Abstract
Hyp mice possess a mutation that inactivates the phosphate-regulating gene, which is homologous to the endopeptidases of the X-chromosome (PHEX). The mutation is associated with severe hypophosphatemia due to excessive urinary phosphate wasting. Such urinary phosphate wasting in Hyp mice is associated with an increased serum accumulation of fibroblast growth factor (FGF) 23. We wanted to determine the biological significance of increased serum FGF23 levels and concomitant hypophosphatemia in Hyp mice and to evaluate whether FGF23 activity could be modified by manipulating klotho (a cofactor of FGF23 signaling). We generated Hyp and klotho double-mutant mice (Hyp/klotho−/−). Severe hypophosphatemia of Hyp mice was reversed to hyperphosphatemia in Hyp/klotho−/− double mutants, despite the fact that the double mutants showed significantly increased serum levels of FGF23. Hyperphosphatemia in Hyp/klotho−/− mice was associated with increased renal expression of sodium/phosphate cotransporter 2a (NaPi2a) protein. Exogenous injection of bioactive parathyroid hormone 1-34 down-regulated renal expression of NaPi2a and consequently reduced serum levels of phosphate in Hyp/klotho−/− mice. Moreover, in contrast to the Hyp mice, the Hyp/klotho−/− mice showed significantly higher serum levels of 1,25-dihydroxyvitamin D and developed extensive calcification in soft tissues and vascular walls. Furthermore, compared with the Hyp mice, Hyp/klotho−/− mice were smaller in size, showed features of generalized tissue atrophy, and generally died by 15–20 wk of age. Our in vivo studies provide genetic evidence for a pathological role of increased FGF23 activities in regulating abnormal phosphate homeostasis in Hyp mice. Moreover, these results suggest that even when serum levels of FGF23 are significantly high, in the absence of klotho, FGF23 is unable to regulate systemic phosphate homeostasis. Our in vivo observations have significant clinical implications in diseases associated with increased FGF23 activity and suggest that the functions of FGF23 can be therapeutically modulated by manipulating the effects of klotho.—Nakatani, Y., Ohnishi, M., Razzaque, M. S. Inactivation of klotho function induces hyperphosphatemia even in presence of high serum fibroblast growth factor 23 levels in a genetically engineered hypophosphatemic (Hyp) mouse model.
Keywords: parathyroid hormone, vitamin D, calcification, survival, kidney, NaPi2a
An understanding of the molecular regulation of phosphate metabolism has both clinical and biological significance (1,2,3). Modifications in phosphate balance can lead to a wide range of disorders, including myopathy, cardiac dysfunction, hematological abnormalities, and compromised bone mineralization processes and can facilitate calcification in vascular and soft tissues (4,5,6). The complex hormonal regulation of phosphate homeostasis is partially mediated by fibroblast growth factor 23 (FGF23). Increased serum levels of FGF23 are associated with renal phosphate wasting disorders (7), whereas reduced FGF23 activity is associated with hyperphosphatemia due to a decrease in the urinary excretion of phosphate, a process that is active in various human diseases (8).
Our understanding of the FGF23-mediated regulation of phosphate homeostasis has been enhanced by the identification of klotho as a cofactor in FGF23 signaling (9). In vitro studies have shown that klotho is essential for FGF23-mediated signaling. In the presence of klotho, FGF receptors (FGFRs) are able to bind FGF23 with a high affinity (9). Despite the presence of FGF23 as a circulatory factor and the ubiquitous presence of its receptors in different tissues, the restricted expression of klotho in kidneys, in parathyroid glands, and in the brain gives a tissue specificity of FGF23 function (10).
PHEX is a phosphate-regulating gene with homology to endopeptidases of the X-chromosome and is a membrane-bound ectoenzyme expressed in bone cells. PHEX shows homology to members of the M13 family of metalloendopeptidases. Mutations in the PHEX gene are associated with X-linked hypophosphatemia (XLH) in humans (11). The endogenous substrates of PHEX are not yet known, and the mechanism by which inactivation of the PHEX gene leads to XLH remains unclear. The Hyp mouse is the murine model of XLH, with an inactivating mutation in the PHEX gene. Hyp mice develop severe hypophosphatemia due to excessive urinary phosphate wasting (12), which leads to rickets and/or osteomalacia. Dietary phosphate supplementation in Hyp mice appears to correct hypophosphatemia but fails to reverse the bone mineralization defects (13). Conversely, osteoblast-targeted PHEX expression in transgenic mice partially corrects skeletal mineralization but does not normalize the hypophosphatemia (14). There are also studies suggesting that absence of PHEX activity can induce both the renal and skeletal phenotypes of Hyp mice (15). Ongoing studies will determine the exact role of PHEX in skeletogenesis and how dysregulation of the PHEX gene results in increased serum accumulation of FGF23. The general consensus, however, is that hypophosphatemia in patients with XLH (11) and in Hyp mice (16) is caused by the increased activity of FGF23. In this study, we used Hyp mice as a model system to determine the role of klotho in FGF23-mediated systemic regulation of phosphate homeostasis.
The klotho gene encodes a type 1 membrane protein, which is predicted to be present on the cell surface of klotho-expressing cells (17). Klotho is an important FGF23 signaling cofactor (3, 9, 18), and the current study was designed to assess the therapeutic potential of klotho to manipulate phosphate regulation functions of FGF23. We generated Hyp and klotho double-mutant (Hyp/klotho−/−) mice to examine whether high serum levels of FGF23 in Hyp mice could regulate phosphate homeostasis in the absence of klotho. In this study, we present direct evidence that klotho is essential in FGF23-mediated systemic regulation of phosphate homeostasis.
MATERIALS AND METHODS
Generation of double-mutant mice
We cross-bred klotho mutants (Lexicon Genetics; Mutant Mouse Regional Resource Centers, University of California at Davis, CA, USA) with Hyp mutants (The Jackson Laboratory, Bar Harbor, ME, USA) to obtain double-mutant mice (Hyp/klotho−/−). Standard PCR procedures were used to identify the genotypes of various mice (19) (Supplemental Fig. 1). All studies were approved by the institutional animal care and use committee at the Harvard Medical School (Boston, MA, USA).
Gross phenotype and body weight
The total body weight of each mouse was assessed weekly from 3 until 25 wk after birth. No klotho−/− or Hyp/klotho−/− survived past 20 wk.
