Abstract
The formation of multiprotein complexes is a repeated theme in biology ranging from the regulation of the extracellular signal-regulated kinase and cAMP signaling pathways to the formation of postsynaptic density complexes or tight junctions. A-kinase anchoring proteins (AKAPs) are well known for their ability to scaffold protein kinase A and components upstream and downstream of cAMP production, including G protein-coupled receptors, cAMP-dependent Rap-exchange factors, and phosphodiesterases. Specific adenylyl cyclase (AC) isoforms have also been identified as components of AKAP complexes, namely AKAP79, Yotiao, and mAKAP. In this review, we summarize recent evidence for AC-AKAP complexes and requirements for compartmentalization of cAMP signaling. The ability of AKAPs to assemble intricate feedback loops to control spatiotemporal aspects of cAMP signaling adds yet another dimension to the classic cAMP pathway.
The generation of cAMP and subsequent activation of protein kinase A (PKA) is one of the best understood signal transduction pathways. However, it remains unclear how the soluble second-messenger cAMP achieves any type of subcellular or molecular specificity. PKA phosphorylates a broad range of substrates but somehow manages to mediate precise phosphorylation events at specific sites within the cell. For example, stimulation of β1 adrenergic receptors (ARs), β2AR, or prostaglandin E1 receptors have clearly distinguishable effects on cardiac myocytes, despite each being coupled to adenylyl cyclase (AC) (Buxton and Brunton, 1983; Steinberg and Brunton, 2001). The follicle-stimulating hormone and luteinizing hormone also seem to use the same intracellular intermediates but activate different sets of genes in granulose cells (Conti, 2002). Measurements of cAMP using cyclic nucleotide-gated channels (CNGs or HCN) or fluorescence resonance energy transfer reporters based on PKA or EPAC have provided direct evidence for limited cAMP diffusion throughout the cell (Fischmeister et al., 2006; Berrera et al., 2008). Where cAMP is produced is also critical, because cAMP generated at the plasma membrane versus cytosol can have opposite effects on endothelial barrier function (Sayner et al., 2006). However, until recently, it was not clear how a restricted pool of cAMP generated by AC was specifically targeted to a select subset of effectors to give rise to distinct physiological outcomes. This problem is solved by tethering AC to complexes containing cAMP effectors and downstream targets. This review focuses on recent evidence that signalosomes formed by A-kinase anchoring proteins (AKAPs) help to coordinate cAMP synthesis and downstream signaling by assembling AC-AKAP complexes.
cAMP Synthesis: Mammalian Adenylyl Cyclase Isoforms
In higher eukaryotes, two basic families of adenylyl cyclase exist: the G protein-regulated transmembrane adenylyl cyclase isoforms, and a soluble adenylyl cyclase. The latter AC is regulated by bicarbonate and calcium and is insensitive to forskolin or activated Gαs (Kamenetsky et al., 2006). The topology of transmembrane ACs consists of a variable in tracellular N terminus and two large cytoplasmic domains separated by two membrane-spanning domains (six transmembranes each) (Sadana and Dessauer, 2009). The transmembrane class of ACs is generally considered the target of most hormone-sensitive cAMP control.
Regulation of AC Isoforms.
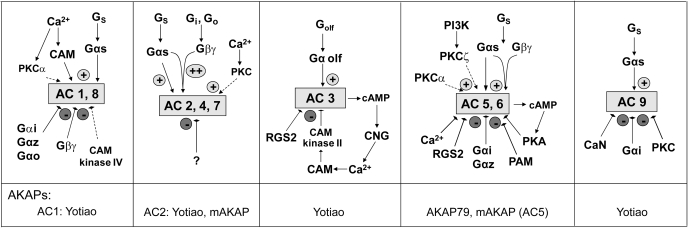
All nine membrane-bound AC isoforms are activated by GTP-bound Gαs and the plant diterpene forskolin, with the exception of AC9. The only other feature shared by all isoforms is the inhibition by adenosine analogs known as P-site inhibitors (Dessauer et al., 1999). Additional regulation among isoforms differs widely, as shown in Fig. 1 (Sadana and Dessauer, 2009). Therefore, one emerging question is how all of the regulation of AC is coordinated? In addition, how does a particular AC isoform generate a pool of cAMP that is positioned near appropriate downstream effectors, given that most cells contain multiple AC isoforms? Possibilities include the localization of ACs, AC regulators, and downstream effectors of cAMP to specific regions of the plasma membrane (i.e., lipid rafts), and the formation of higher-order complexes to facilitate interactions and provide specificity.
Fig. 1.
Regulation of AC isoforms. General patterns of regulation are shown for individual isoforms and, where appropriate, closely related ACs. Broken lines indicate modes of regulation that differ between grouped isoforms. AKAPs known to associate with AC isoforms are indicated. For simplicity, not all forms of regulation are shown. CAM, calmodulin; CNG, cyclic nucleotide-gated channel; Gs, heterotrimeric Gαs · βγ; PAM, protein activator of myc; PI3K, phosphatidylinositol-3-kinase; RGS, regulator of G protein signaling.
Lipid Raft Localization of ACs.
Compartmentalization of signaling can be achieved by localization of select ACs and other signaling molecules in lipid rafts. These highly dynamic structures are rich in cholesterol and sphingolipids, a subset of which also contains the protein caveolin. Lipid rafts are increasingly appreciated for their role in organizing a wide-range of signal-transduction cascades. For example, a growing number of G protein-coupled receptors (GPCRs), ion channels, and receptor tyrosine kinases are localized to lipid rafts (Insel and Patel, 2009). All of the calcium-sensitive ACs (AC 1, 3, 5, 6, and 8) but not the Ca2+-insensitive ACs (AC 2, 4, 7, and 9) are also localized to lipid raft structures, independent of caveolin expression (Cooper and Crossthwaite, 2006). Destruction of lipid rafts by extraction of cholesterol disrupts regulation of AC6 and AC8 by capacitative calcium entry (Fagan et al., 2000; Smith et al., 2002), suggesting that these structures are required for at least some forms of regulation. In addition, AC6 shows differential coupling to GPCRs that cosegregate with AC6 in lipid rafts, enhancing βAR and prostacyclin receptor signaling, but not prostaglandin EP2 or adenosine receptors upon overexpression of AC6 (Bundey and Insel, 2006; Liu et al., 2008). In addition to GPCRs, many other components of the cAMP pathway including G proteins, PDEs, phosphatases, PKC, PKA, and cyclic nucleotide-gated channels can be found within lipid rafts.
