Abstract
Expression levels of the major human sulfotransferases (SULTs) involved in xenobiotic detoxification in a range of human tissues (i.e., SULT “pies”) are not available in a form allowing comparison between tissues and individuals. Here we have determined, by quantitative immunoblotting, expression levels for the five principal human SULTs—SULT1A1, SULT1A3/4, SULT1B1, SULT1E1, and SULT2A1—and determined the kinetic properties toward probe substrates, where available, for these enzymes in cytosol samples from a bank of adult human liver, small intestine, kidney, and lung. We produced new isoform-selective antibodies against SULT1B1 and SULT2A1, which were used alongside antibodies against SULT1A3 and SULT1A1 previously produced in our laboratory or available commercially (SULT1E1). Expression levels were derived using purified recombinant enzymes to construct standard curves for each individual isoform and immunoblot. Substantial intertissue and interindividual differences in expression were observed. SULT1A1 was the major enzyme (>50% of total, range 420-4900 ng/mg cytosol protein) in the liver, followed by SULT2A1, SULT1B1, and SULT1E1. SULT1A3 was completely absent from this tissue. In contrast, the small intestine contained the largest overall amount of SULT of any of the tissues, with SULT1B1 the major enzyme (36%), closely followed by SULT1A3 (31%), and SULT1A1, SULT1E1, and SULT2A1 more minor forms (19, 8, and 6% of total, respectively). The kidney and lung contained low levels of SULT. We provide a unique data set that will add value to the study of the role and contribution of sulfation to drug and xenobiotic metabolism in humans.
Sulfation is an important pathway for detoxification and elimination of xenobiotics, including many drugs, dietary chemicals, and environmental pollutants, and for the biosynthesis and homeostasis of essential endogenous compounds such as steroid/thyroid hormones and catecholamines (Coughtrie, 2002; Strott, 2002; Blanchard et al., 2004; Gamage et al., 2006). These reactions are catalyzed by the sulfotransferase (SULT) superfamily, which in humans comprises at least 13 members (Blanchard et al., 2004; Freimuth et al., 2004; Hildebrandt et al., 2007). SULTs catalyze a sulfuryl transfer reaction, involving the universal cosubstrate 3′-phosphoadenosine 5′-phosphosulfate (PAPS) (Kakuta et al., 1997; Zhang et al., 1998), which generally results in a sulfate conjugate with attenuated biological activity that is readily removed from the body via the urine and/or bile. For some chemicals, including many dietary, industrial, and environmental mutagens, sulfation is a key step in their metabolic activation (Surh and Miller, 1994; Glatt, 2000).
Of the 13 human SULTs known, most have been characterized to some extent, and it can be proposed that the major isoforms for drug metabolism in adults are SULT1A1, SULT1A3/4,2 SULT1B1, SULT1E1, and SULT2A1. SULT1A1 is the major “phenol” SULT, sulfating a wide range of substrates with high affinity (i.e., low Km). SULT1A3, found only in primates, sulfates catecholamines with high selectivity (Dajani et al., 1998; Eisenhofer et al., 1999), and SULT1B1 displays a similar substrate profile to SULT1A1 but with much higher Km values toward most substrates (Tabrett and Coughtrie, 2003; Riches et al., 2007). SULT1E1 and SULT2A1 are both steroid SULTs (selective for estrogens and androgens, respectively), which also sulfate drugs and other xenobiotics (e.g., Forbes-Bamforth and Coughtrie, 1994; Shibutani et al., 1998; Falany and Falany, 2007). The sterol/steroid SULT2B1a and SULT2B1b proteins are not significantly expressed in gastrointestinal or hepatic tissues (Meloche and Falany, 2001; Teubner et al., 2007) (although there is some expression in lung) (He et al., 2005), and SULT1C enzymes are expressed primarily in the fetus (Stanley et al., 2005), with some SULT1C1 in the adult stomach (Teubner et al., 2007). SULT4A1 is a brain-specific protein (Liyou et al., 2003), and SULT6B1 expression has been shown in the testis but not elsewhere (Freimuth et al., 2004). SULT1A2 appears to be expressed at very low levels, if at all, in tissues (Dooley et al., 2000; Teubner et al., 2007). Thus, we believe that SULT1A1, SULT1A3/4, SULT1B1, SULT1E1, and SULT2A1 are the most important and relevant in xenobiotic metabolism in adult humans.
There is considerable variation in the expression patterns of SULTs in different tissues and during development. For example, SULT1A3/4, the catecholamine-sulfating enzyme, is not expressed in adult human liver, whereas it is highly expressed in the upper gastrointestinal tract (Teubner et al., 2007); it is also expressed in the fetal liver (Richard et al., 2001; Stanley et al., 2005). Extensive interindividual variation in expression is also documented for a number of SULT isoforms (e.g., Wood et al., 1996; Raftogianis et al., 1999; Thomae et al., 2002; Adjei et al., 2003), some of which can be directly related to inherited genetic variation, such as the SULT1A1 copy number polymorphism (Hebbring et al., 2007). To date, there has been no systematic analysis of the expression profiles or quantification of the five major SULT enzymes in human tissues important for xenobiotic metabolism (e.g., liver, gastrointestinal tract, kidney, and lung). SULT expression in the gastrointestinal tract has been investigated (Chen et al., 2003; Teubner et al., 2007), confirming that SULT1A3/4 and SULT1B1 are significant enzymes in the gut but much less so in the liver, unlike SULT1A1, which is well expressed in both tissues.
Immunochemical quantification of expression profiles has been carried out for the cytochromes P450 (P450s) in liver (Shimada et al., 1994) and intestine (Paine et al., 2006), generating so-called P450 “pies,” showing the contributions of various isoforms to the total P450 content in these tissues. These detailed expression data are of considerable value in supporting the prediction of the in vivo fate of drugs and other xenobiotics from in vitro/in silico data; a number of commercial software tools (e.g., Simcyp; www.simcyp.com) incorporate such information in their databases. Because many drugs ultimately leave the body conjugated with sulfate (either directly or following phase 1 metabolism), there is a requirement for accurate and detailed expression information for the important xenobiotic metabolizing forms of SULT. In this study, we describe quantification of the expression of the five major xenobiotic-metabolizing SULTs in human liver, small intestine, kidney, and lung cytosol, and present a set of four SULT pies for these tissues.
Materials and Methods
Materials and Chemicals.