Biochemical measurements
Blood was obtained by cheek-pouch bleeding of wild-type, klotho−/−, Hyp, and Hyp/klotho−/− mice. Serum was isolated and stored at −80°C. Serum phosphorus levels were determined using colorimetric measurement with the Stanbio Phosphorus Liqui-UV Test, and calcium levels were obtained using the Calcium (Arsenazo) LiquiColor Test. Urinary phosphorus and creatinine were determined using Stanbio Liqui-UV and creatinine kits, respectively. The level of 1,25-hydroxyvitamin D [1,25(OH)2D3] was measured in serum obtained from wild-type, klotho−/−, Hyp, and Hyp/klotho−/− mice using a kit purchased from IDS (Fountain Hills, AZ, USA). The serum levels of parathyroid hormone (PTH) were measured using a commercial kit (Immutopics International, San Clemente, CA, USA). The serum level of FGF23 was measured by ELISA, as described in a previous publication (19).
Histological analyses
Soft tissues obtained from wild-type, klotho−/−, Hyp, and Hyp/klotho−/− mice were fixed in 10% buffered formalin or Carnoy’s solution and were subsequently embedded in paraffin. Four- to 6-μm paraffin sections of various tissues were mounted on SuperFrost Plus slides. Sections were then stained for histological analysis using standard protocols (20, 21).
Calcification analyses
To determine the effects of hyperphosphatemia on soft tissue and vascular calcification in klotho−/− mice, sections were prepared from the heart, lung, kidney, intestine, and skin and were stained with von Kossa stain to visualize mineralized tissue and calcified foci. The von Kossa-stained sections of Hyp/klotho−/− mice were compared with similarly stained sections of wild-type, klotho−/−, and Hyp mice using light microscopy (22).
Immunohistochemical staining
Immunostaining was performed as described previously (23,24,25,26). In brief, kidneys obtained from wild-type, klotho−/−, Hyp, and Hyp/klotho−/− mice were fixed in 10% formalin and embedded in paraffin. The paraffin sections were deparaffinized and were incubated in a blocking solution for 30 min and then incubated overnight with polyclonal anti-sodium/phosphate cotransporter 2a (NaPi2a) antibody (dilution 1:100) at 4°C. The slides were washed with PBS and incubated with anti-rabbit secondary antibody for 30 min. After a PBS wash, coverslips were placed on slides using mounting medium. The expression of NaPi2a was visualized using a bright-field microscope. Rabbit serum, in place of the primary antibody, was used as a negative control. Kidney sections prepared from NaPi2a−/− mice and incubated with NaPi2a antibody did not show any staining.
Quantitative real-time PCR
Total RNA isolated from the kidneys of wild-type, klotho−/−, Hyp, and Hyp/klotho−/− mice was used to detect the relative expression of 1α-hydroxylase [1α(OH)ase], ennp-1, ANK, Pit-1, and RUNX2 mRNA as described previously (27). Real-time PCR was performed in duplicate. The quantity of mRNA was calculated by normalizing the CT (threshold cycle value) of 1α(OH)ase, ennp-1, ANK, Pit-1, and RUNX2 to the CT of the housekeeping gene GAPDH. The sequences of the primers used to detect expression patterns of various genes are listed in Supplemental Table 1.
TABLE 1.
Phenotypes of various mutant mice compared with those of wild-type mice
| Phenotype | Wild-type | klotho−/− | Hyp | Hyp/klotho−/− |
|---|---|---|---|---|
| Gross appearance | ||||
| Body weight | Normal | Reduced (M) | Reduced (S) | Reduced (M) |
| Growth retardation | Absent | Present | Absent | Present |
| Generalized atrophy | ||||
| Spleen atrophy | Absent | Present | Absent | Present |
| Muscle wasting | Absent | Present | Absent | Present |
| Skin atrophy | Absent | Present | Absent | Present |
| Intestinal atrophy | Absent | Present | Absent | Present |
| Morphological changes | ||||
| Atherosclerosis/arteriosclerosis | Absent | Present | Absent | Present |
| Vascular calcifications | Absent | Present | Absent | Present |
| Ectopic calcifications | Absent | Present | Absent | Present |
| Emphysema | Absent | Present | Absent | Present |
| Biochemical changes | ||||
| Serum 1,25(OH)2D3 | Normal | High | Normal | High |
| Serum phosphate | Normal | High | Low | High |
| Serum calcium | Normal | High | Low | High |
| Serum PTH | Normal | Low | Normal | Low |
| Serum FGF23 | Normal | High | High | High |
| Overall affect | ||||
| Physical activity | Normal | Sluggish | Normal | Sluggish |
| Infertility | Absent | Present | Absent | Present |
| Lifespan | Normal | Short | Normal | Short |
M, marked; S, slight.
PTH injection
Wild-type, klotho−/−, Hyp, and Hyp/klotho−/− mice were subcutaneously injected with bioactive PTH(1-34) (200 μg/kg). Blood was collected by cheek-pouch bleeding before the injections and at 2 h postinjection. Isolated serum was used to determine phosphate levels in animals pre- and postinjection, using the Stanbio Liqui-UV kit. To determine the effects of PTH on NaPi2a expression, kidney sections prepared from PTH-injected mice were also used to detect expression of NaPi2a.
Statistics
Statistically significant differences between groups were evaluated using the Student’s t test of comparisons between two groups or by 1-way ANOVA and Tukey’s test for multiple comparisons. All values are expressed as means ± se. P < 0.05 was considered to be statistically significant. All analyses were performed using Microsoft Excel (Microsoft, Redmond, WA, USA) and StatView (SAS Institute, Cary, NC, USA).
RESULTS
Generation of Hyp/klotho−/− mice
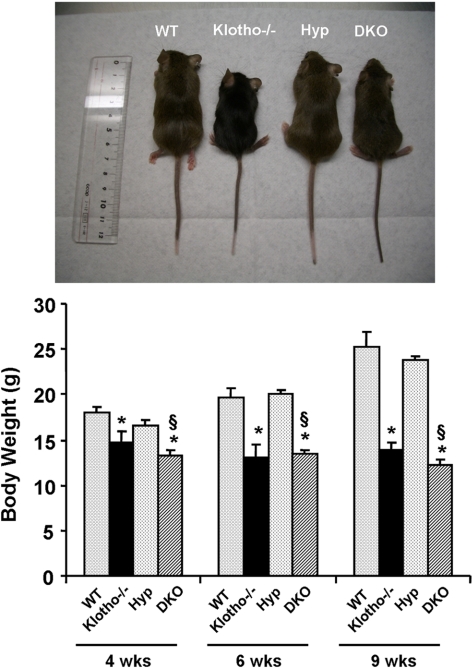
An inactivating mutation of the PHEX gene causes hypophosphatemia in Hyp mice. To determine whether hypophosphatemia in Hyp mice is a FGF23-klotho-dependent process, we generated a new Hyp mouse model deficient in klotho function (Hyp/klotho−/−) by crossing heterozygous klotho mice with Hyp mice. The Hyp/klotho−/− double-mutant mice were viable and were similar in size to klotho−/− mice (Supplemental Fig. 2). At birth, Hyp/klotho−/− mutant mice were indistinguishable from littermates of other genotypes. At 4 wk of age, Hyp/klotho−/− double-mutant mice (13.2±0.6 g) were smaller than wild-type mice (18.1±0.6 g) but were similar in size to klotho−/− mice (14.7±1.2 g) (Fig. 1). At 4 wk, average body weight of Hyp mice was 16.6 ± 0.5 g.