Complexes of AC with G Proteins, GPCRs, and Effectors.
Although lipid raft localization can, in part, explain the selective coupling of GPCRs to AC, the differential ability of ACs to regulate downstream pathways such as PKA, ERK, cell doubling times, or cAMP-mediated cytoskeletal reorganization clearly requires additional mechanisms to generate specificity (Gros et al., 2006). The existence of signaling complexes involving AC was first proposed in 1988 for AC and Gαs·βγ (Levitzki, 1988). Since that time, bioluminescence resonance energy transfer studies suggest that stable complexes occur between Gβγ subunits and AC2 (Rebois et al., 2006), and these complexes are probably assembled before insertion into the plasma membrane (Dupré et al., 2007). There is also evidence for AC complexes containing GPCRs (Lavine et al., 2002) or downstream signaling components. Coimmunoprecipitation of AC1 and ERK1/2 explains the selective activation of ERK signaling in human embryonic kidney 293 cells by AC1 but not AC2, 5, or 6 (Gros et al., 2006). ACs can form even larger complexes containing βAR, G proteins, PKA, phosphatases, and L-type Ca2+ channels to possibly facilitate highly spatially restricted signaling in neurons (Davare et al., 2001). The question becomes how these megacomplexes are assembled. The remainder of this review focuses on AKAPs that scaffold components of the cAMP signaling pathways, including AC, to achieve temporal and spatial specificity.
AKAPs Anchor PKA and Other Components of cAMP Signaling
Compartmentalization of PKA signaling is accomplished by means of AKAPs. Since their initial discovery in 1982 (Theurkauf and Vallee, 1982), more than 50 AKAPs have been identified that are highly divergent, with the exception of a signature PKA regulatory subunit docking motif (Wong and Scott, 2004; McConnachie et al., 2006). AKAPs are localized to numerous cellular sites, including the plasma membrane (AKAP79, Yotiao, AKAP18, and Gravin) (Klauck et al., 1996; Fraser et al., 1998; Lin et al., 1998; Grove and Bruchey, 2001) as well as Golgi, centrosome, nucleus, mitochondria, and cytosol (Feliciello et al., 2001). Those AKAPs that are located at the plasma membrane use a number of different strategies for docking, including myristoylation (AKAP18 and Gravin) (Lin et al., 1996; Fraser et al., 1998), polybasic regions (AKAP79 and Gravin) (Streb and Miano, 2005; Tao et al., 2006), or as-yet-unknown mechanisms (Yotiao). One of the important features of AKAP complexes is the intricate feedback loops that are assembled to control temporal aspects of cAMP signaling. For example, the assembly of protein kinases and phosphatases or PKA and PDEs ensures only local fluctuations in signal output and the possibility for oscillating pulses of activity (Smith et al., 2006a).
Evidence for AC-AKAP Interactions
Our recent evidence suggests that membrane-bound ACs are precoupled to AKAP complexes to potentially generate a local pool of cAMP. Because AKAPs target PKA to specific substrates, and targeting is an important aspect of PKA's ability to sense cAMP gradients, it makes sense that AC might also be in close proximity to these same molecules.
AKAP79/150.
The initial concept of AC-AKAP interactions was tested with AKAP79/150 (AKAP150 is the rat ortholog of human AKAP79, also known as AKAP5). It is highly expressed in neurons, particularly postsynaptic densities of excitatory synapses, and at lower levels in heart and other non-neuronal tissues (Sarkar et al., 1984; Bregman et al., 1989; Carr et al., 1992). AKAP79 anchors calcineurin (CaN, also known as protein phosphatase 2B), PKC, β2AR, and β1AR, in addition to AMPA-type glutamate receptors (via PSD-95 and SAP97), L-type Ca2+ channels, M-type K+ channels, and the transient receptor potential vanilloid receptor 1 channel (capsaicin receptor) (Fig. 2A) (Dodge and Scott, 2000; Smith et al., 2006a; Jeske et al., 2008; Schnizler et al., 2008; Zhang et al., 2008). Because of its multivalent nature, AKAP79 coordinates different enzyme combinations to modulate the activity of anchored channels, tailoring regulation to individual effectors (Hoshi et al., 2005). This provides the ideal scaffold for regulating AC activity as well.
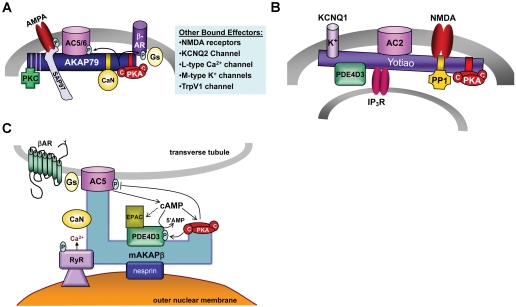
Fig. 2.
AC-AKAP assembled complexes. A, AKAP79 coordinates different protein combinations to tailor effector regulation in different tissues. Anchored PKA can phosphorylate and inhibit bound AC5/6 and desensitize anchored βAR, in addition to regulation of associated downstream effectors. B, Yotiao binds to AC2, in addition to AC 1, 3, and 9. The anchoring of a PKA-regulated PDE sets up potential feedback regulation of cAMP levels independent of Yotiao-mediated inhibition of AC2. C, mAKAPβ complexes assembled on the nuclear envelope. In this model, βAR-stimulated AC5 increases cAMP to activate anchored PKA and potentially EPAC. PKA phosphorylation of the ryanodine receptor (RyR) increases channel activity to allow for Ca2+ activation of CaN. Several feedback loops are also initiated, including PKA-dependent inhibition of AC5 to decrease cAMP synthesis and activation of PDE4D3 by PKA to increase cAMP breakdown. The binding of ERK1/2 to PDE4D3 is not shown. [Adapted from Kapiloff MS, Piggott LA, Sadana R, Li J, Heredia LA, Henson E, Efendiev R, and Dessauer CW (2009) An adenylyl cyclase-mAKAPβ signaling complex regulates cAMP levels in cardiac myocytes. J Biol Chem 284:23540–23546. Copyright © 2009 American Society for Biochemistry and Molecular Biology. Used with permission.]