PAPS (>99% purity) was purchased from HR Glatt (German Institute of Human Nutrition, Potsdam, Germany). [35S]PAPS (PAP35S) (1.5–2.54 Ci/mmol), [3H]17β-estradiol (110 Ci/mmol), [3H]dehydroepiandrosterone (92 Ci/mmol), and scintillation fluid (Emulsifier Safe) were purchased from PerkinElmer Life and Analytical Sciences (Beaconsfield, Buckinghamshire, UK). Dopamine, 17β-estradiol, dehydroepiandrosterone, 4-nitrophenol, and 2-aminophenol were purchased from Sigma-Aldrich (Gillingham, Dorset, UK). All of the other chemicals were of analytical grade and purchased from commonly used suppliers.
Tissue Samples and Preparation of Cytosolic Fractions.
Human liver samples used have been described previously (Thomas and Coughtrie, 2003; Riches et al., 2007). Of the 28 liver samples, 26 were from whites (for two samples that information was not available), and 19 were from male and 9 from female donors. The mean age (±S.D.) of the donors was 45 ± 14 years (minimum, 19 years; maximum, 79 years). Human kidney and intestine samples were obtained from the UK Human Tissue Bank (Leicester, UK). Of the six intestine samples, all were from whites and four were from males. The mean age (±S.D.) of the donors was 50 ± 12 years (minimum, 37 years; maximum, 64 years). The three kidney samples were from male white donors aged 42, 46, and 55 years (mean, 48 ± 7 years). Lung samples were obtained from the local tissue bank, and all were adult white donors—no further information was available. Ethical approval for local use of samples was obtained from the Tayside Research Ethics Committee. Tissue samples were received frozen and were subsequently stored at −70°C. Fresh batches of cytosol were prepared for these experiments. Tissues were weighed and slightly thawed in 1 ml of ice-cold buffer (0.25 M sucrose, 10 mM Hepes, 3 mM 2-mercaptoethanol, pH 7.4) and cut with scissors. In the case of intestine samples, the mucosa was scraped from the muscle layer using a glass microscope slide and then weighed. Sufficient buffer to produce a 20% w/v homogenate was added, and the tissue was homogenized using a Teflon-glass homogenizer. The homogenate was centrifuged at 10,000g for 15 min at 4°C, and the supernatant was removed and centrifuged at 100,000g for 1 h at 4°C. The resulting supernatant (cytosolic fraction) was aspirated and aliquoted into 1-ml tubes, which were then frozen in liquid nitrogen and stored at −70°C until use (normally within 6 months).
Production of Recombinant Human SULTs.
Recombinant human SULT1A1, SULT1A3, SULT1B1, SULT1E1, and SULT2A1 were expressed in Escherichia coli and purified by using ion exchange and affinity chromatography as described previously (Riches et al., 2007 and references therein). Purity was assessed by SDS-polyacrylamide gel electrophoresis (PAGE).
PAP35S Assay for Analysis of SULT1A1 and SULT1A3 Activity.
Sulfation activity was determined with PAP35S using the barium precipitation assay originally described by Foldes and Meek (1973). Reaction mixtures in a total volume of 160 μl were prepared containing 0.1 M phosphate buffer, pH 7.4, human tissue cytosol (liver, 10–30 μg; kidney, 30–40 μg; intestine, 100–150 μg), substrate (Table 1), and PAPS (20 μM) containing 0.09 μCi PAP35S. Reaction mixtures were incubated for 20 min in a circulating water bath at 37°C and were stopped by placing the samples on ice and adding 200 μl of barium acetate (0.1 M). To remove unused PAPS, 200 μl of barium hydroxide (0.1 M) and 200 μl of zinc sulfate (0.1 M) were added. The samples were then mixed and centrifuged at 16,000g for 4 min. A sample (500 μl) of the resulting supernatant was removed and mixed with 4 ml of scintillation fluid, and radioactivity was quantified using liquid scintillation counting for 1 min/vial (Beckman Coulter, High Wycombe, Buckinghamshire, UK). The Michaelis-Menten equation, v * Vmax × [S]/(Km + [S]), was used to analyze the kinetic data except with 4-nitrophenol as substrate, where a modification of the Michaelis-Menten equation to reflect partial substrate inhibition was used: (v * Vmax × [S]/(Km + [S])(1 + [S]/Ki), where Ki is the inhibition constant for the effect). Data were processed by using Excel (Microsoft, Redmond, WA) and Prism 4 (GraphPad Software Inc., San Diego, CA) software.
TABLE 1.
Substrate concentrations used in assays for sulfotransferase enzyme kinetic determination
| Substrate | Concentration Range |
||
|---|---|---|---|
| Liver | Intestine | Kidney | |
| 4-Nitrophenol | 0.5–25 μM | 0.05–25 μM | 0.05–20 μM |
| 2-Aminophenol | 0.5–25 μM | 0.05–25 μM | 0.05–20 μM |
| Dopamine | 0.05–50 μM | 0.05–20 μM | |
| 17β-Estradiol | 0.1–100 nM | 0.1–100 nM | 0.1–100 nM |
| DHEA | 0.5–25 μM | 0.1–7 μM | |
Solvent Extraction Assay for Determining SULT1E1 and SULT2A1 Activity.
[3H]17β-Estradiol was used as substrate to assess SULT1E1 activity, and [3H]dehydroepiandrosterone (DHEA) was used as probe substrate for SULT2A1 activity. Reaction mixtures for SULT1E1 assays were prepared by comprising 0.1 M potassium phosphate buffer, pH 6.0, [3H]17β-estradiol (0.1 μCi), substrate (Table 1), cytosol, and PAPS (20 μM) to a final volume of 200 μl. For the SULT2A1 assays, the reaction mixture (160 μl) comprised 100 mM Tris/HCl, 20 mM MgCl2, pH 7.5, [3H]DHEA (0.1 μCi), substrate (Table 1), cytosol, and PAPS (50 μM). The buffer, substrate, and human cytosol were mixed in and warmed for 2 min at 37°C before the PAPS (or water for blanks) was added to start reactions. The tubes were incubated for 20 min; then 300 μl of ice-cold distilled water and 3 ml of chloroform were added; and the tubes were mixed vigorously, followed by centrifugation for 2 min at 3000 rpm. Two hundred microliters of the aqueous layer was added to 4 ml of scintillation fluid, and radioactivity was quantified by liquid scintillation counting for 1 min/sample. Data were analyzed using Excel (Microsoft) and Prism 4 (GraphPad Software Inc.) software.