Figure 1.
Macroscopic phenotype of Hyp/klotho−/− double mutants. Top panel: gross phenotype of wild-type (WT), klotho−/−, Hyp, and Hyp/klotho−/− (DKO) mice at 6 wk of age. Bottom panel: body weights of 4-, 6-, and 9-wk-old WT, klotho−/−, and Hyp/klotho−/− and Hyp mice, showing that Hyp/klotho−/− mice are smaller than Hyp mice. These observations suggest that inactivation of klotho function in Hyp mice can induce body weight loss in Hyp/klotho−/− mice. *P < 0.01 vs. WT mice; §P < 0.01 vs. Hyp mice.
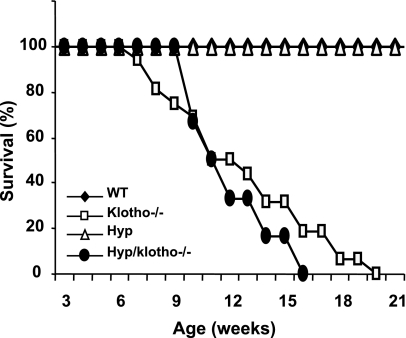
At 6 and 12 wk of age, Hyp/klotho−/− double-mutant mice were still smaller than their wild-type littermates (Hyp/klotho−/− 12.2±0.7 g vs. wild-type 25.3±1.5 g at 9 wk) and were similar in size to klotho−/− mice (13.8±0.8 g at 9 wk). At 9 wk, the average body weight of Hyp mice was 23.7 ± 0.6 g (Fig. 1). Neither Hyp/klotho−/− nor klotho−/− mice survived past 20 wk, whereas all the Hyp and wild-type mice survived to the maximum observed period of 25 wk (Fig. 2).
Figure 2.
Survival of various genotypes. Survival for wild-type (WT) (n=11), klotho−/− (n=13), and Hyp/klotho−/− (DKO, n=7) and Hyp (n=18) mice. Survival of Hyp/klotho−/− double-knockout mice is far lower than that of WT and Hyp mice and is similar to that of klotho−/− mice. Most of the Hyp/klotho−/−and klotho−/−mice died by 15 to 20 wk of age, whereas none of the WT and Hyp mice had died by the end of the 25-wk observation period. Mice used to generate the survival curve were not always littermates but were of similar genetic background.
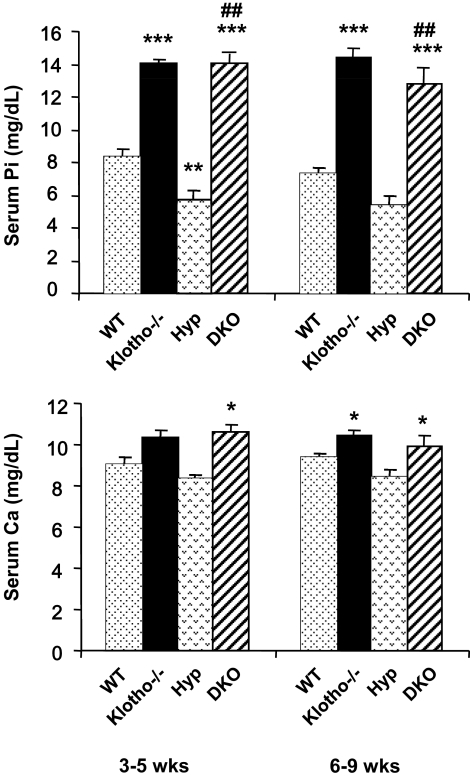
Serum phosphate and calcium levels in Hyp/klotho−/− mice
Serum phosphate and calcium levels were measured in 3- to 5- and 6- to 9-wk-old wild-type, klotho−/−, Hyp, and Hyp/klotho−/− mice. The double-mutant mice were severely hyperphosphatemic by 3–5 wk of age (14±0.6 mg/dl) compared with wild-type mice (8.3±0.4 mg/dl) of similar age. The high serum phosphate levels in Hyp/klotho−/− mice were similar to those seen in age-matched klotho−/− mice (14±0.6 mg/dl) but contrary to those of Hyp mice (5.7±0.5 mg/dl). Similar patterns of serum phosphate levels were observed in 6- to 9-wk-old mice (Fig. 3). Collectively, these findings suggest that inactivation of klotho function from Hyp mice can reverse hypophosphatemia to hyperphosphatemia.
Figure 3.
Biochemical measurements of serum phosphate and calcium levels in Hyp/klotho−/− mice. Note that serum phosphate (top panel) and calcium (bottom panel) levels are higher in klotho−/− mice than in the wild-type (WT) mice. Serum phosphate level is significantly higher in klotho−/− mice (14.±0.2 mg/dl) than in WT mice (8.3±0.4 mg/dl), at 3–5 wk of age. Similar hyperphosphatemia is also observed in klotho−/− mice (14.4±0.5 mg/dl) at 6–9 wk of age, compared with WT mice (7.2±0.4 mg/dl) of the same age. In contrast to the level in Hyp mice at 3–5 wk (5.7±0.57 mg/dl) and 6–9 wk (5.4±0.4 mg/dl), serum phosphate level is significantly increased in Hyp/klotho−/− (DKO) mice, both at 3–5 wk (14±0.6 mg/dl) and 6–9 wk (12.8±0.8 mg/dl) of age. Serum calcium level is higher in klotho−/− mice (10.4±0.3 mg/dl) at 6–9 wk of age than in WT mice (9.4±0.1 mg/dl) (bottom panel). Similar elevated serum calcium levels are also observed in Hyp/klotho−/− double-mutant mice (9.9±0.4 mg/dl) at 6–9 wk of age. In contrast to Hyp/klotho−/− double-mutant mice at 6–9 wk, serum calcium level is lower in Hyp mice at 6–9 wk (8.4±0.3 mg/dl) of age. *P < 0.05, **P < 0.01, ***P < 0.001 vs. WT; ##P < 0.001 vs. Hyp.
