Abstract
Thyrotropin (TSH) regulates thyroid cell proliferation and function through cAMP-mediated signaling pathways that activate protein kinase A (PKA) and Epac/Rap1. The respective roles of PKA versus Epac/Rap1 in TSH signaling remain unclear. We set out to determine whether PKA and/or Rap1 mediate extracellular signal-regulated kinase (ERK) activation by TSH. Neither blocking Rap1 activity nor silencing the expression of Rap1 impaired TSH or forskolin-induced ERK activation in Wistar rat thyroid cells. Direct activation of Epac1 failed to stimulate ERK activity in starved cells, suggesting that Epac-induced Rap1 activity is not coupled to ERK activation in rat thyroid cells. By contrast, PKA activity was required for cAMP-stimulated ERK phosphorylation and was sufficient to increase ERK phosphorylation in starved cells. Expression of dominant-negative Ras inhibited ERK activation by TSH, forskolin, and N6-monobutyryl (6MB)-cAMP, a selective activator of PKA. Silencing the expression of B-Raf also inhibited ERK activation by TSH, forskolin, and 6MB-cAMP, but not that stimulated by insulin or serum. Depletion of B-Raf impaired TSH-induced DNA synthesis, indicating a functional role for B-Raf in TSH-regulated proliferation. Collectively, these results position PKA, Ras, and B-Raf as upstream regulators of ERK activation and identify B-Raf as a selective target of cAMP-elevating agents in thyroid cells. These data provide the first evidence for a functional role for B-Raf in TSH signaling.
Although the TSH receptor couples to multiple G proteins (Laugwitz et al., 1996), most of the effects of TSH are mediated through Gsα and cAMP (Medina and Santisteban, 2000; Kimura et al., 2001). cAMP activates multiple downstream targets, including PKA, guanine nucleotide exchange factors for Rap (de Rooij et al., 1998; Kawasaki et al., 1998) and Ras (Pham et al., 2000; Pak et al., 2002), and certain ion channels. The respective contributions of PKA, Rap1, and Ras to the effects of TSH are unclear. There is general agreement that TSH activates endogenous Rap1 via a PKA-independent mechanism (Dremier et al., 2000; Iacovelli et al., 2001; Tsygankova et al., 2001). Rap1 activation is presumably mediated by Epac1, which is highly expressed in thyroid cells (Iacovelli et al., 2001; Mei et al., 2002; Dremier et al., 2007; Hochbaum et al., 2008). However, the functional significance of Rap1 activation by TSH is controversial. Activation and phosphorylation of Rap1 are required for TSH-stimulated DNA synthesis in rat thyroid PCCL3 cells (Ribeiro-Neto et al., 2002). Roles for both Epac and PKA in TSH-induced DNA synthesis in these cells were reported (Hochbaum et al., 2008). In contrast, studies in canine thyroid cells showed that PKA, and not Epac/Rap1, mediates the effects of TSH, including those on cell proliferation (Dremier et al., 2007).
In cells in which cAMP stimulates ERK activity, Rap1 has been shown to induce B-Raf-dependent activation of ERK (Stork and Schmitt, 2002). Because B-Raf is highly expressed in thyroid cells (Mitsutake et al., 2005), we explored whether Rap1 mediates the effects of TSH on ERK activity in Wistar rat thyroid (WRT) cells. Our studies identified PKA as the primary mediator of cAMP-stimulated ERK activity. They further revealed that Ras and B-Raf function downstream from PKA in the regulation of ERK activity and cell proliferation. These findings identify Ras and B-Raf as important components of TSH-mediated signaling pathways that converge on ERK activation and cell proliferation.
Materials and Methods
Reagents.
Phospho-ERK (Thr202/Tyr204), phospho-MEK1/2 (Ser221), phospho-(serine/threonine) PKA substrate, DYKDDDDK FLAG epitope, Akt and MEK1 antibodies were from Cell Signaling Technology (Danvers, MA). ERK2, B-Raf, Rap1, and Rap1GAP antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Rac1 antibody was from Millipore (Billerica, MA). HA antibody was kindly provided by Dr. Jeffrey Field (Department of Pharmacology, University of Pennsylvania). Sheep anti-BrdU antibody was from BioDesign International (Carmel, NY). Alexa Fluor 488 donkey anti-sheep IgG was from Invitrogen (Carlsbad, CA). Crude bovine TSH and forskolin were from Sigma (St. Louis, MO). Glutathione Sepharose beads were from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK). 8-(4-Chlorophenylthio)-2′-O-methyl-cAMP (EcAMP) and N6-monobutyryl-cAMP (6MB-cAMP) were from Axxora LLC (San Diego, CA). U0126 was purchased from Promega (Madison, WI).
Cell Culture.
WRT, PCCL3, and FRTL5 rat thyroid cells were cultured in Coon's modified Ham's F-12 medium supplemented with calf serum (5%), insulin (10 μg/ml), TSH (1 mU/ml), and transferrin (5 μg/ml), referred to as 3H growth medium. Cells were starved in basal medium (Coon's modified Ham's F-12 medium devoid of growth factors and serum) for 48 h before stimulation with TSH (1 mU/ml), forskolin (10 μM), insulin (10 μg/ml), EcAMP (100 μM), or 6MB-cAMP (1 mM). Starved cells were pretreated with U0126 (10 μM) for 1 h before stimulation.
Viral Infection.
