Abstract
AS1411 is a DNA aptamer that is in phase II clinical trials for relapsed or refractory acute myeloid leukemia and for renal cell carcinoma. AS1411 binds to nucleolin, a protein that is overexpressed in the cytoplasm and on the plasma membrane of some tumor cells compared with normal cells. Studies were performed to determine whether cell surface nucleolin is a receptor for AS1411 in the acute myeloid leukemia cell line MV4-11. Biotinylation of MV4-11 cell surface proteins followed by immunoblotting of the biotinylated proteins showed that full-length (106 kDa) and truncated forms of nucleolin were present on the cell surface. In contrast, K-562 cells, which are 4-fold less sensitive than MV4-11 cells to AS1411, showed no full-length nucleolin and lesser amounts of the truncated forms of nucleolin on the cell surface. Incubation of MV4-11 cells with [32P]AS1411 and immunoprecipitation of the plasma membrane fraction with anti-nucleolin antibody demonstrated the presence of [32P]AS1411-nucleolin complexes. Anti-nucleolin antibody inhibited binding of fluorescein isothiocyanate (FITC)-AS1411 to plasma membrane nucleolin 56 ± 10% SE (P < 0.01) compared with cells incubated with FITC-AS1411 only. Cellular uptake of [32P]AS1411 into MV4-11 cells was blocked by a 20-fold excess of unlabeled AS1411 but not by a 20-fold excess of the biologically inactive oligonucleotide CRO-26. Uptake was approximately 3-fold faster into MV4-11 cells than into K-562 cells. Partial knockdown of plasma membrane and cytosolic nucleolin in MCF-7 cells resulted in a 3-fold decrease in AS1411 uptake. These results provide evidence that plasma membrane nucleolin is a functional receptor for AS1411 in MV4-11 cells.
A major goal of cancer chemotherapy continues to be the eradication of the tumor cell population without inducing toxicity to the normal tissues of the patient. To achieve this goal, emphasis in cancer drug discovery has now shifted somewhat from the identification of new cytotoxic agents to the development of more tumor-targeted therapies. The 26-oligomer DNA aptamer AS1411 is the first aptamer to enter clinical oncology trials. It has shown both promising antitumor activity and a lack of serious systemic toxicity in a phase I clinical trial (Laber et al., 2005). Multi-institutional phase II clinical trials of AS1411 in refractory or relapsed acute myeloid leukemia (AML) (clinicaltrials.gov identifier NCT00512083) and in renal cancer (NCT00740441) are now under way.
AS1411 binds to its target protein with such high affinity and specificity that it has been termed a “chemical antibody” (Ireson and Kelland, 2006). Several studies have identified this target protein in tumor cells as nucleolin (Bates et al., 1999; Soundararajan et al., 2008). Nucleolin has been shown to bind G-quadruplex-forming DNA sequences (Dapic et al., 2003). Because AS1411 forms a stable G-quadruplex structure, this probably contributes to the high affinity and specific binding of the DNA aptamer to nucleolin. We have proposed a model of AS1411 action to explain the selective targeting of AS1411 to tumor cells compared with normal cells (Otake et al., 2007; Soundararajan et al., 2008). According to this model, antitumor selectivity of AS1411 occurs on at least two cellular levels. One basis for the antitumor selectivity relates to the overexpression of nucleolin in the cytoplasm of tumor cells compared with normal cells. Confocal microscopy and flow cytometry studies indicated that at least 90% of the human chronic lymphocytic leukemia cells expressed nucleolin in the cytoplasm, whereas less than 5% of the CD19+ B-cells from healthy human volunteers expressed cytoplasmic nucleolin (Otake et al., 2007). Nucleolin binds to an A + U-rich instability element in the 3′-untranslated region of bcl-2 mRNA and protects the bcl-2 mRNA from degradation (Sengupta et al., 2004; Otake et al., 2007). This results in stabilization of bcl-2 mRNA and allows the tumor cells to overproduce Bcl-2 protein and avoid apoptosis. AS1411, by acting as a molecular decoy (Soundararajan et al., 2008), competes with bcl-2 mRNA for binding to nucleolin and thereby induces bcl-2 mRNA instability and apoptosis. This is proposed to occur to a much greater extent in tumor cells such as AML cells than in normal cells, because normal cells do not overexpress nucleolin in the cytoplasm and may not depend on the stabilization of bcl-2 mRNA for survival. Very recently, it was reported that incubation of human vascular endothelial cells with a polyclonal anti-nucleolin antibody resulted in down-regulation of bcl-2 mRNA levels and induction of apoptosis (Fogal et al., 2009).
Antitumor selectivity may be obtained at an additional cellular level. AS1411 (Soundararajan et al., 2008) and other G-rich DNA aptamers (Bates et al., 1999) are believed to bind to nucleolin that is also overexpressed on the external surface of tumor cells compared with normal cells, and the aptamers gain intracellular access when nucleolin is shuttled from the plasma membrane to the cytoplasm and nucleus. Several lines of experimental evidence are consistent with this hypothesis, although studies which demonstrate directly that plasma membrane nucleolin is the receptor for AS1411 have not been reported. Nucleolin is present on the surface of various types of tumor cells (Hovanessian et al., 2000; Sinclair and O'Brien, 2002; Otake et al., 2007; Chen et al., 2008; Soundararajan et al., 2008), despite its lack of a transmembrane domain or signal sequence (Lapeyre et al., 1987; Srivastava et al., 1989). Cell surface nucleolin is a receptor for DNA nanoparticles (Chen et al., 2008). Results from our confocal microscopy studies are also consistent with the presence of nucleolin on the cell surface of MCF-7 and chronic lymphocytic leukemia cells but not on normal mammary epithelial cells or normal CD19+B cells (Otake et al., 2007; Soundararajan et al., 2008). Thus, according to our model, AS1411 uptake would be more extensive in tumor cells than in normal cells because normal cells lack or have lower levels of the AS1411 receptor (nucleolin) in the plasma membrane.
The identification of a plasma membrane receptor for AS1411 would contribute to our understanding of the mechanism of action and tumor cell selectivity of this drug. The results reported herein show directly that plasma membrane nucleolin functions as a cell surface receptor for AS1411 in MV4-11 cells.
Materials and Methods
Materials.
All antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). PCR primers were synthesized by Operon Technologies (Alameda, CA). Immobilon-P polyvinylidene difluoride transfer membranes and all other chemicals and supplies were purchased from Thermo Fisher Scientific (Waltham, MA). AS1411, having the sequence 5′-d(GGTGGTGGTGGTTGTGGTGGTGGTGG)-3, and 5′-FITC-AS1411 were obtained from Antisoma Research, Ltd. (London, UK). CRO-26 (Integrated DNA Technologies, Coralville, IA) is a cytosine-rich oligonucleotide in which each dG of AS1411 is replaced with dC. The oligonucleotides were dissolved in serum-free tissue culture medium before addition to cell cultures.
