39.0 | <0.0001 |
| LA trspt | 2W | 0.15 ± 0.08 | 0.55 ± 0.09 | 0.0008 | 84.3 ± 33.9 | 221.8 ± 38.9 | 0.0046 |
| (n = 81) | 4W | 0.21 ± 0.10 | 0.55 ± 0.12 | 0.024 | 100.5 ± 35.5 | 269.7 ± 41.4 | 0.0009 |
| L trspt | 2W | 0.23 ± 0.20 | 0.43 ± 0.14 | 0.40 | 188.6 ± 82.3 | 216.2 ± 58.3 | 0.77 |
| (n = 21) | 4W | −0.11 ± 0.25 | 0.28 ± 0.18 | 0.20 | 162.8 ± 85.0 | 134.5 ± 62.7 | 0.78 |
ΔUF, UF of long dwell in wk 2/wk 4–UF of long dwell in baseline; ΔCcr, Ccr of long dwell in wk 2/wk 4–Ccr of long dwell in baseline.
Changes of Ultrafiltration Volume
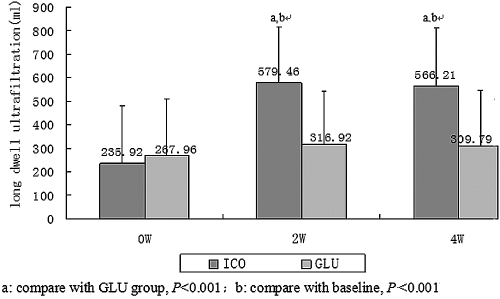
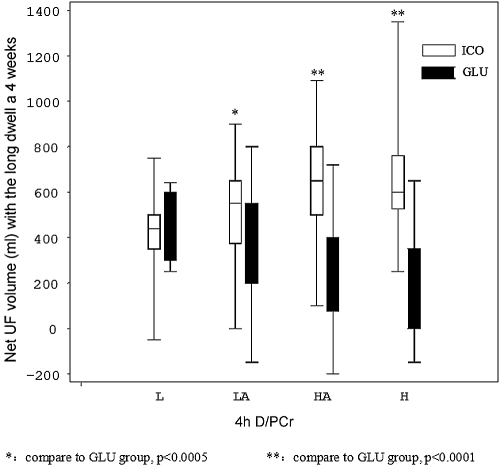
After both 2 and 4 wk, UF was significantly higher in the ICO versus the GLU group (shown in Figure 2, P < 0.001). Data on UF in two groups grouped according to PET are shown in Figure 3. In baseline, UF differed according to peritoneal transport status. High transport patients had the least UF volume. After ICO treatment, patients with low-average (LA), high-average (HA), and high (H) transport all significantly increased compared with baseline and GLU group, while we saw no significant increase in only L transporters. The extent of increased UF in the ICO group was more obvious in high transporters, as shown in Table 3.
Figure 2.
Significant difference in long dwell ultrafiltration volume between the groups and after 4 wk of therapy. Compared with baseline, the long dwell ultrafiltration volume increased significantly in the ICO group and was greater than that of the GLU group.
Figure 3.
Long dwell ultrafiltration volume in the different PET categories after 4 wk of therapy and grouped according to solution.
Metabolic Parameters
While baseline levels of fasting blood glucose, cholesterol, and triglycerides did not vary between the groups following treatment, 4-wk blood cholesterol levels in the ICO group decreased significantly as compared with the GLU group (Table 4). There was no significant difference on fasting plasma glucose between the two groups either at baseline or following treatment.
Table 4.
Changes in metabolic parameters following 4 wk of either icodextrin or glucose-based PD
| ICO Group |
GLU Group |
P valuea | |||
|---|---|---|---|---|---|
| Baseline | Wk 4 | Baseline | Wk 4 | ||
| Glucose (mmol/L) | 5.99 ± 2.37 | 5.70 ± 2.17 | 5.90 ± 2.14 | 6.12 ± 2.65 | 0.238 |
| Cholesterol (mmol/L) | 5.03 ± 1.83 | 4.69 ± 1.12 | 5.19 ± 1.43 | 5.09 ± 1.26 | 0.025 |
| Triglycerides (mmol/L) | 2.65 ± 3.55 | 1.98 ± 1.58 | 2.35 ± 2.11 | 2.35 ± 2.05 | 0.168 |
Parameters of ICO group compare to that of GLU group in wk 4.
Discussion
In the first large-scale randomized study comparing icodextrin to glucose solution for the long dwell in Chinese peritoneal dialysis patients, we report that use of icodextrin was associated with a significant increase in small solute clearance and UF, even in patients with LA transport.