We also measured serum calcium levels in various genotypes. Compared with the wild-type mice (9.4±0.1 mg/dl), klotho−/− mice showed elevated serum calcium levels (10.4±0.3 mg/dl) at 6–9 wk of age, similar to the levels observed in Hyp/klotho−/− mice (9.9±0.4 mg/dl). However, levels were slightly lower in Hyp mice (8.4±0.3 mg/dl) at a similar stage (Fig. 3).
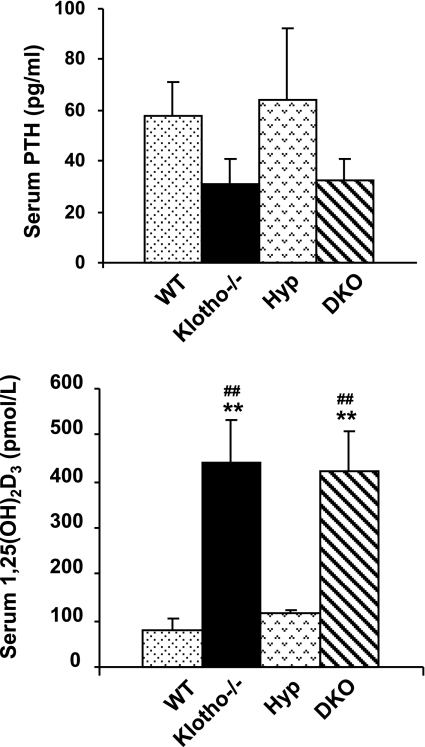
Serum PTH and 1,25(OH)2D3 levels in Hyp/klotho−/− mice
No significant changes in the serum PTH levels were detected in Hyp mice (64.2±28 pg/ml) compared with those in wild-type mice (57.8±13 pg/ml), but PTH levels were reduced in Hyp/klotho−/− mice (32.5±8 pg/ml) and klotho−/− mice (31.3±9 pg/ml) (Fig. 4). Our results suggest that the inactivation of klotho in Hyp mice can significantly reduce serum PTH levels in double-mutant mice. We subsequently measured 1,25(OH)2D3 levels of various genotypes as described previously (27, 28). Compared with those in wild-type (80.7±20.9 pM) and Hyp mice (116.0±9.4 pM) at 6 wk of age, serum 1,25(OH)2D3 levels were markedly elevated in Hyp/klotho−/− (424.6±80 pM) and klotho−/− (441.8±89.7 pM) mice (Fig. 4).
Figure 4.
Biochemical measurements of serum PTH and 1,25(OH)2D3 from various genotypes. Compared with those in wild-type (WT) mice (n=24; 57.8±13 pg/ml), serum PTH levels are reduced in klotho−/− mice (n=22; 31.3±9 pg/ml). Serum PTH levels are similar in WT and Hyp mice (n=11; 64.2±28 pg/ml). In contrast, serum PTH levels are reduced in Hyp/klotho−/− (DKO) mice (n=10; 32.5±8 pg/ml) compared with those in Hyp mice. Compared with levels in WT mice (n=5; 80.7±20 pM) and Hyp mice (n=5; 116.0±9.4 pM), serum 1,25(OH)2D3 levels were markedly increased in both klotho−/− mice (n=5; 441.8±89 pM) and Hyp/klotho−/− mice (n=5; 424.6±80 pM). **P < 0.001 vs. WT; ##P < 0.01 vs. Hyp.
Serum FGF23 levels in Hyp/klotho−/− mice
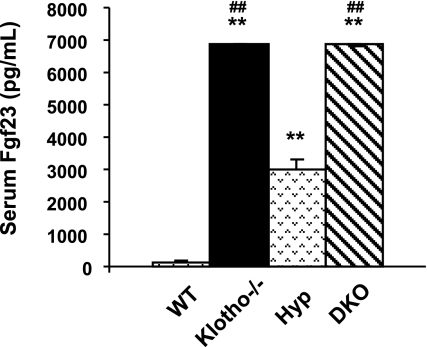
Compared with the control levels (∼150 pg/ml), serum FGF23 levels were extremely high in the klotho−/− mice (∼6500 pg/ml) at 6 wk of age. Markedly increased serum levels of FGF23 were also noted in the Hyp mice (∼3000 pg/ml) and Hyp/klotho−/− mice (∼6500 pg/ml) (Fig. 5). Increased serum levels of FGF23 are associated with hypophosphatemia in Hyp mice. However, in the absence of klotho activity, despite significantly elevated serum levels of FGF23, hypophosphatemia cannot be induced in klotho−/− or Hyp/klotho−/− mice.
Figure 5.
Biochemical measurements of serum FGF23 in various genotypes. Average serum levels of FGF23 (after adjusting dilution factors) are higher in klotho−/− mice (n=5; ∼6500 pg/ml) than in wild-type (WT) mice (n=4; ∼150 pg/ml). Similarly increased FGF23 serum levels are also noted in Hyp mice (n=5; ∼3000 pg/ml) and Hyp/klotho−/− (DKO) mice (n=5; ∼6500 pg/ml). **P < 0.001 vs. WT; ##P < 0.001 vs. Hyp.
Renal expression of NaPi2a and 1α(OH)ase in Hyp/klotho−/− mice
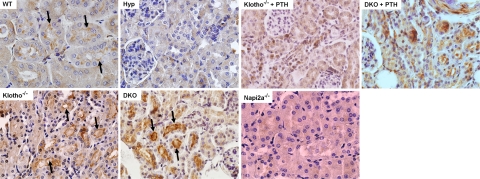
To determine the role of NaPi2a in Hyp/klotho−/− mice, we examined its expression pattern in kidney sections prepared from mice of various genotypes. Compared with wild-type mice, Hyp/klotho−/− mice showed increased expression of NaPi2a protein in the luminal side of the proximal tubules, a pattern of expression very similar to that seen in klotho−/− mice (Fig. 6). Increased expression of NaPi2a is associated with an increase in tubular reuptake of phosphate, causing hyperphosphatemia in Hyp/klotho−/− and klotho−/− mice. In contrast, decreased renal expression of NaPi2a was noted in Hyp mice (Fig. 6). Such reduced NaPi2a expression in the kidneys of Hyp mice may reduce tubular phosphate resorption, causing increased urinary phosphate excretion and hypophosphatemia.
