Abstract
The c-Jun amino-terminal kinase (JNK) is an important player in inflammation, proliferation, and apoptosis. More recently, JNK was found to regulate cell migration by phosphorylating paxillin. Here, we report a novel role of JNK in cell adhesion. Specifically, we provide evidence that JNK binds to E-cadherin/β-catenin complex and phosphorylates β-catenin at serine 37 and threonine 41, the sites also phosphorylated by GSK-3β. Inhibition of JNK kinase activity using dominant-negative constructs reduces phosphorylation of β-catenin and promotes localization of E-cadherin/β-catenin complex to cell-cell contact sites. Conversely, activation of JNK induces β-catenin phosphorylation and disruption of cell contacts, which are prevented by JNK siRNA. We propose that JNK binds to β-catenin and regulates formation of adherens junctions, ultimately controlling cell-to-cell adhesion.—Lee, M.-H., Koria, P., Qu, J., Andreadis, S. T. JNK phosphorylates β-catenin and regulates adherens junctions.
Keywords: cell adhesion, E-cadherin, epithelial development, cell invasion, SP600125, okadaic acid
The c-Jun N-terminal kinases (JNKs), also known as stress-activated protein kinases, belong to the mitogen-activated protein kinase (MAPK) group of serine threonine protein kinases (1, 2). JNKs were originally identified by their ability to phosphorylate c-jun in response to stress induced by a variety of stimuli, such as TNF-α (3) or UV radiation (4). Subsequent studies suggested that JNK signaling may also be important in movement of epithelial sheets (5, 6), wound healing (7), apoptosis (8,9,10), cell survival (11), and tumor development (12). In mammalian cells, JNK is phosphorylated only in cells at the edge of the wound, and inhibition of JNK pathway blocks migration and lamellipodia extension (13). Furthermore, a pool of phosphorylated JNK is localized in focal adhesions (14), where it promotes cell migration by phosphorylating the focal adhesion protein paxillin (15).
Cell-cell adhesion is crucial to many aspects of multicellular existence, including morphogenesis, tissue integrity, and differentiation (16). In epithelial cells, adherens junctions are formed by calcium-dependent homotypic interactions between E-cadherins on the surface of opposing cells. The cytoplasmic domain of E-cadherin forms complexes with plaque proteins known as catenins, namely α-catenin and β-catenin. The C terminus of β-catenin interacts with E-cadherin, whereas its N-terminal portion interacts with α-catenin, which is directly responsible for linking adherens junctions to the actin cytoskeleton (17). Phosphorylation of the cytoplasmic domain of E-cadherin results in enhanced cell adhesion (18), whereas tyrosine phosphorylation of β-catenin has been implicated in adherens junction disassembly (19). On the other hand, serine phosphorylated β-catenin can be incorporated in newly formed adherens junctions but undergoes dephosphorylation as junctions mature (20).
Here, we show that JNK binds to and phosphorylates β-catenin and regulates formation of adherens junctions. These results suggest that JNK may be a novel regulator of cell-cell adhesion and may have wide implications in understanding important biological processes, such as tissue development, tissue regeneration, and cancer metastasis.
MATERIALS AND METHODS
Immunofluorescence and Western blots
Primary keratinocytes were isolated from neonatal human foreskins as described previously (21). Immunofluorescence and Western blots were performed as described previously (22, 23). For immunofluorescence, the following antibodies were used at the indicated conditions: mouse anti-human E-cadherin (1:125 dilution, 4°C overnight; BD Transduction Laboratories, San Diego, CA, USA), mouse anti-human β-catenin (1:200 dilution, 4°C overnight; BD Transduction Laboratories), rabbit anti-human phosphor-β-catenin (1:50 dilution, 4°C overnight; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-human phosphor-JNK (p-JNK) (1:100 dilution, 4°C overnight; Cell Signaling Technology, Danvers, MA, USA), rabbit anti-HA (1:50 dilution, room temperature for 1 h; Sigma, St. Louis, MO, USA) and phalloidin (1:40 dilution, room temperature for 20 min; Invitrogen, Eugene, OR, USA). Images of the stained cells were acquired using an inverted microscope (AxioObserver Z1; Zeiss, New York, NY, USA) or a confocal microscope (LSM 510; Zeiss) equipped with a digital camera.
For Western blots, the following antibodies were used: mouse anti-human E-cadherin (1:2500 in 5% milk; BD Transduction Laboratories); mouse anti-human β-catenin (1:1000 in 5% milk; BD Transduction Laboratories); rabbit anti-human p-JNK (1:500 in 5% BSA; Cell Signaling Technology); rabbit anti-human total JNK (1:1000 in 5% BSA; Cell Signaling Technology); rabbit anti-human phosphor-paxillin (1:1000 in 5% BSA; recognizes phosphorylated protein at Ser-178; Biosource, Camarillo, CA, USA); mouse anti-human paxillin (1:1000 in 5% BSA; BD Transduction Laboratory); rabbit anti-human phosphor-Ser-33/37/Thr41 β-catenin (1:1000 in 5% BSA; Cell Signaling Technology), rabbit anti-human phosphor-Ser-41/45 β-catenin (1:1000 in 5% BSA; Cell Signaling Technology), mouse anti-human phosphor-Ser-37 β-catenin (1:1000 in 5% BSA; Santa Cruz Biotechnology), rabbit anti-human β-catenin (1:1000 in 5% BSA; Cell Signaling Technology), or rabbit anti-HA (1:1000 in 5% dry milk; Sigma) overnight at 4°C.