Rap1GAP adenovirus was constructed as described previously (Tsygankova et al., 2007). The PKI adenovirus was a kind gift from Dr. Mark A. Giembycz (Department of Pharmacology and Therapeutics, University of Calgary, Calgary, AB, Canada). Cells were infected overnight in basal medium, transferred to growth medium for 6 h, and starved in basal medium overnight. On day 2 after infection, cells were stimulated, and total cell lysates were prepared for Western blotting.
Rap Activation Assay.
Rap1 activation was assessed as described previously (Tsygankova et al., 2007), except that lysates were collected in Triton-based lysis buffer (20 mM Tris-HCl, 100 mM NaCl, 10 mM MgCl2, 1.0% Triton-X 100, 100 μM Pefabloc, 1 μg/ml pepstatin, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 200 μg/ml Na3VO4).
Transient Transfection of siRNA.
siRNA duplexes were introduced into cells using the Amaxa Nucleofector (Amaxa Biosystems, Gaithersburg, MD) as described previously (Tsygankova et al., 2007). Cells (1.5 × 106) were transfected with siRNA duplexes (200–1000 nM) in suspension, plated overnight in growth medium, transferred to basal medium for 24 h, and subsequently stimulated. B-Raf siRNAs were from Invitrogen. Scrambled siRNAs, Rap1 siRNAs, and a second set of B-Raf siRNAs were from QIAGEN (Valencia, CA).
Immunoprecipitation.
The Flag-RasN17 plasmid was generously provided by Dr. Phillip Stork (Vollum Institute, Oregon Health and Sciences University, Portland, OR). The HA-ERK1 plasmid was a kind gift from Dr. Margaret Chou (Department of Pathology and Lab Medicine, Children's Hospital of Pennsylvania, Philadelphia, PA). Plasmids (5 μg) were cotransfected using the Amaxa Nucleofector. Cells were starved for 24 h before treatment. At 48 h after transfection, cells were stimulated and then lysed in 20 mM Tris, pH 7.8, 100 mM NaCl, 0.5% Triton, 80 mM β-glycerophosphate, 20 mM NaF, 2 mM EDTA, 100 μM Pefabloc, 1 μg/ml pepstatin, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 200 μg/ml Na3VO4. Lysates were clarified by centrifugation, and the supernatant was precleared with protein G agarose beads (Invitrogen). Proteins (200 μg) were precipitated with HA antibody for 2 h at 4°C, protein G agarose (100 μl) were added for 1 h, and beads were collected by centrifugation. After washing three times in phosphate-buffered saline, 2× Laemmli buffer was added, and the samples were boiled for 5 min and subjected to Western blotting.
Western Blotting.
Western blotting was performed as described previously (Tsygankova et al., 2007). Proteins were visualized via chemiluminescence using the FUJI-LAS 3000 system and Multi Gauge version 3.0 software (Fuji, Tokyo, Japan).
DNA synthesis.
After transfection with siRNAs and plating overnight, cells were starved for 24 h in basal medium supplemented with calf serum (0.2%), stimulated with TSH (1 mU/ml) or insulin (10 μg/ml) for 28 h, and BrdU was added for the final 4 h (24–28 h). In parallel, starved cells were stimulated with 3H growth medium for 20 h and labeled with BrdU for 4 h (16–20 h). Cells were fixed in 3.7% formaldehyde/phosphate-buffered saline and stained with sheep anti-BrdU, Alexa Fluor 488 anti-sheep IgG, and 4′,6-diamidino-2-phenylindole (to stain nuclei). At least six fields (>200 cells) were scored in a blinded fashion. Parallel dishes of cells were harvested and analyzed by Western blotting to confirm silencing in each experiment.
Statistics.
All experiments were performed at least three times with similar results unless otherwise indicated. Statistical significance was determined using the Student's t test.
Results
TSH Stimulates MEK1 and ERK Activity through cAMP.
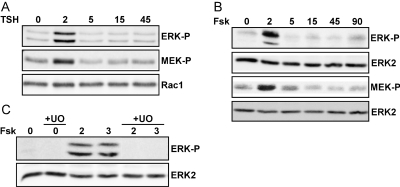
TSH stimulates rapid and transient activating phosphorylation of ERK in rat thyroid cells (Iacovelli et al., 2001). In WRT cells, ERK phosphorylation was stimulated maximally at 2 to 3 min and decreased to near basal levels by 15 min after TSH addition (Fig. 1A). Forskolin elicited similar effects, demonstrating that increased cAMP is sufficient to activate ERK (Fig. 1B). The effects of TSH/cAMP on MEK1 activity have not been reported. TSH and forskolin induced activating phosphorylation of MEK1 with a time course similar to that of ERK phosphorylation. Pretreatment with the MEK inhibitor UO126 blocked ERK activation by cAMP elevating agents (Fig. 1C). These data indicate that the transient activation of ERK by TSH and cAMP is a result of stimulatory effects on MEK1 and focused our analysis on upstream regulators of MEK1 activity.
Fig. 1.
TSH stimulates cAMP-dependent MEK and ERK activation. Starved WRT cells were stimulated with TSH (A) or forskolin (fsk) (B) for the times indicated (in minutes). C, cells were pretreated with U0126 (10 μM) for 30 min before stimulation. Total cell lysates were analyzed for ERK and MEK activation by Western blotting for phospho-ERK (ERK-P) and phospho-MEK1/2 (MEK-P). Equal protein loading was confirmed by Western blotting for Rac1 or ERK2.