Substantial biochemical evidence using forskolin-agarose or immunoprecipitations to purify AC and associated AKAP79/150 and/or PKA activity from rat brain extracts supports a complex between AC5/6 and AKAP79 (Bauman et al., 2006). Reciprocal immunoprecipitations of AKAP79 contain significant AC5 activity. Direct phosphorylation of AC5 or AC6 by PKA inhibits cAMP synthesis (Iwami et al., 1995; Chen et al., 1997). PKA anchoring facilitates the preferential phosphorylation of AC5/6 in rat brain extracts, and AKAP79 expression inhibits AC5 activity in a PKA-dependent manner. Both the inhibition and phosphorylation of AC5 by AKAP79-anchored PKA are abolished upon mutation of the PKA phosphorylation site on AC5.
The PKA-dependent feedback regulation of cAMP synthesis that is assembled on AKAP79 was demonstrated in cells using two different reporters (cyclic nucleotide-gated channels and the PKA AKAR2 fluorescence resonance energy transfer reporter) (Bauman et al., 2006). Knockdown of endogenous AKAP79 increased cAMP levels and sustained PKA activity upon stimulation with β2-AR agonists. Rescue of AKAP79 depletion by the rat ortholog AKAP150 was dependent on the presence of the PKA anchoring site. Thus, PKA phosphorylation of AKAP79-bound AC and/or β2-AR provides negative feedback on AC5 and generates a burst of cAMP synthesis. This is a fairly rapid process, because PKA activity returned to baseline within 4 min of stimulation when AKAP79 was present. Additional AC5 regulation may also occur through anchored PKC and/or calcineurin. Thus, AKAP79/150 clearly shapes the dynamics of cAMP accumulation. Although AKAP79 does not alter the initial rate of PKA activation, it facilitates the subsequent inhibition of AC5/6 by PKA and ultimately the decay of PKA activity.
Yotiao.
Another plasma membrane-associated AKAP is Yotiao (smallest splice variant of AKAP9 family; 250 kDa). It is found in both brain and heart, accumulating near neuromuscular junctions (Lin et al., 1998; Schmidt et al., 1999). Yotiao anchors PKA, protein phosphatase 1, PDE4D3, the NR1 subunit of the NMDA receptor, IP3 receptor, and the K+ channel subunit KCNQ1, which is responsible for IKs currents in the heart (Fig. 2B) (Lin et al., 1998; Westphal et al., 1999; Marx et al., 2002; Tu et al., 2004; Terrenoire et al., 2009). Yotiao is required for sympathetic regulation of IKs currents, which shape the duration of action potentials (Chen and Kass, 2006). Inherited mutations in KCNQ1 and/or Yotiao that disrupt binding to one another are associated with long QT syndrome, a disease characterized by cardiac arrhythmias and sudden death (Chen and Kass, 2006; Chen et al., 2007), emphasizing again the requirement for assembled complexes in the temporal regulation of PKA activity. Yotiao also brings together opposing regulators to control downstream effectors. For example, PKA phosphorylation of NR1 potentiates NMDA receptor activation (Tingley et al., 1997), whereas anchored protein phosphatase 1 reduces channel activity (Westphal et al., 1999).
The tight control of PKA activity described above strongly suggests that AC must also be part of this complex. Immunoprecipitation of Yotiao from brain and heart identified significant associated AC activity (Piggott et al., 2008). Yotiao can associate with AC isoforms 1, 2, 3, and 9 but not 4, 5, and 6. Yotiao binds directly to AC2, as assessed by binding assays using purified fragments of the two proteins. Expression of Yotiao inhibited the activity of AC 2 and 3, but not AC 1 or 9, serving purely as a scaffold for these latter isoforms under the stimulatory conditions tested. The mechanism for inhibition of AC 2 and 3 is unknown, because these isoforms have no reported sensitivity to PKA. However, it is unlikely to be a consequence of direct interaction with Yotiao but rather is due to regulatory proteins recruited to the scaffolding protein. The assembly of both AC and PDE forms another feedback loop to tightly control cAMP-dependent PKA activity (Piggott et al., 2008; Terrenoire et al., 2009).
mAKAPβ.
As discussed above, not all AKAPs are localized to the plasma membrane. The cardiac splice variant of muscle AKAP (mAKAPβ) is anchored to the nuclear envelope by the membrane-spanning protein, nesprin, and is found at lower levels in the sarcoplasmic reticulum of cardiac myocytes (McCartney et al., 1995; Kapiloff et al., 1999; Ruehr et al., 2003; Schulze et al., 2003; Pare et al., 2005b). mAKAPβ anchors a finely tuned series of feedback loops to regulate three cAMP-binding proteins, PKA, EPAC (a cAMP-dependent Rap exchange factor), and PDE4D3 (Fig. 2C) (Dodge-Kafka and Kapiloff, 2006; Bauman et al., 2007). By additionally anchoring calcineurin and components of the ERK pathway, mAKAPβ complexes respond to several classes of intracellular receptors. mAKAPβ expression at the nuclear envelope is required for cytokine-induced hypertrophy, which is sensitive to EPAC activation by cAMP (Dodge-Kafka et al., 2005). In addition, mAKAPβ is required for full induction of cardiac hypertrophy and the activation of calcineurin/nuclear factor of activated T cells transcription by β-adrenergic agonists (Pare et al., 2005a). Thus, mAKAPβ integrates cAMP signaling with that of calcium and MAP kinases.
Despite the intracellular location of mAKAPβ, AC activity strongly associates with mAKAPβ in heart and isolated cardiac myocytes (Kapiloff et al., 2009). Four different antibodies against mAKAPβ, or its nuclear envelope tether nesprin, immunoprecipitate significant AC activity in heart. mAKAPβ associates with AC5 and AC2 but surprisingly not AC6 or AC1. The predominant complex in heart is mAKAPβ-AC5, because mAKAPβ-associated AC activity is completely absent in AC5 knockout hearts. AC5 directly interacts with amino acid 275 to 340 of mAKAPβ, a region that does not overlap with binding sites for other known mAKAP-associated proteins. Similar to the regulation of ACs by other AKAPs, mAKAPβ inhibits AC5 but not AC2 activity. This inhibition is lost upon deletion of the PKA anchoring site on mAKAPβ, consistent with a PKA-dependent mechanism of inhibition observed previously for AKAP79 (Bauman et al., 2006).