Cell Culture.
MV4-11 cells and K-562 leukemia cells were obtained from the American Type Culture Collection (Manassas, VA) and were grown in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 0.1 mg/ml streptomycin, and 100 U/ml penicillin. MCF-7 breast cancer cells transfected with either a nucleolin shRNA or a scrambled shRNA cells were cultured in RPMI 1640 media with 10% heated-inactivated fetal bovine serum and 500 μg/ml G418. All cultures were maintained in a humidified incubator at 37°C with 95% air/5% CO2. Cell counting and measurements of cell viability were carried out as described previously (Otake et al., 2005). AS1411 was dissolved in tissue culture media before the addition to cell cultures and was dissolved in water for the cell-free experiments.
Confocal Microscopy.
MV4-11 cells were grown on MatTek plates (MatTek Corp., Ashland, MA). To stain cell surface nucleolin, the cells were preincubated with blocking buffer (3% bovine serum albumin in PBS) for 30 min on ice, washed twice with PBS, and then incubated with 2 μg/ml anti-nucleolin monoclonal antibody MS-3 for 45 min on ice. The cells were washed twice with ice-cold PBS and then incubated with FITC-conjugated anti-mouse IgG (1:100 dilution in blocking buffer) for 45 min on ice. The cells were washed three times as described above and then incubated with 4 μg/ml FM 4-64, a fluorescent dye (Molecular Probes, Carlsbad, CA), for 10 min on ice to stain the plasma membrane. The cells were again washed three times in PBS and then observed under a Carl Zeiss LSM5 Pa confocal microscope (Carl Zeiss Inc., Thornwood, NY). Confocal images (1024 × 768 pixels) were obtained using a 63× objective lens and the images were overlaid using Carl Zeiss LSM Pascal image browser 4.0 software.
Plasma Membrane Isolation.
MV4-11 cells were placed on ice and washed twice with ice-cold HBS (10 mM HEPES, 137 mM NaCl, 4 mM KCl, and 11 mM glucose) containing a protease inhibitor solution containing 5 μM 4-(2-aminoethyl)benzenesulfonylfluoride · HCl, 1.5 nM aprotinin, 10 nM E-64 protease inhibitor, 5 μM EDTA, and 10 nM leupeptin from Calbiochem (San Diego, CA). The cells were resuspended in ice-cold HBS and then homogenized three times with a Polytron homogenizer (Kinematica, Littau-Lucerne, Switzerland) for 5 s at the maximal setting. The lysate was centrifuged at 450g for 5 min at 4°C, and the supernatant was collected. A sucrose step gradient consisting of 4 ml of 60%, 4 ml of 38%, and 3.2 ml of 15% sucrose (w/v) in 20 mM Tris-HCl, pH 7.4, was prepared. One milliliter of the supernatant from each sample was layered on top of the 15% sucrose and centrifuged for 1 h at 112,000g in a Beckman SW-40 Ti rotor (Beckman Coulter, Fullerton, CA). One milliliter at the interface of the 38%/60% sucrose gradient was collected and diluted with 20 mM Tris-HCl, pH 7.4, and centrifuged at 300,000g for 90 min in a fixed angle Beckman Type Ti70 rotor. The pellet consists of the purified plasma membranes. The purified plasma membranes were resuspended in HBS containing the above protease inhibitor solution and 1% Triton X-100 and then incubated at 4°C overnight with gentle mixing. The suspension was centrifuged at 10,000g for 15 min. The supernatant represents the soluble plasma membrane fraction, and the pellet represents the insoluble fraction.
Cell Surface Biotinylation.
Biotin coupled to the highly reactive N-hydroxysuccinimide ester group was used as a membrane-impermeant probe to covalently tag cell surface proteins. The cells were washed two times with ice-cold PBS containing 0.1 mM CaCl2 and 1 mM MgCl2 and then incubated with sulfosuccinimidyl 2-(biotinamido)-ethyl-1,3-dithiopropionate (EZ-link-sulfo-NHS-biotin; Thermo Fisher Scientific) at a final concentration of 1 mg/ml in PBS/0.1 mM CaCl2/0.1 mM MgCl2 solution. The cells were gently shaken for 1 h at 4°C. The free sulfo-NHS-biotin was removed by washing the cells twice with 100 mM glycine in ice-cold 0.1 mM CaCl2/0.1 mM MgCl2 solution and twice with the above solution lacking glycine. The biotinylated cells were solubilized in 1 ml of RIPA buffer (Niranjanakumari et al., 2002) for 30 min at 4°C with shaking. The samples were centrifuged at 20,000g to pellet-insoluble material, and protein determinations were performed on the supernatants as described previously (Soundararajan et al., 2008). Aliquots of the supernatants were saved for total protein determinations. The biotinylated proteins in the supernatants were separated from nonbiotinylated proteins by batch affinity chromatography using monomeric avidin beads (250 μl of beads/300 μg of protein). Before use, the beads were blocked with 2 mM biotin in PBS for 15 min at room temperature and then washed three times in 1 ml of 0.1 M glycine, pH 2.8. The beads were re-equilibrated in PBS by washing four times in 1 ml of PBS. The supernatant samples were incubated with the beads for 1 h at room temperature with constant rotation, then the beads were centrifuged and the supernatants containing the unbound proteins were stored at 4°C. The beads were washed four times in 1 ml of RIPA buffer, and the biotinylated proteins were eluted from the beads by incubation with an equal volume of SDS-polyacrylamide gel electrophoresis sample buffer (2% SDS, 10% glycerol, 5% mercaptoethanol, 0.001% bromphenol blue, and 62.5 mM Tris-HCl, pH 6.8) for 30 min at room temperature. The samples were subjected to gel electrophoresis and immunoblotted for nucleolin as described previously (Soundararajan et al., 2008). The blots were probed with mouse monoclonal anti-nucleolin antibody. The blots were stripped with 100 mM 2-mercaptoethanol, 2% SDS, and 62.5 mM Tris-HCl, pH 6.7, at 50°C for 30 min and reprobed with polyclonal rabbit anti-calnexin antibody and then with anti-β3-integrin in blocking buffer followed by reaction with anti-rabbit secondary antibody.
AS1411 and CRO-26 Radiolabeling.
AS1411 and CRO were radiolabeled using T4 polynucleotide kinase (Thermo Fisher Scientific) and [γ-32P]ATP (PerkinElmer Life and Analytical Sciences, Waltham, MA). Labeled AS1411 and CRO-26 were purified with MicroSpin G-25 columns (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK).
Coimmunoprecipitation of AS1411 Plasma Membrane Nucleolin Complexes.