Antibody Preparation.
For detection of SULT1A1 and SULT1A3, we used sheep anti-SULT1A3 (Richard et al., 2001) because it reacts with both enzymes; however, they can be easily resolved on SDS-PAGE (Richard et al., 2001). For SULT1E1, we used a rabbit anti-SULT1E1 peptide antibody (raised against amino acids 1–13; PanVera Corp., Madison, WI). To derive an antibody preparation with specificity toward human SULT1B1, we performed amino acid sequence alignment of all the human SULT proteins and selected the N-terminal 17 amino acids of SULT1B1 (MLSPKDILRKDLKLVHG), which show little homology to other SULTs, as the template for synthesis of a Multiple Antigenic Peptide (Alta Biosciences, Birmingham, UK). This peptide was used to immunize a sheep as described previously (Richard et al., 2001). Antibodies against human SULT2A1 were generated by injecting a sheep with purified recombinant SULT2A1 according to the schedule described previously (Richard et al., 2001). Crude IgG preparations of sheep antisera were generated by 50% ammonium sulfate fractionation followed by extensive dialysis, and for anti-SULT1B1 affinity purification against the peptide was performed as described by the manufacturer (Alta Biosciences). Antiserum specificity was evaluated by immunoblot analysis against purified recombinant SULTs and human tissue cytosols.
Quantitative Immunoblot Analysis.
Table 2 summarizes the immunoblotting conditions used. Proteins were resolved on denaturing SDS-PAGE (11% acrylamide monomer) and transferred to polyvinylidene difluoride membranes (Immobilon; Millipore Corporation, Billerica, MA) for immunostaining (Laemmli, 1970; Towbin et al., 1979). Membranes were blocked with 1% (w/v) bovine serum albumin (BSA) (Roche, Burgess Hill, West Sussex, UK) in 50 mM Tris, pH 7.9, 150 mM NaCl, Triton X-100 (0.01% v/v) (TBS-X) for 1 h. Membranes were washed in TBS-X before being exposed to donkey anti-sheep or anti-rabbit (for SULT1E1) IgG-peroxidase conjugate in TBS-X containing 1% (w/v) BSA for 1 h. Finally, the membrane was washed in TBS-X and developed with enhanced chemiluminescence reagents and Hyperfilm (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Standard curves for quantification were generated using purified recombinant SULT and were included on each individual blot. Scans of the developed X-ray film were made on a desktop scanner attached to a personal computer, and band density was calculated by using Quantiscan 3.1 software (BioSoft, Cambridge, Cambridgeshire, UK). Following pilot blots for each antibody and tissue, the quantity of cytosol loaded onto the gels was adjusted such that the densities of the resulting bands were within the linear region of the standard curves on each blot. Each tissue sample was analyzed on three separate blots with each antibody, and therefore individual data points quoted are the means of these three determinations. To estimate percentage contribution of individual SULTs to total SULT present in a given tissue, the mean expression levels for each enzyme were calculated and expressed as a percentage of the sum of all the means of all the SULTs present in that tissue, and it is these data that are plotted in Fig. 3.3
TABLE 2.
Immunostaining conditions for detection and quantification of sulfotransferase isoforms
Incubation time for all the antibodies was 1 h.
| SULT1A1/1A3 | SULT1B1 | SULT2A1 | SULT1E1 | |
|---|---|---|---|---|
| 1° Antibody | ||||
| Antigen | Recombinant human SULT1A3 | Multiple antigenic peptide (MLSPKDILRKDLKLVHG) | Recombinant human SULT2A1 | Commercial antibody (N-terminal peptide) |
| Host | Sheep | Sheep | Sheep | Rabbit |
| Purification | IgG | IgG/peptide affinity | IgG | None |
| Dilution | 1:30,000 | 1:1000 | 1:50,000 | 1:8000 |
| 2° Antibody | ||||
| Dilution | 1:5000 | 1:5000 | 1:5000 | 1:5000 |
Fig. 3.
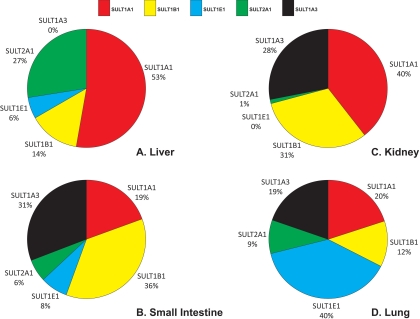
The human SULT pies. The mean expression values for each enzyme are displayed as percentages of the total sum of immunoquantified SULTs (maximum five enzymes) present in each tissue. Expression values are shown for liver (A), small intestine (B), kidney (C), and lung (D).
Protein Concentration Determination.
Quantification of total protein levels in all the tissue cytosols and recombinant protein preparations was carried out using the method originally described by Lowry et al. (1951) using BSA as standard.
Results
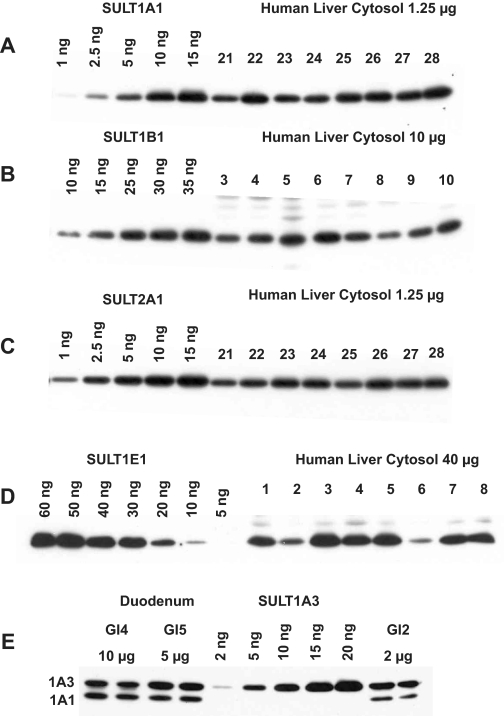
For each tissue and antibody, immunoblotting conditions were optimized such that the resulting bands were within the linear range of the standard curves generated from the purified recombinant SULTs, and that cross-reactivity with other SULT isoforms was minimized. Figure 1 shows a representative blot for each antiserum used in this study. The antibodies all exhibit a high degree of selectivity for the respective SULTs if care is taken with incubation conditions, as we have previously shown (Stanley et al., 2005). The exception is anti-SULT1A3, which recognizes both SULT1A1 and SULT1A3, but these are resolved on SDS-PAGE to such an extent that each protein can be quantified separately (Fig. 1).