Rap1 Is Not Required for cAMP-Stimulated ERK Activity.
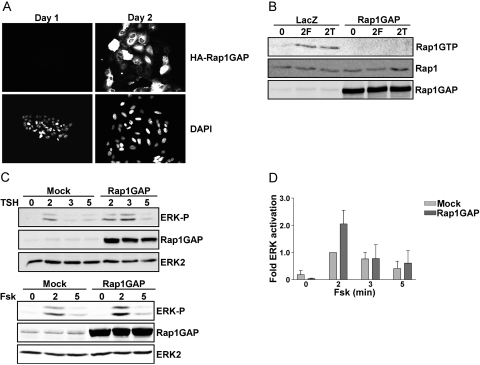
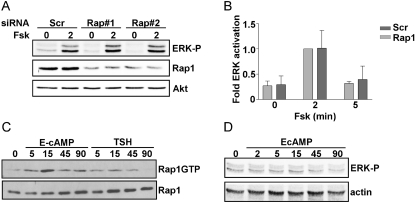
TSH and forskolin activate endogenous Rap1 in canine and rat thyroid cells (Dremier et al., 2000; Iacovelli et al., 2001; Tsygankova et al., 2001). To assess whether Rap mediates cAMP-stimulated ERK activity, Rap activity was inhibited by overexpressing Rap1GAP, a negative regulator of Rap activity. Because the transfection efficiency of rat thyroid cells in transient assays is very low, an adenovirus was used to drive efficient expression of Rap1GAP. Preliminary studies were conducted to determine the lowest dose of virus sufficient to block TSH- and forskolin-stimulated Rap1 activity. When infected at 500 particles/cell, Rap1GAP was expressed in virtually all cells (Fig. 2A) and completely blocked Rap1 activity stimulated by TSH and forskolin (Fig. 2B). Under these conditions, Rap1GAP failed to impair ERK activation by TSH or forskolin (Fig. 2, C and D). This result was unexpected based on an earlier report that cAMP stimulates Rap1-dependent ERK activation in FRTL-5 rat thyroid cells (Iacovelli et al., 2001). Hence, to further explore the contribution of Rap1 to cAMP-stimulated ERK activation, the effects of silencing Rap1 expression were investigated. WRT cells were transfected with two sets of Rap1-directed siRNAs that target different regions of the Rap1 message or scrambled siRNAs as a control. Although the expression of Rap1 was markedly reduced in cells transfected with Rap1-directed siRNAs, ERK activation was not affected (Fig. 3, A and B). To assess whether direct activation of Rap by Epac was sufficient to stimulate ERK activity, starved cells were stimulated with the Epac agonist EcAMP (Enserink, 2002). Similar to the effects of TSH, EcAMP stimulated Rap1 activity (Fig. 3C). Nonetheless, EcAMP failed to stimulate ERK phosphorylation (Fig. 3D). Collectively, these data exclude a major role for Rap1 in the regulation of ERK activity by cAMP in WRT cells.
Fig. 2.
cAMP stimulates Rap-independent ERK activation. A, WRT cells infected with Rap1GAP adenovirus (AdRap1GAP, 500 particles/cell) were fixed and stained for HA-Rap1GAP at days 1 and 2 after infection. Nuclei were stained with 4′,6-diamidino-2-phenylindole. B, cells infected with AdRap1GAP were starved for 24 h, stimulated with forskolin (F) or TSH (T) for 2 min (on day 2 after infection), subjected to RalGDS-RBD pull-down assay, and subsequently blotted for activated Rap1 (Rap1GTP). Total cell lysates were subjected to Western blotting for Rap1 and Rap1GAP. C, cells infected with AdRap1GAP were starved and stimulated with TSH (top) or forskolin (bottom), and total cell lysates were analyzed for ERK phosphorylation and Rap1GAP expression. Equal protein loading was confirmed by Western blotting for ERK2. D, the results from three experiments are summarized.
Fig. 3.
Rap1 is not coupled to ERK activation. A, WRT cells transfected with Rap1 siRNAs (Rap#1 and Rap#2) were starved, stimulated with forskolin for 2 min, and ERK phosphorylation, Rap1, and Akt expression (as a loading control) were analyzed. Rap1 expression was reduced by more than 80% after transfection with Rap1-directed siRNAs. B, the results from three experiments are summarized. C, starved cells were stimulated with EcAMP or TSH for the indicated times (in minutes). Lysates were subjected to RalGDS-RBD pull-down assay and blotted for Rap1GTP. Total cell lysates were analyzed for Rap1 expression. D, starved cells were stimulated with EcAMP for the indicated times (in minutes) and analyzed for ERK phosphorylation. Western blotting for actin was used to ensure equal protein loading.
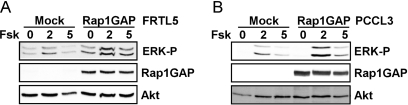
Because our studies were conducted in WRT cells and those reported previously in FRTL-5 cells (Iacovelli et al., 2001), we extended our analysis to FRTL-5 and PCCL3 cells, both continuous lines of rat thyroid follicular cells (Medina and Santisteban, 2000; Kimura et al., 2001). Overexpression of Rap1GAP failed to inhibit cAMP-stimulated ERK activity in both rat thyroid cell lines (Fig. 4). These data differ from the single published report that analyzed the mechanism through which TSH stimulates ERK and concluded that cAMP stimulates Rap1-dependent ERK activity (Iacovelli et al., 2001).