Immunoprecipitation
Cells (8×105/well) were plated on a 6-well plate overnight. After the indicated treatment, the cells were put on ice, washed twice with ice-cold PBS, and lysed using ice-cold RIPA buffer (20 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM sodium orthovanadate, and 1 mM β-glycerophosphate) supplemented with a cocktail of protease inhibitors (Roche Diagnostics, Indianapolis, IN, USA) for 10 min on ice. The lysate centrifuged for 10 min at 4°C, and the supernatant was transferred to another centrifuge tube.
Cell lysate (200 μl) was incubated with rabbit anti-human p-JNK (1:200, Cell Signaling Technology) or rabbit anti-human β-catenin (1:200; Cell Signaling Technology) primary antibodies overnight at 4°C with constant agitation. The next day, protein G-agarose beads (20 μl; Rockland, Gilbertsville, PA, USA) were added to the lysate and incubated at 4°C with constant agitation for 3 h. The beads were spun down at 4°C and washed 5 times with ice-cold RIPA buffer. The pellet was then resuspended in 3× sample buffer, and bound proteins were analyzed using Western blots as described above.
Kinase assays
For in vitro kinase assays, 2 μg of GST β-catenin (Upstate, Charlottesville, VA, USA) was incubated with 0.2 μg of activated JNK1α1 (Upstate) or JNK2α2 (Upstate) in 50 μl of kinase buffer (Cell Signaling Technology) and 50 μM of ATP for 1 h at 37°C. The indicated samples were treated with JNK inhibitor SP600125 (20 μM). After 1 h, the reaction was stopped by the addition of 3× sample buffer, and phosphorylation was examined by Western blots using an antibody specific to phosphor-β-catenin (9561; Cell Signaling Technology).
Dominant-negative and shRNA constructs
293T cells were plated in 6-well plates (106 cells/well) at 24 h before transfection. The next day, the cells were transfected with Flag-JNK1, Flag-JNK2, dominant-negative Flag-JNK1DN (T183A, Y185F), or Flag-JNK2DN (Addgene, Cambridge, MA, USA) using calcium phosphate precipitation. Cells were lysed at 48 h after transfection, and cell lysates were used for immunoprecipitation or Western blots as described above.
Flag-JNK1DN was cloned downstream of the CMV promoter between the NheI and SacII sites of pCS-CG lentiviral vector (Addgene), which also encodes for EGFP under the same promoter using the internal ribosome entry site (IRES) sequence. Lentivirus was produced as described previously (22, 24) and was used to transduce primary human keratinocytes. Transduced (EGFP+) cells were sorted using flow cytometry to obtain a population of flag-JNK1DN-expressing cells.
shRNA targeting the jnk1 and jnk2 mRNAs (target sequence: 5′-AAAGAAUGUCCUACCUUCU-3′) was cloned downstream of the H1 promoter between the MluI and ClaI sites of pLVTHM lentiviral vector. The vector also encodes for EGFP under the EF1-α promoter. Lentivirus was used to transduce A431 cells, which were sorted using flow cytometry to obtain a population of si-JNK1/2-expressing cells (si-JNK1/2 A431).
Mass spectrometry
β-Catenin was phosphorylated by JNK in vitro, and the reaction mixture was precipitated in cold acetone (−20°C) overnight to remove excessive reagents that might compromise nano-LC separation. To improve sequence coverage and achieve a more complete list of phosphosites, the precipitated proteins were digested with two proteolytic enzymes, trypsin, or Glu-C and analyzed using nano-LC/MS/MS. The high-resolution nano-LC system was composed of two Eksigent nanopumps (Eksigent, Dublin, CA, USA); a Spark Endurance autosampler (Spark Holland, Emmen, The Netherlands); a lab-made valveless RP-trap nano-LC flow path; and two Vici 10-port low- dead-volume valves (Valco, Houston, TX, USA). A lab-made dynamic-flow nanospray ion source was employed to couple the nano-LC system to either a LTQ/ETD (Thermo LTQ XL linear ion trap coupled to an electron-transferring dissociation; Thermo, San Jose, CA, USA) for ETD analysis or a LTQ/Orbitrap (Thermo) for CID analysis.
Both MS protocols were run under data-dependent mode with the following scan configuration: 1 microscan cycle comprised a MS1 survey scan, followed by 7 sequential dependent MS2 scans. Typically, digested reaction mixtures were loaded on a reversed-phase peptide trap (5 mm × 300 μm I.D.) at a flow rate of 10 μl/min and washed with 3% acetonitrile in 0.1% formic acid for 3 min to remove salts and other hydrophilic buffer components; then the trap was switched online with a lab-packed reversed-phase nano C-18 column, (3 μm reversed-phase particle packed in a 20-cm, 360-μm O.D., 75-μm I.D. fused silica capillary with a noncoated 2-μm tampered tip). A shallow multistep gradient was used to resolve the samples in 3 h, and the on-column flow rate was 250 nl/min. The raw data were converted to DTA files with ZSA and Combolon filtering algorithms to remove bad DTA files; then the group of DTA files was searched against an indexed tryptic peptide database, which was derived from the National Center for Biotechnology Information protein database, with phosphorylation on STY and oxidation/hydroxylation on M and P assumed. The search results were grouped into an srf file. To ensure high confidence of the results, a group of filters was applied to the result: Xcorr > 2 for z = 1; Xcorr > 2.5 if z = 2; Xcorr > 3 if z = 3; and peptide probability <0.01. For ETD results, the filtering criteria were Xcorr > 2 for z = 1; Xcorr > 2.5 if z = 2; Xcorr > 3 if z = 3; Xcorr > 3.5 if z > 3, and Sf > 0.75. For each of the identified phosphopeptides, a manual examination of the fragment pattern, as well as a high-accuracy Orbitrap precursor measurement (<3-ppm error) is performed to eliminate database searching artifacts.