Fig. 1.
Immunoblot demonstrating anti-SULT antibody specificity and representative standard curves. Cytosols prepared from a number of liver (A–D) or duodenum (E) samples were resolved on SDS-PAGE gels, along with the appropriate purified human SULTs diluted as necessary to construct the standard curves. Following transfer to polyvinylidene difluoride membranes, blots were immunostained with the corresponding anti-SULT antibody and secondary antibody as listed in Table 2.
SULT Protein Expression in Human Tissues.
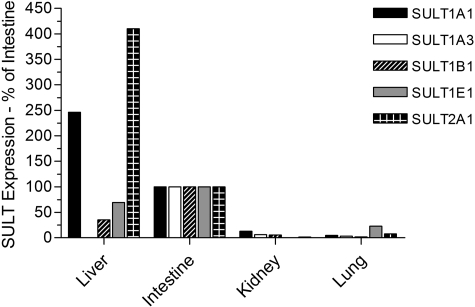
As expected, there were considerable differences between the expression profiles in the various tissues, as well as extensive interindividual differences in expression levels (Table 3). We found expression of SULT1A1, SULT1B1, SULT1E1, and SULT2A1 in all 28 liver samples analyzed, whereas expression of SULT1A3 was not found, which is consistent with other reports (Teubner et al., 2007). There was substantial interindividual variation in the levels of each protein in the liver: 12-fold for SULT1A1, 4-fold for SULT1B1, 13-fold for SULT1E1, and 4-fold for SULT2A1 (Table 3). SULT1A1 was clearly the most abundant SULT in liver, accounting for more than half of the total SULT content (53%), followed by SULT2A1 (27%), with SULT1B1 and SULT1E1 being relatively minor forms (14 and 6% of total, respectively) in this tissue. In the cytosols prepared from the gastrointestinal tract tissues (four samples of duodenum and two of ileum), SULT1B1 was the most abundant enzyme present, accounting for more than one third of the total (36%). SULT1A3 was also a major form in the gastrointestinal tract (31%), with SULT1A1 being much less abundant than in the liver, and accounting for only 19% of total SULT protein. SULT1E1 (8%) and SULT2A1 (6%) were minor forms in the gastrointestinal tract. In the kidney cytosol samples, SULT1A1, SULT1B1, and SULT1A3 were again the main isoforms present (comprising 40, 31, and 28% of the total, respectively), with SULT2A1 expressed at very low levels and no evidence of SULT1E1 expression in this tissue. This was in contrast to the lung, where SULT1E1 was the major expressed isoform (40% of the total SULT protein), followed by SULT1A1 (20%), SULT1A3 (19%), SULT1B1 (12%), and SULT2A1 (9%). Figure 2 illustrates the relative expression of the five enzymes in different tissues by normalizing against the expression in the intestinal samples. This confirms, along with the data presented in Table 3, that for SULT1A3, SULT1B1, and SULT1E1, the small intestine is the major site of expression (of the tissues examined), whereas for SULT1A1 and SULT2A1 the liver is the major tissue. The kidney and lung are, relative to the other tissues, poorly endowed with SULT. Overall, for the five SULTs examined, the small intestine has the highest total expression of SULT, followed by the liver, with the kidney and lung having between 15- and 30-fold less SULT protein per milligram of cytosol than the other tissues (Table 3). Figure 3 shows the SULT pies, with the average percentage contributions of each SULT to the total SULT protein (of the five SULTs investigated) for each of the four tissues investigated. We found no statistically significant differences in SULT expression levels between the liver cytosols from male and female donors or any influence of age on expression (data not shown). The extrahepatic tissue sample numbers were insufficient to carry out these analyses.
TABLE 3.
Quantification of the expression of five SULT isoforms in human tissue cytosols
Data are means ± S.D. for quantification of SULT expression in n tissue samples. Measurements were carried out in triplicate on each sample of cytosol. The range of values obtained with the panel of tissues is given in italics.
| Tissue | SULT Expression |
|||||
|---|---|---|---|---|---|---|
| SULT1A1 | SULT1A3 | SULT1B1 | SULT1E1 | SULT2A1 | Total | |
| ng/mg cytosol protein | ng/mg cytosol protein | ng/mg cytosol protein | ng/mg cytosol protein | ng/mg cytosol protein | ng/mg cytosol protein | |
| Liver (n = 28) | 3200 ± 1100 | BLQ | 840 ± 230 | 340 ± 160 | 1600 ± 420 | 5960 ± 1400 |
| Range | 420–4900 | 390–1600 | 50–660 | 680–2500 | 2300–7900 | |
| Intestine (n = 6) | 1300 ± 650 | 2000 ± 1100 | 2400 ± 1300 | 490 ± 470 | 390 ± 130 | 7800 ± 4600 |
| Range | 330–2200 | 770–3300 | 440–4000 | 250–510 | 50–1300 | 1900–15,900 |
| Kidney (n = 3) | 170 ± 90 | 120 ± 70 | 130 ± 110 | BLQ | 5 ± 10 | 430 ± 270 |
| Range | 100–270 | 70–200 | 60–260 | BLQ-20 | 240–730 | |
| Lung (n = 4) | 60 ± 50 | 60 ± 80 | 40 ± 20 | 110 ± 110 | 30 ± 20 | 290 ± 130 |
| Range | BLQ-120 | 5–170 | 10–50 | BLQ-260 | 4–50 | 100–370 |
BLQ, below limit of quantification.
Fig. 2.
Expression of SULTs in human tissues expressed as a percentage of levels in the small intestine. Mean expression level data are expressed relative to the mean values obtained with the small intestinal cytosols (shown as 100%) because this was the only tissue in which all five enzymes were detected.
