Abstract
Based on animal data, there is speculation that (±)-3,4-methylenedioxymethamphetamine (MDMA) is neurotoxic to humans. Extrapolation of MDMA findings from animals to humans requires assessment of pharmacokinetics in various species, and low-dose administration data from rats are lacking. In this study, we examine MDMA pharmacokinetics in rats given low (2 mg/kg) and high (10 mg/kg) doses of racemic MDMA via intraperitoneal, subcutaneous, and oral routes. Repeated blood specimens were collected from venous catheters, and plasma was assayed for MDMA and its metabolites, 4-hydroxy-3-methoxymethamphetamine (HMMA) and 3,4-methylenedioxyamphetamine (MDA), by gas chromatography-mass spectrometry. After 2 mg/kg, maximum MDMA concentrations (Cmax) were ∼200 ng/ml for intraperitoneal and subcutaneous routes, but less for the oral route. MDMA plasma half-lives were <1 h for low-dose groups, whereas HMMA and MDA half-lives were >2 h. After 10 mg/kg, MDMA areas under the curve (AUCs) were 21-fold (intraperitoneal), 10-fold (subcutaneous), and 36-fold (oral) greater than those at 2 mg/kg. In contrast, HMMA AUC values in high-dose groups were <3-fold above those at 2 mg/kg. Several new findings emerge from this report of low-dose MDMA pharmacokinetics in rats. First, 2 mg/kg MDMA in rats can produce MDMA Cmax values similar to those in humans, perhaps explaining why both species discriminate 1.5 mg/kg MDMA in laboratory paradigms. Second, our data provide additional support for nonlinear kinetics of MDMA in rats, and, analogous to humans, this phenomenon appears to involve impaired drug metabolism. Finally, given key similarities between MDMA pharmacokinetics in rats and humans, data from rats may be clinically relevant when appropriate dosing conditions are used.
(±)-3,4-Methylenedioxymethamphetamine [(MDMA) Ecstasy] is an illicit drug that stimulates the transporter-mediated release of monoamine transmitters—serotonin (5-HT), norepinephrine, and dopamine—from nerve cells (Johnson et al., 1991; Verrico et al., 2007). Administration of MDMA to rats and other animals can cause long-term deficits in brain 5-HT neurons (Ricaurte et al., 2000), prompting speculation that the drug is neurotoxic to humans. However, extrapolation of MDMA data from animals to humans is complicated by a variety of factors, especially differences in dosing across species (Baumann et al., 2007). For example, rats are given single or multiple injections of 10 to 20 mg/kg MDMA by the intraperitoneal or subcutaneous route to produce 5-HT depletions (Ricaurte et al., 2000; Green et al., 2003), whereas humans ingest 1 to 2 mg/kg by the oral route for recreational purposes (Cole et al., 2002; Parrott, 2005). More importantly, rats and humans readily discriminate 1.5 mg/kg MDMA in laboratory paradigms (Schechter, 1988; Johanson et al., 2006), suggesting that psychoactive effects occur over the same dose range in both species.
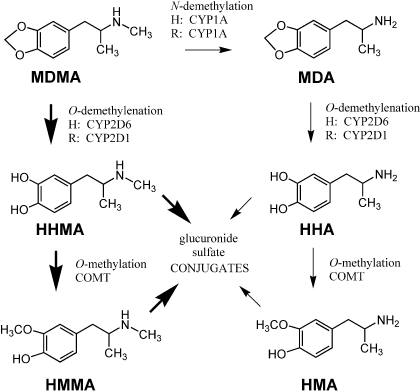
Drug metabolism is another factor that can complicate the extrapolation of MDMA findings across species (Maurer et al., 2000; de la Torre and Farré, 2004). MDMA is metabolized by hepatic mechanisms in humans and other mammals, and two main pathways of biotransformation are shown in Fig. 1 (Maurer et al., 2000; de la Torre et al., 2004). In the major pathway, MDMA is O-demethylenated to form (±)-3,4 dihydroxymethamphetamine (HHMA), which is O-methylated to generate (±)-4-hydroxy-3-methoxymethamphetamine (HMMA). In the minor pathway, MDMA is N-demethylated to form (±)-3,4-methylenedioxyamphetmaine (MDA), a potent and efficacious monoamine-releasing agent (Johnson et al., 1986). MDA is further metabolized to (±)-3,4-dihydroxyamphetamine (HHA) and (±)-4-hydroxy-3-methoxyamphetamine (HMA). There are notable differences between humans and rats with regard to MDMA metabolism. In particular, the main cytochrome P450 isoform responsible for O-demethylenation in humans is CYP2D6 (Tucker et al., 1994), whereas in rats an analogous but distinct isoform, CYP2D1, is involved (Kumagai et al., 1994). Humans also tend to generate less MDA than do rats (de la Torre and Farré, 2004). Species differences in MDMA metabolism would be predicted to influence the extent of toxicity, because certain metabolites are implicated in causing 5-HT deficits (Monks et al., 2004).
Fig. 1.
MDMA metabolism in humans and rats. Thick arrows represent major pathways of biotransformation, and thin arrows indicate minor pathways. The main cytochrome P450 isoforms responsible for specific reactions in humans (H) and rats (R) are noted. COMT, catechol-O-methyltransferase.
Perhaps the optimal strategy for relating MDMA data from animals to humans is to compare pharmacokinetic parameters across species (de la Torre and Farré, 2004; Green et al., 2009). Clinical investigations have examined plasma concentrations of MDMA and its metabolites after the administration of psychoactive doses (Mas et al., 1999; de la Torre et al., 2000; Kolbrich et al., 2008b), and comparable low-dose experiments have been performed in monkeys (Banks et al., 2007; Mueller et al., 2008). In contrast, few studies in rats have examined MDMA pharmacokinetics after administration of doses less than 10 mg/kg. In one notable exception, Chu et al. (1996) administered various doses of subcutaneous (+)-MDMA (5–40 mg/kg) to rats and discovered that increases in plasma and brain concentrations of (+)-MDMA were greater than the proportional increases in dose administered, suggesting nonlinear pharmacokinetics. The paucity of low-dose pharmacokinetic data from rats is problematic because the majority of MDMA neurotoxicity findings have been obtained using this species. Recent reports have described MDMA metabolism in rats given high-dose treatments that produce 5-HT depletions in brain (Valtier et al., 2007; Goni-Allo et al., 2008; Meyer et al., 2008; Upreti and Eddington, 2008), but no pharmacokinetic data are available for direct comparison to existing human data.
In the present work, the pharmacokinetics and metabolism of MDMA were examined in male rats. Because MDMA evokes pharmacological effects in rats and humans at doses of 1 to 2 mg/kg (reviewed in Baumann et al., 2007), we hypothesized that low-dose MDMA administration to rats would yield pharmacokinetic parameters comparable to those in humans. Our study design had several unique features. First, we used a within-subjects approach in which MDMA was administered to rats bearing indwelling jugular catheters, thereby allowing repeated blood sampling (Baumann et al., 2008). Second, plasma concentrations of MDMA and its metabolites, HMMA, MDA, and HMA, were measured using a sensitive gas chromatography-mass spectrometry (GCMS) method developed for small-volume specimens (Scheidweiler et al., 2008). Third, MDMA pharmacokinetic parameters were compared in groups of rats that received a low pharmacologically active dose (i.e., 2 mg/kg, low dose) or a 5-fold higher amount known to produce 5-HT syndrome (i.e., 10 mg/kg, high dose) (Slikker et al., 1989). Finally, to assess possible effects of route of administration, doses of MDMA were administered via intraperitoneal, subcutaneous, or oral route.
Materials and Methods
Drugs and Reagents.
Racemic MDMA HCl was generously provided by the National Institute on Drug Abuse, Drug Supply Program (Rockville, MD). MDMA solutions for injection were prepared in sterile 0.9% NaCl (saline) immediately before administration. Reagents required for GCMS and high-performance liquid chromatography (HPLC) assays were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Animals and Surgery.