Approximately 1 × 106 MV4-11 cells were incubated for 2 h in serum-free medium with either no drug, 400 nM [32P]AS1411 containing 2 × 105 cpm, or 400 nM [32P]CRO-26 containing 2 × 105 cpm. The cells were resuspended in PBS, and formaldehyde was added to the cell suspension to a final concentration of 1.0% (v/v). The reaction was incubated at room temperature for 10 min with slow mixing. Cross-linking was quenched by the addition of glycine (pH 7.0, 0.125 M final concentration) followed by incubation of the mixture at room temperature for 5 min. The 1% Triton X-100-soluble plasma membrane fraction was isolated as described above. Aliquots containing equal amounts of protein (200 μg) were mixed with 20 μl of Protein A-Sepharose beads (Santa Cruz) for 1 h at 4°C followed by centrifugation at 400g for 5 min. The precleared supernatant was diluted with 250 μl of RIPA buffer containing RNasin and protease inhibitors, mixed with either monoclonal anti-nucleolin antibody (3 μg/ml) or isotype-matched IgG antibody (3 μg/ml), and incubated for 3.5 h at 4°C. Protein A-Sepharose beads were added, and the samples were incubated with shaking for 3 h at 4°C. The immunoprecipitates were washed twice, run on an agarose/TAE gel, which was then dried on nitrocellulose paper, and analyzed with the use of a Typhoon PhosphorImager (GE Healthcare).
Inhibition of AS1411 Binding to Plasma Membrane Nucleolin by Anti-Nucleolin Antibody.
MV4-11 cells (106 cells/sample) were preincubated with either PBS or 10 μg/ml monoclonal anti-nucleolin antibody MS-3 for 45 min on ice before the incubation of the cells for an additional 45 min on ice with 10 μM FITC-AS1411. As a negative control, the untreated cells were incubated with 10 μg/ml isotype-matched (to the MS-3 antibody) mouse IgG or with 10 μg/ml monoclonal anti-cytochrome c oxidase antibody, followed by the FITC-conjugated secondary antibody. As a positive control, the untreated cells were incubated with 10 μg/ml mouse monoclonal anti-β3-integrin antibody. The cells were then washed twice and resuspended in 50 μl of PBS, and the fluorescence intensity (485 nm/530 nm) was quantitated using a fluorescence plate reader.
Drug Uptake Studies.
MV4-11 cells and K562 cells were seeded in 12-well culture plates at 4 × 105 cells/well and were used 48 h later when the cells were in the exponential growth phase. MCF-7 cells previously transfected with either a nucleolin shRNA or a scrambled shRNA were seeded at 3 × 105 cells/ml and used 48 h later. Unlabeled AS1411 was mixed with [32P]AS1411 to achieve a final concentration of 400 nM containing 2 × 105 cpm and incubated with the cells for 0, 1, 2, or 4 h. After drug incubation, the cells were washed three times with ice-cold PBS and once with PBS-EDTA. The cells were then lysed for 10 min on ice in lysis buffer (10 mM HEPES-KOH buffer, pH8.0, 200 mM mannitol, 68 mM sucrose, 10 mM KCl, 1.5 mM MgCl2, protease inhibitor solution, and 1 mM activated sodium orthovanadate) followed by sonication for 5 s at 20% output with a microtip. The lysates were then centrifuged at 10,000g for 10 min at 4°C to yield S10 extracts. The S10 extracts were then centrifuged at 100,000g for 1 h to yield S100 extracts. Protein concentrations were determined by BCA assay (Pierce, Rockford, IL). Radioactivity in the S100 extracts was counted using a liquid scintillation counter.
Measurement of Bcl-2 mRNA Stability.
MV4-11 cells were incubated with either no drug, 5 μM AS1411, or 10 μM CRO-26 for 72 h. The cells were then incubated with either 0.5% ethanol or 3 μg/ml actinomycin D in 0.5% ethanol. Aliquots were removed from the cultures at times 0, 2, 4, and 8 h. Actinomycin D at this concentration did not induce any DNA fragmentation during this time period. At the various time points, 2 × 107 cells were harvested by centrifugation and washed with PBS. Total RNA was isolated using TRIzol (Invitrogen), and the RNA concentrations were determined spectrophotometrically at 260 nm. Equal amounts of total RNA (2–5 μg) from each sample were reverse-transcribed using Moloney murine leukemia virus reverse transcriptase and random hexamers. PCR amplification of the cDNAs was carried out with primer pairs for the bcl-2 message (5′-GGAAGTGAACATTTCGGTGAC-3′; 5′-GCCTCTCCTCACGTTCCC-3′) and the β-actin message (5′-GCGGGAAATCGTGCGTGACAT-3′; 5′-GATGGAGTTGAAGGTA-GTTC-3′). All PCR primers were obtained from Integrated DNA Technologies, Inc. (Coralville, IA). The reaction mixture contained 200 nM dATP, dCTP, dGTP, and dTTP primers at 200 nM each, 2.5 units of HotStar Taq DNA polymerase (QIAGEN, Valencia, CA), which lacks 3′5′-exonuclease activity, 2.5 mM MgCl2, and 1 μl of cDNA product in a final volume of 25 μl. The HotStar Taq DNA polymerase was activated by a 15-min incubation at 95°C in the thermal cycler. This was followed by template denaturation for 1 min at 94°C, primer-template annealing for 1 min at 57°C, and then primer extension for 1 min at 72°C. After 26 cycles for bcl-2 and 24 cycles for β-actin, the extension reactions were continued for an additional 7 min at 72°C. The PCR products were separated on a 1% agarose gel, stained with ethidium bromide, and product formation quantitated by determining the integrated density value of each band. Product formation was linear over the range of the amounts of cDNA and PCR cycles used.
Results
Nucleolin Is Present on the Cell Surface of MV4-11 Cells.
If nucleolin serves as a receptor for AS1411 on MV4-11 cells, then it would be expected to be present on the surface of these cells. The initial studies used confocal microscopy to examine the colocalization of nucleolin with the cell membrane marker dye FM 4-64. At 4°C, FM 4-64 inserts into the outer leaflet of the plasma membrane and thus serves as a cell surface marker dye. Figure 1A shows in duplicate that nucleolin (green fluorescence) colocalized with FM 4-64 (red fluorescence) in nonpermeabilized MV4-11 cells. These results suggest that nucleolin is present on the surface of these cells.
Fig. 1.
A, confocal images of plasma membrane nucleolin in MV4-11 cells. Nucleolin localization was determined by indirect immunofluorescence using an anti-nucleolin primary antibody and an FITC-conjugated secondary antibody. Colocalization of nucleolin staining with the cell membrane marker dye, FM 4-64, demonstrates the presence of nucleolin in the plasma membrane of MV4-11 cells. The magnification used to capture the images was 88×. Results from two separate experiments are shown. B, effect of AS1411 and CRO-26 on the growth (cell counting) and viability [ 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium assay] of MV4-11 and K-562 cells. ♦, untreated control; ○, 10 μM CRO-26; ▴, 5 μM AS1411; ●, 10 μM AS1411; ■, 20 μM AS1411.