To confirm that protein expression levels as measured here are representative of enzyme function, we also carried out assays for SULT activity. We used a series of probe substrates for the different enzymes: 4-nitrophenol and 2-aminophenol for SULT1A1, dopamine for SULT1A3, 17β-estradiol for SULT1E1, and dehydroepiandrosterone for SULT2A1, and carried out kinetic analysis for all the samples of liver, intestine, and kidney—we did not have sufficient lung samples to carry out these analyses. There is no known selective substrate for SULT1B1; however, it does metabolize 4-nitrophenol and, to a lesser extent, 2-aminophenol, with considerably higher Km values than for SULT1A1 (Riches et al., 2007).
The results of these analyses are provided in Table 4 and are in broad agreement with the immunoquantification data. No dopamine sulfation was observed in liver cytosol, consistent with the absence of SULT1A3 protein expression in that tissue, and the kidney displayed no sulfation of DHEA, again an observation that is entirely consistent with the immunoblotting experiments. Kidney cytosol retained a small amount of activity toward 17β-estradiol, but the Vmax/Km values were only between 1 and 2% of those found in intestine and liver. This suggests either that the enzyme activity assay is more sensitive than the immunoblotting assay (which is likely given the very low 17β-estradiol concentration used) or that one of the other enzymes is carrying out the sulfation of 17β-estradiol. We believe this latter explanation is unlikely given the very low Km obtained with kidney cytosol, consistent with the involvement of SULT1E1, which displays low nanomolar Km values toward this substrate. We also correlated expression in the individual liver cytosols with enzyme activity measurements in the same samples. As expected, strong correlations were observed between SULT1A1 expression and both 4-nitrophenol (2 μM) and 2-aminophenol (2 μM) SULT activity (Pearson's r * 0.76; p < 0.0001), between SULT1E1 expression and SULT activity toward 0.1 μM 17β-estradiol (r * 0.86; p < 0.0001), and between SULT2A1 expression and sulfation of 2 μM DHEA (r * 0.64; p < 0.0001). Expression of SULT1B1 did not show strong correlation (r < 0.5) with any of the enzyme activities measured.
TABLE 4.
SULT enzyme kinetics for five probe substrates in human tissue cytosols
Assays were performed in duplicate on each sample of cytosol for each substrate. Data represent the mean ± S.D. of the values obtained for n tissue cytosols. All the data points were fitted to the Michaelis-Menten equation, with the exception of 4-nitrophenol in the liver and kidney, where substantial substrate inhibition is observed. In these cases, the Michaelis-Menten equation modified for partial substrate inhibition was used (see under Materials and Methods).
| Substrate | Liver (n = 28) |
Intestine (n = 6) |
Kidney (n = 3) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Km | Vmax | Vmax/Km | Ki | Km | Vmax | Vmax/Km | Km | Vmax | Vmax/Km | Ki | |
| μM | pmol/min/mg | μM | μM | pmol/min/mg | μM | pmol/min/mg | μM | ||||
| 4-Nitrophenol | 3.9 ± 3.5 | 1865 ± 1787 | 478 | 4.5 ± 5.8 | 0.9 ± 0.6 | 33 ± 20 | 37 | 1.5 ± 0.8 | 37 ± 15 | 25 | 8.8 ± 7.5 |
| 2-Aminophenol | 1.3 ± 0.6 | 590 ± 251 | 453 | 2.0 ± 1.3 | 49 ± 27 | 25 | 0.3 ± 0.1 | 27 ± 16 | 90 | ||
| Dopamine | BLQ | BLQ | 2.2 ± 1.2 | 215 ± 180 | 98 | 2.4 ± 1.3 | 10 ± 12 | 4.2 | |||
| 17β-Estradiol | 0.14 ± 0.23 | 20 ± 10 | 142 | 0.03 ± 0.01 | 7.0 ± 6.1 | 233 | 0.08 ± 0.05 | 0.23 ± 0.02 | 2.9 | ||
| DHEA | 2.7 ± 0.7 | 234 ± 86 | 87 | 1.3 ± 0.7 | 15 ± 10.3 | 12 | BLQ | BLQ | |||
BLQ, below the limit of quantification of the assay method used.
Discussion
The ability to reliably predict the in vivo fate of chemicals such as drugs and other xenobiotics would be of considerable value to the pharmaceutical and other chemical industries, as well as to regulatory agencies. To support development of the tools necessary to underpin such predictions, it is essential to have (among other things) a thorough understanding of the contribution the different enzyme systems and transporters that influence the fate of xenobiotics. Sulfation is an important pathway of biotransformation, and a number of isoforms are involved in the metabolism of xenobiotics; however, we do not as yet have a detailed picture of the relative contributions of the different isoforms in the major tissues responsible for chemical defense. Therefore, we sought to quantify the expression of key SULT enzymes in human liver, small intestine, kidney, and lung with the aim of identifying the major contributing enzymes and tissues involved in the sulfation of xenobiotics.
The spectrum of SULTs expressed varied markedly between the different organs, as did the total amount of SULT protein (expressed per milligram of cytosolic protein), and, as expected, there was substantial interindividual variation in expression. The small intestine had a higher total SULT content than the liver, and both of these had substantially greater amounts (generally at least 10-fold) of SULT protein than either the kidney or the lung. This reinforces the potentially significant role that the gastrointestinal tract could play in the presystemic sulfation of xenobiotics, as well as indicating that, for the elimination of xenobiotics via sulfation at least, the kidneys and lungs may not play a particularly important role in humans. However, it should be noted that given the complex cellular composition of these tissues, metabolic activation via sulfation may play a significant role in the organ-specific toxicity of many xenobiotics. It is also important to recognize that the sample size, particularly for the extrahepatic tissues, was limited; therefore, some caution should be applied in drawing conclusions from the results presented. However, this is the only study to date comparing expression levels of key SULT enzymes in all these human tissues.
SULT1A1 is generally recognized as the major xenobiotic-metabolizing SULT, as a result of its capacity to accept a very wide range of substrates, and this enzyme accounted for more than 50% of total SULT protein in the liver, consistent with its presumed primary role in detoxification and chemical defense. However, in the small intestine, SULT1A1 was much less prevalent and was present at less than half the level of the liver (representing less than 20% of total SULT protein). In kidney cytosol, SULT1A1 was again the major isoform, although the amount was only 5% of that found in the liver, and in the lung it comprised 20% of the total SULT measured. Common genetic variants in SULT1A1 are known, including a coding region single nucleotide polymorphism (resulting in the amino acid change R213H) and a gene copy number polymorphism, both of which have independently been related to interindividual variation in expression levels (Raftogianis et al., 1999; Hebbring et al., 2007). We were not able to make a meaningful analysis of the influence of SULT1A1 genetic variants on expression/activity levels on the samples used for the present study.