In the cardiac myocyte, the transverse tubular system consists of invaginations within the plasma membrane that bring it adjacent to the sarcoplasmic reticulum, which is contiguous with the outer nuclear membrane. The model presented in Fig. 2C suggests that AC5, located on transverse tubules or the plasma membrane (Gao et al., 1997), interacts with mAKAPβ on the nuclear envelope when these structures are close in space. This is perhaps the simplest explanation for the organization of this complex. However, there are also reports of AC activity in nuclear membrane preparations, which is supported by immunocytochemistry of AC5 on the nuclear envelope of cardiac myocytes (Belcheva et al., 1995; Boivin et al., 2006). In addition, numerous GPCRs are also targeted to the perinuclear region, including α- and β-adrenergic receptors, angiotensin II type 1 receptors, endothelin receptors, metabotropic glutamate receptors, and prostaglandin receptors (Boivin et al., 2008). What remains unclear is the orientation of these receptors and/or AC components. If receptors maintain the topology found within the endoplasmic reticulum, then the C-terminal tail containing G-protein interaction sites would face the cytoplasm (Boivin et al., 2008). The same would seem to be true for AC5. However the physiological relevance of this localization is unknown, particularly in terms of the activation of GPCRs and ACs on the nuclear membrane by membrane-impermeable agonists such as catecholamines.
What Dictates Specificity for AC-AKAPs?
Each AKAP seems to bind a unique subset of AC isoforms. There are few common threads among the ACs recognized by Yotiao and/or mAKAP. Yotiao bound AC 1, 2, 3, and 9, and each displays very different regulatory patterns (Fig. 1) (Piggott et al., 2008). Because the C1 and C2 domains of ACs are highly conserved and form the catalytic site, the N terminus is the most logical binding site for obtaining specificity. This region is highly variable and could serve to differentiate AC isoforms. Both Yotiao and mAKAPβ interact with the N terminus of AC2 and AC5, respectively, providing a means for Yotiao to selectively interact with AC2 over the closely related isoform AC4 or mAKAPβ to bind AC5 versus AC6 (Piggott et al., 2008; Kapiloff et al., 2009). However, additional domains of AC (C1 and C2) participate in interactions between AC5 and mAKAPβ (Kapiloff et al., 2009). Thus, interactions with the N termini probably dictate the observed specificity among AC isoforms, but clear differences in the mechanism of binding exist.
A general “AC binding motif” on AKAPs has also not been identified. This is in part due to the limited sequence homology between AKAPs. Although the AC binding domain has been identified on both Yotiao (for AC2, 808–957) and mAKAP (for AC5, 245–275), no sequence similarity exists between these regions or with AKAP79. Different AKAPs seem to use different mechanisms to interact with the same AC isoform, because the mAKAP binding site for AC5 cannot compete for AKAP79 interactions with AC5 (Kapiloff et al., 2009). In addition, it is clear that different ACs interact with different regions on the same AKAP. For example, the N terminus of AC2 effectively competes for Yotiao-AC2 binding and inhibition but not for Yotiao-AC3 inhibition, indicating unique binding sites for the two ACs (Piggott et al., 2008). With clearly distinct AC binding domains on Yotiao, the question arises as to whether multiple ACs can bind at once. Although steric hindrance may be an issue, this is certainly a possibility that cannot be ruled out. In fact, there are reports of homo- and heterodimerization of AC isoforms that might be facilitated by AKAP interactions (Willoughby and Cooper, 2007).
Physiological Relevance for AC-AKAP Complexes
AKAP79-AC5/6.
PKA-anchoring to AKAP79 plays an important role in hippocampal long-term potentiation, phosphorylation of L-type Ca2+ channels, and TrpV1 regulation, because deletion of the PKA binding site on AKAP79/150 (AKAP150Δ36) resulted in a significant disruption of these functions (Lu et al., 2007, 2008; Schnizler et al., 2008). Deletion of the entire AKAP79 scaffold results in mislocalization of PKA in neurons, altered AMPA receptor modulation, reductions in memory retention, defects in motor coordination and strength, resistance to muscarinic-induced seizures, and protection against angiotensin II-induced hypertension (Navedo et al., 2008; Tunquist et al., 2008). Thus, although AKAP79 has no catalytic activity of its own, it is required to facilitate the coordinated regulation of many physiological events.
Several of the AKAP79 phenotypes have similarities with knockouts of AC5 or AC6. For example, both AKAP150Δ36 mice and AC5 knockouts show reduced inflammatory thermal hypersensitivity in response to prostaglandin E2 or formalin, respectively (Kim et al., 2007; Schnizler et al., 2008). These effects correlate with the loss of transient receptor potential vanilloid receptor 1 regulation by PKA in AKAP150Δ36 dorsal root ganglia. AC5 and AKAP150 are highly expressed in striatum, and both exhibit defects in motor coordination when deleted (Iwamoto et al., 2003; Tunquist et al., 2008). In heart, AKAP150Δ36 mice lack persistent Ca2+ sparklets and have lower intracellular calcium because of a loss of PKA regulation of L-type Ca2+ channels. Deletion of AC6 results in reduced Ca2+ transients and other defects associated with calcium handling in cardiac myocytes (Tang et al., 2008). The significant overlap between a subset of AC5/6 and AKAP79 phenotypes in brain and to a lesser extent heart suggests that many, but not all, of the cAMP-dependent processes associated with these proteins may require scaffolding of AC5/6 to AKAP79/150 to properly regulate cAMP dynamics.
Yotiao-AC2.
Yotiao plays a clear role in the sympathetic regulation of the IKs current that is responsible for the normal repolarization of the heart (Chen and Kass, 2006; Chen et al., 2007). Of the Yotiao-interacting ACs, only AC2 and AC9 are expressed in the adult cardiac myocytes, albeit at lower levels than AC5/6. Thus, these AC isoforms may participate in the temporal PKA regulation of IKs function that is balanced by the anchored PDE and phosphatase present in the Yotiao complex.