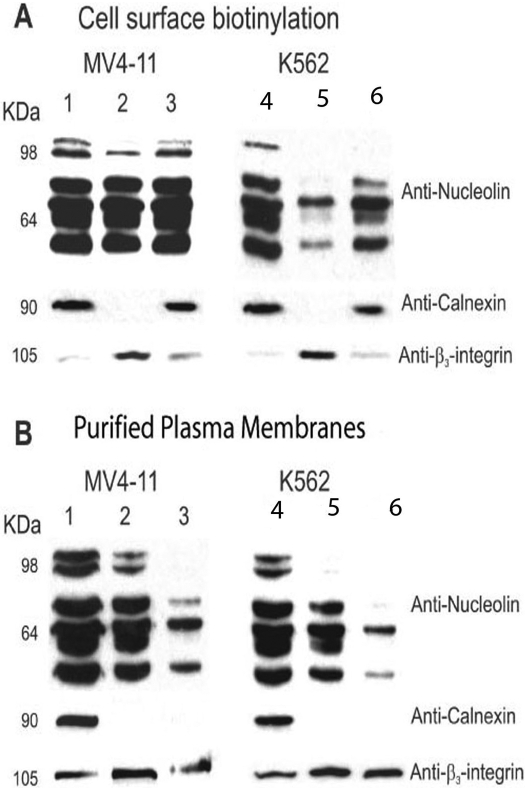
Results from 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium assays showed that MV4-11 cells were more sensitive (IC50 = 4 μM) than K-562 leukemia cells (IC50 = 16 μM) to AS1411 after a 72-h continuous drug exposure (Fig. 1B). To further investigate the expression of nucleolin on the cell surface and to relate this expression to the cytotoxic effects of AS1411, the cell surface proteins of MV4-11 cells and K-562 leukemia cells were biotinylated using EZ-link-NHS-biotin. This reagent reacts only with cell surface proteins and is not internalized. Figure 2A (lane 2, avidin-bound fraction) shows that full-length nucleolin (>98 kDa) and lower molecular mass forms of nucleolin were expressed on the cell surface of MV4-11 cells. The truncated forms of nucleolin are probably fragments that result from the self-cleaving activity of nucleolin (Chen et al., 1991; Fang and Yeh, 1993). In contrast, K-562 cells, which are less sensitive than MV4-11 cells to AS1411, showed no full-length nucleolin and smaller amounts of the lower molecular mass forms of nucleolin in the avidin-bound fraction (lane 5). Full-length nucleolin was detected only in the total cytosolic fraction of K-562 cells (lane 4). β3-Integrin served as a membrane-positive control, because it is expressed at high levels in plasma membranes. Calnexin, an intrinsic membrane protein of the endoplasmic reticulum, was not detected in the avidin-bound fraction indicating a lack of contamination of the plasma membrane fractions with cytoplasmic components. Likewise, immunoblotting with an anti-human nucleolin antibody of the Triton X-100-soluble plasma membranes purified by sucrose density gradient centrifugation revealed that full-length nucleolin was present in the plasma membrane of MV4-11 cells (Fig. 2B, lane 2) but was undetectable in the Triton X-100 soluble plasma membranes from K-562 cells (lane 5). These results indicate that expression of nucleolin on the cell surface correlates with the sensitivity of these leukemia cells to AS1411.
Fig. 2.
Cell surface and plasma membrane nucleolin in MV4-11 and K-562 cells. A, biotinylation of cell surface nucleolin in MV4-11 and K-562 cells. Cell surface proteins were labeled with the membrane-impermeant probe EZ-link-sulfo-NHS-biotin and the biotinylated plasma membrane were purified by avidin affinity chromatography. Nucleolin, calnexin, and β3-integrin were identified by immunoblotting. Lanes 1 and 4, total soluble nucleolin; lanes 2 and 5, avidin-bound fraction; lanes 3 and 6, avidin-unbound fraction. B, immunoblot of nucleolin, calnexin, and β3-integrin in the plasma membrane fractions purified by sucrose density gradient centrifugation. Lanes 1 and 4, total soluble nucleolin; lanes 2 and 5, 1% Triton X-100 soluble plasma membrane nucleolin; lanes 3 and 6, 1% Triton X-100-insoluble plasma membrane nucleolin. The results shown in A and B are representative of three separate experiments, all of which yielded similar results.
Secretion of nucleolin by either MV4-11 (lane 2) or K-562 (lane 3) cells into the tissue culture medium was not detected despite a 50-fold concentration of the medium before immunoblot analysis (Supplemental Fig. S1). In contrast, nucleolin was readily detected in the positive control samples consisting of either 1 μg of recombinant nucleolin added to tissue culture media (lane 1) or endogenous nucleolin present in S10 extracts of MV4-11 (lane 4) or K-562 cells (lane 5). These results suggest that the presence of nucleolin on the cell surface is not the result of adsorption of secreted nucleolin to cell surface macromolecules.
AS1411 Induces bcl-2 mRNA Instability in M4-11 Cells.
A major biochemical effect of AS1411 observed in tumor cells that overexpress plasma membrane nucleolin compared with normal cells is the induction of bcl-2 mRNA instability and subsequent down-regulation of Bcl-2 protein (Sengupta et al., 2004; Soundararajan et al., 2008). It was of interest to determine whether incubation of MV4-11 cells with AS1411 also leads to destabilization of bcl-2 mRNA. PhosphorImaging and analysis of the data in Supplemental Fig. S2 revealed that the half-lives of bcl-2 mRNA were 8.0 and 1.1 h in MV4-11 cells incubated for 72 h with either 10 μM CRO-26 or 5 μM AS1411, respectively. Thus, bcl-2 mRNA also becomes highly unstable in MV4-11 cells after exposure to AS1411.
AS1411 Binds to Plasma Membrane Nucleolin.
MV4-11 cells were incubated with either [32P]AS1411 or [32P]CRO-26 and cross-linked with formaldehyde. CRO-26 is a control oligonucleotide in which each dG of AS1411 is replaced by dC. It does not form a stabilized quadruplex structure and lacks antitumor activity (Bates et al., 1999). Immunoprecipitation of the purified soluble plasma membrane fraction with anti-nucleolin antibody followed by SDS-polyacrylamide gel electrophoresis of the solubilized immunoprecipitates revealed that nearly all of the [32P]AS1411 migrated as a [32P]AS1411-nucleolin complex of approximately 100 kDa (Fig. 3). Free [32P]AS1411 migrated near the solvent front on the gel. The minor [32P]AS1411 bands that migrated slower than the 98-kDa marker protein probably represent [32P]AS1411 that was cross-linked to other membrane proteins larger than full-length nucleolin. No [32P]AS1411-protein complexes were detected when immunoprecipitation was carried out using an irrelevant mouse IgG antibody. Likewise no CRO-26-protein complexes were seen when MV4-11 cells were incubated with [32P]CRO-26 and immunoprecipitated with either an anti-nucleolin or an irrelevant IgG antibody.
Fig. 3.
