Abstract
Cystic fibrosis (CF) is most frequently associated with deletion of phenylalanine at position 508 (ΔF508) in the CF transmembrane conductance regulator (CFTR) protein. The ΔF508-CFTR mutant protein exhibits a folding defect that affects its processing and impairs chloride-channel function. This study aimed to determine whether CFTR fragments approximately half the size of wild-type CFTR and complementary to the portion of CFTR bearing the mutation can specifically rescue the processing of endogenous ΔF508-CFTR in vivo. cDNA encoding CFTR fragments were delivered to human airway epithelial cells and mice harboring endogenous ΔF508-CFTR. Delivery of small CFTR fragments, which do not act as chloride channels by themselves, rescue ΔF508-CFTR. Therefore, we can speculate that the presence of the CFTR fragment, which does not harbor a mutation, might facilitate intermolecular interactions. The rescue of CFTR was evident by the restoration of chloride transport in human CFBE41o- bronchial epithelial cells expressing ΔF508-CFTR in vitro. More important, nasal administration of an adenovirus expressing a complementary CFTR fragment restored some degree of CFTR activity in the nasal airways of ΔF508 homozygous mice in vivo. These findings identify complementary protein fragments as a viable in vivo approach for correcting disease-causing misfolding of plasma membrane proteins.—Cormet-Boyaka, E., Hong, J. S., Berdiev, B. K., Fortenberry, J. A., Rennolds, J., Clancy, J. P., Benos, D. J., Boyaka, P. N., Sorscher, E. J. A truncated CFTR protein rescues endogenous ΔF508-CFTR and corrects chloride transport in mice.
Keywords: cystic fibrosis, in vivo rescue, processing, transcomplementation
Cystic fibrosis (CF) is caused by inherited mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR), a cAMP-regulated chloride channel expressed in epithelial tissues (1). CF affects numerous organs, including the lungs, small intestine, pancreas, and sweat glands. The most prevalent disease-causing mutation—present in ∼80% of all CF chromosomes worldwide—is deletion of a single phenylalanine residue at position 508 (ΔF508) (2, 3). Of CF patients, 70% are homozygous for the ΔF508 mutation, and >80% of patients carry ≥1 ΔF508 cftr allele. Wild-type (WT)-CFTR moves out of the endoplasmic reticulum (ER) to the Golgi apparatus, where it acquires complex-glycosylation (mature or band C CFTR), and is targeted to the apical plasma membrane, where it functions as a chloride channel. In contrast to WT-CFTR, ΔF508-CFTR is retained in the ER, presumably due to misfolding. The CFTR molecules retained in the ER are immature core-glycosylated and commonly referred to as band B. Immature CFTR is degraded by the ER-associated degradation (ERAD) pathway through which CFTR is ubiquitinated and retrotranslocated to the cytosol, where it undergoes proteasomal degradation (4, 5). The ubiquitin-proteasome-mediated degradation is the dominant pathway for disposal of misfolded CFTR in mammalian cells (5).
Physical and chemical treatments have been reported to partially restore CFTR chloride-channel function in cells expressing ΔF508-CFTR. We and others have shown that incubation of cells at low temperature (i.e., 26°C) helps redirect some ΔF508-CFTR to the cell membrane (6,7,8). The ΔF508-CFTR that reaches the membrane forms regulated chloride channels but exhibits a defect in phosphorylation, an observation that could help explain the 30–100% decrements in ΔF508-CFTR functional activity compared to wild-type (WT) (9). In addition, ΔF508-CFTR is less stable at the plasma membrane than WT-CFTR (6, 10). Certain chemicals (i.e., DMSO, 4-phenyl-butyrate, CPX), that act on cellular chaperones (i.e., Hsp70) also rescue processing of ΔF508-CFTR (11, 12). These chemicals are not specific to CFTR and therefore affect other proteins in the ER.
Provision of a functional exogenous CFTR protein in cells represent an attractive therapeutic approach for this disease due to the protein misfolding and maturation defect. However, no proven method currently exists for delivery of transmembrane proteins. Administration of DNA encoding CFTR protein could be an alternative, although a major limitation of this approach remains the size of the full-length (∼4.5 kb) CFTR, which is close to the maximum size currently achievable for transfer strategies using adeno-associated virus (AAV) (13). One should also consider that, even if successful, expression of any WT-CFTR transgene would be governed by the transgene promoter. In contrast, nonfunctional corrective fragments should allow endogenous (chromosomal) ΔF508-CFTR regulation at the promoter level to remain intact. We and others have recently shown that coexpression of small CFTR fragments can specifically rescue the processing of ΔF508-CFTR, resulting in functional CFTR chloride channels at the cell surface in vitro (14, 15). Using this approach, we now show for the first time that these CFTR fragments can rescue ΔF508-CFTR and restore chloride transport both in human airway epithelial cells and, more important, in vivo in mice. These results provide new molecular insight into the nature of the misfolding defect elicited by ΔF508. Moreover, protein misfolding is a common cause of several diseases, and our findings suggest that expression of complementary protein fragments is a viable in vivo approach for correcting disease-causing misfolding of plasma membrane proteins.