RESULTS
The JNK inhibitor SP600125 induces cell-cell adhesion
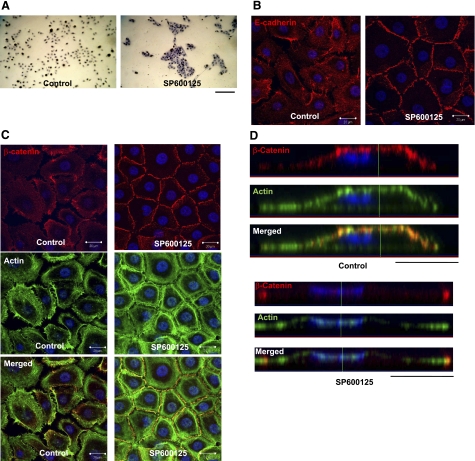
When grown in serum-free, low Ca2+-containing medium, keratinocytes grow as individual cells or cell colonies with no adherens junctions. Treatment of these cells with the JNK inhibitor SP600125 (10 μM) led to enhanced cell-cell adhesion and formation of compact colonies within 30 min (Fig. 1A), suggesting a possible role of JNK in cell adhesion. Indeed, confocal microscopy showed that inhibition of JNK kinase activity by SP600125 led to localization of E-cadherin and its partner β-catenin to cell-cell contact sites (Fig. 1B, C). Costaining with β-catenin (red), actin phalloidin (green), and cell nucleus (blue) showed that under control conditions (serum-free, low calcium), β-catenin colocalized with cortical actin right under the cell surface (Fig. 1D; side view). Interestingly, on SP600125 treatment, β-catenin was detected only at cell-cell contact sites and actin organized in thick bundles underneath β-catenin and parallel to the cell borders (Fig. 1C, D). These data clearly showed that inhibition of JNK-induced translocation of β-catenin from the apical and basal cell surface to cell-cell adhesion sites, as well as significant actin-reorganization.
Figure 1.
Inhibition of JNK activity promotes translocation of E-cadherin and β-catenin to the cell-cell adhesion sites. A) Human epidermal keratinocytes were treated with 10 μM SP600125 for 30 min. Then the cells were fixed with 4% paraformaldehyde and stained with hematoxylin (view ×10). B, C) Confocal immunofluorescence microscopy (bottom z section) of keratinocytes that were similarly treated and immunostained for E-cadherin (red) (B), or β-catenin (red) and actin phalloidin (green) (C). Cell nuclei were stained with Hoechst (blue) (view ×63). D) Lateral images were formed by maximum intensity projection from confocal z-stack images. Scale bars = 100 μm (A); 20 μm (B, C).
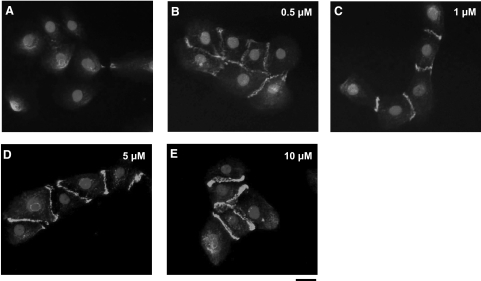
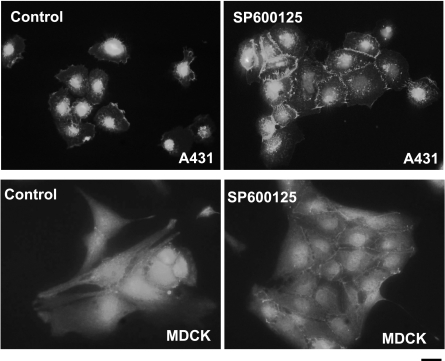
In addition, the amount of β-catenin at the cell-cell contact sites appeared to increase with increasing SP600125 concentration (Fig. 2). Similar results were also obtained with two other epithelial cell lines, A431 epidermoid carcinoma and Madin-Darby canine kidney (MDCK) cells (Fig. 3). Since SP600125 is a reversible inhibitor of JNK, we examined whether SP600125-mediated translocation of E-cadherin to cell adhesion sites was also reversible. To this end, keratinocytes were treated with SP600125 for 30 min and fixed immediately or after 24-h incubation in the absence of SP600125. As shown in Supplemental Fig. 1, E-cadherin was present at the cell borders as early as 30 min after SP600125 treatment but disappeared from cell-cell contacts by 24 h after removal of the inhibitor, suggesting a possible role of JNK in loss of E-cadherin-mediated cell adhesion.
Figure 2.
SP600125 causes localization of β-catenin to cell-cell borders in a dose-dependent manner. Primary keratinocytes were treated with either vehicle (control) (A) or different concentrations of JNK inhibitor SP600125; 0.5 μM (B), 1 μM (C), 5 μM (D), or 10 μM (E). After 30 min, cells were fixed with 4% paraformaldehyde and immunostained for β-catenin. Nuclei were visualized with Hoechst 33258 (solid circles; view ×40). Scale bar = 20 μm.
Figure 3.
JNK inhibition leads to cell adhesion in A431 and MDCK cells. Cells were plated in tissue culture-treated glass slides and were then treated with either vehicle (control) or SP600125 (10 μM) for 30 min. After 30 min, the cells were fixed and then stained for E-cadherin. Nuclei were visualized with Hoechst 33258 (solid circles; view ×40). Scale bar = 20 μm.