Figure 6.
Expression of NaPi2a. Immunostaining for NaPi2a in the kidney sections prepared from the wild-type (WT), klotho−/−, Hyp, and Hyp/klotho−/− (DKO) mice. Expression of NaPi2a protein is significantly increased in the luminal side of the proximal tubular epithelial cells of klotho−/− and Hyp/klotho−/− mice, in contrast with the reduced expression seen in Hyp mice. Note that PTH injection suppressed NaPi2a expression in both klotho−/− and Hyp/klotho−/− mice. Kidney sections prepared from NaPi2a−/− mice did not show any specific staining.
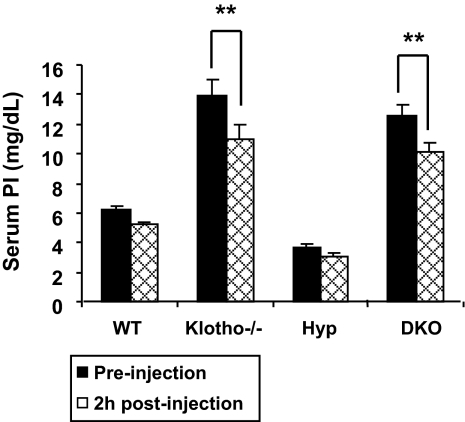
Studies have shown an inverse correlation between PTH and renal expression of NaPi2a (29, 30). We believe that hyperphosphatemia in klotho−/− and Hyp/klotho−/− mice is due to increased activity of NaPi2a, which is partially regulated by reduced serum PTH levels in these mutant mice (Fig. 4). This view is further substantiated by our observations that serum phosphate levels decrease significantly within 2 h of PTH injection in klotho−/− and Hyp/klotho−/− mice (Fig. 7). Moreover, PTH injection suppressed NaPi2a expression in both klotho−/− and Hyp/klotho−/− mice (Fig. 6). In combination, these results suggest that compared with the hypophosphatemia of Hyp mice, the hyperphosphatemia of Hyp/klotho−/− mice is caused primarily by an increase in renal phosphate resorption due to increased NaPi2a activity, despite significantly high serum FGF23 levels.
Figure 7.
Bioactive PTH injection: serum phosphate levels before and 2 h after injection with bioactive PTH(1-34) protein. PTH protein injection resulted in significantly lowered serum phosphate levels in klotho−/− mice (13.8±1.0 vs. 10.9±0.94 mg/dl; P<0.01) and Hyp/klotho−/− (DKO) mice (12.5±0.7 vs. 10.1±0.6 mg/dl; P<0.01), suggesting that PTH controls systemic phosphate homeostasis by influencing NaPi2a activities. **P < 0.01 vs. corresponding preinjection value.
Using real-time PCR, we determined that renal expression of 1α(OH)ase, the enzyme that converts inactive vitamin-D metabolites into active 1,25(OH)2D3, was ∼6-fold higher in Hyp/klotho−/− mice than in wild-type mice. Similarly, compared with wild-type kidneys, in klotho−/− kidneys 1α(OH)ase expression was almost 10-fold increased (Supplemental Fig. 3). Increased renal expression of 1α(OH)ase was associated with increased 1,25(OH)2D3 serum levels in klotho−/− and Hyp/klotho−/− mice (Fig. 4). Mild increased renal expression of 1α(OH)ase was in accord with slightly elevated serum 1,25(OH)2D3 levels in the Hyp mice, although such elevation was not statistically significant.
Soft tissue anomalies of Hyp/klotho−/− mice
Histological examination of various soft tissues from Hyp/klotho−/− mice showed generalized atrophy of the skin, skeletal muscle, reproductive organs, and intestine. This atrophy was obvious by 6 wk of age and remained evident throughout the life of the animals. Severe gonadal atrophy in Hyp/klotho−/− mice was associated with infertility of both male and female homozygous mutants. In addition, severe lung emphysema was consistently observed in Hyp/klotho−/− mice. All of the soft tissue changes noted in Hyp/klotho−/− mice were absent in Hyp mice but were present in klotho−/− mice (Table 1), suggesting an important role of klotho in the Hyp mice phenotype.
Vascular and soft tissue calcification of Hyp/klotho−/− mice
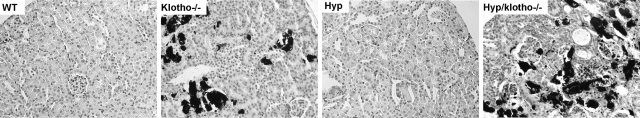
We examined the calcification in various murine genotypes by von Kossa staining. We detected extensive vascular and soft tissue calcification in the lung, kidney, aorta, and other organs in klotho−/− mice by von Kossa staining (Fig. 8). The extensive calcification noted in klotho−/− mice was absent in Hyp mice. However, inactivation of klotho function from Hyp mice resulted in extensive soft tissue and vascular calcification in Hyp/klotho−/− mice (Fig. 8), similarly suggesting a crucial role of klotho in Hyp mice phenotype.
Figure 8.
Von Kossa staining of kidney tissues. Renal sections prepared from wild-type (WT), klotho−/−, Hyp, and Hyp/klotho−/− mice, with extensive calcification seen in kidneys of klotho−/− and Hyp/klotho−/− mice. No such renal calcification is found in Hyp or WT mice (×20).
DISCUSSION
To determine whether in vivo FGF23 and klotho interactions are necessary for the hypophosphatemic phenotype characteristic of the Hyp mouse, in this study we examined the role of klotho in FGF23-mediated systemic regulation of phosphate homeostasis. Our genetic manipulation studies showed that FGF23 cannot regulate systemic phosphate homeostasis without klotho. Inactivation of the klotho gene from Hyp mice resulted in severe hyperphosphatemia in the Hyp/klotho−/− double-mutant mice, despite significantly higher serum levels of FGF23.
Studies have shown that FGF23 requires the cofactor klotho to interact with the FGFR. FGF23 can bind to multiple FGFRs, including FGFR1c, FGFR3c, and FGFR4 (9). In the presence of klotho, FGF23 can activate downstream signaling events, evident in the phosphorylation of FGF receptor substrate-2a, extracellular signal-regulated kinase, p38, c-Jun NH2-terminal kinase, and AKT proteins (9, 18). The dependence of FGF23 signaling on klotho is further underscored by fact that the physical, morphological, and biochemical phenotypes of Fgf23−/−/klotho−/− double-mutant mice are similar to those of the Fgf23−/− and the klotho−/− single-knockout mice (3, 19, 28, 31). Such similarities in phenotypes of Fgf23−/−, klotho−/−, and Fgf23−/−/klotho−/− mice suggest that a common signaling pathway has been disrupted in each of these mutants (32).
