Abstract
Bietti's crystalline dystrophy is an ocular disease that is strongly associated with polymorphisms in the CYP4V2 gene. CYP4 enzymes are typically microsomal fatty acid ω-hydroxylases that function together with mitochondrial and peroxisomal β-oxidation enzymes to degrade cellular lipids. Indeed, ocular and peripheral cells cultured from patients with Bietti's have been reported to exhibit abnormal lipid metabolism. However, CYP4V2 possesses low sequence homology to other members of the CYP4 family. Therefore, we cloned and expressed CYP4V2 and analyzed the functional characteristics of this new cytochrome P450 enzyme. We find that CYP4V2 is a selective ω-hydroxylase of saturated, medium-chain fatty acids with relatively high catalytic efficiency toward myristic acid. Moreover, N-hydroxy-N′-(4-n-butyl-2-methylphenyl formamidine) (HET0016) is a nanomolar inhibitor of the enzyme. Therefore, CYP4V2 exhibits catalytic functions typical of a human CYP4 enzyme, but with a distinctive chain-length selectivity coupled with high ω-hydroxylase specificity. Consequently, defective ω-oxidation of ocular fatty acids/lipids secondary to mutations in the CYP4V2 gene appears to be a plausible mechanism underlying Bietti's crystalline dystrophy.
CYP4V2, a relatively new member of the pantheon of human cytochrome P450 (P450) enzymes, is termed an “orphan” P450 because its substrate specificity and physiological roles are unknown (Stark and Guengerich, 2007). The issue of CYP4V2's substrate specificity is important because an intriguing genetic association has emerged between CYP4V2 and a rare ocular disorder known as Bietti's corneoretinal crystalline dystrophy (BCD) (Bietti, 1937). BCD is a progressive disease that leads to atrophy of the retinal epithelium, constriction of the visual field, and night blindness. Potentially disruptive exonic and intronic mutations in the CYP4V2 gene were identified originally in nearly two dozen BCD patients, and subsequently many investigators have corroborated this gene defect (Li et al., 2004; Wada et al., 2005; Nakamura et al., 2006). Given the well recognized role of other CYP4 enzymes in fatty acid metabolism, a deficiency in this catalytic function of CYP4V2 in BCD patients is an attractive hypothesis.
The prototypic enzymatic reaction of the CYP4 enzymes is fatty acid ω-hydroxylation. However, CYP4V2 is an unusual CYP4 enzyme in that gene resides on human chromosome 4, separate from the CYP4ABXZ and CYP4F gene clusters on chromosomes 1 and 19 (Hsu et al., 2007), respectively, and has very low sequence identity (31–37%) to other CYP4 enzymes (Rettie and Kelly, 2008). These observations raise the question of how closely the catalytic capabilities of CYP4V2 resemble those of other human CYP4 enzymes, delineation of which is a first step toward elucidating the role of the enzyme in BCD. Therefore, the goals of the present study were to clone and express CYP4V2 and to characterize the enzyme's functional behavior toward saturated fatty acids and the formamidine derivative, HET0016, which is emerging as a high-affinity inhibitor of many of the human CYP4 isoforms (Miyata et al., 2005).
Materials and Methods
Chemicals.
NADPH, BSTFA, octanoic acid, lauric acid, myristic acid, palmitic acid, 8-hydroxy octanoic acid, 12-hydroxy lauric acid, 16-hydroxy palmitic acid, 14-hydroxytetradeca-10, 12-diynoic acid, Ex-Cell 420 media, and δ-aminolevulinic acid were obtained from Sigma-Aldrich (St. Louis, MO). The ω-1, ω-2, and ω-3 hydroxy metabolites of lauric acid, myristic acid, and palmitic acid metabolites were generated biologically using recombinant CYP102, which was kindly provided by Dr. Rheem Totah (University of Washington, Seattle, WA). ω-1 Hydroxy octanoic acid was generated from recombinant rabbit CYP4B1. 14-Hydroxy myristic acid was synthesized as described previously (Lillemin et al., 1984), and the product was confirmed by gas chromatography-mass spectrometry (GC-MS) and NMR. HET0016 was obtained from Cayman Chemical (Ann Arbor, MI).
CYP4V2 Cloning and Baculovirus Production.