Association of JNK with β-catenin in the same protein complex
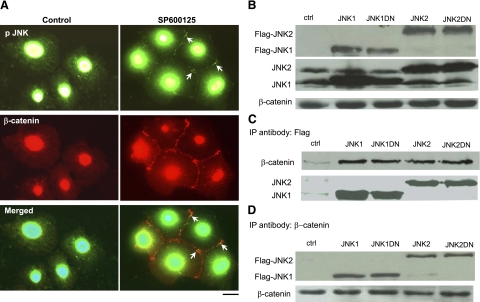
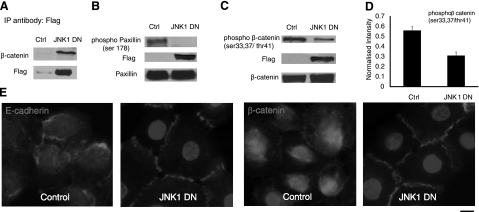
Immunofluorescence microscopy showed that in control cells, most of the p-JNK was in the cytoplasm and around the nucleus (Fig. 4A). However, in cells treated with SP600125, there was a small pool of p-JNK that colocalized with β-catenin at the cell border (Fig. 4A). To investigate whether JNK interacts directly with adherens junctions, we expressed either Flag-JNK1, Flag-JNK2, dominant-negative (kinase dead) Flag-JNK1DN, or Flag-JNK2DN in HEK 293T cells (Fig. 4B). Immunoprecipitation with anti-Flag or anti-β-catenin followed by Western blot for β-catenin or Flag, respectively, showed that JNK associated with β-catenin (Fig. 4C, D).
Figure 4.
JNK is associated with β-catenin. Human epidermal keratinocytes were treated with 10 μM SP600125 for 30 min. A) Immunostaining for phosphorylated (p)-JNK (green) and β-catenin (red). B) 293T cells were transfected with plasmids encoding for Flag-JNK1, Flag-JNK2, or the kinase-dead Flag-JNK1DN, Flag-JNK2DN. Cell lysates were subjected to Western blotting for Flag and β-catenin. C) Cell lysates were immunoprecipitated with antibody against Flag, and β-catenin was detected using Western blotting. Total JNK was used as loading control. D) Cell lysates were immunoprecipitated with antibody against β-catenin, and Flag was detected using Western blotting. β-catenin was used as loading control. Nontransfected cells were used as negative control (ctrl; B–D). This experiment was repeated 3 times with similar results; a representative experiment is shown.
JNK phosphorylates β-catenin at Ser-37 and Thr41
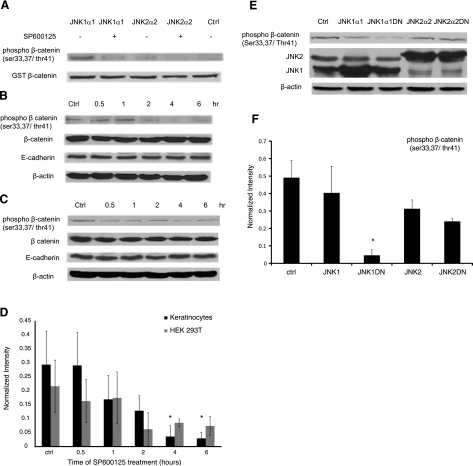
Since JNK coimmunoprecipitated with β-catenin, we reasoned that β-catenin might be a possible substrate of JNK. To test this hypothesis, we evaluated the phosphorylation of purified GST-β-catenin in vitro. Using an antibody that recognizes phosphorylated β-catenin at Ser-33/37/Thr41 (9561; Cell Signaling Technology), we found that JNK1 strongly phosphorylated β-catenin and that phosphorylation was significantly reduced by SP600125 (Fig. 5A). JNK2 also phosphorylated β-catenin, albeit to a much lesser extent.
Figure 5.
JNK phosphorylates β-catenin. A) In vitro kinase assay using purified activated JNK1α1 or JNK2α2 and GST-β-catenin in the presence or absence of SP600125 (20 μM). Phosphorylated GST-β-catenin was detected using Western blotting, and total GST-β-catenin served as a loading control. This experiment was repeated 5 times with similar results; a representative experiment is shown. B, C) Human keratinocytes (B) and 293T cells (C) were treated with SP600125 for the indicated times, and cell lysates were subjected to Western blotting for phosphor-β-catenin, total β-catenin, and E-cadherin; β-actin served as a loading control. Blot is representative of 3 independent experiments with similar results. D) Lane intensity of phosphor-β-catenin was determined using Kodak gel documentation software and normalized to total β-catenin. Data from 3 independent experiments were plotted as average ± se. E) 293T cells were transfected with plasmids encoding for Flag-JNK1, Flag-JNK2, or the kinase-dead Flag-JNK1DN, and Flag-JNK2DN, and cell lysates were subjected to Western blotting for phosphor-β-catenin and total JNK; β-actin served as loading control. F) Lane intensity of phosphor-β-catenin was normalized to β-actin loading control; data from 3 independent experiments were plotted as average ± se. *P < 0.05 vs. control.
To determine whether endogenous β-catenin was also phosphorylated by JNK, primary keratinocytes and 293T cells were treated with SP600125, and phosphor-β-catenin was detected at the indicated times. SP600125 treatment reduced phosphorylation of β-catenin in both cell types (Fig. 5B–D). To further corroborate this result, the amount of phosphor-β-catenin was determined in HEK 293T cells that were transfected with Flag-JNK1, Flag-JNK2, or dominant-negative Flag-JNK1DN- or Flag-JNK2DN-encoding plasmids. As shown in Fig. 5E, F, the level of phosphorylated β-catenin was significantly reduced in JNK1DN-expressing cells by 10-fold as compared to mock transfected cells. In agreement with the in vitro kinase assay, JNK1DN blocked phosphorylation but JNK2DN did not.