Male Sprague-Dawley rats weighing 320 to 360 g were double-housed (lights on: 7:00 AM–7:00 PM) in conditions of controlled temperature (22 ± 2°C) and humidity (45 ± 5%), with free access to food and water. Experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996). Vivarium facilities were fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and study procedures were approved by the National Institute on Drug Abuse Intramural Research Program, Animal Care and Use Committee. After 2 weeks of acclimation to the vivarium, rats were transported to the surgery room and anesthetized with sodium pentobarbital (60 mg/kg i.p.). Catheters made of Silastic tubing (Dow Corning, Midland, MI) were filled with sterile saline and implanted into the jugular vein as described previously (Baumann et al., 2008). In brief, the proximal end of the catheter was inserted into the right jugular vein and advanced to the atrium, whereas the distal end was exteriorized on the nape and plugged with a metal stylet. Rats were single-housed postoperatively and allowed at least 1 week to recover.
Blood Sampling Procedures.
On the morning of an experiment, rats were moved to the testing room in their home cages and given 1 h to acclimate to the surroundings. Feeding trays were removed, and wire lids were place atop the cages. Polyethylene extension tubes (30 cm) were filled with sterile saline, connected to intravenous catheters, and threaded outside the cages. Catheters were flushed with 0.3 ml of 48 IU/ml heparin saline to facilitate blood withdrawal. Groups of four to five rats received 2 or 10 mg/kg MDMA in a volume of 1 ml/kg by the intraperitoneal, subcutaneous, or oral route of administration. Intraperitoneal injections were given in the lower right quadrant of the abdomen, subcutaneous injections were administered posterior to the shoulder blades on the back, and oral injections were delivered into the gut using an oral dosing needle. Blood specimens (0.25 ml) were withdrawn at 0.8, 0.16, 0.25, 0.5, 1, 1.5, 2.5, 3.5, 5, 7, and 10 h after treatments. Blood was collected into 1-ml disposable tuberculin syringes, transferred to 1.5-ml plastic tubes on ice, and spun for 10 min at 1500 rpm; plasma was decanted and stored at −80°C. An equal volume of sterile saline was infused after each blood withdrawal to maintain volume and osmotic homeostasis. The occurrence of 5-HT behavioral syndrome was evaluated during the first 5 h of blood sampling (Baumann et al., 2008). Specific elements of the syndrome, namely flat body posture and forepaw treading, were used as indicators of syndrome severity. Before each blood withdrawal, rats were observed for 1 min, and the presence of flat body posture and forepaw treading was scored using the graded scale: 0, absent; 1, equivocal; 2, present; and 3, intense. Rats were given a single score for each behavior that consisted of the summed scores over all nine time points.
Analysis of MDMA and Its Metabolites.
MDMA, HMMA, MDA, and HMA were measured concurrently in individual plasma specimens using a GCMS method optimized for small specimen volume (Scheidweiler et al., 2008). HHMA and HHA were not included in our analyses because high-purity reference standards for these metabolites were not available. Eight calibrators were prepared in 100 μl of blank plasma to yield final concentrations of 10 to 2500 ng/ml MDMA, HMMA, MDA, and HMA. In addition, four quality control samples containing 30, 225, 900, and 1800 ng/ml MDMA, HMMA, MDA, and HMA were prepared in blank plasma. Each calibrator, quality control, and plasma specimen was spiked with an internal standard solution to yield a final concentration of 100 ng/ml MDMA-d5 and 50 ng/ml MDA-d5. Plasma specimens were subjected to protein precipitation by addition of 0.5 ml of ice-cold 200 mM trichloroacetic acid and were centrifuged at 3000g for 10 min at 4°C. Supernatants were collected, and protein pellets were resuspended in 0.5 ml of 200 mM trichloroacetic acid and repelleted via centrifugation, and the resulting supernatants were combined with the first fraction. Acid hydrolysis was performed by adding 100 μl of 12 M hydrochloric acid to the supernatants and incubating for 45 min at 100°C; hydrolysis was performed to deconjugate glucuronidated and sulfated metabolites of MDMA. It is noteworthy that these hydrolysis conditions have been demonstrated to achieve 60% efficiency for cleaving conjugated HMMA and HMA in mouse plasma relative to enzyme hydrolysis with β-glucuronidase from Helix pomatia (Scheidweiler et al., 2008). On the other hand, Mueller et al. (2009b) used glucuronidase hydrolysis methods to quantify plasma concentrations of HMMA in rats given 20 mg/kg (±)-MDMA, and the absolute concentrations of HMMA reported by these investigators are similar to those found in our study. Erring on the side of caution, we suggest that plasma concentrations of HMMA and HMA reported in our study could be lower than actual concentrations.
Acidified samples were neutralized by addition of 6 M sodium hydroxide and were diluted with 4 ml of 0.2 M sodium acetate buffer, pH 4.5, before solid-phase extraction using 10 ml/70 mg SPEC MP1 columns (Varian, Inc., Lake Forest, CA). Analytes were eluted from columns using 1.5 ml of ethyl acetate-methanol-ammonium hydroxide (77:20:3, v/v/v). Eluates were completely dried at 37°C under nitrogen after addition of 15 μl of 0.12 M hydrochloric acid in methanol. Derivatization at 60°C for 20 min was performed by using 100 μl of triethylamine (0.05 M) in heptane and 10 μl of heptafluorobutyric acid anhydride. Liquid-liquid extraction was performed by addition of 200 μl of 0.1 M potassium phosphate buffer, pH 7.4, and the organic layer was transferred to autosampler vials after centrifugation for 10 min at 25°C and 3000g.
Analysis was performed on an Agilent 6890 gas chromatograph (Agilent Technologies, Wilmington, DE) with a mass selective detector (Agilent 5975) operated in electron impact mode. The injection port temperature was 250°C. A 1-μl aliquot of sample was injected in pulsed splitless mode with a pulse pressure of 34 kPa (5 psi). The GC capillary column was an Agilent DB-17 ms (30-m length by 0.32 mm i.d.; 0.25 μm film thickness). The GC oven temperature program began at 70°C, was immediately increased by 20°C/min to 150°C (no hold time), 15°C/min to 195°C (no hold time), 5°C/min to 205°C, and finally at 35°C/min to 320°C and held for 2 min. High-purity helium (99.999%) was the carrier gas with a flow rate of 1.5 ml/min operated in constant flow mode. Quadrupole, ion source, and mass selective interface temperatures were 150, 230, and 280°C, respectively. The MS system was operated in the selected ion monitoring mode with the electron multiplier voltage set at the daily tune value. The following ions were monitored (quantification ions in parentheses): 162, 210, and (254) for MDMA-d0; 213 and (258) for MDMA-d5; 135, (162), and 375 for MDA-d0; (167) and 380 for MDA-d5; (254), 333, and 360 for HMMA-d0, and 163, (240), and 360 for HMA-d0. Extraction efficiencies of MDMA, MDA, HMMA, and HMA were greater than 85%. Linear ranges were 10 to 1000 ng/ml for MDA, HMMA, and HMA and 10 to 2500 ng/ml for MDMA. Duplicate quality control samples were prepared and analyzed on 8 different days to characterize assay performance. The lower limit of assay quantification was 10 ng/ml for all analytes. Interassay analytical recoveries (bias) for MDMA, MDA, HMMA, and HMA in plasma were between 95.4 and 108.4% of target concentrations (n = 16). Interassay imprecision ranged from 3.2 to 8.2% coefficient of variation for all four analytes (n = 16).
Analysis of Monoamines in Brain Tissue.