MATERIALS AND METHODS
Material and reagents
Human embryonic kidney (HEK293T) cells were cultured in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10% bovine growth serum (HyClone, Logan, UT, USA) and penicillin/streptomycin (Invitrogen) in 5% CO2 at 37°C. Human CF bronchial epithelial (CFBE) cells stably expressing ΔF508-CFTR, CFBE41o-ΔF, were cultured as described previously and grown on filters at an air-liquid interface (16). The CFBE41o- cells are homozygous ΔF508/ΔF508.
Adenoviral vectors
cDNA encoding CFTR fragments (1-633 and 1-614KKAA) were cloned into the pShuttle-CMV adenovirus vector (Stratagene, La Jolla, CA, USA). This vector allows transgene to be expressed under the control of the CMV promoter. E1A-deleted recombinant adenovirus expressing the fragment was produced by using the Ad-Easy Adenovirus System (Stratagene) according to manufacturer’s instructions. Linearized shuttle vectors bearing 1-633 or 1-614KKAA CFTR were cotransformed with pAdEasy-1(a plasmid containing most of the adenoviral genome) into BJ5183 Escherichia coli competent cells, and a double homologous recombination event was used to produce full-length adenovirus genome containing the desired CFTR fragment. Transformants were selected by kanamycin resistance, and correct recombinants were screened by restriction digestion. Correct plasmid was amplified and transfected into HEK293 cells (Microbix, Toronto, ON, Canada) to generate recombinant adenovirus. The viruses were screened by CFTR-specific PCR and then amplified. Ad-WT-CFTR virus (WT-CFTR expressed under the control of CMV promoter) was a generous gift from Dr. J. Zabner (University of Iowa, Iowa City, IA, USA). Large-scale adenovirus stocks were produced by CsCl gradient centrifugation (17). The purified viruses were portioned into aliquots and stored at −80°C.
Transient transfection and Western blot
HEK293T cells were transfected using the Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions. Three days after transfection, HEK293T cells were lysed in PBS-0.2% Triton X-100 containing a cocktail of protease inhibitors (Roche Diagnostics, Indianapolis, IN, USA). The lysates were subjected to immunoprecipitation using a C-CFTR monoclonal antibody (R&D Systems, Minneapolis, MN, USA; 24-1) cross-linked on A/G agarose beads. After extensive washings, bound proteins were analyzed by Western blot by transfer to PVDF membranes (Bio-Rad, Hercules, CA, USA). Primary antibody [C-CFTR (24-1)] was added at a dilution of 1:2000. HRP-conjugated secondary antibody (Pierce, Rockford, IL, USA) was used at 1:10,000. The signal was detected using West Pico (Pierce).
Immunofluorescence
HEK293T cells were seeded on coverslips and transfected with the CFTR constructs described above. The cells were rinsed in PBS and fixed in methanol for 30 min at −20°C. After blocking for 30 min with 1% BSA in PBS, cells were incubated with CFTR antibody raised against the amino-terminal tail of CFTR (dilution 1:100; Chemicon MAB 3482) and rabbit polyclonal calreticulin antibody (dilution 1:100; Affinity Bioreagents, Rockford, IL, USA) for 1 h at 37°C. After several washings, cells were exposed for 45 min at 37°C to secondary antibody Alexa Fluor 488 anti-mouse IgG and Alexa Fluor 594 anti-rabbit at a dilution of 1:100 (Invitrogen). At the end of the experiment, cells were washed with PBS 1×, and coverslips were mounted with Vectashield (Vector Laboratories, Burlingame, CA, USA).