Fig. 4.
ERK activation in FRTL5 and PCCL3 cells does not require Rap activity. FRTL-5 (A) and PCCL3 cells (B) infected with Rap1GAP adenovirus (500 particles/cell) were starved for 24 h, stimulated with forskolin for the indicated times (in minutes), and subjected to Western blotting for ERK phosphorylation, Rap1GAP, and Akt expression, the latter to confirm equal protein loading. Similar results were obtained in two experiments in each cell line.
PKA Mediates cAMP-Stimulated ERK Activation.
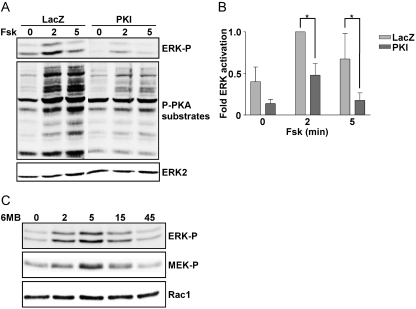
Having excluded a role for Rap in cAMP-regulated ERK activity, we next investigated the role of PKA. PKI, a highly specific inhibitor of PKA, was used in these experiments. Overexpression of PKI using an adenovirus markedly reduced PKA activity as determined by Western blotting for substrates phosphorylated by PKA using a PKA-phospho-substrate antibody (Fig. 5A, middle). Under these conditions, PKI significantly impaired cAMP-stimulated ERK activity (Fig. 5, A and B). To determine whether PKA activity was sufficient to stimulate ERK activity, starved cells were treated with the selective PKA agonist, 6MB-cAMP. 6MB-cAMP stimulated activating phosphorylation of both ERK and MEK1 with a similar time course (Fig. 5C). Thus, PKA activity is required for cAMP-stimulated ERK activity and is sufficient to increase MEK1 and ERK activity.
Fig. 5.
ERK activation is PKA-dependent. A, WRT cells infected with LacZ or PKI adenoviruses were starved overnight and stimulated with forskolin for the indicated times (in minutes). ERK and PKA substrate phosphorylation were analyzed by Western blotting. ERK2 expression documented equal protein loading. B, PKI significantly reduced forskolin-stimulated ERK activation (*, p < 0.05 at 2 and 5 min). C, starved cells were stimulated with 6MB-cAMP for the indicated times (in minutes) and analyzed by Western blotting for phospho-ERK, phospho-MEK, and Rac1 as a loading control. MEK1 activation by 6-MB-cAMP was analyzed in a single experiment.
Ras Is Required for cAMP-Stimulated ERK Activity.
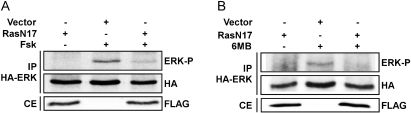
Studies were next conducted to elucidate the mechanism through which PKA stimulates ERK activity. Having excluded a role for Rap in the regulation of ERK activity, the contribution of Ras was investigated. Expression vectors encoding FLAG-tagged dominant-negative RasN17 and HA-tagged ERK1 were cotransfected into WRT cells, and ERK activation was assessed in HA immunoprecipitates. This approach circumvented difficulties associated with low transfection efficiency by monitoring ERK activity selectively in transfected cells. Forskolin stimulated activating phosphorylation of HA-ERK1, and cotransfection with FLAG-RasN17 but not empty vector impaired ERK activation (Fig. 6A). To determine whether the requirement for Ras was upstream or downstream of PKA, the effects of RasN17 on 6MB-cAMP-stimulated ERK activation were examined. Expression of RasN17 inhibited ERK phosphorylation by 6MB-cAMP (Fig. 6B), supporting a role for Ras downstream from PKA in the regulation of ERK activity.
Fig. 6.
Ras activity is required for ERK activation. A, WRT cells transfected with HA-ERK and FLAG-RasN17 or empty vector were starved, stimulated with forskolin for 2 min, and HA-ERK immunoprecipitated (IP) using an HA antibody and subjected to Western blotting for phospho-ERK and HA-ERK expression. Whole-cell extracts (CE) were analyzed for FLAG-RasN17 expression. B, cells transfected as described in A were stimulated with 6MB-cAMP for 2 min. Two experiments using forskolin and 6-MB-cAMP and a single experiment using TSH were performed with similar results.
ERK Activation by cAMP Requires B-Raf.
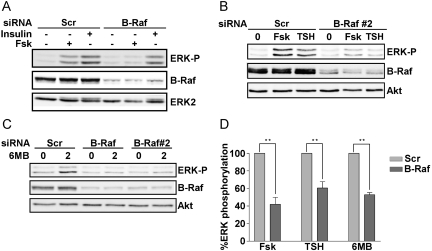
WRT cells express Raf-1 (data not shown) and B-Raf (Fig. 7). Although B-Raf plays a major role in the regulation of ERK activity in human thyroid tumor cells (Melillo et al., 2005), the role of B-Raf in the regulation of ERK by TSH is unknown. To assess whether B-Raf mediates the effects of TSH on ERK activity, the expression of B-Raf was silenced. Decreasing the expression of B-Raf using two different sets of siRNAs significantly impaired TSH- and forskolin-stimulated ERK phosphorylation (Fig. 7, A, B, and D). Silencing B-Raf induced a similar reduction in MEK1 phosphorylation by forskolin and TSH (data not shown). It is noteworthy that in contrast to the effects observed using cAMP elevating agents, silencing B-Raf did not impair insulin-stimulated ERK activation (Fig. 7A). Similar results were observed for serum-stimulated ERK activity (data not shown).