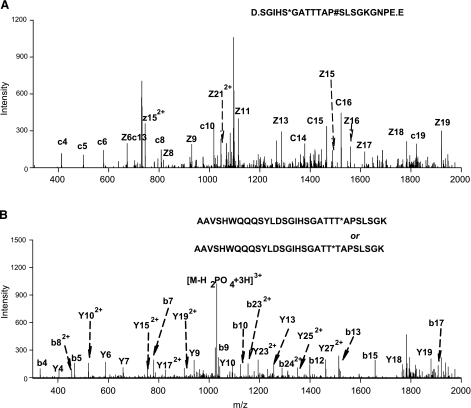
These data were further confirmed by nano-LC/tandem mass spectrometry. To this end, β-catenin was phosphorylated by JNK in vitro, and the reaction mixture was digested with either of two proteases, trypsin or Staphylococcus aureus V8 to generate two sets of proteolytic peptides and achieve a more complete list of phosphosites (25, 26). The digested samples were analyzed by a nano-LC coupled to a high accuracy Orbitrap mass analyzer (<2 ppm mass error). Using two complementary fragmentation technologies, CID and ETD, we identified two phosphosites with high confidence. Phosphorylation of Ser-37 was identified by ETD fragmentation of a triply charged precursor in the V8-digested sample (Fig. 6A), with a complete ladder of c and z ions. Another phosphosite was identified at either Thr41 or Thr42 (Fig. 6B) by CID fragmentation of a triply charged precursor in the trypsin-digested sample. Although the identification of the phosphopeptide is highly confident, as suggested by the Xcorr and P values, we could not determine which of the two sites were phosphorylated solely from the nano-LC/Obitrap data, because the domain contains 3 adjacent Thr residues, and the y8 and b22 ions, which could otherwise eliminate this ambiguity, were not observed.
Figure 6.
Phosphosite identification by mass spectrometry. Two phoshosites were identified on human β-catenin using nano-LC coupled to a LTQ/Orbitrap tandem MS with either CID or ETD activation. GST-catenin was phosphorylated by JNK in vitro, and the reaction mixture was separately digested with either trypsin (cuts after K and R) or S. aureus V8 (cuts after D and E). A) Phosphorylation at Ser-37 was identified by ETD in a V8-treated sample; precursor mass error = 0.02 Da, Xcorr = 3.4. B) Another phosphosite at either Thr41 or Thr42 was identified by CID in a trypsin-treated sample; precursor mass error = 0.01 Da, Xcorr = 3.1.
Expression of JNK1DN decreases phosphorylation of β-catenin and induces translocation of β-catenin and E-cadherin to the cell surface
Next, we examined the results of inhibition of JNK1 kinase activity in primary keratinocytes by overexpressing JNK1DN using a lentivirus encoding for Flag-JNK1DN and EGFP. The latter was used to purify transduced cells using flow cytometry sorting. As with 293T cells (Fig. 4C, D), JNK1DN also associated with β-catenin in primary keratinocytes (Fig. 7A). In agreement with previous results (15), phosphorylation of paxillin was completely blocked in JNK1DN cells (Fig. 7B), suggesting that overexpression of JNK1DN blocked the kinase activity of JNK1. In addition, expression of JNK1DN significantly decreased phosphorylation of β-catenin by ∼50% (Fig. 7C, D) and induced translocation of E-cadherin and β-catenin to the cell borders in the presence of serum-free, low-calcium-containing medium (Fig. 7E).
Figure 7.
Expression of JKN1DN decreases β-catenin phosphorylation and promotes formation of adherens junctions. Human epidermal keratinocytes were transduced with recombinant lentivirus encoding for Flag-JNK1DN and EGFP. Transduced cells were sorted by flow cytometry and termed JNK1DN cells. A) Cell lysates were immunoprecipitated with antibody against Flag and subjected to Western blotting for β-catenin and Flag. B) Western blot for phosphor-paxillin (Ser-178, Flag, and total paxillin using lysates of control and JNK1DN keratinocytes. C) Same lysates were blotted for phosphor-β-catenin, Flag, and β-catenin. D) Total amount of phosphor-β-catenin (C) was determined using Kodak gel documentation software and normalized to β-catenin loading control. Data from 3 independent experiments were plotted as means ± se; n = 3. E) Immunostaining of control and JNK1DN keratinocytes for E-cadherin or β-catenin. Cell nuclei were counterstained with Hoechst (solid circles) (view ×63). Scale bar = 10 μm.
To determine the importance of β-catenin phosphorylation at Ser-37 or Thr41 in formation of adherens junctions, wild-type (HA-β-catenin) or mutated β-catenin that could not be phosphorylated at Ser-37 or Thr41 [denoted as HA-β-catenin(S37A) or HA-β-catenin(T41A), respectively] were overexpressed in A431 cells using recombinant lentivirus (Supplemental Fig. 3A). In contrast to native β-catenin that was colocalized with cortical actin and in agreement with previous reports (27,28,29,30), all ectopically expressed β-catenin variants—wild type and mutants—were mainly localized in the nucleus (Supplemental Fig. 3B, C). Notably, immunoprecipitation with an antibody against the HA tag showed that in contrast to native β-catenin, overexpressed β-catenin did not bind to E-cadherin (Supplemental Fig. 3D); therefore, it is unlikely to participate in formation of adherens junctions. These data suggested that ectopically expressed β-catenin mutants could not be used to determine the importance of β-catenin phosphorylation in adherens junction formation. On the other hand, immunostaining showed that on SP600125 treatment, β-catenin localized at the cell-cell adhesion sites, but Ser-37 phosphorylated β-catenin remained cytoplasmic, suggesting that phosphorylated β-catenin may not be able to participate in the formation of adherens junctions (Supplemental Fig. 3E).