In vitro studies, however, have suggested that FGF23 can exert functions on certain cells independent of klotho (33,34,35). Indeed, FGF23 treatment has been shown to suppress osteoblast differentiation and bone mineralization in fetal rat calvaria cells. Because klotho is not expressed in osteoblasts, any effect of FGF23 on these culture cells would suggest a klotho-independent function of FGF23. To address whether FGF23 has klotho-independent function in vivo, we generated Hyp/klotho−/− double-mutant mice. Because double-mutant mice have significantly elevated serum FGF23 levels, these mice were a way to examine the possible effects of FGF23 without klotho in a developing mammal. Inactivation of klotho from Hyp mice resulted in hyperphosphatemic Hyp/klotho−/− double-mutant mice, even though these mice showed significantly elevated serum levels of FGF23, compared with the Hyp mice with high serum FGF23 levels and hypophosphatemia. In Hyp/klotho−/− mice lacking endogenous klotho, the high serum level of FGF23 is unable to lower the serum phosphate levels. In addition to abnormal phosphate homeostasis, Hyp/klotho−/− mice have decreased life expectancy, pulmonary emphysema, infertility, extensive soft tissue calcifications, skin atrophy, and skeletal muscle wasting. Thus, the phenotype of Hyp/klotho−/− mice is largely indistinguishable from that of klotho single-knockout mice.
Hyperphosphatemia in klotho−/− and Hyp/klotho−/− mice is associated with extensive soft tissue calcification. An imbalance between phosphate and pyrophosphate usually determines the ectopic calcification process (36, 37). We have found that the expression of ennp-1 (a pyrophosphate generator) and ANK (a pyrophosphate transporter) is slightly reduced in the kidneys of Hyp mice; no major changes in the expression of ennp-1 and ANK are noted in klotho−/− mice, suggesting that extensive calcification in these mutant mice is mostly associated with high serum phosphate levels (Supplemental Fig. 4). Further studies will be required to determine whether FGF23 or klotho individually or in combination can influence pyrophosphate activities.
Our newly generated Hyp/klotho−/− mice have increased components of vitamin D metabolism, as reflected by the presence of increased renal expression of 1α(OH)ase and a concomitant increase in the serum levels of 1,25(OH)2D3 levels. We did not found any statistical difference in serum 1,25(OH)2D3 levels in the wild-type and Hyp mice. The slight differences in the serum 1,25(OH)2D3 levels between our study and earlier published studies may be related to the different methods of measurement. Bai et al. (38) in their study used an RIA, whereas we used a nonisotopic assay with a commercially available kit. Compared with kidneys in the wild-type control mice, in the Hyp mice kidneys a mild increase in the expression of 1α(OH)ase mRNA was detected. Of relevance, Yuan et al. (30) also found increased transcripts of 1α(OH)ase in the Hyp mice and phosphate-depleted normal mice; it is, however, important to mention that increased 1α(OH)ase mRNA transcripts in phosphate-depleted normal mice was associated with increased enzyme activity, whereas a similar increment in mRNA transcripts in Hyp mice failed to alter enzyme activity, suggesting a possible translational dysregulation in Hyp mice (30).
Extremely high serum 1,25(OH)2D3 levels in klotho−/− and Hyp/klotho−/− mice is likely to induce indistinguishable phenotypes in these mutant mice. Genetic inactivation of 1α(OH)ase from klotho−/− mice can reverse biochemical phenotypes, including lowering of serum phosphate and calcium levels, while increasing serum PTH levels in klotho−/−/1α(OH)ase−/− double-knockout mice. Genetic inactivation of vitamin D activities from klotho−/− mice resulted in elimination of calcification and prolonged survival of klotho−/−/1α(OH)ase−/− double-knockout mice compared with those for the klotho−/− single-knockout mice (Supplemental Fig. 5). An identical response was also noted in klotho−/− mice that were fed a vitamin D-deficient diet; such a modified diet delayed the appearance of numerous phenotypes with a better survival rate than that of klotho mutant mice (39, 40).
Despite significantly high serum levels of FGF23, the opposing phenotypes of Hyp and Hyp/klotho−/− mice suggest that that the dissimilarities arise from the disruption of klotho-mediated pathways (3, 41). This study provides compelling genetic evidence that klotho has an important in vivo role in the regulation of systemic phosphate homeostasis by FGF23 in Hyp mice. Our in vivo experimental results are similar to human diseases; a patient with a homozygous loss-of-function mutation in the Klotho gene exhibits tumoral calcinosis, severe hyperphosphatemia, and ectopic calcification despite high serum levels of FGF23 (42).
Hyp/klotho−/− mice show decreased serum levels of PTH. The PTH deficit may account for the increased renal expression of NaPi2a in the Hyp/klotho−/− double-mutant mice, as PTH can influence renal endocytosis of NaPi2a transporters from the luminal side of proximal tubular epithelial cells and can eventually cause lysosomal degradation of the transporters (43,44,45). A decreased level of PTH, therefore, can facilitate an increase in NaPi2a concentration in the brush-border membrane of proximal tubular epithelial cells, causing an increase in renal phosphate reabsorption. This increase in phosphate uptake can eventually lead to hyperphosphatemia in Hyp/klotho−/− mice as well as in klotho−/− mice. We have found that injection of bioactive PTH to either Hyp/klotho−/− mice or klotho−/− mice can reduce serum phosphate levels. Moreover, injection of PTH in either klotho−/− or Hyp/klotho−/− mice can reduce the concentration of NaPi2a at the brush-border membrane of proximal tubular epithelial cells, again suggesting that PTH is the potent regulator of NaPi2a in the mutant mice. The Hyp/klotho−/− mice show reduced serum PTH and increased serum FGF23, calcium, and phosphate levels, characteristics strikingly similar to the increased serum FGF23 levels, hyperphosphatemia, and hypercalcemia seen in Hyp/pth−/− mice (38).