Fig. 7.
B-Raf is required for cAMP-stimulated ERK activation. WRT cells transfected with scrambled- (Scr) versus B-Raf-directed siRNAs were starved, stimulated with forskolin (2 min), insulin (5 min) (A) or TSH (2 min) (B) and subjected to Western blotting for phospho-ERK and B-Raf. Equal protein loading was confirmed by blotting for ERK2 or Akt. Depletion of B-Raf using an independent set of siRNAs (B-Raf#2) inhibited ERK activation by forskolin and TSH. C, siRNA-transfected cells were stimulated with 6MB-cAMP for the times indicated (in minutes) and analyzed for phospho-ERK, B-Raf, and Akt as a loading control. D, the decrease in ERK activation in B-Raf-depleted cells was statistically significant (**, p < 0.01).
To assess whether activation of ERK by PKA required B-Raf, the consequences of silencing B-Raf on ERK activation by 6MB-cAMP were examined. As for the cAMP-elevating agents, ERK activation by 6MB-cAMP was reduced in cells depleted of B-Raf (Fig. 7, C and D). These findings indicate that PKA lies upstream from B-Raf in the regulation of ERK activity and that the requirement for B-Raf in ERK activation seems to be selective to cAMP-elevating agents in these cells.
B-Raf Is Required for TSH-Stimulated DNA Synthesis.
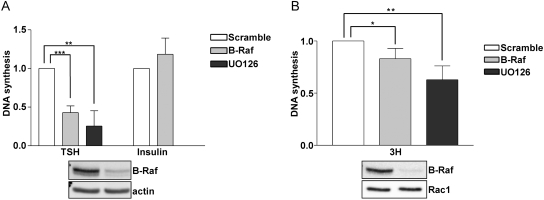
To assess the contribution of B-Raf to the biological effects of TSH, the consequences of silencing B-Raf expression on DNA synthesis were examined. Because TSH fails to stimulate proliferation in the absence of cooperating growth factors (Medina and Santisteban, 2000; Kimura et al., 2001), DNA synthesis was examined in cells starved in the presence of 0.2% calf serum (Fig. 8A). TSH-stimulated DNA synthesis was significantly reduced in the presence of the MEK1 inhibitor (U0126). Depletion of B-Raf also inhibited TSH-induced DNA synthesis. In contrast, silencing B-Raf did not impair insulin-stimulated DNA synthesis. Thyroid cell proliferation is maximal in growth medium containing TSH, insulin, and serum (3H). It is noteworthy that although modest in magnitude, silencing the expression of B-Raf or treatment with the MEK1 inhibitor significantly decreased 3H-stimulated DNA synthesis (Fig. 8B). We did not anticipate a more marked decrease in 3H-stimulated DNA synthesis, because other pathways, most notably those mediated through phosphatidylinositol 3-kinase and the mammalian target of rapamycin, make substantial contributions to thyroid cell proliferation in vitro (Cass and Meinkoth, 1998; Cass et al., 1999; Medina and Santisteban, 2000; Kimura et al., 2001) and in vivo (Yeager et al., 2008; Miller et al., 2009). These data confirm that B-Raf contributes to the proliferation of nontransformed thyroid cells, possibly through the ability of cAMP-elevating agents to stimulate B-Raf-dependent ERK activation.
Fig. 8.
B-Raf is required for TSH-dependent DNA synthesis. Cells pretreated with U0126 (10 μM) for 1 h or depleted of B-Raf were stimulated with TSH, insulin (A), or 3H growth medium (B), and DNA synthesis was analyzed. The percentage of BrdU-positive nuclei (13.2% for TSH, 19.1% for insulin, 52.6% for 3H versus 2.0% for starved cells) in mitogen-stimulated cells was set to 1. The decrease in DNA synthesis in U0126-treated (**, p < 0.01 for TSH; **, p < 0.01 for 3H) and B-Raf-depleted (***, p < 0.001 for TSH; *, p < 0.05 for 3H) cells was statistically significant.
Discussion
Given the important role played by Rap1 in the activation of B-Raf (Stork and Schmitt, 2002), together with the frequent mutational activation of B-Raf in thyroid tumors (Xing, 2005; Kondo et al., 2006), we investigated whether Rap1 contributes to ERK activation by TSH/cAMP. Our findings indicate that PKA, and not Rap1, mediates B-Raf-dependent ERK activation by TSH. A role for endogenous Rap1 in the regulation of ERK by TSH/cAMP was excluded based on two major lines of evidence. Overexpression of Rap1GAP failed to impair TSH/cAMP-stimulated ERK activity, and silencing the expression of Rap1 had no effect on ERK activation. In addition, direct activation of Epac with the specific agonist EcAMP (Enserink, 2002) failed to stimulate ERK activity in starved cells. Collectively, these data show that Rap1 activation is neither required for nor sufficient to induce ERK activation in WRT cells. These results differ from the single report published to date that concluded that Rap1 was required for TSH/cAMP-induced ERK activity in FRTL-5 cells (Iacovelli et al., 2001). The reason for these differences remains to be determined. In our hands, overexpression of Rap1GAP had no effect on cAMP-stimulated ERK activity in either FRTL-5 or rat thyroid PCCL3 cells, another widely used model of rat thyroid cells (Medina and Santisteban, 2000; Kimura et al., 2001). Further work is clearly required before an accepted model for the regulation of ERK by TSH can be derived.