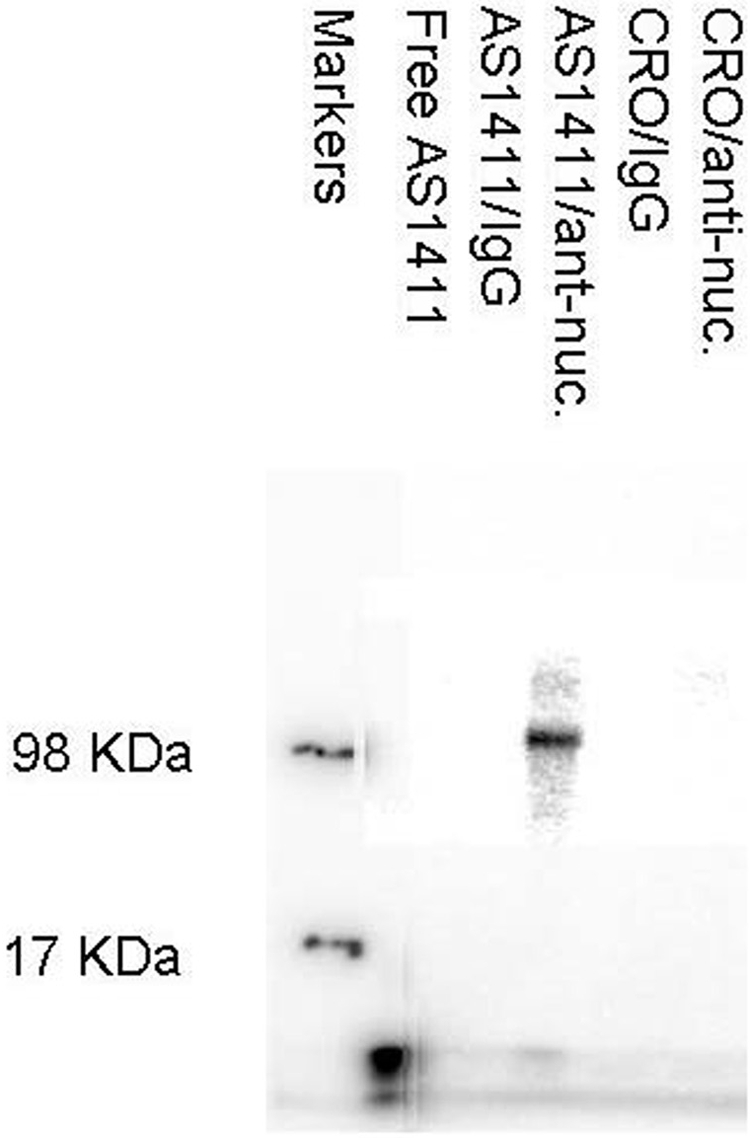
PhosphorImage of immunoprecipitated [32P]AS1411-nucleolin complexes. MV4-11 cells were incubated with either [32P]AS1411 or [32P]CRO-26 and cross-linked with formaldehyde. Immunoprecipitation of the purified soluble plasma membrane fraction with anti-nucleolin antibody or nonspecific IgG antibody was followed by analysis of the solubilized immunoprecipitates on an agarose/TAE gel, and the radioactivity was detected by PhosphorImaging. Protein molecular mass markers were labeled with a 32P solution. Lanes from left to right: lane 1, molecular mass markers; lane 2, free [32P]AS1411; lane 3, control immunoprecipitation of [32P]AS1411-treated cells with an irrelevant IgG antibody; lane 4, immunoprecipitation of [32P]AS1411-treated cells with anti-nucleolin antibody; lane 5, immunoprecipitation of [32P]CRO-26-treated cells with an irrelevant IgG antibody; lane 6, immunoprecipitation of [32P]CRO-26 -treated cells with anti-nucleolin antibody.
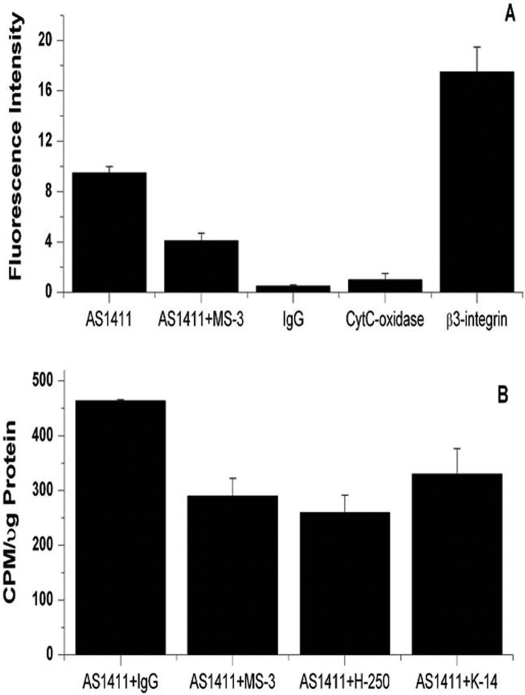
Preincubation of MV4-11 cells with the MS-3 anti-nucleolin antibody inhibited binding of FITC-AS1411 to plasma membrane nucleolin 56 ± 10% SE (n = 3, P < 0.01) compared with cells incubated with FITC-AS1411 only (Fig. 4A). In all experiments, background fluorescence emitted by control samples consisting of MV4-11 cells plus FITC-IgG was less than 4% of that emitted by an equal number of cells incubated with FITC-AS1411. The cells were incubated with antibodies and FITC-AS1411 on ice to prevent their internalization. Under these conditions, mitochondrial cytochrome c oxidase was barely detectable (Fig. 4A), and no FITC-AS1411 was detected by confocal microscopy in either the cytoplasm or nucleus of the cells (data not shown). The positive control, anti-β3 integrin, gave a strong fluorescence signal, reflecting a high cell surface expression of this integrin subunit on MV4-11 cells. Thus, the partial inhibition (56%) of FITC-AS1411 binding to nucleolin by the MS-3 monoclonal anti-nucleolin antibody was not the result of binding of AS1411 to nucleolin inside the cell in which it is inaccessible to the anti-nucleolin antibody. The effect of various anti-nucleolin antibodies on the direct binding of [32P]AS1411 to nucleolin within purified plasma membranes was also examined (Fig. 4B). Plasma membranes were isolated from MV4-11 cells, and aliquots containing 5 μg of membrane protein were preincubated with the indicated anti-nucleolin antibodies (10 μg/ml) for 1 h at room temperature and then incubated with 2 nM [32P]AS1411 for an additional hour at room temperature. The MS-3 monoclonal antibody was raised against full-length human nucleolin, the H-250 rabbit polyclonal antibody was raised against amino acids 251 to 520 of human nucleolin, and the goat polyclonal antibody was raised against an unspecified internal sequence of human nucleolin (Santa Cruz Biotechnology, product literature). Despite the diversity of antibodies that were used, only partial inhibition of [32P]AS1411 binding to nucleolin in isolated plasma membranes was observed compared with an irrelevant IgG antibody. These results seem consistent with the possibility that cell surface nucleolin is heterogeneous with respect to its association with the plasma membrane and recognition by various antibodies (see Discussion).