Postmortem brain tissue was obtained from all MDMA-treated rats to assess the possibility of long-term neurotoxic depletions of 5-HT. Two weeks after MDMA treatments, rats were decapitated, and brain tissue was dissected to obtain the frontal cortex and striatum. Brain tissue was also collected from a group of control rats that received 1 ml/kg i.p. saline 2 weeks before decapitation. Tissue specimens were weighed, homogenized in 0.1 N HClO4, and centrifuged at 15,000 rpm for 15 min. Concentrations of 5-HT, dopamine, and their respective metabolites were quantified in supernatants using HPLC with electrochemical detection (Baumann et al., 2008). In brief, 20-μl aliquots of supernatant were injected onto a C18 reverse-phase HPLC column linked to a coulometric detector (Coulochem II; ESA Laboratories, Inc., Bedford, MA). The mobile phase consisting of 50 mM sodium phosphate monobasic, 250 μM Na2EDTA, 0.03% sodium octanesulfonic acid, and 25% methanol (final pH = 2.75) was recirculated at 0.9 ml/min. Data were acquired by a Millennium software system (Waters, Milford, MA), in which peak heights of unknowns were compared with those of standards. The lower limit of assay sensitivity was 10 pg/injection.
Data Analysis and Statistics.
Behavioral, pharmacokinetic, and neurochemical data were analyzed using Prism (version 4.0; GraphPad Software, Inc., San Diego, CA). In particular, behavioral scores and neurochemical measures were subjected to two-factor (dose × route) analysis of variance (ANOVA) followed by Newman-Keuls post hoc tests. Concentration-time curves for plasma MDMA, HMMA, MDA, and HMA at each dose were analyzed by one-factor (route) ANOVA with repeated measures. Plasma pharmacokinetic data were analyzed with WinNonlin (version 5.2; Pharsight, Mountain View, CA) to determine various pharmacokinetic constants including maximum concentration (Cmax), time of maximum concentration (Tmax), area under the curve (AUC), and elimination half-life (t1/2). Noncompartmental modeling was used. At least three data points on the descending linear limb of the concentration-time curve were used to calculate t1/2 values. Pharmacokinetic parameters for each analyte at a given dose of MDMA were subjected to one-way ANOVA (route) followed by Newman-Keuls post hoc tests. In some cases, pharmacokinetic constants could not be calculated because analyte concentrations were below the limits of quantification (i.e., <10 ng/ml). P < 0.05 was considered the minimal criterion for statistical significance.
Results
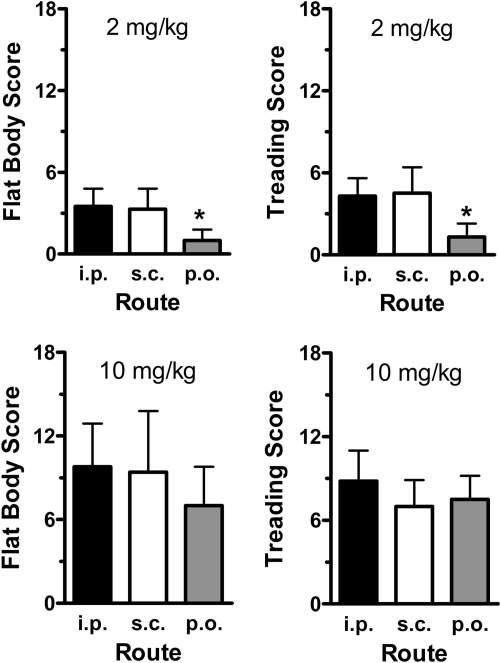
Figure 2 illustrates the effects of 2 and 10 mg/kg MDMA on specific elements of the 5-HT behavioral syndrome. MDMA dose significantly influenced scores for flat body posture [F(1, 20) = 31.58, P < 0.0001] and forepaw treading [F(1, 20) = 40.56, P < 0.0001]. Scores for both behaviors were 2- to 5-fold greater after 10 mg/kg than after 2 mg/kg. At the low dose of MDMA, signs of 5-HT syndrome were minimal, and the oral route of administration resulted in significantly less flat body posture [F(2, 11) = 5.06, P < 0.03] and forepaw treading [F(2, 11) = 6.12, P < 0.02] with respect to the intraperitoneal and subcutaneous routes. After the high MDMA dose, rats displayed a robust 5-HT syndrome, but route of administration did not affect behavioral scores.
Fig. 2.
Effects of 2 and 10 mg/kg MDMA on 5-HT behavioral syndrome in rats. Rats bearing indwelling jugular catheters received MDMA by the intraperitoneal, subcutaneous, or oral route. Subjects were observed for 1 min immediately before the withdrawal of each blood specimen for the first 5 h after treatment (nine time points). Flattened body posture and forepaw treading were scored as described under Materials and Methods, and rats were given a single summed score for each behavior. Data are the mean ± S.D. for n = 4 to 5 rats/group. *, P < 0.05, compared with intraperitoneal and subcutaneous routes at 2 mg/kg MDMA.
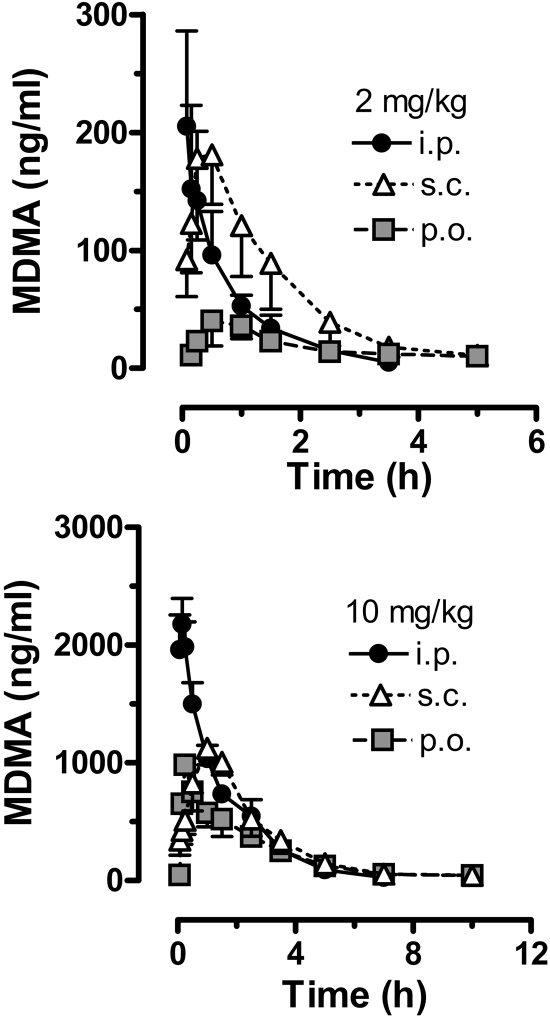
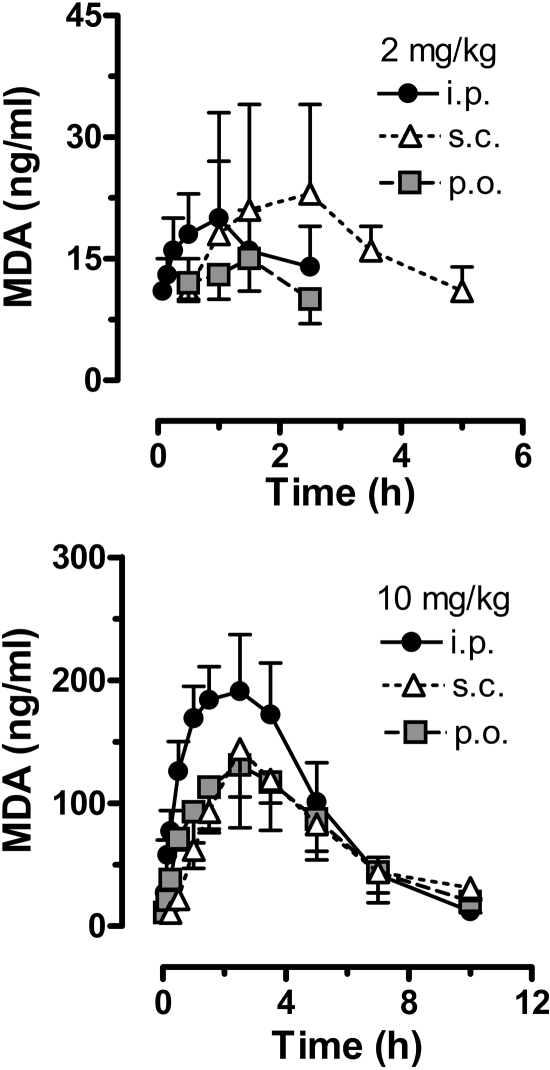
The data in Fig. 3 show the time-concentration profiles for plasma MDMA after administration of 2 and 10 mg/kg MDMA by the intraperitoneal, subcutaneous, and oral routes. Pharmacokinetic constants derived from the MDMA curves are summarized in Table 1. For all dosing conditions, peak MDMA concentrations were achieved rapidly, usually within 1 h, and declined to undetectable levels by 5 to 10 h. At the 2 mg/kg dose, oral administration of MDMA produced significantly lower circulating levels of MDMA over the sampling period, compared with the intraperitoneal and subcutaneous routes [F(2, 24) = 4.26, P < 0.01]. Accordingly, rats treated with 2 mg/kg p.o. had markedly diminished MDMA Cmax [F(2, 11) = 6.89, P < 0.01] and AUC values [F(2, 11) = 19.10, P < 0.001]. Rats treated with 2 mg/kg i.p. displayed a significantly faster Tmax [F(2, 11) = 6.27, P < 0.01], with peak concentrations achieved by 8 min after injection. Route of administration did not alter t1/2 for plasma MDMA in the 2 mg/kg dose condition, with values ranging from 46 to 48 min.