Our findings indicate that PKA mediates cAMP-stimulated ERK activation. Inhibition of PKA activity using the highly selective inhibitor PKI impaired TSH/cAMP-stimulated ERK activity. Moreover, treatment with the selective PKA analog 6-MB-cAMP was sufficient to stimulate activating phosphorylation of MEK1 and ERK in starved cells. The molecular mechanism through which PKA regulates ERK activity was explored. Silencing the expression of B-Raf impaired ERK activation by TSH, forskolin, and 6MB-cAMP, documenting a role for B-Raf in the regulation of ERK by cAMP and PKA. Having excluded a role for Rap1 in the regulation of ERK activity, our analysis focused on Ras. TSH activates Ras in rat thyroid cells (Tsygankova et al., 2000; Iacovelli et al., 2001). Expression of dominant-negative Ras inhibited TSH-, forskolin- and 6MB-cAMP-stimulated ERK activity. Although there are precedents for PKA-dependent Ras activation (Ambrosini et al., 2000; Yang et al., 2003; Obara et al., 2007), we reported that TSH activates Ras through a PKA-independent mechanism, experiments that were conducted in thyroid cells overexpressing human H-Ras (Tsygankova et al., 2000). Unfortunately, we have been unable to detect the activation of endogenous Ras in WRT cells, even in response to serum mitogens (L.A. Vuchak, unpublished data). Iacovelli et al. (2001) reported that TSH activates endogenous Ras in FRTL-5 cells and that Ras is required for ERK activation. Unfortunately, the mechanism through which Ras was activated was not explored (Iacovelli et al., 2001). It is conceivable that PKA-dependent and -independent mechanisms of Ras activation coexist in thyroid cells, similar to what has been reported for Rap1, in which PKA-dependent and -independent modes of activation have been described. Alternatively, the requirement for Ras in PKA-dependent ERK activation could reflect the effects of PKA on Ras signaling. TSH influences downstream events in Ras signaling. TSH transiently impaired ERK activation by RasV12S35, an effector domain mutant that signals preferentially through Raf, whereas it enhanced ERK activation by RasV12G37, a mutant that signals via RalGDS and Ral proteins (Miller et al., 1998). In the absence of TSH, Raf-1 was required for DNA synthesis stimulated by microinjected cellular Ras protein, whereas in the presence of TSH, Ras-stimulated DNA synthesis did not require Raf1 (al-Alawi et al., 1995). Ciullo et al. (2001) reported that TSH stimulated the association of Ras with the p85 regulatory subunit of phosphatidylinositol 3-kinase through PKA-mediated phosphorylation of p85. Moreover, these authors showed that cAMP disrupted Ras/Raf-1 complexes at least partly through PKA (Ciullo et al., 2001; De Gregorio et al., 2007). Collectively, these studies indicate that there are multiple sites of cross-talk between PKA- and Ras-mediated signaling.
Our data are the first to ascribe a functional role to B-Raf in TSH signaling. Silencing the expression of B-Raf impaired DNA synthesis stimulated by TSH. Depleting B-Raf also induced a modest decrease in DNA synthesis stimulated by 3H growth medium, which contains TSH, insulin, and serum. In that depletion of B-Raf failed to inhibit insulin- or serum-induced ERK activation, these data suggest that the requirement for B-Raf in cell proliferation resides downstream of TSH. This is consistent with previous studies showing that the expression of dominant-negative Ras (Kupperman et al., 1993; Medina and Santisteban, 2000; Ciullo et al., 2001) or treatment with MEK1 inhibitors (Iacovelli et al., 2001; this report) impairs TSH-stimulated DNA synthesis. Even in canine thyroid cells, in which PKA mediates all or many of the effects of TSH (Dremier et al., 2007), treatment with an MEK1 inhibitor impaired TSH-stimulated DNA synthesis (Vandeput et al., 2003). Other ligands that activate Gs-coupled receptors have been shown to signal through B-Raf. Parathyroid hormone stimulated PKA- and B-Raf-dependent ERK activation in Chinese hamster ovary cells expressing the parathyroid hormone 1 receptor (Wang et al., 2008). Silencing the expression of B-Raf impaired MSH-induced ERK activation in melanocytes (Dumaz et al., 2006).
In conclusion, our findings highlight important roles for PKA, Ras, and B-Raf in TSH signaling to ERK. The requirement for Ras in TSH-induced ERK activation provides further evidence that Ras functions in TSH signaling (al-Alawi et al., 1995; Ciullo et al., 2001; Iacovelli et al., 2001; Kupperman et al., 1993; Medina and Santisteban, 2000; Tsygankova et al., 2000). Mutations in Ras and B-Raf are prevalent in thyroid tumors. Patients with Carney's syndrome, caused by inactivating mutations in the gene for the PKA regulatory subunit R1α, exhibit an increased frequency of thyroid tumors (Boikos and Stratakis, 2007). Loss of heterozygosity for PRKAR1A and increased PKA activity have been observed in thyroid tumors (Sandrini et al., 2002). Elucidating sites of cross-talk between these important signaling molecules in nontransformed thyroid cells may reveal novel insight into the molecular basis of thyroid cancer.