Yotiao is also highly expressed in brain. The AC2 binding site on Yotiao (Tyr808–957) effectively competes for AC2-Yotiao interactions and reverses inhibition of AC2 by Yotiao when added to membranes (Piggott et al., 2008). Disruption of Yotiao-AC interactions in brain using purified Tyr808– 957 gives rise to a 40% increase in AC activity upon stimulation. Thus, AC activity is clearly regulated by association with Yotiao in brain tissue. Interactions with other AKAPs, such as AKAP79, may give rise to similar modes of regulation. The interaction of ACs and Yotiao may play a role in NMDA regulation. Depending on the associated AC, either feed-forward (AC1), feed-back (AC9), or calcium-insensitive pathways (AC2) could be assembled on Yotiao to regulate downstream effector activity.
mAKAPβ-AC5.
The deletion of AC5 results in protection from cardiac stress and hypertrophy as a result of age-induced cardiomyopathy or in response to pressure overload through aortic banding (Okumura et al., 2003; Yan et al., 2007). Knockdown of mAKAPβ in cardiac myocytes also protects against cytokine- or adrenergic-induced hypertrophy (Dodge-Kafka et al., 2005; Pare et al., 2005a). Therefore, binding of AC5 to mAKAPβ may be required for the transduction of sympathetic hypertrophic cAMP signaling in cardiac myocytes. This concept was tested using the AC-mAKAP binding domain (245–340 of mAKAPβ) to disrupt association between AC5 and mAKAPβ (Kapiloff et al., 2009). Overexpression of AC-mAKAP binding domain in cardiac myocytes using adenoviral expression resulted in increased basal and isoproterenol-stimulated cAMP, presumably because of a relief of mAKAPβ inhibition of AC5 and/or loss of PDE4D3 control of cAMP levels at the complex. This is analogous to the increase in AC activity exhibited by disruption of AC2-Yotiao interactions in brain (Piggott et al., 2008). Disruption of AC5-mAKAPβ interactions also led to an increase in basal protein synthesis and cardiac myocyte cell size, consistent with the increased levels of cAMP. Thus, the regulation of AC5 via mAKAP-anchored proteins seems to be critical for maintaining a delicate balance between cAMP production (via AC5) and potentially degradation (via anchored PDE4D3) to control anchored PKA signaling and ultimately the hypertrophic response.
Concluding Remarks and Future Directions
It is increasingly appreciated that cAMP is restricted in its diffusion throughout the cell and that AKAP scaffolding proteins contribute to the temporal and spatial regulation of cAMP signaling (Fischmeister et al., 2006; Smith and Scott, 2006; Berrera et al., 2008). When an AKAP tethers both PKA and its substrate, the rate of substrate phosphorylation by PKA is enhanced (Zhang et al., 2001). The addition of AC to this complex not only provides added feedback regulation of cAMP production but may also alter the kinetics of PKA signaling, as demonstrated for AKAP79-AC complexes (Bauman et al., 2006). In addition, scaffolding of AC may provide spatial resolution for cAMP effector proteins such as PKA, EPACs, and cyclic nucleotide-gated ion channels. For mAKAPβ-AC5, this results in cAMP generation near the nuclear envelope, perhaps when appropriate membrane surfaces are in close proximity (Kapiloff et al., 2009). However, what about other cellular sites? There are more than 30 mammalian AKAPs located on structures as diverse as Golgi, microtubules, centrosomes, peroxisomes, nucleus, mitochondria, or even within bulk cytosol. Does scaffolding of ACs represent a general paradigm for AC functions, or is it a unique property of a subset of ACs and AKAPs? If cAMP diffusion is truly limited, how does AKAP-anchored PKA present at cellular sites other than the plasma membrane sense cAMP generated (presumably) at the cell surface? Does the bicarbonate-sensitive soluble AC, which can be found in mitochondria, nuclei, and other subcellular organelles, scaffold to AKAPs (Zippin et al., 2003)? One future challenge is to overcome the limitations of AC antibodies and to define other possible membranes and organelles in which transmembrane ACs may reside and the modes of AC regulation that occur at these sites.
In addition, how dynamic are AKAP-generated complexes? Microdomains created as a result of lipid rafts are highly transient in nature, but it is unclear whether AC association with scaffolds exhibit dynamic or stable interactions. AKAPs such as AKAP79 and Gravin are known to shuttle on and off the plasma membrane. For example, AKAP79 is targeted to postsynaptic membranes via associations with the actin cytoskeleton, phosphatidylinositol-4,5-bisphosphate, and cadherins (Dell'Acqua et al., 2006). Brief NMDA activation leads to persistent redistribution of AKAP79/PKA and dissociation from cadherin and F-actin complexes and release of calcineurin (Smith et al., 2006b). The ability of AKAPs to suppress endogenous AC activity suggests that these complexes may be quite stable under at least some conditions, but what about after stimulation? One of the real challenges for the future is to determine what combination of ACs, PKA substrates, and AKAPs are required to control the numerous cAMP-dependent physiological events.
Acknowledgments
I thank Dr. Richard Clark for thoughtful comments of this review.
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant GM060419] and the American Heart Association [Grant 09GRNT2200034].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.059345
- PKA
- protein kinase A
- AC
- adenylyl cyclase
- AKAP
- A-kinase anchoring protein
- AR
- adrenergic receptor
- CaN
- calcineurin
- EPAC
- exchange protein activated by cAMP
- GPCR
- G protein-coupled receptor
- PDE
- phosphodiesterase
- PKC
- protein kinase C
- ERK
- extracellular signal-regulated kinase.