Fig. 3.
Time-concentration profiles for plasma MDMA after administration of 2 and 10 mg/kg MDMA by the intraperitoneal, subcutaneous, or oral route. Rats bearing indwelling jugular catheters received MDMA at time 0, and blood specimens (0.25 ml) were withdrawn via catheters at 0.8, 0.16, 0.25, 0.5, 1, 1.5, 2.5, 3.5, 5, 7, and 10 h after treatment. Plasma was assayed for MDMA by GCMS as described under Materials and Methods. Data are mean ± S.D. for n = 4 to 5 rats/group.
TABLE 1.
Pharmacokinetic constants for plasma MDMA after administration of 2 and 10 mg/kg MDMA to rats
Parameters were calculated from MDMA time-concentration profiles depicted in Fig. 3, with WinNonlin noncompartmental modeling. Data are the mean ± S.D. for n = 4–5 rats/group.
| MDMA Treatment | MDMA Cmax | MDMA Tmax | MDMA AUC | MDMA t1/2 |
|---|---|---|---|---|
| ng/ml | h | h·ng/ml | h | |
| 2 mg/kg i.p. | 210 ± 108 | 0.14 ± 0.08* | 163 ± 56 | 0.80 ± 0.16 |
| 2 mg/kg s.c. | 196 ± 50 | 0.75 ± 0.29 | 304 ± 65 | 0.79 ± 0.14 |
| 2 mg/kg p.o. | 46 ± 15* | 0.56 ± 0.31 | 61 ± 42* | 0.77 ± 0.11 |
| 10 mg/kg i.p. | 2257 ± 131# | 0.13 ± 0.04 | 3432 ± 278 | 1.08 ± 0.14 |
| 10 mg/kg s.c. | 1130 ± 138 | 1.10 ± 0.22# | 3146 ± 514 | 1.27 ± 0.39 |
| 10 mg/kg p.o. | 966 ± 49 | 0.31 ± 0.13 | 2226 ± 301# | 1.62 ± 0.41 |
P < 0.05 compared with other routes at 2 mg/kg MDMA.
P < 0.05 compared with other routes at 10 mg/kg MDMA.
After the 10 mg/kg dose, intraperitoneal administration produced significantly higher circulating levels of MDMA over time, compared with those for the subcutaneous and oral routes [F(2, 31) = 4.15, P < 0.02]. The data in Table 1 show that MDMA Cmax after 10 mg/kg i.p. was greater than Cmax for the oral and subcutaneous routes [F(2, 13) = 169.6, P < 0.0001], whereas AUC for the oral route was significantly less than AUC for other routes [F(2, 13) = 11.53, P < 0.002]. Rats treated with 10 mg/kg s.c. MDMA exhibited a significantly slower Tmax [F(2, 13 = 11.97, P < 0.001], with peak concentrations achieved after more than 1 h. It is noteworthy that MDMA AUC values after 10 mg/kg were greater than expected compared with the 2 mg/kg dose. In theory, AUC should increase 5-fold when the dose is increased from 2 to 10 mg/kg. However, MDMA AUC values for 10 mg/kg were 21-fold (intraperitoneal), 10-fold (subcutaneous), and 36-fold (oral) greater than corresponding values for the 2 mg/kg dose. These markedly elevated AUCs suggest that MDMA displays nonlinear kinetics at high doses in rats, effects observed to some degree after all routes of administration.
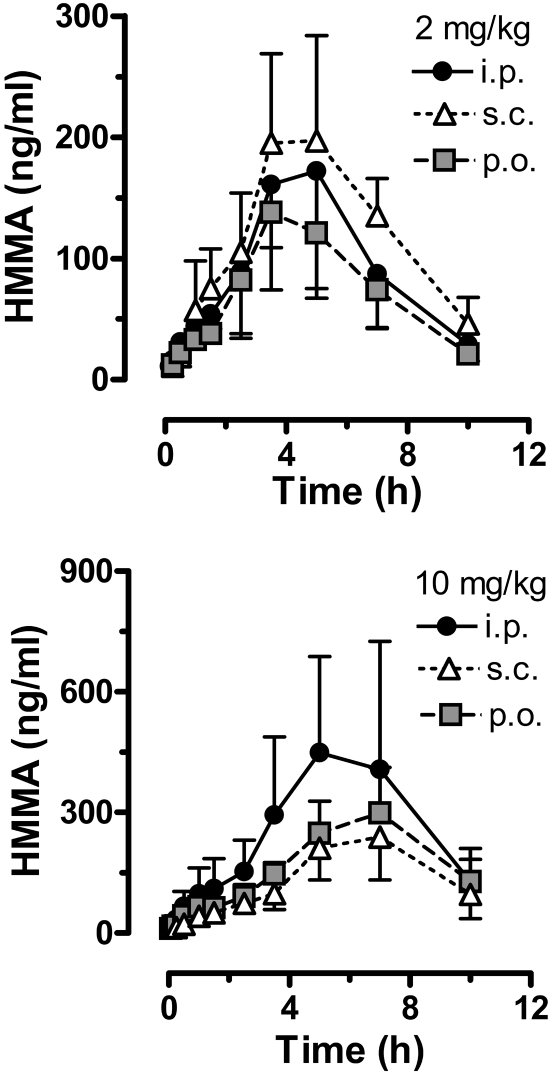
Figure 4 illustrates the time-concentration data for plasma HMMA in rats given 2 and 10 mg/kg MDMA via the intraperitoneal, subcutaneous, and oral routes. HMMA pharmacokinetic constants derived from these curves are summarized in Table 2. For all dosing conditions, plasma HMMA concentrations increased slowly over time, gradually reaching Tmax at 4 to 6 h after injection and declined thereafter. Plasma HMMA levels were still detectable 10 h after both doses of MDMA. After 2 mg/kg MDMA, route of administration did not significantly affect circulating HMMA concentrations, with comparable Cmax, Tmax, AUC, and t1/2 across intraperitoneal, subcutaneous, and oral groups. For the low-dose condition, HMMA AUCs were always greater than MDMA AUCs. After 10 mg/kg MDMA, route of administration did not affect HMMA time-concentration curves or other pharmacokinetic parameters. HMMA AUCs after 10 mg/kg MDMA were much lower than expected. In particular, HMMA AUC values after 10 mg/kg MDMA were only 2.6-fold (intraperitoneal), 1.2-fold (subcutaneous), and 2.5-fold (oral) greater than corresponding AUCs for 2 mg/kg. These data indicate an impaired ability to form HMMA after administration of high-dose MDMA, a finding that could be related to the elevated plasma concentrations of MDMA described above (Fig. 3; Table 1).