The cDNA for CYP4V2 (I.M.A.G.E. clone ID 30333559) was purchased from the American Type Culture Collection (Manassas, VA), and a recombinant baculovirus containing CYP4V2 with a hexahistidine tag was generated by using the Bac-to-Bac Baculovirus expression system (Invitrogen, Carlsbad, CA). In brief, to obtain the full-length cDNA with the hexahistidine tag, CYP4V2 was amplified by PCR with the specific primers, 5′-GCG CGA ATT CAT GGC GGG GCT CTG GCT GGG G-3′ and 5′-GCG CGT CGA CTT AAT GAT GAT GAT GAT GAT GGC GTT CAT CTG C-3′. This PCR product was double digested and subcloned into the pFastBac1 vector via the cloning sites EcoRI and Sal1 to generate the construct pFastbacCYP4V2HIS. DH10Bac Escherichia coli was transfected with pFastbacCYP4V2HIS to obtain the recombinant bacmid DNA. Recombinant baculovirus was produced in sf9 cells (Spodoptera frugiperda) by transformation with bacmid DNA. The presence of CYP4V2 in both bacmid and recombinant baculovirus was confirmed by PCR and sequence analysis.
Baculovirus Expression of CYP4V2.
Hexahistidine-tagged CYP4V2 was expressed in sf9 cells after infection with the recombinant baculovirus. Mock-infected cells were also prepared as a control. Sf9 cells were grown in the suspension culture by using Ex-Cell 420 media containing 2.5% fetal bovine serum and antibiotics-antimycotics. The expression culture was supplemented with 0.3 mM δ-aminolevulinic acid and 0.2 mM ferric citrate 24 h after infection with the recombinant virus. Cells were harvested 72 h after infection. The microsomal fraction of cells was prepared by differential ultracentrifugation, and CYP4V2 expression was confirmed by SDS-polyacrylamide gel electrophoresis analysis (9%) with Coomassie blue staining and Western blotting with an hexa-histidine antibody (QIAGEN, Valencia, CA).
Reduced CO-Bound Spectra.
Reduced, CO-bound spectra were recorded on a Cary 300 UV/VIS Spectrometer (Varian Inc., Palo Alto, CA). In brief, dithionite and 1.2 μM methyl viologen were added to microsomal samples diluted in 100 mM potassium phosphate, pH 7.4, containing 0.5 mM EDTA and 20% glycerol. After bubbling with CO gas, holo-P450 concentrations were measured by using an extinction coefficient of 91 mM−1cm−1 (450–500 nm).
Metabolic Incubations.
Microsomal CYP4V2 was mixed with purified NADPH-cytochrome P450 reductase and cytochrome b5 at a molar ratio of 1:2:1, respectively, in a total volume of 500 μl of potassium phosphate buffer (100 mM) (pH 7.4). Saturated fatty acids, dissolved in dimethyl sulfoxide, were added and then preincubated for 2 min at 37°C in a water bath. Metabolic reactions were initiated by the addition of 1 mM NADPH and allowed to proceed for 20 min. Reactions were quenched with 500 μl of chilled 10% hydrochloric acid. Samples were spiked with 2.5 μg of the internal standard (15-hydroxy pentadecanoic acid for lauric acid, myristic acid, and palmitic acid metabolite assays, or 12-hydroxy lauric acid for the octanoic acid metabolite assay) and extracted twice with ethyl acetate. Pooled organic extracts were dried under a N2 stream and reconstituted with ethyl acetate (50 μl). The same volume of BSTFA was added to the samples, which were heated at 90°C for 45 min and analyzed by GC-MS.
GC-MS Analysis.
Derivatized extracts were analyzed on a Shimadzu GCMS-QP5050A (Shimadzu, Kyoto, Japan) or HP5986 GC-FID (Agilent Technologies, Foster City, CA) fitted with a fused silica capillary column coated with a DB-1 stationary phase. Lauric acid metabolism was assayed as described previously (Guan et al., 1998). Myristic acid and palmitic acid incubation extracts were injected at a temperature of 100°C. After 2 min, the oven temperature was raised at 40°C/min to 200°C, held for 1 min, then raised at 4°C/min to 245°C, and finally at 30°C/min to 290°C. Under these conditions, the derivatized ω, ω-1, ω-2, and ω-3 hydroxylated metabolites of myristic acid (and palmitic acid) eluted at 12.4 (15.3), 11.3 (14.2), 11.0 (13.9), and 10.6 (13.5) min, respectively. Derivatized extracts from octanoic acid incubations were also injected at an initial temperature of 100°C. After 2 min, the oven temperature was raised at 40°C/min to 130°C, held for 2 min, then raised at 4°C/min to 210°C, and finally at 30°C/min to 290°C. Under these conditions, the derivatized ω and ω-1 hydroxylated metabolites of octanoic acid eluted at 12.9 and 11.2 min, respectively. Rate data were calculated from standard curves generated with authentic ω-hydroxy metabolites.