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK55757] and the National Institutes of Health National Cancer Institute Cancer Education and Career Development Program, Training Program in Cancer Pharmacology [Grant 5R25-CA101871].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.060129
- TSH
- thyrotropin
- EcAMP
- 8-(4-chlorophenylthio)-2′-O-methyl-cAMP
- Epac
- exchange protein activated by cAMP
- ERK
- extracellular signal-regulated kinase
- PKA
- protein kinase A
- PKI
- heat-stable protein kinase A inhibitor
- WRT
- Wistar rat thyroid
- 6MB-cAMP
- N6-monobutyryl-cAMP
- WRT
- Wistar rat thyroid
- MEK
- mitogen-activated protein kinase kinase
- HA
- hemagglutinin
- siRNA
- short interfering RNA
- U0126
- 1,4-diamino-2,3-dicyano-1,4-bis(methylthio)butadiene.
References
- al-Alawi N, Rose DW, Buckmaster C, Ahn N, Rapp U, Meinkoth J, Feramisco JR. (1995) Thyrotropin-induced mitogenesis is Ras dependent but appears to bypass the Raf-dependent cytoplasmic kinase cascade. Mol Cell Biol 15:1162–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini A, Tininini S, Barassi A, Racagni G, Sturani E, Zippel R. (2000) cAMP cascade leads to Ras activation in cortical neurons. Brain Res Mol Brain Res 75:54–60 [DOI] [PubMed] [Google Scholar]
- Boikos SA, Stratakis CA. (2007) Carney complex: the first 20 years. Curr Opin Oncol 19:24–29 [DOI] [PubMed] [Google Scholar]
- Cass LA, Meinkoth JL. (1998) Differential effects of cyclic adenosine 3′,5′-monophosphate on p70 ribosomal S6 kinase. Endocrinology 139:1991–1998 [DOI] [PubMed] [Google Scholar]
- Cass LA, Summers SA, Prendergast GV, Backer JM, Birnbaum MJ, Meinkoth JL. (1999) Protein kinase A-dependent and -independent signaling pathways contribute to cyclic AMP-stimulated proliferation. Mol Cell Biol 19:5882–5891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciullo I, Diez-Roux G, Di Domenico M, Migliaccio A, Avvedimento EV. (2001) cAMP signaling selectively influences Ras effectors pathways. Oncogene 20:1186–1192 [DOI] [PubMed] [Google Scholar]
- De Gregorio G, Coppa A, Cosentino C, Ucci S, Messina S, Nicolussi A, D'Inzeo S, Di Pardo A, Avvedimento EV, Porcellini A. (2007) The p85 regulatory subunit of PI3K mediates TSH-cAMP-PKA growth and survival signals. Oncogene 26:2039–2047 [DOI] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. (1998) Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396:474–477 [DOI] [PubMed] [Google Scholar]
- Dremier S, Milenkovic M, Blancquaert S, Dumont JE, Døskeland SO, Maenhaut C, Roger PP. (2007) Cyclic adenosine 3′,5′-monophosphate (cAMP)-dependent protein kinases, but not exchange proteins directly activated by cAMP (Epac), mediate thyrotropin/cAMP-dependent regulation of thyroid cells. Endocrinology 148:4612–4622 [DOI] [PubMed] [Google Scholar]
- Dremier S, Vandeput F, Zwartkruis FJ, Bos JL, Dumont JE, Maenhaut C. (2000) Activation of the small G protein Rap1 in dog thyroid cells by both cAMP-dependent and -independent pathways. Biochem Biophys Res Commun 267:7–11 [DOI] [PubMed] [Google Scholar]
- Dumaz N, Hayward R, Martin J, Ogilvie L, Hedley D, Curtin JA, Bastian BC, Springer C, Marais R. (2006) In melanoma, RAS mutations are accompanied by switching signaling from BRAF to CRAF and disrupted cyclic AMP signaling. Cancer Res 66:9483–9491 [DOI] [PubMed] [Google Scholar]
- Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Døskeland SO, Blank JL, Bos JL. (2002) A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 4:901–906 [DOI] [PubMed] [Google Scholar]
- Hochbaum D, Hong K, Barila G, Ribeiro-Neto F, Altschuler DL. (2008) Epac, in synergy with cAMP-dependent protein kinase (PKA), is required for cAMP-mediated mitogenesis. J Biol Chem 283:4464–4468 [DOI] [PubMed] [Google Scholar]
- Iacovelli L, Capobianco L, Salvatore L, Sallese M, D'Ancona GM, De Blasi A. (2001) Thyrotropin activates mitogen-activated protein kinase pathway in FRTL-5 by a cAMP-dependent protein kinase A-independent mechanism. Mol Pharmacol 60:924–933 [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. (1998) A family of cAMP-binding proteins that directly activate Rap1. Science 282:2275–2279 [DOI] [PubMed] [Google Scholar]
- Kimura T, Van Keymeulen A, Golstein J, Fusco A, Dumont JE, Roger PP. (2001) Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocr Rev 22:631–656 [DOI] [PubMed] [Google Scholar]
- Kondo T, Ezzat S, Asa SL. (2006) Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer 6:292–306 [DOI] [PubMed] [Google Scholar]