Fig. 4.
Time-concentration profiles for plasma HMMA after administration of 2 and 10 mg/kg MDMA by the intraperitoneal, subcutaneous, or oral route. Rats bearing indwelling jugular catheters received MDMA at time 0, and blood specimens (0.25 ml) were withdrawn via catheters at 0.8, 0.16, 0.25, 0.5, 1, 1.5, 2.5, 3.5, 5, 7, and 10 h after treatment. Plasma was assayed for HMMA by GCMS as described under Materials and Methods. Data are the mean ± S.D. for n = 4 to 5 rats/group.
TABLE 2.
Pharmacokinetic constants for plasma HMMA after administration of 2 and 10 mg/kg MDMA to rats
Parameters were calculated from HMMA time-concentration profiles depicted in Fig. 4, with WinNonlin noncompartmental modeling. Data are the mean ± S.D. for n = 4–5 rats/group.
| MDMA Treatment | HMMA Cmax | HMMA Tmax | HMMA AUC | HMMA t1/2 |
|---|---|---|---|---|
| ng/ml | h | h·ng/ml | h | |
| 2 mg/kg i.p. | 186 ± 88 | 4.25 ± 0.87 | 926 ± 417 | 2.25 ± 0.23 |
| 2 mg/kg s.c. | 236 ± 87 | 3.88 ± 0.75 | 1192 ± 251 | 2.66 ± 0.70 |
| 2 mg/kg p.o. | 141 ± 59 | 3.86 ± 0.75 | 702 ± 257 | 2.57 ± 0.88 |
| 10 mg/kg i.p. | 474 ± 268 | 5.50 ± 1.50 | 2478 ± 1433 | 2.73 ± 0.57 |
| 10 mg/kg s.c. | 270 ± 106 | 6.20 ± 1.10 | 1373 ± 375 | 3.42 ± 2.23 |
| 10 mg/kg p.o. | 309 ± 95 | 6.13 ± 1.75 | 1747 ± 467 | 3.26 ± 0.95 |
Data in Fig. 5 depict time-concentration profiles for plasma MDA in rats given 2 and 10 mg/kg MDMA via the intraperitoneal, subcutaneous, and oral routes. MDA pharmacokinetic constants from these curves are summarized in Table 3 depict time-concentration profiles for plasma MDA in rats given 2 and 10 mg/kg MDMA via the intraperitoneal, subcutaneous, and oral routes. MDA pharmacokinetic constants from these curves are summarized in Table 3. In general, MDA concentrations were much lower that those of MDMA and HMMA, suggesting that N-demethylation is a minor pathway of metabolism in rats. After 2 mg/kg MDMA, route of administration did not significantly influence circulating levels of MDA over time, and Cmax, AUC, and t1/2 were similar across groups. MDA t1/2 could not be calculated for the 2 mg/kg p.o. dosing condition because of a lack of specimens with detectable metabolite levels (i.e., <10 ng/ml). MDA Tmax was significantly delayed for the subcutaneous route compared with the intraperitoneal and oral routes [F(2, 10) = 7.44, P < 0.01]. For low-dose MDMA, MDA AUCs were 23% (intraperitoneal), 24% (subcutaneous), and 34% (oral) of AUCs for the parent compound, indicating that ∼20 to 30% of MDMA is metabolized via N-demethylation. After 10 mg/kg MDMA, route of administration had no effect on MDA time-concentration profiles, and pharmacokinetic constants were similar across routes. AUCs for MDA were much greater than expected after high-dose MDMA, specifically, 25-fold (intraperitoneal), 9-fold (subcutaneous), and 32-fold (oral) higher than corresponding 2 mg/kg AUCs.
Fig. 5.
Time-concentration profiles for plasma MDA after administration of 2 and 10 mg/kg MDMA by the intraperitoneal, subcutaneous, or oral route. Rats bearing indwelling jugular catheters received MDMA at time 0, and blood specimens (0.25 ml) were withdrawn via catheters at 0.8, 0.16, 0.25, 0.5, 1, 1.5, 2.5, 3.5, 5, 7, and 10 h after treatment. Plasma was assayed for MDA by GCMS as described under Materials and Methods. Data are the mean ± S.D. for n = 4 to 5 rats/group.
TABLE 3.
Pharmacokinetic constants for plasma MDA after administration of 2 and 10 mg/kg MDMA to rats
Parameters were calculated from MDA time-concentration profiles depicted in Fig. 5, with WinNonlin version 5.2. Data are the mean ± S.D. for n = 4–5 rats/group.
| MDMA Treatment | MDA Cmax | MDA Tmax | MDA AUC | MDA t1/2 |
|---|---|---|---|---|
| ng/ml | h | h·ng/ml | h | |
| 2 mg/kg i.p. | 21 ± 13 | 0.75 ± 0.29 | 38 ± 20 | 2.51 ± 1.01 |
| 2 mg/kg s.c. | 22 ± 12 | 2.00 ± 0.58* | 73 ± 41 | 2.91 ± 1.23 |
| 2 mg/kg p.o. | 13 ± 4 | 1.00 ± 0.54 | 24 ± 11 | N.D. |
| 10 mg/kg i.p. | 201 ± 36 | 2.00 ± 0.71 | 987 ± 218 | 2.18 ± 0.48 |
| 10 mg/kg s.c. | 145 ± 38 | 2.70 ± 0.45 | 685 ± 177 | 2.62 ± 1.17 |
| 10 mg/kg p.o. | 132 ± 50 | 2.25 ± 0.50 | 727 ± 232 | 2.79 ± 1.22 |
N.D., not determined.
P < 0.05 compared with other routes at 2 mg/kg.
Table 4 summarizes HMA pharmacokinetic constants. At both doses of MDMA, HMA concentrations increased slowly over time, reaching maxima at 4 to 6 h and declined thereafter. For all conditions, HMA plasma concentrations were nominal, in agreement with the low MDA concentrations noted above. After 2 mg/kg MDMA, HMA concentrations were near the limit of quantification (i.e., 10 ng/ml), precluding calculation of HMA t1/2. For the low-dose oral condition, HMA was undetectable at all time points after injection. After 10 mg/kg MDMA, HMA concentrations were quantifiable but low. Route of administration did not affect HMA Cmax, Tmax, or AUC after high-dose MDMA. HMA t1/2 could not be determined because less than three specimens with detectable levels were available on the descending limb of the time-concentration curve.
TABLE 4.
Pharmacokinetic constants for plasma HMA after administration of 2 and 10 mg/kg MDMA to rats
Parameters were calculated using WinNonlin version 5.2, and data are the mean ± S.D. for n = 4–5 rats/group. t1/2 values could not be determined.
| MDMA Treatment | HMA Cmax | HMA Tmax | HMA AUC |
|---|---|---|---|
| ng/ml | h | h·ng/ml | |
| 2 mg/kg i.p. | 13 ± 4 | 4.00 ± 0.87 | 67 ± 13 |
| 2 mg/kg s.c. | 15 ± 2 | 4.00 ± 0.88 | 85 ± 12 |
| 2 mg/kg p.o. | N.D. | N.D. | N.D. |
| 10 mg/kg i.p. | 33 ± 12 | 6.60 ± 0.89 | 212 ± 67 |
| 10 mg/kg s.c. | 23 ± 5 | 6.60 ± 1.01 | 146 ± 36 |
| 10 mg/kg p.o. | 25 ± 8 | 6.00 ± 1.15 | 157 ± 57 |
N.D., not determined.