Kinetic Analysis.
Kinetic parameters for CYP4V2-dependent fatty acid ω-hydroxylation were obtained by nonlinear regression analysis of rate data generated from substrate concentrations between 1.6 and 300 μM by using GraphPad Prism.
Inhibition by HET0016.
Reconstituted microsomal CYP4V2 was incubated, as described above, with lauric acid (100 μM) and NADPH in the presence of 1 to 1000 nM HET0016. The IC50 of HET0016 for CYP4V2-dependent lauric acid ω-hydroxylation was also determined using GraphPad Prism (GraphPad Software Inc., San Diego, CA).
Results and Discussion
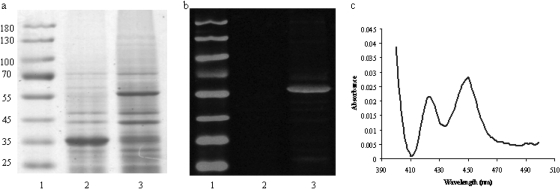
CYP4V2 was successfully expressed in insect cell microsomes as evidenced by the protein band near 55 kDa that is visualized by Coomassie staining in Fig. 1a and by immunoblotting with an anti-His6 antibody in Fig. 1b. The presence of holo-CYP4V2 was confirmed spectrophotometrically by the CO difference spectrum obtained in Fig. 1c. The specific content of CYP4V2 insect cell microsomes was 0.2 nmol/mg protein, and total expression levels of holo-CYP4V2 in culture were estimated at ∼50 nmol/l.
Fig. 1.
Analysis of CYP4V2 expressed in insect cells by SDS-polyacrylamide gel electrophoresis with Coomassie staining (a), and Western blotting with an anti-His6 antibody (b). Lane 1, molecular weight markers. Lane 2, microsomes from mock-infected Sf9 cells. Lane 3, microsomes from Sf9 cells infected with baculovirus-containing CYP4V2. Lanes 2 and 3 were loaded with 10 μg of protein. c, CO difference spectrum of CYP4V2-containing insect cell microsomes demonstrating absorbance of holo-P450 at ∼450 nm.
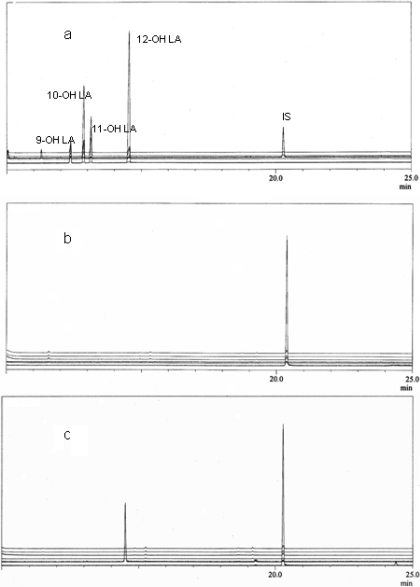
The catalytic activity of CYP4V2 was first evaluated against lauric acid because ω-hydroxylation of this substrate is the prototypical reaction of the CYP4A family of enzymes (Okita and Okita, 2001). Figure 2 demonstrates that CYP4V2 is an NADPH-dependent lauric acid hydroxylase with high selectivity for ω-hydroxylation. Kinetic parameters (Km and Vmax) for 12-hydroxy lauric acid formation by CYP4V2 were 140 ± 20 μM and 3.7 ± 0.27 pmol/pmol/min, respectively.
Fig. 2.
GC-MS analysis of lauric acid metabolites. a, bis-TMS derivatives of hydroxy lauric acid standards monitored at m/z 345, 117, 131, and 145 for 12-, 11-, 10-, and 9-hydroxy lauric acid, respectively. The derivatized internal standard (IS), 15-hydroxypentadecanoic acid, was monitored at m/z 387. b, metabolites produced from CYP4V2 microsomes in the absence of NADPH. Similar data were obtained with NADPH and mock-transfected insect cells. c, metabolites produced from CYP4V2 microsomes in the presence of NADPH. A trace amount of 11-hydroxy lauric acid was formed, but this is not evident in the chromatographic traces because the ion current intensities are normalized to the major product.