References
- Bauman AL, Michel JJ, Henson E, Dodge-Kafka KL, Kapiloff MS. (2007) The mAKAP signalosome and cardiac myocyte hypertrophy. IUBMB Life 59:163–169 [DOI] [PubMed] [Google Scholar]
- Bauman AL, Soughayer J, Nguyen BT, Willoughby D, Carnegie GK, Wong W, Hoshi N, Langeberg LK, Cooper DM, Dessauer CW, et al. ( 2006) Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol Cell 23:925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcheva MM, Gucker S, Chuang DM, Clark WG, Jefcoat LB, McHale RJ, Toth G, Borsodi A, Coscia CJ. (1995) Modulation of opioid binding associated with nuclear matrix and nuclear membranes of NG108–15 cells. J Pharmacol Exp Ther 274:1513–1523 [PubMed] [Google Scholar]
- Berrera M, Dodoni G, Monterisi S, Pertegato V, Zamparo I, Zaccolo M. (2008) A toolkit for real-time detection of cAMP: insights into compartmentalized signaling. Handb Exp Pharmacol 186:285–298 [DOI] [PubMed] [Google Scholar]
- Boivin B, Lavoie C, Vaniotis G, Baragli A, Villeneuve LR, Ethier N, Trieu P, Allen BG, Hébert TE. (2006) Functional beta-adrenergic receptor signalling on nuclear membranes in adult rat and mouse ventricular cardiomyocytes. Cardiovasc Res 71:69–78 [DOI] [PubMed] [Google Scholar]
- Boivin B, Vaniotis G, Allen BG, Hébert TE. (2008) G protein-coupled receptors in and on the cell nucleus: a new signaling paradigm? J Recept Signal Transduct Res 28:15–28 [DOI] [PubMed] [Google Scholar]
- Bregman DB, Bhattacharyya N, Rubin CS. (1989) High affinity binding protein for the regulatory subunit of cAMP-dependent protein kinase II-B. Cloning, characterization, and expression of cDNAs for rat brain P150. J Biol Chem 264:4648–4656 [PubMed] [Google Scholar]
- Bundey RA, Insel PA. (2006) Adenylyl cyclase 6 overexpression decreases the permeability of endothelial monolayers via preferential enhancement of prostacyclin receptor function. Mol Pharmacol 70:1700–1707 [DOI] [PubMed] [Google Scholar]
- Buxton IL, Brunton LL. (1983) Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J Biol Chem 258:10233–10239 [PubMed] [Google Scholar]
- Carr DW, Stofko-Hahn RE, Fraser ID, Cone RD, Scott JD. (1992) Localization of the cAMP-dependent protein kinase to the postsynaptic densities by A-kinase anchoring proteins. Characterization of AKAP 79. J Biol Chem 267:16816–16823 [PubMed] [Google Scholar]
- Chen L, Kass RS. (2006) Dual roles of the A kinase-anchoring protein Yotiao in the modulation of a cardiac potassium channel: a passive adaptor versus an active regulator. Eur J Cell Biol 85:623–626 [DOI] [PubMed] [Google Scholar]
- Chen L, Marquardt ML, Tester DJ, Sampson KJ, Ackerman MJ, Kass RS. (2007) Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc Natl Acad Sci U S A 104:20990–20995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Harry A, Li J, Smit MJ, Bai X, Magnusson R, Pieroni JP, Weng G, Iyengar R. (1997) Adenylyl cyclase 6 is selectively regulated by protein kinase A phosphorylation in a region involved in Gαs stimulation. Proc Natl Acad Sci U S A 94:14100–14104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M. (2002) Specificity of the cyclic adenosine 3′,5′-monophosphate signal in granulosa cell function. Biol Reprod 67:1653–1661 [DOI] [PubMed] [Google Scholar]
- Cooper DM, Crossthwaite AJ. (2006) Higher-order organization and regulation of adenylyl cyclases. Trends Pharmacol Sci 27:426–431 [DOI] [PubMed] [Google Scholar]
- Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, Horne MC, Hoshi T, Hell JW. (2001) A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science 293:98–101 [DOI] [PubMed] [Google Scholar]
- Dell'Acqua ML, Smith KE, Gorski JA, Horne EA, Gibson ES, Gomez LL. (2006) Regulation of neuronal PKA signaling through AKAP targeting dynamics. Eur J Cell Biol 85:627–633 [DOI] [PubMed] [Google Scholar]
- Dessauer CW, Tesmer JJ, Sprang SR, Gilman AG. (1999) The interactions of adenylate cyclases with P-site inhibitors. Trends Pharmacol Sci 20:205–210 [DOI] [PubMed] [Google Scholar]
- Dodge-Kafka KL, Kapiloff MS. (2006) The mAKAP signaling complex: integration of cAMP, calcium, and MAP kinase signaling pathways. Eur J Cell Biol 85:593–602 [DOI] [PubMed] [Google Scholar]
- Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, Scott JD. (2005) The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature 437:574–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge K, Scott JD. (2000) AKAP79 and the evolution of the AKAP model. FEBS Lett 476:58–61 [DOI] [PubMed] [Google Scholar]
- Dupré DJ, Baragli A, Rebois RV, Ethier N, Hébert TE. (2007) Signalling complexes associated with adenylyl cyclase II are assembled during their biosynthesis. Cell Signal 19:481–489 [DOI] [PubMed] [Google Scholar]
- Fagan KA, Smith KE, Cooper DM. (2000) Regulation of the Ca2+-inhibitable adenylyl cyclase type VI by capacitative Ca2+ entry requires localization in cholesterol-rich domains. J Biol Chem 275:26530–26537 [DOI] [PubMed] [Google Scholar]
- Feliciello A, Gottesman ME, Avvedimento EV. (2001) The biological functions of A-kinase anchor proteins. J Mol Biol 308:99–114 [DOI] [PubMed] [Google Scholar]
- Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, Leroy J, Vandecasteele G. (2006) Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res 99:816–828 [DOI] [PubMed] [Google Scholar]
- Fraser ID, Tavalin SJ, Lester LB, Langeberg LK, Westphal AM, Dean RA, Marrion NV, Scott JD. (1998) A novel lipid-anchored A-kinase anchoring protein facilitates cAMP-responsive membrane events. EMBO J 17:2261–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T, Puri TS, Gerhardstein BL, Chien AJ, Green RD, Hosey MM. (1997) Identification and subcellular localization of the subunits of L-type calcium channels and adenylyl cyclase in cardiac myocytes. J Biol Chem 272:19401–19407 [DOI] [PubMed] [Google Scholar]