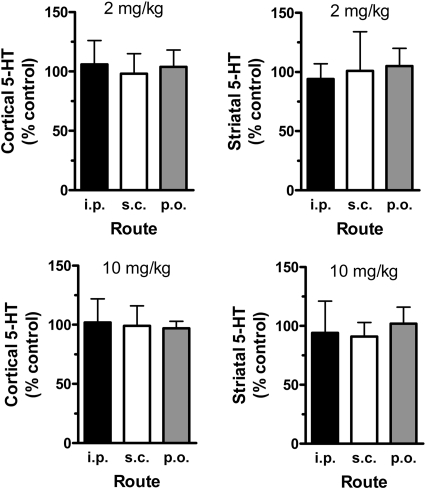
Figure 6 depicts the effects of 2 and 10 mg/kg MDMA on postmortem 5-HT concentrations in the cortex and striatum 2 weeks after drug treatments. It is important to note that neurochemical data from MDMA-treated rats are presented as a percentage of data from saline-treated control rats. MDMA treatments did not cause significant changes in 5-HT levels in either brain region after the 2 or 10 mg/kg dose.
Fig. 6.
Effects of 2 and 10 mg/kg MDMA on postmortem tissue concentrations of 5-HT in rat cortex and striatum. Rats bearing indwelling jugular catheters received MDMA by the intraperitoneal, subcutaneous, or oral route. Subjects were returned to the vivarium after blood sampling procedures, and a group of control rats received 1 ml/kg i.p. saline. Two weeks later, all rats were decapitated, brain regions were dissected, and tissue was frozen until 5-HT assay by HPLC-electrochemical detection as described under Materials and Methods. Data are the mean ± S.D. for n = 4 to 5 rats/group expressed as a percentage of the saline-treated control. Tissue concentrations of 5-HT in control rats were 210 ± 52 and 319 ± 77 pg/mg of tissue for cortex and striatum, respectively.
Discussion
The findings presented here are the first to characterize MDMA pharmacokinetics after low-dose administration in rats. Substantial evidence shows that MDMA evokes pharmacological effects at 1 to 2 mg/kg in rats and humans (Schechter, 1988; Johanson et al., 2006; Baumann et al., 2008; Kolbrich et al., 2008a), yet no pharmacokinetic studies with these doses have been reported in rats. Administration of 2 mg/kg MDMA by intraperitoneal and subcutaneous routes produced an MDMA Cmax of ∼200 ng/ml, a concentration close to the MDMA Cmax in humans who receive 1.3 to 1.7 mg/kg by the oral route under controlled conditions (Mas et al., 1999; de la Torre et al., 2000; Kolbrich et al., 2008b). The similarities between MDMA Cmax in rats and humans may explain why both species discriminate 1.5 mg/kg in laboratory test paradigms (Schechter, 1988; Johanson et al., 2006). Administration of 2 mg/kg MDMA by the oral route produced a lower MDMA Cmax in rats, indicating reduced absorption and/or significant first-pass metabolism in the liver and/or gut. MDMA t1/2 was ∼45 min for rats given 2 mg/kg, and this interval is much shorter than the t1/2 of 7 to 9 h observed in humans (Mas et al., 1999; de la Torre et al., 2000; Kolbrich et al., 2008b). Given the rapid MDMA clearance in rats, repeated intraperitoneal or subcutaneous injections of low-dose MDMA in this species might be an acceptable model for single oral doses in humans.
When the MDMA dose was increased to 10 mg/kg, MDMA Cmax was ∼1000 ng/ml for the subcutaneous and oral routes but >2000 ng/ml for the intraperitoneal route. Our MDMA Cmax data agree with those of others who reported Cmax of 1000 to 2500 ng/ml in rats given 10 to 20 mg/kg by the intraperitoneal, subcutaneous, or oral route (Valtier et al., 2007; Meyer et al., 2008; Upreti and Eddington, 2008). Administration of 10 mg/kg MDMA in our study produced a robust 5-HT syndrome but no long-term 5-HT depletion, suggesting that this dose is not neurotoxic under the conditions used here. MDMA t1/2 was longer than 1 h after administration of 10 mg/kg, compared with 45 min after 2 mg/kg, indicating that drug elimination is prolonged at high MDMA doses. Accordingly, MDMA AUCs after 10 mg/kg were much greater than the predicted 5-fold increase above those after 2 mg/kg. Table 1 reveals that MDMA AUCs after 10 mg/kg were 10- to 36-fold higher than those after 2 mg/kg. Our AUC data are consistent with nonlinear kinetics of MDMA, a phenomenon demonstrated in monkeys and humans (de la Torre et al., 2000; Kolbrich et al., 2008b; Mueller et al., 2008). Chu et al. (1996) provided evidence for nonlinear pharmacokinetics after administration of (+)-MDMA to rats, but no rat studies have addressed this issue using racemic MDMA. In fact, Green et al. (2009) suggested recently that MDMA may not exhibit nonlinear kinetics in the rat, based on a comparison of published pharmacokinetic data from high-dose treatments in rats (5–20 mg/kg) versus low-dose treatments in humans (0.5–2 mg/kg). The present data, showing side-by-side comparison of MDMA pharmacokinetics after 2 and 10 mg/kg, provide definitive evidence for nonlinear MDMA kinetics in rats and indicate the generality of this effect across species (Mueller et al., 2008, 2009a).
Previous findings showed that HHMA and HMMA are major MDMA metabolites in rats and humans (Lim et al., 1992; Segura et al., 2001; de la Torre et al., 2004; Valtier et al., 2007; Goni-Allo et al., 2008; Kolbrich et al., 2008b). HHMA was not assayed in our study because of the lack of commercially available standards and unsuitability of measurement by GCMS, but HMMA was readily quantified. After 2 mg/kg MDMA, HMMA Cmax ranged from 141 to 236 ng/ml, comparable to HMMA Cmax in humans given recreational doses of MDMA under controlled conditions (de la Torre et al., 2000; Segura et al., 2001; Kolbrich et al., 2008b). It must be reiterated that the HMMA concentrations reported here may be less than actual amounts because of incomplete acid hydrolysis of conjugated metabolites (Scheidweiler et al., 2008). Nonetheless, HMMA AUC was always greater than MDMA AUC in rats given 2 mg/kg MDMA, confirming that HMMA is a principal metabolite in the rat (Goni-Allo et al., 2008; Mueller et al., 2009b). HMMA Tmax was ∼4 h for the low-dose MDMA condition. The delayed appearance of HMMA in rats differs from that in humans, in whom MDMA and HMMA achieve Cmax concurrently and follow the same time course of elimination (de la Torre et al., 2000; Segura et al., 2001; Kolbrich et al., 2008b). The slow kinetics of HMMA in rats suggests that this species might be especially vulnerable to toxic effects of hydroxylated metabolites, and further studies are warranted to address this hypothesis.