Next, we evaluated the chain-length specificity over the range C8 to C16 for CYP4V2-dependent metabolism of saturated fatty acids (Table 1). Only trace amounts of internally hydroxylated metabolites were detected. Octanoic acid was not detectably ω-hydroxylated by CYP4V2, but palmitic acid, like lauric acid, was selectively ω-hydroxylated by CYP4V2 at a comparable rate, but with a 3-fold higher Km. Likewise, myristic acid was selectively ω-hydroxylated by CYP4V2, a reaction that proceeded with the highest catalytic efficiency of all the substrates examined (Table 1). This metabolic profile differs from CYP4B1 and CYP4F enzymes that show a preference for shorter chain (Fisher et al., 1998) and longer-chain (Hardwick, 2008) fatty acids, respectively. CYP4V2 displays a similar chain-length specificity to CYP4A11 (Hardwick, 2008), but it differs from this latter isoform in its selectivity for ω-hydroxylation of myristic acid and palmitic acid. These data demonstrate that CYP4V2 is a saturated fatty acid hydroxylase with regioselectivity and chain-length specificities that distinguish the enzyme from other well characterized human CYP4 isoforms.
TABLE 1.
CYP4V2-dependent metabolism of saturated fatty acids
| Fatty acid | Metabolite* | Km | Vmax | Vmax/Km | ω/ω-1*** |
|---|---|---|---|---|---|
| μM | nmol/nmol/min | μl/nmol/min | |||
| Octanoic acid | 8-OH octanoate | —** | <0.1 | —** | —** |
| Lauric acid | 12-OH laurate | 140 ± 20 | 3.7 ± 0.27 | 26 | >20:1 |
| Myristic acid | 14-OH myristate | 65 ± 7.4 | 2.3 ± 0.07 | 35 | >20:1 |
| Palmitic acid | 16-OH palmitate | 430 ± 89 | 3.5 ± 0.26 | 8.1 | >10:1 |
ω-Hydroxy metabolites of the C8 to C16 substrates were detected as the molecular ions of the TMS derivatives at m/z 289, 345, 373, and 401, respectively.
Reaction velocities were below quantifiable levels.
Regioselectivity was determined by GC-FID analysis of the TMS derivatives, assuming an equal detector response for the ω and ω-1 metabolites.
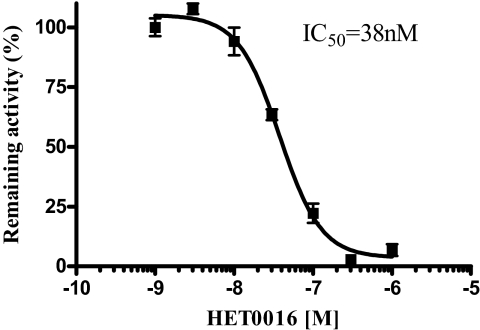
The P450 inhibitor, HET0016, has been used extensively in renal P450 physiology for its ability to inhibit 20-HETE, a metabolite of arachidonic acid that regulates renal vascular function (Maier and Roman, 2001). HET0016 is a potent and selective inhibitor of CYP4A and CYP4F isoforms exhibiting IC50s in the low nanomolar region (Miyata et al., 2005). This compound also inhibits CYP2C9, CYP2D6, and CYP3A4 but with ∼1000-fold lower potency (Miyata et al., 2001). Therefore, we evaluated the inhibitory potency of HET0016 toward CYP4V2-mediated lauric acid metabolism for further evidence of a ligand selectivity indicative of a CYP4 family enzyme. Inhibition of 12-hydroxylauric acid metabolite formation was concentration-dependent with an IC50 of 38 nM (Fig. 3). This finding extends the array of P450 CYP4 subfamilies susceptible to potent inhibition by HET0016.
Fig. 3.
Effect of HET0016 (1–1000 nM) on CYP4V2-catalyzed ω-hydroxylation of lauric acid (100 μM). HET0016 is a potent inhibitor of CYP4V2, as evidenced by the IC50 value of 38 nM.
In summary, we report the first functional analysis of recombinant human CYP4V2. Despite its low sequence homology to other members of the CYP4 family, CYP4V2 displays catalytic properties that are characteristic of other members of the human CYP4 family in that the enzyme is a selective fatty acid ω-hydroxylase and is potently inhibited by HET0016. Expressed sequence tag analysis reveals that CYP4V2 is widely distributed and present in the eye (http://www.ncbi.nlm.nih.gov/UniGene/ESTProfileViewer.cgi?uglist = Hs.237642). Taken together, these observations suggest that ocular CYP4V2 may play a role in fatty acid homeostasis in the eye. Moreover, it seems plausible that defective ω-oxidation of ocular fatty acids/lipids secondary to mutations in the CYP4V2 gene could contribute to the etiology of BCD. Functional studies with polyunsaturated fatty acids, found at high concentrations in the eye, are underway.