While PD with icodextrin will soon be available to China's 13,000 PD patients, there is a paucity of data regarding the performance of this compound in this patient group. Altogether, there are some randomized controlled trials (RCTs) of icodextrin, and these mainly include Caucasian and Latin patients. Wolfson et al. (5) showed in a multicenter study in North Americans (2% Asians) that icodextrin is superior in Ccr and UF to glucose in this population, data that the present study now extends to Chinese patients. Finkelstein et al. (6) reported a significant increase in both UF and Ccr after switching to icodextrin in North Americans and Australians (4.3% Asian). Finally, a small number of nonrandomized studies looking at icodextrin in Asian patients have all suggested various benefits but are limited by their designs (11–13).
Our result showed the extents of increased UF volume were different among four transportors after using icodextrin. Hwang et al. (14) evaluated the difference in the peritoneal equilibrium test with icodextrin and glucose. They found that the higher D/PCr patients were, the more ultrafiltration they got. Araújo Teixeira (15) evaluated UF of long dwell (10 h) in different transport patients between icodextrin and 3.86% glucose dialysate. They found H and HA patients achieved a higher ultrafiltration with 7.5% ICO than with 3.86% GLU. On the opposite, low and low-average transport patients in ICO got a significantly lower ultrafiltration compared with 3.86% GLU solution. So now icodextrin's excellent effects on UF were only showed in higher transport patients. As a result, icodextrin has been used as a salvage therapy in high transport peritoneal dialysis patients who often suffered with refractory fluid overload until now (6–9).
The most important finding of the present RCT study is that icodextrin can improve UF and small solute clearance not only in high and high-average transport but also in low-average transport patients as compared with 2.5% glucose. That means icodextrin can also be used in lower transport patients with fluid overload. Some RCT studies only select special transport patients. In Finkelstein's study (6), only higher transport patients (D/PCr>0.7) were included. Low and low-average transport patients were excluded. Wolfson et al. (4) did include all kinds of transport categories, but found no effect of icodextrin in those with low or low-average transporter. The reasons are not clear. This is of particular interest as the proportion of LA transporters appears to be greater in Asian PD patients than in other groups (1,2). Whether this is due to genetic factors (16) or unknown factors needs further studies.
Besides a beneficial effect of icodextrin versus glucose in solute and fluid removal, we found that blood cholesterol levels decreased significantly following 4 wk of icodextrin therapy, but did not change following glucose therapy. Although the decrease of serum cholesterol is rather small, it has statistical significance. If we prolong duration of the study, the benefit may be more obvious and have clinical significance. These data thus support previous studies showing an increased prevalence of the metabolic syndrome in Chinese patients treated with glucose-based PD for 1 yr (17), as well as other long-term studies in other ethnic groups demonstrating an improvement in dyslipidemia, insulin resistance, and metabolic syndrome with icodextrin (18–20).
A number of weaknesses should be acknowledged and considered when interpreting the results of the present study. Most important is the short study duration (4 wk), which precludes us from knowing if the observed benefits are stable over time, as well as reporting on any putative long-term side effects. Also, we did not assess the potential impact of the observed biochemical changes on residual renal function, patient morbidity, and mortality.
In conclusion, in the first large-scale randomized trial comparing icodextrin and glucose in Chinese PD patients, we report that icodextrin may also improve the UF profile in Chinese patients, even those with a low-average peritoneal transport.
Disclosures
None.
Acknowledgments
Members of the Icodextrin National Multi-center Cooperation Group. Jiaqi Qian, Aiwu Lin, Wei Fang, Liou Cao (Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai Center for Peritoneal Dialysis Research, Shanghai), Xiaomei Li, Hongbing Gan (Peking University First Hospital, Peking), Xueqing Yu, Qunying Guo (The First Affiliated Hospital, Sun Yat-sen University, Guangzhou), Wenhu Liu, Gang Wang (Beijing Friendship Hospital, Capital Medical University, Peking), Yang Sun, Hong Xu (Peking Union Medical College Hospital, Beijing), Nan Chen, Hong Ren Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai), Changlin Mei, Zhiguo Mao (Shanghai Changzheng Hospital, Shanghai).