Increasing the MDMA dose to 10 mg/kg in rats did not cause a corresponding rise in HMMA Cmax or AUC. As shown in Table 2, HMMA AUCs after 10 mg/kg MDMA were only 1.5- to 2.6-fold higher than those after 2 mg/kg. The unexpectedly low HMMA concentrations are consistent with saturable kinetics and possible enzyme inhibition. Incomplete acid hydrolysis of conjugated HMMA may complicate interpretation of our HMMA results; however, incomplete hydrolysis alone is not likely to account for the large discrepancy between observed and predicted HMMA AUCs. Furthermore, the HMMA concentrations that we report after 10 mg/kg MDMA are similar to those reported by Mueller et al. (2009b) who used enzymatic hydrolysis methods to deconjugate plasma HMMA in rats that were treated with 20 mg/kg (±)-MDMA. Figure 1 shows that biotransformation of MDMA to HMMA in the rat involves two steps: 1) O-demethylenation of MDMA by CYP2D1 to form HHMA and 2) O-methylation of HHMA to form HMMA (Maurer et al., 2000; de la Torre and Farré, 2004). The factors responsible for saturable metabolism of MDMA in rats have not been determined, but clinical data may provide clues. In humans, nonlinear elevations in MDMA are accompanied by impaired ability to form HHMA and its downstream catechol-O-methyltransferase product HMMA (de la Torre et al., 2000, 2004) secondary to inhibition of CYP2D6 (Wu et al., 1997). MDMA interacts with CYP2D6 to form an enzyme-metabolite complex, which irreversibly blocks further catalytic activity. Whether an analogous inhibition of rat CYP2D1 occurs in vivo is not clear, but MDMA and MDA can form inhibitory complexes with certain rat cytochrome P450 isoforms in vitro (Brady et al., 1986; Delaforge et al., 1999; de la Torre and Farré, 2004). Future investigations to quantify plasma MDMA, HHMA, and HMMA concentrations in rats may help resolve this question.
It is well established that MDA is an important bioactive metabolite of MDMA in rats and humans (Maurer et al., 2000; de la Torre et al., 2004). In addition to being a potent 5-HT releaser (Johnson et al., 1986), MDA is an efficacious agonist at 5-HT2B receptors (Setola et al., 2003), potentially contributing to dangerous cardiac side effects (Droogmans et al., 2007). After 2 mg/kg MDMA, MDA Cmax ranged from 13 to 21 ng/ml, a somewhat higher concentration than that reported for humans given 1.3 to 1.7 mg/kg MDMA (Mas et al., 1999; de la Torre et al., 2000; Kolbrich et al., 2008b). Based on a comparison of low-dose AUC data shown in Tables 1 and 3, approximately 23 to 34% of administered MDMA is N-demethylated to form MDA, confirming that MDA is a minor metabolite in rats (Valtier et al., 2007; Meyer et al., 2008; Upreti and Eddington, 2008). On the other hand, humans typically convert less than 10% of MDMA to MDA, demonstrating that rats produce more MDA than observed in humans (de la Torre and Farré, 2004). When the dose of MDMA was increased to 10 mg/kg, MDA AUC values rose 9- to 32-fold higher than those at 2 mg/kg, consistent with nonlinear kinetics. The failure of plasma HMA concentrations to increase after high-dose MDMA suggests that enzyme inhibition may underlie the nonlinear kinetics of MDA, but more studies are required to characterize this phenomenon.
In summary, the present results reveal a number of new and important findings with regard to MDMA pharmacokinetics in rats. First, when rats receive 2 mg/kg MDMA by the intraperitoneal or subcutaneous route, MDMA and HMMA Cmax are comparable to those in humans given recreational doses of the drug. Our Cmax data may explain why rats and humans discriminate the same dose of MDMA and argue against the arbitrary use of interspecies scaling to adjust MDMA doses across species (de la Torre and Farré, 2004; Baumann et al., 2007). Second, we provide further support for nonlinear kinetics of MDMA in the rat, as first reported by Chu et al. (1996). Our AUC data clearly show that high-dose MDMA causes greater-than-expected elevations in plasma MDMA, in conjunction with impaired ability to form HMMA. Thus, nonlinear MDMA kinetics is a cross-species phenomenon observed in rats, monkeys, and humans (Chu et al., 1996; de la Torre et al., 2000; Mueller et al., 2008, 2009a). Finally, given the similarities between MDMA kinetics in rats and humans, we suggest that MDMA data obtained from rats can be clinically relevant if the appropriate dosing conditions are used.
This work was supported by the Intramural Research Program of the National Institutes of Health National Institute of Drug Abuse.
A portion of the data shown herein was previously presented as follows: Baumann MH, Zolkowska D, Kim I, Scheidweiler KA, Rothman RB, and Huestis MA (2009) Nonlinear kinetics of MDMA in rats. Annual Meeting of the College on Problems of Drug Dependence; 2009 Jun 20–25; Reno, NV. College on Problems of Drug Dependence, Philadelphia, PA.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.109.028506
- MDMA
- (±)-3,4-methylenedioxymethamphetamine
- 5-HT
- serotonin
- HHMA
- (±)-3,4-dihydroxymethamphetamine
- HMMA
- (±)-4-hydroxy-3-methoxymethamphetamine
- MDA
- (±)-3,4-methylenedioxyamphetamine
- HHA
- (±)-3,4-dihydroxyamphetamine
- HMA
- (±)-4-hydroxy-3-methoxyamphetamine
- GC
- gas chromatography
- MS
- mass spectrometry
- HPLC
- high-performance liquid chromatography
- ANOVA
- analysis of variance
- Cmax
- maximum concentration
- AUC
- area under the curve
- Tmax
- time of maximum concentration
- t1/2
- elimination half-life.
References
- Banks ML, Sprague JE, Kisor DF, Czoty PW, Nichols DE, Nader MA. (2007) Ambient temperature effects on 3,4-methylenedioxymethamphetamine-induced thermodysregulation and pharmacokinetics in male monkeys. Drug Metab Dispos 35: 1840–1845 [DOI] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Franken FH, Rutter JJ, Rothman RB. (2008) Tolerance to 3,4-methylenedioxymethamphetamine in rats exposed to single high-dose binges. Neuroscience 152: 773–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Wang X, Rothman RB. (2007) 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology (Berl) 189: 407–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JF, Di Stefano EW, Cho AK. (1986) Spectral and inhibitory interactions of (±)-3,4-methylenedioxyamphetamine (MDA) and (±)-3,4-methylenedioxymethamphetamine (MDMA) with rat hepatic microsomes. Life Sci 39: 1457–1464 [DOI] [PubMed] [Google Scholar]
- Chu T, Kumagai Y, DiStefano EW, Cho AK. (1996) Disposition of methylenedioxymethamphetamine and three metabolites in the brains of different rat strains and their possible roles in acute serotonin depletion. Biochem Pharmacol 51: 789–796 [DOI] [PubMed] [Google Scholar]
- Cole JC, Bailey M, Sumnall HR, Wagstaff GF, King LA. (2002) The content of ecstasy tablets: implications for the study of their long-term effects. Addiction 97: 1531–1536 [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farré M. (2004) Neurotoxicity of MDMA (ecstasy): the limitations of scaling from animals to humans. Trends Pharmacol Sci 25: 505–508 [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farré M, Ortuño J, Mas M, Brenneisen R, Roset PN, Segura J, Camí J. (2000) Non-linear pharmacokinetics of MDMA (‘ecstasy’) in humans. Br J Clin Pharmacol 49: 104–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre R, Farré M, Roset PN, Pizarro N, Abanades S, Segura M, Segura J, Camí J. (2004) Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit 26: 137–144 [DOI] [PubMed] [Google Scholar]