Activation of JNK increases β-catenin phosphorylation
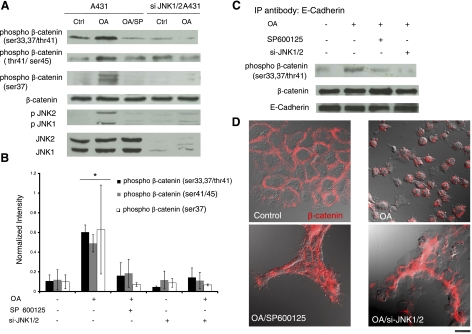
Following previous studies (31, 32), we employed okadaic acid (OA) to activate JNK transiently and examined the phosphorylation status of β-catenin. As shown in Fig. 8A, B, exposure to OA induced phosphorylation of JNK as well as β-catenin at Ser-33/37/Thr41, but SP600125 or JNK1/JNK2 siRNA (si-JNK1/2) decreased β-catenin phosphorylation significantly by ∼3.4- or 3.9-fold, respectively. Similar results were also observed with two additional antibodies, one recognizing phosphor-Ser-37 and another against phosphor-Thr41/Ser-45 of β-catenin. Interestingly, immunoprecipitation with anti-E-cadherin after OA treatment showed that the phosphorylated β-catenin was associated with E-cadherin and that SP600125 or si-JNK1/2 diminished phosphorylation of β-catenin in the E-cadherin/β-catenin complex (Fig. 8C). Consistent with these data, OA disrupted cell-cell adhesion, which was prevented by SP600125 or si-JNK1/2 (Fig. 8D).
Figure 8.
JNK activation induces β-catenin phosphorylation and disruption of cell-cell adhesion. A431 cells were transduced with recombinant lentivirus encoding for EGFP and shRNA targeting JNK1/2. EGFP+ cells were sorted using flow cytometry and denoted as si-JNK1/2 A431 cells. Control or si-JNK1/2 A431 cells were treated with OA (500 nM) for 30 min in the presence or absence of SP600125 (10 μM) as indicated. SP600125 was added for 30 min before as well as during OA treatment. A) Cell lysates were subjected to Western blotting using 3 different antibodies against phosphor-β-catenin at Ser-33,37/Thr41, Thr41/Ser-45, or Ser-37, as well as p-JNK, total JNK, and total β-catenin. B) The band intensity of phosphor-β-catenin was determined using Kodak gel documentation software and normalized to total β-catenin. Data from 3 independent experiments were plotted as means ± se; n = 3. C) Cell lysates were immunoprecipitated with an antibody against E-cadherin, and phosphor-β-catenin and total β-catenin were detected using Western blotting. E-cadherin served as a loading control. D) Immunostaining of control or si-JNK1/2 A431 cells for β-catenin (red) under the indicated conditions. Fluorescence and differential interference contrast images were overlaid to highlight β-catenin localization and cell morphology (view ×63). Scale bar = 20 μm.
DISCUSSION
In summary, we showed that blocking JNK led to translocation of E-cadherin and β-catenin to cell-cell contact sites and formation of compact colonies, suggesting a possible role of JNK in cell adhesion. Indeed, immunostaining and coimmunoprecipitation showed that a pool of JNK colocalized and associated with β-catenin. In vitro kinase assays and mass spectrometry determined that JNK phosphorylated β-catenin at serine 33/37 and threonine 41. The JNK inhibitor SP600125 and overexpression of dominant-negative JNK1 decreased phosphorylation of β-catenin and promoted translocation of β-catenin and E-cadherin at cell-cell contact sites. In addition, knockdown of JNK prevented β-catenin phosphorylation and disruption of cell-cell adhesion in response to OA treatment. Collectively, our results showed for the first time that JNK binds to and phosphorylates β-catenin and regulates adherens junctions. These findings contribute to our understanding of cell-cell adhesion and may have wider implications in wound healing, embryonic development, and cancer metastasis.
Previous studies showed that confluence of epidermal cells was accompanied by increased expression of E-cadherin and β-catenin, as well as translocation of β-catenin from the cytoplasm to the plasma membrane (33). Cell confluence was also accompanied by loss of p-JNK and c-jun (34), while disruption of cell adhesion was accompanied by JNK activation (35). Our data may provide a possible explanation of these seemingly unrelated observations as blocking the kinase activity of JNK decreased β-catenin phosphorylation and promoted translocation of β-catenin and E-cadherin from the apical and basal cell surface to the cell borders and formation of cell-cell contacts. This result suggests that upon JNK inhibition, adherens junctions may be formed by E- cadherin/β-catenin complexes that have already translocated to the cell surface and not those remaining in cell cytoplasm. In addition, immunofluorescence microscopy showed that phosphor-β-catenin at Ser-37 did not localize with β-catenin at cell-cell contact sites on JNK inhibition. This observation is in agreement with a recent study showing that nonphosphorylated β-catenin localized to sites of cell-cell contacts (36). Taken together, our data suggest that JNK may be a molecular switch that regulates cell adhesion in a swift and dynamic manner. Certain biological circumstances, such as acute wounds, may require such dynamic regulation of cell junctions to ensure a quick response. In other circumstances, e.g., cancer, JNK may regulate tumor cell invasion and migration.
Surprisingly, JNK1 overexpression did not increase phosphorylation of β-catenin in HEK 293T cells. It is possible that the number of binding sites holding p-JNK, E-cadherin, and β-catenin together may be limited compared to the total number of p-JNK molecules, which are abundant in 293T cells (not shown). As a result JNK1 overexpression may have no additional effect in these cells. In contrast, keratinocytes exhibited low level of p-JNK1, which was significantly elevated by OA treatment, leading to increased β-catenin phosphorylation and disruption of cell adhesion. The limited binding sites hypothesis may also explain why ectopically expressed β-catenin does not bind to E-cadherin but localizes mainly in the nucleus. If true, this hypothesis would suggest that caution must be exercised in interpreting experimental results obtained with overexpression approaches.