Fig. 4.
Anti-nucleolin antibody inhibits binding of AS1411 to plasma membrane nucleolin. A, MV4-11 cells (106 cells/sample) were preincubated with either PBS or 10 μg/ml monoclonal anti-nucleolin antibody MS-3 for 45 min on ice before the incubation of the cells for an additional 45 min on ice with 10 μM FITC-AS1411. As a negative control, the untreated cells were incubated with 10 μg/ml MS-3 isotype-matched mouse IgG or with 10 μg/ml monoclonal anti-cytochrome c oxidase antibody, followed by the FITC-conjugated secondary antibody. As a positive control, the untreated cells were incubated with 10 μg/ml MS-3 isotype-matched mouse monoclonal anti–β3-integrin antibody. The cells were then washed twice and resuspended in 50 μl of PBS, and the fluorescence intensity (485/530 nm) was quantitated using a plate reader. B, plasma membranes from MV4-11 cells were isolated by sucrose density gradient centrifugation and resuspended in HBS containing protease inhibitors. Samples containing 5 μg of membrane protein were preincubated with the indicated antibodies (10 μg/ml, 67 nM) for 1 h at room temperature and then incubated with 2 nM [32P]AS1411 for an additional hour at room temperature. The plasma membranes were washed twice with HBS, and the radioactivity was counted by liquid scintillation counting.
Nucleolin-Dependent Uptake of AS1411 into Tumor Cells.
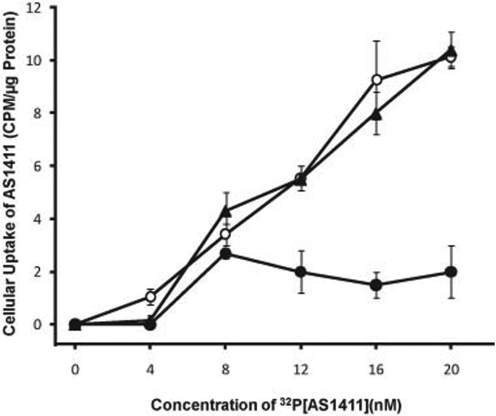
Additional studies were carried out to determine whether plasma membrane nucleolin has a functional role in the cellular uptake of AS1411. The uptake of [32P]AS1411 into the S100 cytoplasmic fraction of MV4-11 cells was concentration-dependent over the range of 4 to 168 nM (8 nM [32P]AS1411 plus 160 nM unlabeled AS1411) (Fig. 5). PhosphorImaging analysis of the S100 fraction run on a 5% polyacrylamide gel revealed that essentially all of the radioactivity was associated with intact [32P]AS1411 and a negligible amount of free 32P was detected in the solvent front. It is important to note that [32P]AS1411 uptake at concentrations greater than 8 nM was blocked by a 20-fold excess of unlabeled AS1411 but not by a 20-fold excess of unlabeled CRO-26. This indicated that the cellular uptake of AS1411 into MV4-11 cells was mediated by a specific, high-affinity receptor.
Fig. 5.
Inhibition of [32P]AS1411 uptake into MV4-11 cells by excess unlabeled AS1411. Aliquots of MV4-11 cells were incubated for 2 h with increasing concentrations of [32P]AS1411 only (○) or with the same concentrations of [32P]AS1411 plus a 20-fold excess of either unlabeled AS1411 (●) or unlabeled CRO-26 (▴). The amounts of radioactivity in the S100 cytoplasmic fractions were determined by liquid scintillation counting, and the results were normalized to the amount of protein in each sample. Results are expressed as the means of three determinations ± S.E. Similar results were obtained when the experiment was repeated.
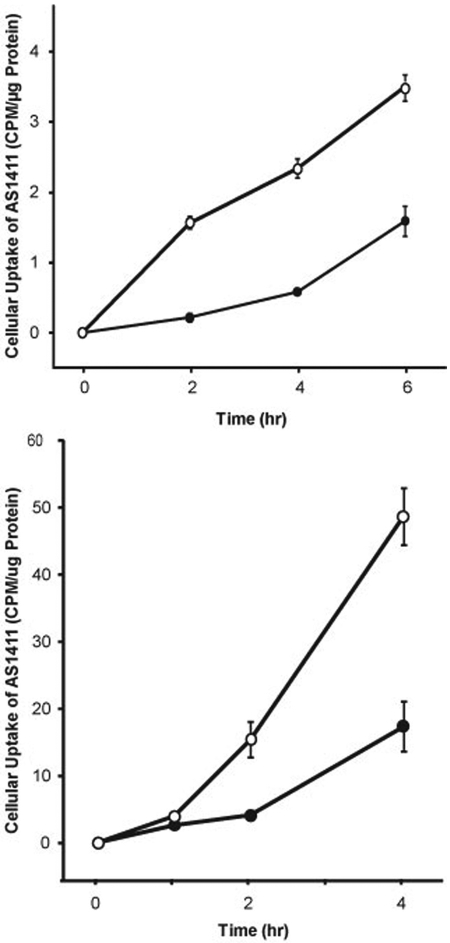
The following studies provide further evidence that the protein responsible for the specific uptake of AS1411 is nucleolin. As indicated above, K-562 cells are less sensitive to the cytotoxic effect AS1411 than MV4-11 cells. No full-length nucleolin (>98 kDa) and lesser amounts of the lower molecular mass forms of nucleolin were present in the plasma membrane fraction of K-562 cells compared with MV4-11 cells (Fig. 2). MV4-11 and K-562 cells were incubated with 400 nM [32P]AS1411 to ensure that cellular uptake was not limited by the extracellular drug concentration (Fig. 5). Uptake of [32P]AS1411 into the cytoplasm of MV4-11 cells was approximately 3-fold faster (slope = 0.59 ± 0.02 SE cpm/μg protein/h, r = 0.99) than in K-562 cells (slope = 0.21 ± 0.03 cpm/μg protein/h, r = 0.93) (Fig. 6A).
Fig. 6.
Uptake of AS1411 into MV4-11 and K-562 into human leukemia cells. A, aliquots of MV4-11 (○) and K-562 cells (●) were incubated for either 0, 2, 4, or 6 h with 2 × 105 cpm [32P]AS1411 and sufficient unlabeled AS1411 to achieve a final concentration of 400 nM. Uptake [32P]AS1411 into the S100 cytoplasmic fraction was determined by liquid scintillation counting, and the results were normalized to the amount of protein in each sample. Results are expressed as the means of six determinations ± S.E. B, uptake of AS1411 into nucleolin knockdown cells. MCF-7 cells previously transfected with either a nucleolin shRNA (●) or a scrambled shRNA (○) were seeded at 3 × 105 cells/ml. Unlabeled AS1411 was mixed with [32P]AS1411 to achieve a final concentration of 400 nM containing 2 × 105 cpm and incubated with the cells for either 0, 1, 2, or 4 h. Uptake of [32P]AS1411 into the S100 cytoplasmic fraction was determined by liquid scintillation counting, and the results were normalized to the amount of protein in each sample. Results are expressed as the means of three separate experiments ± S.E.