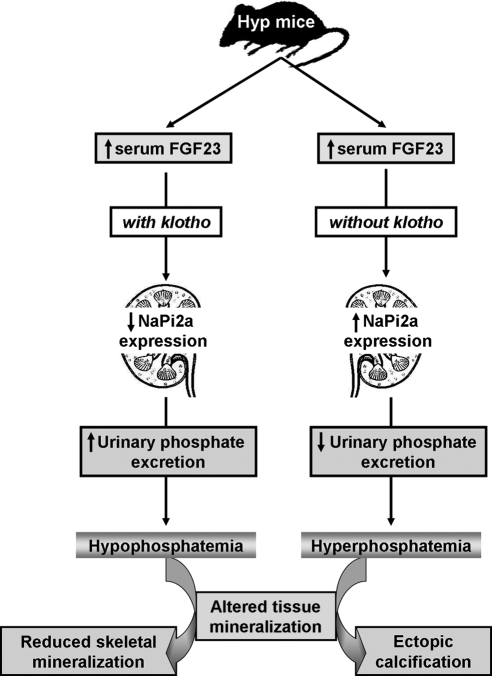
Our results with Hyp/klotho−/− double-mutant mice suggest that FGF23-mediated hypophosphatemia in Hyp mice is a klotho-dependent process, and genetic inactivation of klotho activities results in severe hyperphosphatemia and impaired vitamin D metabolism in Hyp mice despite elevated serum levels of FGF23. Furthermore, Hyp/klotho−/− mice provide a unique in vivo tool to study local and systemic effects of mouse FGF23 without interfering with endogenous mouse klotho activities. Using Hyp mice as an in vivo model system, our results clearly suggest that klotho is essential in the regulation of FGF23-mediated phosphate homeostasis, an action that may contribute to the development of the hypophosphatemic diseases. Despite the crucial biological and clinical importance of maintaining physiological mineral ion balance, our knowledge of in vivo coordinated regulations of NaPi systems, FGF23, and klotho is limited and requires additional carefully designed studies (Fig. 9).
Figure 9.
Regulation of phosphate homeostasis in Hyp mice. Schematic diagram outlining the possible molecular and biochemical events that may be involved in inducing different phenotypes in the Hyp mice in the presence or absence of klotho.
Finally, our results suggest that klotho may potentially be used as a therapeutic tool to manipulate the functions of FGF23 and that directly manipulating the effects of klotho may prove a novel therapeutic strategy for FGF23-related disorders and diseases associated with abnormal mineral ion metabolism (32, 41, 46). In conclusion, FGF23 does not show a klotho-independent function in the regulation of systemic phosphate homeostasis in live mammalian systems.
Supplementary Material
Acknowledgments
Parts of this research were supported by grant R01-DK077276 (to M.S.R.) from the National Institute of Diabetes and Digestive and Kidney Diseases, U.S. National Institutes of Health.
References
- Yu X, White K E. FGF23 and disorders of phosphate homeostasis. Cytokine Growth Factor Rev. 2005;16:221–232. doi: 10.1016/j.cytogfr.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Fukumoto S. Physiological regulation and disorders of phosphate metabolism—pivotal role of fibroblast growth factor 23. Intern Med. 2008;47:337–343. doi: 10.2169/internalmedicine.47.0730. [DOI] [PubMed] [Google Scholar]
- Razzaque M S, Lanske B. The emerging role of the fibroblast growth factor-23-klotho axis in renal regulation of phosphate homeostasis. J Endocrinol. 2007;194:1–10. doi: 10.1677/JOE-07-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque M S, St-Arnaud R, Taguchi T, Lanske B. FGF-23, vitamin D and calcification: the unholy triad. Nephrol Dial Transplant. 2005;20:2032–2035. doi: 10.1093/ndt/gfh991. [DOI] [PubMed] [Google Scholar]
- Gaasbeek A, Meinders A E. Hypophosphatemia: an update on its etiology and treatment. Am J Med. 2005;118:1094–1101. doi: 10.1016/j.amjmed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Memon F, El-Abbadi M, Nakatani T, Taguchi T, Lanske B, Razzaque M S. Does Fgf23-klotho activity influence vascular and soft tissue calcification through regulating mineral ion metabolism? Kidney Int. 2008;74:566–570. doi: 10.1038/ki.2008.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADHR Consortium Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- Benet-Pages A, Orlik P, Strom T M, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14:385–390. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt K P, Baum M G, Schiavi S, Hu M C, Moe O W, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S A, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29:91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- Jonsson K B, Zahradnik R, Larsson T, White K E, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang I M, Miyauchi A, Econs M J, Lavigne J, Juppner H. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H S. X-linked hypophosphataemia: a homologous disorder in humans and mice. Nephrol Dial Transplant. 1999;14:333–341. doi: 10.1093/ndt/14.2.333. [DOI] [PubMed] [Google Scholar]
- Ecarot B, Glorieux F H, Desbarats M, Travers R, Labelle L. Effect of dietary phosphate deprivation and supplementation of recipient mice on bone formation by transplanted cells from normal and X-linked hypophosphatemic mice. J Bone Miner Res. 1992;7:523–530. doi: 10.1002/jbmr.5650070508. [DOI] [PubMed] [Google Scholar]
- Bai X, Miao D, Panda D, Grady S, McKee M D, Goltzman D, Karaplis A C. Partial rescue of the Hyp phenotype by osteoblast-targeted PHEX (phosphate-regulating gene with homologies to endopeptidases on the X chromosome) expression. Mol Endocrinol. 2002;16:2913–2925. doi: 10.1210/me.2002-0113. [DOI] [PubMed] [Google Scholar]
- Yuan B, Takaiwa M, Clemens T L, Feng J Q, Kumar R, Rowe P S, Xie Y, Drezner M K. Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia. J Clin Invest. 2008;118:722–734. doi: 10.1172/JCI32702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Zhou J, Tang W, Jiang X, Rowe D W, Quarles L D. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291:E38–E49. doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- Goetz R, Beenken A, Ibrahimi O A, Kalinina J, Olsen S K, Eliseenkova A V, Xu C, Neubert T A, Zhang F, Linhardt R J, Yu X, White K E, Inagaki T, Kliewer S A, Yamamoto M, Kurosu H, Ogawa Y, Kuro-o M, Lanske B, Razzaque M S, Mohammadi M. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol. 2007;27:3417–3428. doi: 10.1128/MCB.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani T, Bara S, Ohnishi M, Densmore M J, Taguchi T, Goetz R, Mohammadi M, Lanske B, Razzaque M S. In vivo genetic evidence of klotho-dependent functions of FGF23 in regulation of systemic phosphate homeostasis. FASEB J. 2009;23:433–441. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha Y, Taguchi T, Nazneen A, Shimokawa I, Higami Y, Razzaque M S. Genetic suppression of GH-IGF-1 activity, combined with lifelong caloric restriction, prevents age-related renal damage and prolongs the life span in rats. Am J Nephrol. 2008;28:755–764. doi: 10.1159/000128607. [DOI] [PubMed] [Google Scholar]