This work was supported in part by the National Institutes of Health National Institute of General Medical Sciences [Grant GM49054].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.109.028530
- P450
- cytochrome P450
- BCD
- Bietti's corneoretinal crystalline dystrophy
- HET0016
- N-hydroxy-N′-(4-n-butyl-2-methylphenyl formamidine)
- BSTFA
- N,O-bis(trimethylsilyl)trifluoroacetate
- GC-MS
- gas chromatography-mass spectrometry
- TMS
- trimethylsyl.
References
- Bietti G. (1937) Ueber familiaeres vorkommen von ‘retinitis punctata albescens’ (verbunden mit dystrophia marginalis cristallinea corneae), glitzern des glaskoerpers und anderen degenerativen augenveraenderungen. Klin Mbl Augenheilk 99: 737–757 [Google Scholar]
- Fisher MB, Zheng YM, Rettie AE. (1998) Positional specificity of rabbit CYP4B1 for omega-hydroxylation1 of short-medium chain fatty acids and hydrocarbons. Biochem Biophys Res Commun 248: 352–355 [DOI] [PubMed] [Google Scholar]
- Guan X, Fisher MB, Lang DH, Zheng YM, Koop DR, Rettie AE. (1998) Cytochrome P450-dependent desaturation of lauric acid: isoform selectivity and mechanism of formation of 11-dodecenoic acid. Chem Biol Interact 110: 103–121 [DOI] [PubMed] [Google Scholar]
- Hardwick JP. (2008) Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochem Pharmacol 75: 2263–2275 [DOI] [PubMed] [Google Scholar]
- Hsu MH, Savas U, Griffin KJ, Johnson EF. (2007) Human cytochrome P450 family 4 enzymes: function, genetic variation and regulation. Drug Metab Rev 39: 515–538 [DOI] [PubMed] [Google Scholar]
- Li A, Jiao X, Munier FL, Schorderet DF, Yao W, Iwata F, Hayakawa M, Kanai A, Shy Chen M, Alan Lewis R, et al. ( 2004) Bietti crystalline corneoretinal dystrophy is caused by mutations in the novel gene CYP4V2. Am J Hum Genet 74: 817–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillemin D, Cadiot P, Kuetagan P. (1984) A new synthesis of ω-hydroxyalkanoic acids via copper catalysis. Synthesis 3: 230–231 [Google Scholar]
- Maier KG, Roman RJ. (2001) Cytochrome P450 metabolites of arachidonic acid in the control of renal function. Curr Opin Nephrol Hypertens 10: 81–87 [DOI] [PubMed] [Google Scholar]
- Miyata N, Seki T, Tanaka Y, Omura T, Taniguchi K, Doi M, Bandou K, Kametani S, Sato M, Okuyama S, et al. ( 2005) Beneficial effects of a new 20-hydroxyeicosatetraenoic acid synthesis inhibitor, TS-011 [N-(3-chloro-4-morpholin-4-yl) phenyl-N′-hydroxyimido formamide], on hemorrhagic and ischemic stroke. J Pharmacol Exp Ther 314: 77–85 [DOI] [PubMed] [Google Scholar]
- Miyata N, Taniguchi K, Seki T, Ishimoto T, Sato-Watanabe M, Yasuda Y, Doi M, Kametani S, Tomishima Y, Ueki T, et al. ( 2001) HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol 133: 325–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Lin J, Nishiguchi K, Kondo M, Sugita J, Miyake Y. (2006) Bietti crystalline corneoretinal dystrophy associated with CYP4V2 gene mutations. Adv Exp Med Biol 572: 49–53 [DOI] [PubMed] [Google Scholar]
- Okita RT, Okita JR. (2001) Cytochrome P450 4A fatty acid omega hydroxylases. Curr Drug Metab 2: 265–281 [DOI] [PubMed] [Google Scholar]
- Rettie AE, Kelly EJ. (2008) The CYP4 family, in Cytochrome P450: Role in the Metabolism and Toxicity of Drugs and Other Xenobiotics ( Ioannides C. ed), Royal Society of Chemistry, London [Google Scholar]
- Stark K, Guengerich FP. (2007) Characterization of orphan human cytochromes P450. Drug Metab Rev 39: 627–637 [DOI] [PubMed] [Google Scholar]
- Wada Y, Itabashi T, Sato H, Kawamura M, Tada A, Tamai M. (2005) Screening for mutations in CYP4V2 gene in Japanese patients with Bietti's crystalline corneoretinal dystrophy. Am J Ophthalmol 139: 894–899 [DOI] [PubMed] [Google Scholar]