Surprisingly, JNK phosphorylates β-catenin on the same residues (Ser-37, Thr41) as GSK3β, which is responsible for proteosomal degradation of cytoplasmic β-catenin (37). Wnt signaling inhibits GSK3β, stabilizing β-catenin, which then translocates to the nucleus, where it binds TCF-Lef1 and initiates transcription of a large number of growth-related genes (38). Interestingly, OA induced phosphorylation of GSK3β at Ser-9, which was not blocked by SP600125 or si-JNK1/2 (Supplemental Fig. 2A). Although phosphorylation at Ser-9 is thought to inactivate GSK3β (39), phosphorylation of β-catenin increased (instead of decreasing as would be expected with an inactive GSK3β), and total β-catenin remained unchanged, suggesting that another kinase might phosphorylate β-catenin. Indeed, treatment with SP600125 or si-JNK1/2 significantly decreased phosphorylation of E-cadherin-associated β-catenin (Fig. 8A–C), implicating JNK in this process. Although our in vitro kinase assay suggests that JNK can phosphorylate β-catenin directly, the possibility that JNK may mediate β-catenin phosphorylation by inhibiting GSK3β phosphorylation at Ser-9 cannot be excluded. This intriguing hypothesis warrants further investigation.
At present, it is unclear whether JNK-phosphorylated β-catenin is also targeted for degradation via proteosomal pathways. Nevertheless, immunoprecipitation with E-cadherin revealed that JNK phosphorylates β-catenin in adherens junction complexes, suggesting the following intriguing hypothesis: while GSK-3β regulates cytoplasmic β-catenin, JNK may regulate β-catenin in adherens junctions, ultimately controlling cell-cell adhesion. Therefore, phosphorylation of β-catenin by more than one kinase may provide a mechanism by which cells decouple the adhesion and signaling functions of β-catenin. The role, if any, that Wnt ligands may play in this process is presently unclear.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH; R01 EB 000876-01) and the National Science Foundation (BES-0354626) to S.T.A. We thank the Pharmaceutical Sciences Instrumentation Facility at the University at Buffalo (UB) for the use of the liquid chromatography/mass spectrometry systems. The MS instruments were obtained from shared instrumentation grants S10-RR14592 and S10-RR021221 from the National Center for Research Resources, NIH. We also thank Dr. Wade J. Sigurdson (Confocal Microscopy and Flow Cytometry Facility, UB) for his help with confocal microscopy.
References
- Davis R J. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Weston C R, Davis R J. The JNK signal transduction pathway. Curr Opin Genet Dev. 2002;12:14–21. doi: 10.1016/s0959-437x(01)00258-1. [DOI] [PubMed] [Google Scholar]
- Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Devary Y, Gottlieb R A, Lau L F, Karin M. Rapid and preferential activation of the c-jun gene during the mammalian UV response. Mol Cell Biol. 1991;11:2804–2811. doi: 10.1128/mcb.11.5.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glise B, Bourbon H, Noselli S. hemipterous encodes a novel Drosophila MAP kinase kinase, required for epithelial cell sheet movement. Cell. 1995;83:451–461. doi: 10.1016/0092-8674(95)90123-x. [DOI] [PubMed] [Google Scholar]
- Riesgo-Escovar J R, Jenni M, Fritz A, Hafen E. The Drosophila Jun-N-terminal kinase is required for cell morphogenesis but not for DJun-dependent cell fate specification in the eye. Genes Dev. 1996;10:2759–2768. doi: 10.1101/gad.10.21.2759. [DOI] [PubMed] [Google Scholar]
- Ramet M, Lanot R, Zachary D, Manfruelli P. JNK signaling pathway is required for efficient wound healing in Drosophila. Dev Biol. 2002;241:145–156. doi: 10.1006/dbio.2001.0502. [DOI] [PubMed] [Google Scholar]
- Iordanov M S, Wong J, Newton D L, Rybak S M, Bright R K, Flavell R A, Davis R J, Magun B E. Differential requirement for the stress-activated protein kinase/c-Jun NH(2)-terminal kinase in RNAdamage-induced apoptosis in primary and in immortalized fibroblasts. Mol Cell Biol Res Commun. 2000;4:122–128. doi: 10.1006/mcbr.2000.0266. [DOI] [PubMed] [Google Scholar]
- Tang G, Minemoto Y, Dibling B, Purcell N H, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-κB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Yamaguchi T, Natsume T, Kufe D, Miki Y. JNK phosphorylation of 14–3-3 proteins regulates nuclear targeting of c-Abl in the apoptotic response to DNA damage. Nat Cell Biol. 2005;7:278–285. doi: 10.1038/ncb1228. [DOI] [PubMed] [Google Scholar]
- Lamb J A, Ventura J J, Hess P, Flavell R A, Davis R J. JunD mediates survival signaling by the JNK signal transduction pathway. Mol Cell. 2003;11:1479–1489. doi: 10.1016/s1097-2765(03)00203-x. [DOI] [PubMed] [Google Scholar]
- Kennedy N J, Davis R J. Role of JNK in tumor development. Cell Cycle. 2003;2:199–201. [PubMed] [Google Scholar]
- Altan Z M, Fenteany G. c-Jun N-terminal kinase regulates lamellipodial protrusion and cell sheet migration during epithelial wound closure by a gene expression-independent mechanism. Biochem Biophys Res Commun. 2004;322:56–67. doi: 10.1016/j.bbrc.2004.07.079. [DOI] [PubMed] [Google Scholar]
- Almeida E A, Ilic D, Han Q, Hauck C R, Jin F, Kawakatsu H, Schlaepfer D D, Damsky C H. Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase. J Cell Biol. 2000;149:741–754. doi: 10.1083/jcb.149.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Rajfur Z, Borchers C, Schaller M D, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature. 2003;424:219–223. doi: 10.1038/nature01745. [DOI] [PubMed] [Google Scholar]