SULT1A3 has evolved to selectively sulfate catecholamines in primates, with the vast majority of circulating dopamine, adrenaline, and noradrenaline in the form of the sulfate conjugate (Eisenhofer et al., 1999; Blanchard et al., 2004). The enzyme also sulfates a number of drugs, including salbutamol and apomorphine (Morgan et al., 1986; Thomas and Coughtrie, 2003). We could not detect SULT1A3 in any of the liver cytosol samples; however, it was a major enzyme in the small intestine, second only to SULT1B1. The absence of this enzyme from the adult liver is entirely consistent with previous studies from this and other laboratories (e.g., Richard et al., 2001; Teubner et al., 2007), as is the fact that this enzyme is a major extrahepatic form, particularly in the small intestine (Teubner et al., 2007), where it accounted for more than 30% of the total SULT protein. The presence of this enzyme at high levels in the small intestine has implications for the oral bioavailability of a number of drugs, including salbutamol (Morgan et al., 1986), as well as many dietary compounds (such as biogenic amines), which are substrates (Eisenhofer et al., 1999). In addition, it is shown that the mesenteric organs are capable of dopamine sulfate production in vivo, and indeed the gastrointestinal tract is believed to be the principal source of sulfated dopamine (Eisenhofer et al., 1999).
Of the major SULT enzymes involved in xenobiotic metabolism, we probably understand least about the function of SULT1B1. It has a broadly similar substrate profile to that of SULT1A1, although in general SULT1B1 exhibits a lower affinity for its substrates; indeed to date no “probe” substrate for this enzyme has been identified, and the presence of this enzyme in liver cytosol interferes with accurate kinetic measurements of the sulfation of certain phenolic molecules such as 4-nitrophenol (Tabrett and Coughtrie, 2003; Riches et al., 2007). It was originally suggested that the enzyme was selective for various iodothyronines (Wang et al., 1998), although it has been shown subsequently that other SULTs are able to efficiently sulfate these compounds in vitro (Kester et al., 1999a,b; Richard et al., 2001). In this study, we have shown that SULT1B1 represents only a small portion of the total SULT protein in the liver (14%), whereas it is the major SULT in the gastrointestinal tract, at 36% of the total. This finding suggests that SULT1B1 could play an important role in determining the oral bioavailability of phenolic drugs and other xenobiotics, although the dose may be important because of the generally poorer kinetic properties of this enzyme compared with SULT1A1. SULT1B1 is also present at high levels in the fetal small intestine (Stanley et al., 2005).
SULT1E1 displays very high selectivity and affinity for endogenous estrogens, in particular 17β-estradiol (Forbes-Bamforth and Coughtrie, 1994; Zhang et al., 1998), and is believed to play an important role in modulating the biological function of these hormones. Certainly there is evidence that estrogen-responsive tissues such as the endometrium use sulfation as a sensitive and specific mechanism of controlling estrogenic stimulation (Rubin et al., 1999), and this is supported by the strong inhibition of SULT1E1 by environmental pollutants such as hydroxylated polychlorinated biphenyls, a potential mechanism for the endocrine-disrupting effects of these chemicals (Kester et al., 2000). The enzyme also sulfates a number of important drugs, including 17α-ethinylestradiol. We found SULT1E1 was expressed at relatively low levels in the liver and small intestine and was absent from the kidney. In the lung, it was the major isoform present, although at lower levels than found in the liver and small intestine. It seems that SULT1E1 is generally expressed at higher levels during fetal development than in the adult (Stanley et al., 2005; Duanmu et al., 2006), perhaps suggesting a requirement to protect the developing fetus from the actions of 17β-estradiol. It is also possible that the low levels of expression are related to the fact that the Km of the enzyme for its main substrate, 17β-estradiol, is so low (in the low nanomolar range, commensurate with normal plasma levels) that a large turnover may not be required for efficient sulfation.
The hydroxysteroid SULT2A1 plays an important role in the biosynthesis of sex steroids because it sulfates the major adrenal steroid DHEA and many other hydroxylated steroid hormones and bile acids. In this regard, it is expressed at particularly high levels in the fetal adrenal gland (Barker et al., 1994). In addition, it can metabolize a wide range of xenobiotics, in particular alcohols (Shibutani et al., 1998; Sheng and Duffel, 2003). We found SULT2A1 to be a major form in the liver, second in abundance to SULT1A1, although levels in the extrahepatic tissues were low compared with the other SULTs. This suggests that the liver would be an important site of metabolism for xenobiotics that are substrates for SULT2A1, as well as reflecting the important role that this enzyme plays in bile acid homeostasis and protection against the toxic effects of bile acids (Kitada et al., 2003; Hofmann, 2004).
In summary, we present a detailed analysis of the expression levels of five key SULT enzymes in four human tissues, relative to each other within and between tissues—the SULT pies. We showed that SULT1A1 is the major hepatic xenobiotic-sulfating enzyme but that there are substantial quantities of SULT present in the small intestine, which has significant implications for the oral bioavailability of many drugs. The importance of the minor isoforms in the kidney and lung cannot be clearly identified from the results presented here; however, it is certainly possible that in, for example, the lung, significant ability to both detoxify and bioactivate airborne procarcinogens exists. The data presented here will also provide a significant expansion in the information available for modeling the metabolic fate of drugs cleared by sulfation.
Acknowledgments.
We thank Nerys Thomas for help with producing anti-SULT2A1 antibody and Dr. Sheila Sharp for helpful advice.
This work was supported in part by the Biotechnology and Biological Sciences Research Council [Grant BBS/S/B/2004/11767]; GlaxoSmithKline; the Medical Research Council [Grant G0000267]; and an equipment grant from Tenovus Tayside.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.109.028399
Two genes, SULT1A3 and SULT1A4, are extremely closely related and, despite minor sequence difference, code for identical SULT proteins (Freimuth et al., 2004). Here, for the sake of simplicity, we use SULT1A3, although of course the protein measured will represent the products of both genes.
We also calculated these contributions by taking the average expression of the individual enzymes in each tissue sample, and the values obtained (and the relative standings of each enzyme) were in very close agreement and did not alter the conclusions drawn.