- Kupperman E, Wen W, Meinkoth JL. (1993) Inhibition of thyrotropin-stimulated DNA synthesis by microinjection of inhibitors of cellular Ras and cyclic AMP-dependent protein kinase. Mol Cell Biol 13:4477–4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugwitz KL, Allgeier A, Offermanns S, Spicher K, Van Sande J, Dumont JE, Schultz G. (1996) The human thyrotropin receptor: a heptahelical receptor capable of stimulating members of all four G protein families. Proc Natl Acad Sci U S A 93:116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DL, Santisteban P. (2000) Thyrotropin-dependent proliferation of in vitro rat thyroid cell systems. Eur J Endocrinol 143:161–178 [DOI] [PubMed] [Google Scholar]
- Mei FC, Qiao J, Tsygankova OM, Meinkoth JL, Quilliam LA, Cheng X. (2002) Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J Biol Chem 277:11497–11504 [DOI] [PubMed] [Google Scholar]
- Melillo RM, Castellone MD, Guarino V, De Falco V, Cirafici AM, Salvatore G, Caiazzo F, Basolo F, Giannini R, Kruhoffer M, et al. ( 2005) The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J Clin Invest 115:1068–1081 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Miller KA, Yeager N, Baker K, Liao XH, Refetoff S, Di Cristofano A. (2009) Oncogenic Kras requires simultaneous PI3K signaling to induce ERK activation and transform thyroid epithelial cells in vivo. Cancer Res 69:3689–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Rioux L, Prendergast GV, Cannon S, White MA, Meinkoth JL. (1998) Differential effects of protein kinase A on Ras effector pathways. Mol Cell Biol 18:3718–3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsutake N, Knauf JA, Mitsutake S, Mesa C, Jr., Zhang L, Fagin JA. (2005) Conditional BRAFV600E expression induces DNA synthesis, apoptosis, dedifferentiation, and chromosomal instability in thyroid PCCL3 cells. Cancer Res 65:2465–2473 [DOI] [PubMed] [Google Scholar]
- Obara Y, Horgan AM, Stork PJ. (2007) The requirement of Ras and Rap1 for the activation of ERKs by cAMP, PACAP, and KCl in cerebellar granule cells. J Neurochem 101:470–482 [DOI] [PubMed] [Google Scholar]
- Pak Y, Pham N, Rotin D. (2002) Direct binding of the beta1 adrenergic receptor to the cyclic AMP-dependent guanine nucleotide exchange factor CNrasGEF leads to Ras activation. Mol Cell Biol 22:7942–7952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham N, Cheglakov I, Koch CA, de Hoog CL, Moran MF, Rotin D. (2000) The guanine nucleotide exchange factor CNrasGEF activates ras in response to cAMP and cGMP. Curr Biol 10:555–558 [DOI] [PubMed] [Google Scholar]
- Ribeiro-Neto F, Urbani J, Lemee N, Lou L, Altschuler DL. (2002) On the mitogenic properties of Rap1b: cAMP-induced G(1)/S entry requires activated and phosphorylated Rap1b. Proc Natl Acad Sci U S A 99:5418–5423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini F, Matyakhina L, Sarlis NJ, Kirschner LS, Farmakidis C, Gimm O, Stratakis CA. (2002) Regulatory subunit type I-alpha of protein kinase A (PRKAR1A): a tumor-suppressor gene for sporadic thyroid cancer. Genes Chromosomes Cancer 35:182–192 [DOI] [PubMed] [Google Scholar]
- Stork PJ, Schmitt JM. (2002) Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol 12:258–266 [DOI] [PubMed] [Google Scholar]
- Tsygankova OM, Kupperman E, Wen W, Meinkoth JL. (2000) Cyclic AMP activates Ras. Oncogene 19:3609–3615 [DOI] [PubMed] [Google Scholar]
- Tsygankova OM, Prendergast GV, Puttaswamy K, Wang Y, Feldman MD, Wang H, Brose MS, Meinkoth JL. (2007) Downregulation of Rap1GAP contributes to Ras transformation. Mol Cell Biol 27:6647–6658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsygankova OM, Saavedra A, Rebhun JF, Quilliam LA, Meinkoth JL. (2001) Coordinated regulation of Rap1 and thyroid differentiation by cyclic AMP and protein kinase A. Mol Cell Biol 21:1921–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeput F, Perpete S, Coulonval K, Lamy F, Dumont JE. (2003) Role of the different mitogen-activated protein kinase subfamilies in the stimulation of dog and human thyroid epithelial cell proliferation by cyclic adenosine 5′-monophosphate and growth factors. Endocrinology 144:1341–1349 [DOI] [PubMed] [Google Scholar]
- Wang B, Yang Y, Friedman PA. (2008) Na/H exchange regulatory factor 1, a novel AKT-associating protein, regulates extracellular signal-regulated kinase signaling through a B-Raf-mediated pathway. Mol Biol Cell 19:1637–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing M. (2005) BRAF mutation in thyroid cancer. Endocr Relat Cancer 12:245–262 [DOI] [PubMed] [Google Scholar]
- Yang H, Cooley D, Legakis JE, Ge Q, Andrade R, Mattingly RR. (2003) Phosphorylation of the Ras-GRF1 exchange factor at Ser916/898 reveals activation of Ras signaling in the cerebral cortex. J Biol Chem 278:13278–13285 [DOI] [PubMed] [Google Scholar]
- Yeager N, Brewer C, Cai KQ, Xu XX, Di Cristofano A. (2008) Mammalian target of rapamycin is the key effector of phosphatidylinositol-3-OH-initiated proliferative signals in the thyroid follicular epithelium. Cancer Res 68:444–449 [DOI] [PubMed] [Google Scholar]