- Gumbiner B M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson W J, Weis W I. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickert H, Bauer A, Kemler R, Stappert J. Casein kinase II phosphorylation of E-cadherin increases E-cadherin/β-catenin interaction and strengthens cell-cell adhesion. J Biol Chem. 2000;275:5090–5095. doi: 10.1074/jbc.275.7.5090. [DOI] [PubMed] [Google Scholar]
- Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel M M, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadot E, Conacci-Sorrell M, Zhurinsky J, Shnizer D, Lando Z, Zharhary D, Kam Z, Ben-Ze'ev A, Geiger B. Regulation of S33/S37 phosphorylated β-catenin in normal and transformed cells. J Cell Sci. 2002;115:2771–2780. doi: 10.1242/jcs.115.13.2771. [DOI] [PubMed] [Google Scholar]
- Koria P, Andreadis S T. Epidermal morphogenesis: the transcriptional program of human keratinocytes during stratification. J Invest Dermatol. 2006;126:1834–1841. doi: 10.1038/sj.jid.5700325. [DOI] [PubMed] [Google Scholar]
- Tian J, Lei P, Laychock S G, Andreadis S T. Regulated insulin delivery from human epidermal cells reverses hyperglycemia. Mol Ther. 2008;16:1146–1153. doi: 10.1038/mt.2008.79. [DOI] [PubMed] [Google Scholar]
- Liu J Y, Peng H F, Andreadis S T. Contractile smooth muscle cells derived from hair-follicle stem cells. Cardiovasc Res. 2008;79:1–2. doi: 10.1093/cvr/cvn059. [DOI] [PubMed] [Google Scholar]
- Tian J, Andreadis S T. Independent and high-level dual-gene expression in adult stem-progenitor cells from a single lentiviral vector. Gene Ther. 2009;16:874–884. doi: 10.1038/gt.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen H, Jebanathirajah J A, Rush J, Morrice N, Kirschner M W. Phosphorylation analysis by mass spectrometry: myths, facts, and the consequences for qualitative and quantitative measurements. Mol Cell Proteomics. 2006;5:172–181. doi: 10.1074/mcp.M500135-MCP200. [DOI] [PubMed] [Google Scholar]
- Qu J, Straubinger R M. Improved sensitivity for quantification of proteins using triply charged cleavable isotope-coded affinity tag peptides. Rapid Commun Mass Spectrom. 2005;19:2857–2864. doi: 10.1002/rcm.2138. [DOI] [PubMed] [Google Scholar]
- Kim K, Pang K M, Evans M, Hay E D. Overexpression of β-catenin induces apoptosis independent of its transactivation function with LEF-1 or the involvement of major G1 cell cycle regulators. Mol Biol Cell. 2000;11:3509–3523. doi: 10.1091/mbc.11.10.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyssen G, Li T H, Lehmann L, Zhuo M, Sharma M, Sun Z. LZTS2 is a novel β-catenin-interacting protein and regulates the nuclear export of beta-catenin. Mol Cell Biol. 2006;26:8857–8867. doi: 10.1128/MCB.01031-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao G, Tao Q, Kofron M, Chen J S, Schloemer A, Davis R J, Hsieh J C, Wylie C, Heasman J, Kuan C Y. Jun NH2-terminal kinase (JNK) prevents nuclear beta-catenin accumulation and regulates axis formation in Xenopus embryos. Proc Natl Acad Sci U S A. 2006;103:16313–16318. doi: 10.1073/pnas.0602557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Leung L, Brocardo M, Henderson J, Flegg C, Henderson B R. Membrane localization of adenomatous polyposis coli protein at cellular protrusions: targeting sequences and regulation by beta-catenin. J Biol Chem. 2006;281:17140–17149. doi: 10.1074/jbc.M513027200. [DOI] [PubMed] [Google Scholar]
- Serres M, Grangeasse C, Haftek M, Durocher Y, Duclos B, Schmitt D. Hyperphosphorylation of beta-catenin on serine-threonine residues and loss of cell-cell contacts induced by calyculin A and okadaic acid in human epidermal cells. Exp Cell Res. 1997;231:163–172. doi: 10.1006/excr.1996.3443. [DOI] [PubMed] [Google Scholar]
- Avdi N J, Malcolm K C, Nick J A, Worthen G S. A role for protein phosphatase-2A in p38 mitogen-activated protein kinase-mediated regulation of the c-Jun NH(2)-terminal kinase pathway in human neutrophils. J Biol Chem. 2002;277:40687–40696. doi: 10.1074/jbc.M204455200. [DOI] [PubMed] [Google Scholar]
- Dietrich C, Scherwat J, Faust D, Oesch F. Subcellular localization of beta-catenin is regulated by cell density. Biochem Biophys Res Commun. 2002;292:195–199. doi: 10.1006/bbrc.2002.6625. [DOI] [PubMed] [Google Scholar]
- Lallemand D, Ham J, Garbay S, Bakiri L, Traincard F, Jeannequin O, Pfarr C M, Yaniv M. Stress-activated protein kinases are negatively regulated by cell density. EMBO J. 1998;17:5615–5626. doi: 10.1093/emboj/17.19.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani Y, Wheelock M J, Johnson K R. Phosphoinositide-3 kinase-Rac1-c-Jun NH2-terminal kinase signaling mediates collagen I-induced cell scattering and up-regulation of N-cadherin expression in mouse mammary epithelial cells. Mol Biol Cell. 2006;17:2963–2975. doi: 10.1091/mbc.E05-12-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi C J, Gumbiner B M. Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol. 2004;167:339–349. doi: 10.1083/jcb.200402153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of β-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- Zhang F, Phiel C J, Spece L, Gurvich N, Klein P S. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3. J Biol Chem. 2003;278:33067–33077. doi: 10.1074/jbc.M212635200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.