- Gros R, Ding Q, Chorazyczewski J, Pickering JG, Limbird LE, Feldman RD. (2006) Adenylyl cyclase isoform-selective regulation of vascular smooth muscle proliferation and cytoskeletal reorganization. Circ Res 99:845–852 [DOI] [PubMed] [Google Scholar]
- Grove BD, Bruchey AK. (2001) Intracellular distribution of gravin, a PKA and PKC binding protein, in vascular endothelial cells. J Vasc Res 38:163–175 [DOI] [PubMed] [Google Scholar]
- Hoshi N, Langeberg LK, Scott JD. (2005) Distinct enzyme combinations in AKAP signalling complexes permit functional diversity. Nat Cell Biol 7:1066–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel PA, Patel HH. (2009) Membrane rafts and caveolae in cardiovascular signaling. Curr Opin Nephrol Hypertens 18:50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwami G, Kawabe J, Ebina T, Cannon PJ, Homcy CJ, Ishikawa Y. (1995) Regulation of adenylyl cyclase by protein kinase A. J Biol Chem 270:12481–12484 [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Okumura S, Iwatsubo K, Kawabe J, Ohtsu K, Sakai I, Hashimoto Y, Izumitani A, Sango K, Ajiki K, et al. ( 2003) Motor dysfunction in type 5 adenylyl cyclase-null mice. J Biol Chem 278:16936–16940 [DOI] [PubMed] [Google Scholar]
- Jeske NA, Diogenes A, Ruparel NB, Fehrenbacher JC, Henry M, Akopian AN, Hargreaves KM. (2008) A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain 138:604–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetsky M, Middelhaufe S, Bank EM, Levin LR, Buck J, Steegborn C. (2006) Molecular details of cAMP generation in mammalian cells: a tale of two systems. J Mol Biol 362:623–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapiloff MS, Piggott LA, Sadana R, Li J, Heredia LA, Henson E, Efendiev R, Dessauer CW. (2009) An adenylyl cyclase-mAKAPβ signaling complex regulates cAMP levels in cardiac myocytes. J Biol Chem 284:23540–23546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapiloff MS, Schillace RV, Westphal AM, Scott JD. (1999) mAKAP: an A-kinase anchoring protein targeted to the nuclear membrane of differentiated myocytes. J Cell Sci 112:2725–2736 [DOI] [PubMed] [Google Scholar]
- Kim KS, Kim J, Back SK, Im JY, Na HS, Han PL. (2007) Markedly attenuated acute and chronic pain responses in mice lacking adenylyl cyclase-5. Genes Brain Behav 6:120–127 [DOI] [PubMed] [Google Scholar]
- Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD. (1996) Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science 271:1589–1592 [DOI] [PubMed] [Google Scholar]
- Lavine N, Ethier N, Oak JN, Pei L, Liu F, Trieu P, Rebois RV, Bouvier M, Hebert TE, Van Tol HH. (2002) G protein-coupled receptors form stable complexes with inwardly rectifying potassium channels and adenylyl cyclase. J Biol Chem 277:46010–46019 [DOI] [PubMed] [Google Scholar]
- Levitzki A. (1988) From epinephrine to cyclic AMP. Science 241:800–806 [DOI] [PubMed] [Google Scholar]
- Lin JW, Wyszynski M, Madhavan R, Sealock R, Kim JU, Sheng M. (1998) Yotiao, a novel protein of neuromuscular junction and brain that interacts with specific splice variants of NMDA receptor subunit NR1. J Neurosci 18:2017–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Tombler E, Nelson PJ, Ross M, Gelman IH. (1996) A novel src- and ras-suppressed protein kinase C substrate associated with cytoskeletal architecture. J Biol Chem 271:28430–28438 [DOI] [PubMed] [Google Scholar]
- Liu X, Thangavel M, Sun SQ, Kaminsky J, Mahautmr P, Stitham J, Hwa J, Ostrom RS. (2008) Adenylyl cyclase type 6 overexpression selectively enhances beta-adrenergic and prostacyclin receptor-mediated inhibition of cardiac fibroblast function because of colocalization in lipid rafts. Naunyn Schmiedebergs Arch Pharmacol 377:359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Allen M, Halt AR, Weisenhaus M, Dallapiazza RF, Hall DD, Usachev YM, McKnight GS, Hell JW. (2007) Age-dependent requirement of AKAP150-anchored PKA and GluR2-lacking AMPA receptors in LTP. EMBO J 26:4879–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhang M, Lim IA, Hall DD, Allen M, Medvedeva Y, McKnight GS, Usachev YM, Hell JW. (2008) AKAP150-anchored PKA activity is important for LTD during its induction phase. J Physiol 586:4155–4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Kurokawa J, Reiken S, Motoike H, D'Armiento J, Marks AR, Kass RS. (2002) Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science 295:496–499 [DOI] [PubMed] [Google Scholar]
- McCartney S, Little BM, Scott JD. (1995) Analysis of a novel A-kinase anchoring protein-100, (AKAP 100). Biochem Soc Trans 23:268S. [DOI] [PubMed] [Google Scholar]
- McConnachie G, Langeberg LK, Scott JD. (2006) AKAP signaling complexes: getting to the heart of the matter. Trends Mol Med 12:317–323 [DOI] [PubMed] [Google Scholar]
- Navedo MF, Nieves-Cintrón M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS, Santana LF. (2008) AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res 102:e1–e11 [DOI] [PubMed] [Google Scholar]
- Okumura S, Takagi G, Kawabe J, Yang G, Lee MC, Hong C, Liu J, Vatner DE, Sadoshima J, Vatner SF, et al. ( 2003) Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proc Natl Acad Sci U S A 100:9986–9990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare GC, Bauman AL, McHenry M, Michel JJ, Dodge-Kafka KL, Kapiloff MS. (2005a) The mAKAP complex participates in the induction of cardiac myocyte hypertrophy by adrenergic receptor signaling. J Cell Sci 118:5637–5646 [DOI] [PubMed] [Google Scholar]
- Pare GC, Easlick JL, Mislow JM, McNally EM, Kapiloff MS. (2005b) Nesprin-1alpha contributes to the targeting of mAKAP to the cardiac myocyte nuclear envelope. Exp Cell Res 303:388–399 [DOI] [PubMed] [Google Scholar]