Repeated attempts at knocking down nucleolin with short interfering RNAs in MV4-11 cells were unsuccessful. Alternatively, we used MCF-7 cells that were transfected previously with either a nucleolin shRNA or with a scrambled shRNA having limited homology to any known human genomic sequence (Soundararajan et al., 2008). The nucleolin shRNA transfectants have decreased plasma membrane and cytoplasmic nucleolin compared with the MCF-7 cells that were transfected with the scrambled shRNA. At 2 and 4 h after the start of the drug incubation, the levels of [32P]AS1411 were approximately 3-fold higher, respectively, in the S100 cytoplasmic fraction of the scrambled shRNA transfectants than in the nucleolin shRNA transfectants (Fig. 6B). In summary, the results presented herein indicate that plasma membrane nucleolin functions as a cellular receptor for AS1411.
Discussion
The ability of nucleolin to perform numerous and diverse functions within the cell is related to the multiple structural domains within the protein. Its negatively charged N-terminal domain regulates rDNA transcription by inducing nucleolar chromatin decondensation (Srivastava et al., 1989), whereas the central globular domain contains four RNA binding domains (Serin et al., 1997). It has been proposed that nucleolin, via binding of its RNA binding domain and its RGG-rich C-terminal domains to preribosomal RNA, functions as an assembly factor by bringing together the correctly folded rRNA and other components necessary for rRNA maturation and ribosome assembly (Ginisty et al., 2001). Nucleolin may also be involved in exporting ribosomes to the cytoplasm while shuttling between the cytoplasm and nucleus (Srivastava and Pollard, 1999). AS1411 is known to interfere with the stabilization of bcl-2 mRNA by nucleolin in human breast cancer cells (Soundararajan et al., 2008) and in various types of human leukemia cells, including MV4-11 cells (Supplemental Figure S2). We have reported previously that the binding of AS1411 to nucleolin in MCF-7 breast cancer cells interferes with the ability of nucleolin to protect bcl-2 mRNA from degradation. This leads to bcl-2 mRNA instability and induction of apoptosis (Soundararajan et al., 2008).
It seems that the shuttling of nucleolin between the cell surface, cytoplasm, and nucleus (Said et al., 2002; Christian et al., 2003) is of particular importance with regard to the pharmacokinetics of AS1411. Nucleolin has been isolated as a phosphoprotein from the surface of various types of proliferating cells (Pfeifle and Anderer, 1983; Hovanessian et al., 2000). In some of these cells, plasma membrane nucleolin has been reported to function as a receptor for different ligands, including intimin-γ of Escherichia coli (Sinclair and O'Brien, 2002), the anti-HIV agent midkine (Said et al., 2002), laminin-1 (Kibbey et al., 1995), DNA nanoparticles (Chen et al., 2008), and the antiangiogenic pseudopeptide HB-19 (Destouches et al., 2008). In proliferating tumor cells, nucleolin is often associated with endocytotic vesicles that invaginate from the plasma membrane (Hovanessian et al., 2000). Ligands bound to nucleolin within these vesicles become internalized in a temperature-dependent process. Bates et al. (1999) speculated that nucleolin may serve as a receptor for certain G-rich oligonucleotides having antiproliferative activity, because nucleolin in plasma membrane extracts bound the active G-rich oligonucleotides when analyzed by Southwestern blotting. The results reported herein show directly that nucleolin present in the plasma membrane of MV4-11 cells is a functional receptor for AS1411. It is interesting that CRO-26, a noncytotoxic oligonucleotide, did not form an immunoprecipitatable complex with plasma membrane nucleolin. In addition, CRO-26 did not inhibit AS1411 uptake when MV4-11 cells were incubated with concentrations of CRO-26 that were 20-fold greater than the corresponding AS1411 concentrations. K-562 leukemia cells, which have lower levels of plasma membrane nucleolin than MV4-11 cells and a slower rate of AS1411 uptake, were also less sensitive to the cytotoxic effects of AS1411. Complete knockdown of nucleolin was incompatible with cell survival. However, partial knockdown of plasma membrane nucleolin in MCF-7 cells resulted in slower cellular uptake of AS1411. When taken together, these data indicate that binding of AS1411 to plasma membrane nucleolin is a determinant of the cellular uptake and subsequent antitumor effect of AS1411.
The anti-nucleolin monoclonal antibody MS-3 partially inhibited binding of FITC-AS1411 to plasma membrane nucleolin of intact MV4-11 cells (56% inhibition). Likewise, anti-nucleolin antibodies MS-3, H-250, and K-14 only partially blocked binding of [32P]AS1411 to nucleolin in purified plasma membranes. These results are interesting in light of the diversity of the nucleolin epitopes recognized by the above antibodies. One explanation for these results is that cell surface nucleolin is heterogeneous with respect to its association with the plasma membrane and recognition by anti-nucleolin antibodies. Nucleolin lacks a transmembrane domain or signal sequence but undergoes extensive post-translational modification (Lapeyre et al., 1987; Srivastava et al., 1989). This could result in a fraction of the nucleolin epitopes being masked within the plasma membranes without interfering with the ability of the protein to bind AS1411. Palmitoylation, prenylation, or myristoylation of nucleolin may allow for heterogeneous insertion or anchoring of these hydrophobic regions of the protein into the plasma membrane (Fig. 2B, Triton X-100 soluble fraction). Because AS1411 is proposed to form a compact G-quadruplex structure (Dapic et al., 2003), it may have better access to its binding site on nucleolin than the more bulky anti-nucleolin antibodies. Our results show that nucleolin is not secreted from either MV4-11 cells or K-562 cells into the tissue culture medium. This suggests that the presence of nucleolin on the cell surface is not the result of adsorption of secreted nucleolin by macromolecules on the cell surface of tumor cells. Although AS1411 binds to nucleolin with high affinity and specificity (Ireson and Kelland, 2006), the data reported herein do not rule out completely the possibility that the surface of MV4-11 cells contains more that one type of receptor for AS1411. However, the results do provide a biochemical rationale for the use of AS1411 as a novel targeted therapeutic for patients whose AML cells express cell surface nucleolin.
Supplementary Material
Acknowledgments
We acknowledge the assistance with the AS1411-anti-nucleolin antibody competition experiments that was provided by Rick Pepler of the Hollings Cancer Center Flow Cytometry Facility. Confocal microscopy was carried out in the Cell and Molecular Imaging Shared Resource of the Hollings Cancer Center.