We sincerely thank Dr. Jonas Axelsson for his constructive comments and help in revising the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Fang W, Qian JQ, Lin AW, Rowaie F, Ni ZH, Yao Q, Bargman JM, Oreopoulos DG: Comparison of peritoneal dialysis practice patterns and outcomes between a Canadian and a Chinese centre. Nephrol Dial Transplant 1– 8, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Chung SH, Heimburger O, Lindholm B, Lee HB: Peritoneal dialysis patient survival: A comparison between a Swedish and a Korean centre. Nephrol Dial Transplant 20: 1207– 1213, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Churchill DN, Thorpe KE, Nolph KD, Keshaviah PR, Oreopoulos DG, Pagé D: Increased peritoneal membrane transport is associated with decreased patient and technique survival for continuous peritoneal dialysis patients. The Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol 9: 1285– 1292, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Mujais S, Nolph K, Gokal R, Blake P, Burkart J, Coles G, Kawaguchi Y, Kawanishi H, Korbet S, Krediet R, Lindholm B, Oreopoulos D, Rippe B, Selgas R: Evaluation and management of ultrafiltration problems in peritoneal dialysis. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Perit Dial Int 20[ Suppl 4]: S5– S21, 2000 [PubMed] [Google Scholar]
- 5.Wolfson M, Piraino B, Hamburger RJ, Morton AR; Icodextrin Study Group A randomized controlled trial to evaluate the efficacy and safety of icodextrin in peritoneal dialysis. Am J Kidney Dis 40: 1055– 1065, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Finkelstein F, Healy H, Abu-Alfa A, Ahmad S, Brown F, Gehr T, Nash K, Sorkin M, Mujais S: Superiority of icodextrin compared with 4.25% dextrose for peritoneal ultrafiltration. J Am Soc Nephrol 16: 546– 554, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Woodrow G, Stables G, Oldroyd B, Gibson J, Turney JH, Brownjohn AM: Comparison of icodextrin and glucose solutions for the daytime dwell in automated peritoneal dialysis. Nephrol Dial Transplant 14: 1530– 1535, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Wilkie ME, Plant MJ, Edwards L, Brown CB: Icodextrin 7.5% dialysate solution (glucose polymer) in patients with ultrafiltration failure: Extension of CAPD technique survival. Perit Dial Int 17: 84– 87, 1997 [PubMed] [Google Scholar]
- 9.Johnson DW, Arndt M, O'Shea A, Watt R, Hamilton J, Vincent K: Icodextrin as salvage therapy in peritoneal dialysis patients with refractory fluid overload. BMC Nephrology 2: 2, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Twardowski ZJ: Peritoneal equilibration test. Perit Dial Bull 7: 138– 147, 1987 [Google Scholar]
- 11.Nakamoto H, Babazono T, Kasai K, Kuriyama S, Sugimoto T, Nakayama M, Hamada C, Furuya R, Hasegawa H, Kasahara M, Moriishi M, Tomo T, Miyazaki M, Yorioka N, Sato M, Yamabe K, Kawaguchi Y: Successful use of icodextrin in elderly patients on continuous ambulatory peritoneal dialysis. Adv Perit Dial 21: 168– 174, 2005 [PubMed] [Google Scholar]
- 12.Hiramatsu T, Furuta S, Kakuta H: Favorable changes in lipid metabolism and cardiovascular parameters after icodextrin use in peritoneal dialysis patients. Adv Perit Dial 23: 58– 61, 2007 [PubMed] [Google Scholar]
- 13.Furuya R, Odamaki M, Kumagai H, Hishida A: Beneficial effects of icodextrin on plasma level of adipocytokines in peritoneal dialysis patients. Nephrol Dial Transplant 21: 494– 498, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Hwang JC, Wang HY, Wang CT, Chen HC: Comparison of peritoneal equilibrium test with icodextrin and 2.5% glucose dialysis solutions. J Nephrol 19: 758– 763, 2006 [PubMed] [Google Scholar]
- 15.Araújo Teixeira MR, Pecoits-Filho RF, Romão Junior JE, Sabbaga E, Marcondes MM, Abensur H: The relationship between ultrafiltrate volume with icodextrin and peritoneal transport pattern according to the peritoneal equilibration test. Perit Dial Int 22: 229– 233, 2002 [PubMed] [Google Scholar]
- 16.Gillerot G, Goffin E, Michel C, Evenepoel P, Biesen WV, Tintillier M, Stenvinkel P, Heimbürger O, Lindholm B, Nordfors L, Robert A, Devuyst O: Genetic and clinical factors influence the baseline permeability of the peritoneal membrane. Kidney Int 67: 2477– 2487, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Jiang N, Qian JQ, Lin AW, Lindholm B, Axelsson J, Yao Q: Initiation of glucose-based peritoneal dialysis is associated with increased prevalence of metabolic syndrome in nondiabetic patients with end-stage renal disease. Blood Purif 26: 423– 428, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Gokal R, Moberly J, Lindholm B, Mujais S: Metabolic and laboratory effects of icodextrin. Kidney Int 61[ suppl 81]: S62– S71, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Bredie SJ, Bosch FH, Demacker PN, Stalenhoef AF, van Leusen R: Effects of peritoneal dialysis with an overnight icodextrin dwell on parameters of glucose and lipid metabolism. Perit Dial Int 21: 275– 281, 2001 [PubMed] [Google Scholar]
- 20.Babazono T, Nakamoto H, Kasai K, Kuriyama S, Sugimoto T, Nakayama M, Hamada C, Furuya R, Hasegawa H, Kasahara M, Moriishi M, Tomo T, Miyazaki M, Sato M, Yorioka N, Kawaguchi Y. Japanese Extraneal Collaborated Study Group. Effects of icodextrin on glycemic and lipid profiles in diabetic patients undergoing peritoneal dialysis. Am J Nephrol 27: 409– 415, 2007 [DOI] [PubMed] [Google Scholar]