- Piggott LA, Bauman AL, Scott JD, Dessauer CW. (2008) The A-kinase anchoring protein Yotiao binds and regulates adenylyl cyclase in brain. Proc Natl Acad Sci U S A 105:13835–13840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebois RV, Robitaille M, Galés C, Dupré DJ, Baragli A, Trieu P, Ethier N, Bouvier M, Hébert TE. (2006) Heterotrimeric G proteins form stable complexes with adenylyl cyclase and Kir3.1 channels in living cells. J Cell Sci 119:2807–2818 [DOI] [PubMed] [Google Scholar]
- Ruehr ML, Russell MA, Ferguson DG, Bhat M, Ma J, Damron DS, Scott JD, Bond M. (2003) Targeting of protein kinase A by muscle A kinase-anchoring protein (mAKAP) regulates phosphorylation and function of the skeletal muscle ryanodine receptor. J Biol Chem 278:24831–24836 [DOI] [PubMed] [Google Scholar]
- Sadana R, Dessauer CW. (2009) Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals 17:5–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Erlichman J, Rubin CS. (1984) Identification of a calmodulin-binding protein that co-purifies with the regulatory subunit of brain protein kinase II. J Biol Chem 259:9840–9846 [PubMed] [Google Scholar]
- Sayner SL, Alexeyev M, Dessauer CW, Stevens T. (2006) Soluble adenylyl cyclase reveals the significance of cAMP compartmentation on pulmonary microvascular endothelial cell barrier. Circ Res 98:675–681 [DOI] [PubMed] [Google Scholar]
- Schmidt PH, Dransfield DT, Claudio JO, Hawley RG, Trotter KW, Milgram SL, Goldenring JR. (1999) AKAP350, a multiply spliced protein kinase A-anchoring protein associated with centrosomes. J Biol Chem 274:3055–3066 [DOI] [PubMed] [Google Scholar]
- Schnizler K, Shutov LP, Van Kanegan MJ, Merrill MA, Nichols B, McKnight GS, Strack S, Hell JW, Usachev YM. (2008) Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci 28:4904–4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze DH, Muqhal M, Lederer WJ, Ruknudin AM. (2003) Sodium/calcium exchanger (NCX1) macromolecular complex. J Biol Chem 278:28849–28855 [DOI] [PubMed] [Google Scholar]
- Smith FD, Langeberg LK, Scott JD. (2006a) The where's and when's of kinase anchoring. Trends Biochem Sci 31:316–323 [DOI] [PubMed] [Google Scholar]
- Smith FD, Scott JD. (2006) Anchored cAMP signaling: onward and upward—a short history of compartmentalized cAMP signal transduction. Eur J Cell Biol 85:585–592 [DOI] [PubMed] [Google Scholar]
- Smith KE, Gibson ES, Dell'Acqua ML. (2006b) cAMP-dependent protein kinase postsynaptic localization regulated by NMDA receptor activation through translocation of an A-kinase anchoring protein scaffold protein. J Neurosci 26:2391–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Gu C, Fagan KA, Hu B, Cooper DM. (2002) Residence of adenylyl cyclase type 8 in caveolae is necessary but not sufficient for regulation by capacitative Ca2+ entry. J Biol Chem 277:6025–6031 [DOI] [PubMed] [Google Scholar]
- Steinberg SF, Brunton LL. (2001) Compartmentation of G protein-coupled signaling pathways in cardiac myocytes. Annu Rev Pharmacol Toxicol 41:751–773 [DOI] [PubMed] [Google Scholar]
- Streb JW, Miano JM. (2005) Cross-species sequence analysis reveals multiple charged residue-rich domains that regulate nuclear/cytoplasmic partitioning and membrane localization of a kinase anchoring protein 12 (SSeCKS/Gravin). J Biol Chem 280:28007–28014 [DOI] [PubMed] [Google Scholar]
- Tang T, Gao MH, Lai NC, Firth AL, Takahashi T, Guo T, Yuan JX, Roth DM, Hammond HK. (2008) Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation 117:61–69 [DOI] [PubMed] [Google Scholar]
- Tao J, Shumay E, McLaughlin S, Wang HY, Malbon CC. (2006) Regulation of AKAP-membrane interactions by calcium. J Biol Chem 281:23932–23944 [DOI] [PubMed] [Google Scholar]
- Terrenoire C, Houslay MD, Baillie GS, Kass RS. (2009) The cardiac IKs potassium channel macromolecular complex includes the phosphodiesterase PDE4D3. J Biol Chem 284:9140–9146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf WE, Vallee RB. (1982) Molecular characterization of the cAMP-dependent protein kinase bound to microtubule-associated protein 2. J Biol Chem 257:3284–3290 [PubMed] [Google Scholar]
- Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. (1997) Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-d-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem 272:5157–5166 [DOI] [PubMed] [Google Scholar]
- Tu H, Tang TS, Wang Z, Bezprozvanny I. (2004) Association of type 1 inositol 1,4,5-trisphosphate receptor with AKAP9 (Yotiao) and protein kinase A. J Biol Chem 279:19375–19382 [DOI] [PubMed] [Google Scholar]
- Tunquist BJ, Hoshi N, Guire ES, Zhang F, Mullendorff K, Langeberg LK, Raber J, Scott JD. (2008) Loss of AKAP150 perturbs distinct neuronal processes in mice. Proc Natl Acad Sci U S A 105:12557–12562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal RS, Tavalin SJ, Lin JW, Alto NM, Fraser ID, Langeberg LK, Sheng M, Scott JD. (1999) Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science 285:93–96 [DOI] [PubMed] [Google Scholar]
- Willoughby D, Cooper DM. (2007) Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev 87:965–1010 [DOI] [PubMed] [Google Scholar]
- Wong W, Scott JD. (2004) AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol 5:959–970 [DOI] [PubMed] [Google Scholar]
- Yan L, Vatner DE, O'Connor JP, Ivessa A, Ge H, Chen W, Hirotani S, Ishikawa Y, Sadoshima J, Vatner SF. (2007) Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell 130:247–258 [DOI] [PubMed] [Google Scholar]
- Zhang J, Ma Y, Taylor SS, Tsien RY. (2001) Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proc Natl Acad Sci U S A 98:14997–15002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li L, McNaughton PA. (2008) Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 59:450–461 [DOI] [PubMed] [Google Scholar]
- Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, Levin LR, Buck J. (2003) Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J 17:82–84 [DOI] [PubMed] [Google Scholar]