- Delaforge M, Jaouen M, Bouille G. (1999) Inhibitory metabolite complex formation of methylenedioxymethamphetamine with rat and human cytochrome P450: particular involvement of CYP 2D. Environ Toxicol Pharmacol 7: 153–158 [DOI] [PubMed] [Google Scholar]
- Droogmans S, Cosyns B, D'haenen H, Creeten E, Weytjens C, Franken PR, Scott B, Schoors D, Kemdem A, Close L, et al. ( 2007) Possible association between 3,4-methylenedioxymethamphetamine abuse and valvular heart disease. Am J Cardiol 100: 1442–1445 [DOI] [PubMed] [Google Scholar]
- Goni-Allo B, O Mathúna B, Segura M, Puerta E, Lasheras B, de la Torre R, Aguirre N. (2008) The relationship between core body temperature and 3,4-methylenedioxymethamphetamine metabolism in rats: implications for neurotoxicity. Psychopharmacology (Berl) 197: 263–278 [DOI] [PubMed] [Google Scholar]
- Green AR, Gabrielsson J, Marsden CA, Fone KC. (2009) MDMA: On the translation from rodent to human dosing. Psychopharmacology (Berl) 204: 375–378 [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. (2003) The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol Rev 55: 463–508 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Johanson CE, Kilbey M, Gatchalian K, Tancer M. (2006) Discriminative stimulus effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans trained to discriminate among d-amphetamine, meta-chlorophenylpiperazine and placebo. Drug Alcohol Depend 81: 27–36 [DOI] [PubMed] [Google Scholar]
- Johnson MP, Conarty PF, Nichols DE. (1991) [3H]Monoamine releasing and uptake inhibition properties of 3,4-methylenedioxymethamphetamine and p-chloroamphetamine analogues. Eur J Pharmacol 200: 9–16 [DOI] [PubMed] [Google Scholar]
- Johnson MP, Hoffman AJ, Nichols DE. (1986) Effects of the enantiomers of MDA, MDMA and related analogues on [3H]serotonin and [3H]dopamine release from superfused rat brain slices. Eur J Pharmacol 132: 269–276 [DOI] [PubMed] [Google Scholar]
- Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. (2008a) Physiological and subjective responses to controlled oral 3,4-methylenedioxymethamphetamine administration. J Clin Psychopharmacol 28: 432–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. (2008b) Plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine after controlled oral administration to young adults. Ther Drug Monit 30: 320–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai Y, Lin LY, Hiratsuka A, Narimatsu S, Suzuki T, Yamada H, Oguri K, Yoshimura H, Cho AK. (1994) Participation of cytochrome P450–2B and -2D isozymes in the demethylenation of methylenedioxymethamphetamine enantiomers by rats. Mol Pharmacol 45: 359–365 [PubMed] [Google Scholar]
- Lim HK, Zeng S, Chei DM, Foltz RL. (1992) Comparative investigation of disposition of 3,4-(methylenedioxy)methamphetamine (MDMA) in the rat and the mouse by a capillary gas chromatography-mass spectrometry assay based on perfluorotributylamine-enhanced ammonia positive ion chemical ionization. J Pharm Biomed Anal 10: 657–665 [DOI] [PubMed] [Google Scholar]
- Mas M, Farré M, de la Torre R, Roset PN, Ortuño J, Segura J, Camí J. (1999) Cardiovascular and neuroendocrine effects and pharmacokinetics of 3,4-methylenedioxymethamphetamine in humans. J Pharmacol Exp Ther 290: 136–145 [PubMed] [Google Scholar]
- Maurer HH, Bickeboeller-Friedrich J, Kraemer T, Peters FT. (2000) Toxicokinetics and analytical toxicology of amphetamine-derived designer drugs (‘Ecstasy’). Toxicol Lett 112–113: 133–142 [DOI] [PubMed] [Google Scholar]
- Meyer JS, Piper BJ, Vancollie VE. (2008) Development and characterization of a novel animal model of intermittent MDMA (“Ecstasy”) exposure during adolescence. Ann N Y Acad Sci 1139: 151–163 [DOI] [PubMed] [Google Scholar]
- Monks TJ, Jones DC, Bai F, Lau SS. (2004) The role of metabolism in 3,4-(+)-methylenedioxyamphetamine and 3,4-(+)-methylenedioxymethamphetamine (ecstasy) toxicity. Ther Drug Monit 26: 132–136 [DOI] [PubMed] [Google Scholar]
- Mueller M, Kolbrich EA, Peters FT, Maurer HH, McCann UD, Huestis MA, Ricaurte GA. (2009a) Direct comparison of (±) 3,4-methylenedioxymethamphetamine (“ecstasy”) disposition and metabolism in squirrel monkeys and humans. Ther Drug Monit 31: 367–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M, Peters FT, Maurer HH, McCann UD, Ricaurte GA. (2008) Nonlinear pharmacokinetics of (±)3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) and its major metabolites in squirrel monkeys at plasma concentrations of MDMA that develop after typical psychoactive doses. J Pharmacol Exp Ther 327: 38–44 [DOI] [PubMed] [Google Scholar]
- Mueller MA, Yuan J, Neudorffer A, Peters F, Maurer H, McCann U, Felim AF, Largeron M, Ricaurte GA. (2009b) Further studies on the role of metabolites in MDMA-induced serotonergic neurotoxicity. Drug Metab Dispos doi: 10.1124/dmd.109.028340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC. (2005) Chronic tolerance to recreational MDMA (3,4-methylenedioxymethamphetamine) or Ecstasy. J Psychopharmacol 19: 71–83 [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Yuan J, McCann UD. (2000) (±)3,4-Methylenedioxymethamphetamine (‘Ecstasy’)-induced serotonin neurotoxicity: studies in animals. Neuropsychobiology 42: 5–10 [DOI] [PubMed] [Google Scholar]
- Schechter MD. (1988) Serotonergic-dopaminergic mediation of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol Biochem Behav 31: 817–824 [DOI] [PubMed] [Google Scholar]
- Scheidweiler KB, Barnes AJ, Huestis MA. (2008) A validated gas chromatographic-electron impact ionization mass spectrometric method for methamphetamine, methylenedioxymethamphetamine (MDMA), and metabolites in mouse plasma and brain. J Chromatogr B Analyt Technol Biomed Life Sci 876: 266–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura M, Ortuño J, Farré M, McLure JA, Pujadas M, Pizarro N, Llebaria A, Joglar J, Roset PN, Segura J, et al. ( 2001) 3,4-Dihydroxymethamphetamine (HHMA). A major in vivo 3,4-methylenedioxymethamphetamine (MDMA) metabolite in humans. Chem Res Toxicol 14: 1203–1208 [DOI] [PubMed] [Google Scholar]
- Setola V, Hufeisen SJ, Grande-Allen KJ, Vesely I, Glennon RA, Blough B, Rothman RB, Roth BL. (2003) 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol Pharmacol 63: 1223–1229 [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr, Holson RR, Ali SF, Kolta MG, Paule MG, Scallet AC, McMillan DE, Bailey JR, Hong JS, Scalzo FM. (1989) Behavioral and neurochemical effects of orally administered MDMA in the rodent and nonhuman primate. Neurotoxicology 10: 529–542 [PubMed] [Google Scholar]
- Tucker GT, Lennard MS, Ellis SW, Woods HF, Cho AK, Lin LY, Hiratsuka A, Schmitz DA, Chu TY. (1994) The demethylenation of methylenedioxymethamphetamine (“ecstasy”) by debrisoquine hydroxylase (CYP2D6). Biochem Pharmacol 47: 1151–1156 [DOI] [PubMed] [Google Scholar]
- Upreti VV, Eddington ND. (2008) Fluoxetine pretreatment effects pharmacokinetics of 3,4-methylenedioxymethamphetamine (MDMA, ECSTASY) in rat. J Pharm Sci 97: 1593–1605 [DOI] [PubMed] [Google Scholar]
- Valtier S, Phelix CF, Cody JT. (2007) Analysis of MDMA and its metabolites in urine and plasma following a neurotoxic dose of MDMA. J Anal Toxicol 31: 138–143 [DOI] [PubMed] [Google Scholar]
- Verrico CD, Miller GM, Madras BK. (2007) MDMA (Ecstasy) and human dopamine, norepinephrine, and serotonin transporters: implications for MDMA-induced neurotoxicity and treatment. Psychopharmacology (Berl) 189: 489–503 [DOI] [PubMed] [Google Scholar]
- Wu D, Otton SV, Inaba T, Kalow W, Sellers EM. (1997) Interactions of amphetamine analogs with human liver CYP2D6. Biochem Pharmacol 53: 1605–1612 [DOI] [PubMed] [Google Scholar]