- SULT
- sulfotransferase
- PAPS
- 3′-phosphoadenosine 5′-phosphosulfate
- P450
- cytochrome P450
- PAP35S
- [35S]PAPS
- PAGE
- polyacrylamide gel electrophoresis
- DHEA
- dehydroepiandrosterone
- BSA
- bovine serum albumin
- TBS-X
- Tris-buffered saline/Triton X-100.
References
- Adjei AA, Thomae BA, Prondzinski JL, Eckloff BW, Wieben ED, Weinshilboum RM. (2003) Human estrogen sulfotransferase (SULT1E1) pharmacogenomics: gene resequencing and functional genomics. Br J Pharmacol 139: 1373–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker EV, Hume R, Hallas A, Coughtrie WH. (1994) Dehydroepiandrosterone sulfotransferase in the developing human fetus: quantitative biochemical and immunological characterization of the hepatic, renal, and adrenal enzymes. Endocrinology 134: 982–989 [DOI] [PubMed] [Google Scholar]
- Blanchard RL, Freimuth RR, Buck J, Weinshilboum RM, Coughtrie MW. (2004) A proposed nomenclature system for the cytosolic sulfotransferase (SULT) superfamily. Pharmacogenetics 14: 199–211 [DOI] [PubMed] [Google Scholar]
- Chen G, Zhang D, Jing N, Yin S, Falany CN, Radominska-Pandya A. (2003) Human gastrointestinal sulfotransferases: identification and distribution. Toxicol Appl Pharmacol 187: 186–197 [DOI] [PubMed] [Google Scholar]
- Coughtrie MW. (2002) Sulfation through the looking glass—recent advances in sulfotransferase research for the curious. Pharmacogenomics J 2: 297–308 [DOI] [PubMed] [Google Scholar]
- Dajani R, Hood AM, Coughtrie MW. (1998) A single amino acid, Glu146, governs the substrate specificity of a human dopamine sulfotransferase, SULT1A3. Mol Pharmacol 54: 942–948 [DOI] [PubMed] [Google Scholar]
- Dooley TP, Haldeman-Cahill R, Joiner J, Wilborn TW. (2000) Expression profiling of human sulfotransferase and sulfatase gene superfamilies in epithelial tissues and cultured cells. Biochem Biophys Res Commun 277: 236–245 [DOI] [PubMed] [Google Scholar]
- Duanmu Z, Weckle A, Koukouritaki SB, Hines RN, Falany JL, Falany CN, Kocarek TA, Runge-Morris M. (2006) Developmental expression of aryl, estrogen, and hydroxysteroid sulfotransferases in pre- and postnatal human liver. J Pharmacol Exp Ther 316: 1310–1317 [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Coughtrie MWH, Goldstein DS. (1999) Dopamine sulphate: an enigma resolved. Clin Exp Pharmacol Physiol 26: S41–S53 [PubMed] [Google Scholar]
- Falany JL, Falany CN. (2007) Interactions of the human cytosolic sulfotransferases and steroid sulfatase in the metabolism of tibolone and raloxifene. J Steroid Biochem Mol Biol 107: 202–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldes A, Meek JL. (1973) Rat brain phenolsulfotransferase: partial purification and some properties. Biochim Biophys Acta 327: 365–374 [DOI] [PubMed] [Google Scholar]
- Forbes-Bamforth KJ, Coughtrie MW. (1994) Identification of a new adult human liver sulfotransferase with specificity for endogenous and xenobiotic estrogens. Biochem Biophys Res Commun 198: 707–711 [DOI] [PubMed] [Google Scholar]
- Freimuth RR, Wiepert M, Chute CG, Wieben ED, Weinshilboum RM. (2004) Human cytosolic sulfotransferase database mining: identification of seven novel genes and pseudogenes. Pharmacogenomics J 4: 54–65 [DOI] [PubMed] [Google Scholar]
- Gamage N, Barnett A, Hempel N, Duggleby RG, Windmill KF, Martin JL, McManus ME. (2006) Human sulfotransferases and their role in chemical metabolism. Toxicol Sci 90: 5–22 [DOI] [PubMed] [Google Scholar]
- Glatt H. (2000) Sulfotransferases in the bioactivation of xenobiotics. Chem Biol Interact 129: 141–170 [DOI] [PubMed] [Google Scholar]
- He D, Frost AR, Falany CN. (2005) Identification and immunohistochemical localization of Sulfotransferase 2B1b (SULT2B1b) in human lung. Biochim Biophys Acta 1724: 119–126 [DOI] [PubMed] [Google Scholar]
- Hebbring SJ, Adjei AA, Baer JL, Jenkins GD, Zhang J, Cunningham JM, Schaid DJ, Weinshilboum RM, Thibodeau SN. (2007) Human SULT1A1 gene: copy number differences and functional implications. Hum Mol Genet 16: 463–470 [DOI] [PubMed] [Google Scholar]
- Hildebrandt MA, Carrington DP, Thomae BA, Eckloff BW, Schaid DJ, Yee VC, Weinshilboum RM, Wieben ED. (2007) Genetic diversity and function in the human cytosolic sulfotransferases. Pharmacogenomics J 7: 133–143 [DOI] [PubMed] [Google Scholar]
- Hofmann AF. (2004) Detoxification of lithocholic acid, a toxic bile acid: relevance to drug hepatotoxicity. Drug Metab Rev 36: 703–722 [DOI] [PubMed] [Google Scholar]
- Kakuta Y, Pedersen LG, Carter CW, Negishi M, Pedersen LC. (1997) Crystal structure of estrogen sulphotransferase. Nature Struct Biol 4: 904–908 [DOI] [PubMed] [Google Scholar]
- Kester MH, Bulduk S, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MW, Bergman A, Safe SH, Kuiper GG, et al. ( 2000) Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs. Endocrinology 141: 1897–1900 [DOI] [PubMed] [Google Scholar]
- Kester MH, Kaptein E, Roest TJ, van Dijk CH, Tibboel D, Meinl W, Glatt H, Coughtrie MW, Visser TJ. (1999a) Characterization of human iodothyronine sulfotransferases. J Clin Endocrinol Metab 84: 1357–1364 [DOI] [PubMed] [Google Scholar]
- Kester MH, van Dijk CH, Tibboel D, Hood AM, Rose NJ, Meinl W, Pabel U, Glatt H, Falany CN, Coughtrie MW, et al. ( 1999b) Sulfation of thyroid hormone by estrogen sulfotransferase. J Clin Endocrinol Metab 84: 2577–2580 [DOI] [PubMed] [Google Scholar]