 The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the National Institutes of Health National Cancer Institute [Grant CA109254]; the Leukemia and Lymphoma Society [Grant 6006-06]; and an unrestricted grant from Antisoma Ltd., London, UK.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.055947
- AML
- acute myeloid leukemia
- HBS
- HEPES-buffered saline
- FITC
- fluorescein isothiocyanate
- PCR
- polymerase chain reaction
- shRNA
- short hairpin RNA
- PBS
- phosphate-buffered saline
- RIPA
- radioimmunoprecipitation assay
- HB-19
- 5[Kψ(CH2N)PR]-TASP.
References
- Bates PJ, Kahlon JB, Thomas SD, Trent JO, Miller DM. (1999) Antiproliferative activity of G-rich oligonucleotides correlates with protein binding. J Biol Chem 274:26369–26377 [DOI] [PubMed] [Google Scholar]
- Chen CM, Chiang SY, Yeh NH. (1991) Increased stability of nucleolin in proliferating cells by inhibition of its self-cleaving activity. J Biol Chem 266:7754–7758 [PubMed] [Google Scholar]
- Chen X, Kube DM, Cooper MJ, Davis PB. (2008) Cell surface nucleolin serves as receptor for DNA nanoparticles composed of pegylated polylysine and DNA. Mol Ther 16:333–342 [DOI] [PubMed] [Google Scholar]
- Christian S, Pilch J, Akerman ME, Porkka K, Laakkonen P, Ruoslahti E. (2003) Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J Cell Biol 163:871–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapić V, Abdomerović V, Marrington R, Peberdy J, Rodger A, Trent JO, Bates PJ. (2003) Biophysical and biological properties of quadruplex oligodeoxyribonucleotides. Nucleic Acids Res 31:2097–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destouches D, El Khoury D, Hamma-Kourbali Y, Krust B, Albanese P, Katsoris P, Guichard G, Briand JP, Courty J, Hovanessian AG. (2008) Suppression of tumor growth and angiogenesis by a specific antagonist of the cell-surface expressed nucleolin. PLoS ONE 3:e2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang SH, Yeh NH. (1993) The self-cleaving activity of nucleolin determines its molecular dynamics in relation to cell proliferation. Exp Cell Res 208:48–53 [DOI] [PubMed] [Google Scholar]
- Fogal V, Sugahara KN, Ruoslahti E, Christian S. (2009) Cell surface nucleolin antagonist causes endothelial cell apoptosis and normalization of tumor vasculature. Angiogenesis 12:91–100 [DOI] [PubMed] [Google Scholar]
- Ginisty H, Amalric F, Bouvet P. (2001) Two different combinations of RNA-binding domains determine the RNA binding specificity of nucleolin. J Biol Chem 276:14338–14343 [DOI] [PubMed] [Google Scholar]
- Hovanessian AG, Puvion-Dutilleul F, Nisole S, Svab J, Perret E, Deng JS, Krust B. (2000) The cell-surface-expressed nucleolin is associated with the actin cytoskeleton. Exp Cell Res 261:312–328 [DOI] [PubMed] [Google Scholar]
- Ireson CR, Kelland LR. (2006) Discovery and development of anticancer aptamers. Mol Cancer Ther 5:2957–2962 [DOI] [PubMed] [Google Scholar]
- Kibbey MC, Johnson B, Petryshyn R, Jucker M, Kleinman HK. (1995) A 110-kD nuclear shuttling protein, nucleolin, binds to the neurite-promoting IKVAV site of laminin-1. J Neurosci Res 42:314–322 [DOI] [PubMed] [Google Scholar]
- Laber DA, Sharma VR, Bhupalam L, Taft B, Hendler FJ, Barnhart KM. (2005) Update on the first phase I study of AGRO100 in advanced cancer (Abstract). J Clin Oncol 23 ( 16 Suppl): 3064 [Google Scholar]
- Lapeyre B, Bourbon H, Amalric F. (1987) Nucleolin, the major nucleolar protein of growing eukaryotic cells: an unusual protein structure revealed by the nucleotide sequence. Proc Natl Acad Sci U S A 84:1472–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niranjanakumari S, Lasda E, Brazas R, Garcia-Blanco MA. (2002) Reversible cross-linking combined with immunoprecipitation to study RNA-protein interactions in vivo. Methods 26:182–190 [DOI] [PubMed] [Google Scholar]
- Otake Y, Sengupta TK, Bandyopadhyay S, Spicer EK, Fernandes DJ. (2005) Retinoid-induced apoptosis in HL-60 cells is associated with nucleolin down-regulation and destabilization of Bcl-2 mRNA. Mol Pharmacol 67:319–326 [DOI] [PubMed] [Google Scholar]
- Otake Y, Soundararajan S, Sengupta TK, Kio EA, Smith JC, Pineda-Roman M, Stuart RK, Spicer EK, Fernandes DJ. (2007) Overexpression of nucleolin in chronic lymphocytic leukemia cells induces stabilization of bcl2 mRNA. Blood 109:3069–3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifle J, Anderer FA. (1983) Isolation and characterization of phosphoprotein pp 105 from simian virus 40-transformed mouse fibroblasts. Biochim Biophys Acta 762:86–93 [DOI] [PubMed] [Google Scholar]
- Said EA, Krust B, Nisole S, Svab J, Briand JP, Hovanessian AG. (2002) The anti-HIV cytokine midkine binds the cell surface-expressed nucleolin as a low affinity receptor. J Biol Chem 277:37492–37502 [DOI] [PubMed] [Google Scholar]
- Sengupta TK, Bandyopadhyay S, Fernandes DJ, Spicer EK. (2004) Identification of nucleolin as an AU-rich element binding protein involved in bcl-2 mRNA stabilization. J Biol Chem 279:10855–10863 [DOI] [PubMed] [Google Scholar]
- Serin G, Joseph G, Ghisolfi L, Bauzan M, Erard M, Amalric F, Bouvet P. (1997) Two RNA-binding domains determine the RNA-binding specificity of nucleolin. J Biol Chem 272:13109–13116 [DOI] [PubMed] [Google Scholar]
- Sinclair JF, O'Brien AD. (2002) Cell surface-localized nucleolin is a eukaryotic receptor for the adhesin intimin-γ of enterohemorrhagic Escherichia coli O157:H7. J Biol Chem 277:2876–2885 [DOI] [PubMed] [Google Scholar]
- Soundararajan S, Chen W, Spicer EK, Courtenay-Luck N, Fernandes DJ. (2008) The nucleolin targeting aptamer AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer cells. Cancer Res 68:2358–2365 [DOI] [PubMed] [Google Scholar]
- Srivastava M, Fleming PJ, Pollard HB, Burns AL. (1989) Cloning and sequencing of the human nucleolin cDNA. FEBS Lett 250:99–105 [DOI] [PubMed] [Google Scholar]
- Srivastava M, Pollard HB. (1999) Molecular dissection of nucleolin's role in growth and cell proliferation: new insights. FASEB J 13:1911–1922 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.