- Liu D, Nazneen A, Taguchi T, Razzaque M S. Low-dose local kidney irradiation inhibits progression of experimental crescentic nephritis by promoting apoptosis. Am J Nephrol. 2008;28:555–568. doi: 10.1159/000115290. [DOI] [PubMed] [Google Scholar]
- Razzaque M S, Soegiarto D W, Chang D, Long F, Lanske B. Conditional deletion of Indian hedgehog from collagen type 2α1-expressing cells results in abnormal endochondral bone formation. J Pathol. 2005;207:453–461. doi: 10.1002/path.1870. [DOI] [PubMed] [Google Scholar]
- Razzaque M S, Taguchi T. Collagen-binding heat shock protein (HSP) 47 expression in anti-thymocyte serum (ATS)-induced glomerulonephritis. J Pathol. 1997;183:24–29. doi: 10.1002/(SICI)1096-9896(199709)183:1<24::AID-PATH1106>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Zha Y, Le V T, Higami Y, Shimokawa I, Taguchi T, Razzaque M S. Life-long suppression of growth hormone-insulin-like growth factor I activity in genetically altered rats could prevent age-related renal damage. Endocrinology. 2006;147:5690–5698. doi: 10.1210/en.2006-0302. [DOI] [PubMed] [Google Scholar]
- Razzaque M S, Kumatori A, Harada T, Taguchi T. Coexpression of collagens and collagen-binding heat shock protein 47 in human diabetic nephropathy and IgA nephropathy. Nephron. 1998;80:434–443. doi: 10.1159/000045217. [DOI] [PubMed] [Google Scholar]
- Razzaque M S, Nazneen A, Taguchi T. Immunolocalization of collagen and collagen-binding heat shock protein 47 in fibrotic lung diseases. Mod Pathol. 1998;11:1183–1188. [PubMed] [Google Scholar]
- Razzaque M S, Sitara D, Taguchi T, St-Arnaud R, Lanske B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006;20:720–722. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque M S, Lanske B. Hypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol Med. 2006;12:298–305. doi: 10.1016/j.molmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Segawa H, Yamanaka S, Ohno Y, Onitsuka A, Shiozawa K, Aranami F, Furutani J, Tomoe Y, Ito M, Kuwahata M, Imura A, Nabeshima Y, Miyamoto K. Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in klotho mice. Am J Physiol Renal Physiol. 2007;292:F769–F779. doi: 10.1152/ajprenal.00248.2006. [DOI] [PubMed] [Google Scholar]
- Yuan B, Xing Y, Horst R L, Drezner M K. Evidence for abnormal translational regulation of renal 25-hydroxyvitamin D-1α-hydroxylase activity in the hyp-mouse. Endocrinology. 2004;145:3804–3812. doi: 10.1210/en.2004-0192. [DOI] [PubMed] [Google Scholar]
- Lanske B, Razzaque M S. Premature aging in klotho mutant mice: cause or consequence? Ageing Res Rev. 2007;6:73–79. doi: 10.1016/j.arr.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque M S. Does FGF23 toxicity influence the outcome of chronic kidney disease? Nephrol Dial Transplant. 2009;24:4–7. doi: 10.1093/ndt/gfn620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yoshiko Y, Yamamoto R, Minamizaki T, Kozai K, Tanne K, Aubin J E, Maeda N. Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. J Bone Miner Res. 2008;23:939–948. doi: 10.1359/jbmr.080220. [DOI] [PubMed] [Google Scholar]
- Yu X, Ibrahimi O A, Goetz R, Zhang F, Davis S I, Garringer H J, Linhardt R J, Ornitz D M, Mohammadi M, White K E. Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology. 2005;146:4647–4656. doi: 10.1210/en.2005-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitara D, Kim S, Razzaque M S, Bergwitz C, Taguchi T, Schuler C, Erben R G, Lanske B. Genetic evidence of serum phosphate-independent functions of FGF-23 on bone. PLoS Genet. 2008;4:e1000154. doi: 10.1371/journal.pgen.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demer L L, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty T M, Fitzpatrick L A, Inoue D, Qiao J H, Fishbein M C, Detrano R C, Shah P K, Rajavashisth T B. Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocr Rev. 2004;25:629–672. doi: 10.1210/er.2003-0015. [DOI] [PubMed] [Google Scholar]
- Bai X, Miao D, Goltzman D, Karaplis A C. Early lethality in Hyp mice with targeted deletion of Pth gene. Endocrinology. 2007;148:4974–4983. doi: 10.1210/en.2007-0243. [DOI] [PubMed] [Google Scholar]
- Ohnishi M, Nakatani T, Lanske B, Razzaque M S. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1α-hydroxylase. Kidney Int. 2009;75:1166–1172. doi: 10.1038/ki.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol. 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- Razzaque M S. FGF23-mediated regulation of systemic phosphate homeostasis: is Klotho an essential player? Am J Physiol Renal Physiol. 2009;296:F470–F476. doi: 10.1152/ajprenal.90538.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa S, Imel E A, Kreiter M L, Yu X, Mackenzie D S, Sorenson A H, Goetz R, Mohammadi M, White K E, Econs M J. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117:2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister M F, Ruf I, Stange G, Ziegler U, Lederer E, Biber J, Murer H. Parathyroid hormone leads to the lysosomal degradation of the renal type II Na/Pi cotransporter. Proc Natl Acad Sci U S A. 1998;95:1909–1914. doi: 10.1073/pnas.95.4.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch I, Traebert M, Lotscher M, Kaissling B, Murer H, Biber J. Parathyroid hormone and dietary phosphate provoke a lysosomal routing of the proximal tubular Na/Pi-cotransporter type II. Kidney Int. 1998;54:1224–1232. doi: 10.1046/j.1523-1755.1998.00115.x. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Ito M, Tatsumi S, Kuwahata M, Segawa H. New aspect of renal phosphate reabsorption: the type IIc sodium-dependent phosphate transporter. Am J Nephrol. 2007;27:503–515. doi: 10.1159/000107069. [DOI] [PubMed] [Google Scholar]
- Razzaque M S. Can fibroblast growth factor 23 fine-tune therapies for diseases of abnormal mineral ion metabolism? Nat Clin Pract Endocrinol Metab. 2007;3:788–789. doi: 10.1038/ncpendmet0667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.