- Kitada H, Miyata M, Nakamura T, Tozawa A, Honma W, Shimada M, Nagata K, Sinal CJ, Guo GL, Gonzalez FJ, et al. ( 2003) Protective role of hydroxysteroid sulfotransferase in lithocholic acid-induced liver toxicity. J Biol Chem 278: 17838–17844 [DOI] [PubMed] [Google Scholar]
- Laemmli UK. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Liyou NE, Buller KM, Tresillian MJ, Elvin CM, Scott HL, Dodd PR, Tannenberg AE, McManus ME. (2003) Localization of a brain sulfotransferase, SULT4A1, in the human and rat brain: an immunohistochemical study. J Histochem Cytochem 51: 1655–1664 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275 [PubMed] [Google Scholar]
- Meloche CA, Falany CN. (2001) Expression and characterization of the human 3beta-hydroxysteroid sulfotransferases (SULT2B1a and SULT2B1b). J Steroid Biochem Mol Biol 77: 261–269 [DOI] [PubMed] [Google Scholar]
- Morgan DJ, Paull JD, Richmond BH, Wilson-Evered E, Ziccone SP. (1986) Pharmacokinetics of intravenous and oral salbutamol and its sulphate conjugate. Br J Clin Pharmacol 22: 587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. (2006) The human intestinal cytochrome P450 “pie.” Drug Metab Dispos 34: 880–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftogianis RB, Wood TC, Weinshilboum RM. (1999) Human phenol sulfotransferases SULT1A2 and SULT1A1: genetic polymorphisms, allozyme properties, and human liver genotype-phenotype correlations. Biochem Pharmacol 58: 605–616 [DOI] [PubMed] [Google Scholar]
- Richard K, Hume R, Kaptein E, Stanley EL, Visser TJ, Coughtrie MW. (2001) Sulfation of thyroid hormone and dopamine during human development: ontogeny of phenol sulfotransferases and arylsulfatase in liver, lung, and brain. J Clin Endocrinol Metab 86: 2734–2742 [DOI] [PubMed] [Google Scholar]
- Riches Z, Bloomer JC, Coughtrie MW. (2007) Comparison of 2-aminophenol and 4-nitrophenol as in vitro probe substrates for the major human hepatic sulfotransferase, SULT1A1, demonstrates improved selectivity with 2-aminophenol. Biochem Pharmacol 74: 352–358 [DOI] [PubMed] [Google Scholar]
- Rubin GL, Harrold AJ, Mills JA, Falany CN, Coughtrie MW. (1999) Regulation of sulphotransferase expression in the endometrium during the menstrual cycle, by oral contraceptives and during early pregnancy. Mol Hum Reprod 5: 995–1002 [DOI] [PubMed] [Google Scholar]
- Sheng JJ, Duffel MW. (2003) Enantioselectivity of human hydroxysteroid sulfotransferase ST2A3 with naphthyl-1-ethanols. Drug Metab Dispos 31: 697–700 [DOI] [PubMed] [Google Scholar]
- Shibutani S, Shaw PM, Suzuki N, Dasaradhi L, Duffel MW, Terashima I. (1998) Sulfation of alpha-hydroxytamoxifen catalyzed by human hydroxysteroid sulfotransferase results in tamoxifen-DNA adducts. Carcinogenesis 19: 2007–2011 [DOI] [PubMed] [Google Scholar]
- Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. (1994) Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther 270: 414–423 [PubMed] [Google Scholar]
- Stanley EL, Hume R, Coughtrie MW. (2005) Expression profiling of human fetal cytosolic sulfotransferases involved in steroid and thyroid hormone metabolism and in detoxification. Mol Cell Endocrinol 240: 32–42 [DOI] [PubMed] [Google Scholar]
- Strott CA. (2002) Sulfonation and molecular action. Endocr Rev 23: 703–732 [DOI] [PubMed] [Google Scholar]
- Surh YJ, Miller JA. (1994) Roles of electrophilic sulfuric acid ester metabolites in mutagenesis and carcinogenesis by some polynuclear aromatic hydrocarbons. Chem Biol Interact 92: 351–362 [DOI] [PubMed] [Google Scholar]
- Tabrett CA, Coughtrie MW. (2003) Phenol sulfotransferase 1A1 activity in human liver: kinetic properties, interindividual variation and re-evaluation of the suitability of 4-nitrophenol as a probe substrate. Biochem Pharmacol 66: 2089–2097 [DOI] [PubMed] [Google Scholar]
- Teubner W, Meinl W, Florian S, Kretzschmar M, Glatt H. (2007) Identification and localization of soluble sulfotransferases in the human gastrointestinal tract. Biochem J 404: 207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomae BA, Eckloff BW, Freimuth RR, Wieben ED, Weinshilboum RM. (2002) Human sulfotransferase SULT2A1 pharmacogenetics: genotype-to-phenotype studies. Pharmacogenomics J 2: 48–56 [DOI] [PubMed] [Google Scholar]
- Thomas NL, Coughtrie MW. (2003) Sulfation of apomorphine by human sulfotransferases: evidence of a major role for the polymorphic phenol sulfotransferase, SULT1A1. Xenobiotica 33: 1139–1148 [DOI] [PubMed] [Google Scholar]
- Towbin H, Stehilin T, Gordon J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc Natl Acad Sci U S A 76: 4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Falany JL, Falany CN. (1998) Expression and characterization of a novel thyroid hormone-sulfating form of cytosolic sulfotransferase from human liver. Mol Pharmacol 53: 274–282 [DOI] [PubMed] [Google Scholar]
- Wood TC, Her C, Aksoy I, Otterness DM, Weinshilboum RM. (1996) Human dehydroepiandrosterone sulfotransferase pharmacogenetics: quantitative Western analysis and gene sequence polymorphisms. J Steroid Biochem Mol Biol 59: 467–478 [DOI] [PubMed] [Google Scholar]
- Zhang H, Varlamova O, Vargas FM, Falany CN, Leyh TS, Varmalova O. (1998) Sulfuryl transfer: the catalytic mechanism of human estrogen sulfotransferase. J Biol Chem 273: 10888–10892 [DOI] [PubMed] [Google Scholar]