Planar lipid bilayers and single-channel recordings
Small vesicles (microsomes) were prepared from membranes of HEK293T cells as previously reported (18). Microsomes were fused with Mueller-Rudin planar lipid bilayers comprised of a 1:1 (wt:wt) diphytanoyl-phosphatidyl-ethanolamine/diphytanoyl-phosphatidylserine solution in n-octane (final lipid concentration 25 mg/ml). The bilayers were bathed with symmetrical (in mM) 140 N-methyl-d-glucamine, 10 TES, 3 MgCl2, and 1 EGTA (pH 7.3). Bathing solution also contained 200 μM niflumic acid (Ca2+-activated chloride-channel blocker). Holding potential was −100 mV. CFTR channels were activated with 25 U/ml protein kinase A catalytic subunit (Promega, Madison, WI, USA) and 1 mM Mg-ATP added to both compartments of the bilayer chamber. Identity of CFTR channels was tested at the end of each experiment by adding 150 μM glybenclamide to both sides of the bilayer. Probability of CFTR channels in an open state was estimated assuming two active channels in the bilayer. Single-channel currents were measured using a conventional current-to-voltage converter with a 1-GΩ feedback resistor (Eltec, Daytona Beach, FL, USA). Single-channel analyses were performed using pCLAMP 5.6 software (Axon Instruments, Burlingame, CA, USA) on current records low-pass filtered at 300 Hz through an 8-pole Bessel filter (902 LPF; Frequency Devices, Haverhill, MA, USA) prior to acquisition using a Digidata 1200 interface (Axon Instruments). For illustration purposes, records were filtered at 100 Hz using the built-in digital filtering option of the pCLAMP software.
Transepithelial short-circuit currents
Human CFBE41o-ΔF cells were cultured on permeable membranes (Transwell; Costar, Milpitas, CA, USA). Short-circuit current (Isc) measurements were done as described previously by mounting the filters in Ussing chambers (19). Briefly, Ringer’s buffer (115 mM NaCl, 25 mM NaHCO3, 2.4 KH2PO4, 1.24 K2HPO4, 1.2 CaCl2, 1.2 mM MgCl2, and 10 mM d-glucose; pH 7.4) was added in the basal compartment of the Ussing chamber, whereas a low-chloride solution (1.2 mM NaCl and 115 mM Na gluconate replacing 115 mM NaCl) was added to the apical chamber. Bath solutions were stirred vigorously and gassed with 5% CO2. Solutions and chambers were maintained at 37°C. Amiloride (100 μM) was added to the apical side of the monolayers to block sodium absorption. Forskolin (10 μM) and genistein (50 μM) were added to both sides of the monolayer to evaluate cAMP-stimulated currents. Glibenclamide (200 μM) was added to the apical side of the bilayers at the end of each experiment to block CFTR channels.
Measurement of nasal potential difference (NPD)
Mice received 10 μl containing 109 plaque-forming units (PFU) per nostril of an adenovirus expressing WT-CFTR or 1-614KKAA 2×/wk. A 3-step protocol was used, as described previously (20). First, the nasal cavities of anesthetized mice (Cftrtm1Kth C57BL/6J mice) were perfused with Ringer’s solution containing 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, and 50 μM amiloride (pH 7.3). Next, a low-chloride-containing solution (6 mM) was perfused (NMDG solution, pH 7.3). CFTR chloride channels were activated with forskolin (20 μM) and genistein (50 μM) in the perfusate. Because of the continuous presence of amiloride (50 μM) and the complete replacement of Na+ with a membrane-impermeant cation (140 mM NMDG in the perfusion solution), hyperpolarization reflects only chloride secretion rather than cation absorption. The traces were interpreted in a blind fashion. All mouse protocols were reviewed and approved by the University of Alabama at Birmingham institutional animal care and use committee.
Data analysis
Data were expressed as means ± sd and tested for significance using the paired or unpaired Student’s t test with analysis of variance as appropriate. Results with P < 0.05 were considered significant.
RESULTS
Construction and intracellular localization of a CFTR fragment containing an ER retention signal
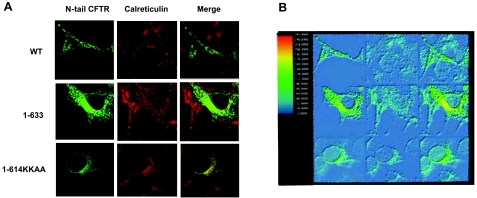
While it is generally accepted that misfolded ΔF508-CFTR is degraded in the cytoplasm, it also has been proposed that a fraction of ΔF508-CFTR protein reaches the plasma membrane (21). To better understand whether plasma membrane and/or ER is the cellular compartment in which rescue of CFTR can occur, we generated a CFTR1-614 fragment containing an ER retention signal at the carboxyl terminus. We took advantage of a partial ER retention/retrieval consensus sequence KKXX present at position 611-614 in the CFTR1-614 molecule (KKAD). This sequence was mutated to a carboxyl terminus KKAA (CFTR1-614KKAA), which has been shown to exhibit a stronger ER retention/retrieval capacity (22). After transient transfection in HEK293T cells, the ER localization of CFTR1-614KKAA was confirmed by immunofluorescence and indicated exclusive colocalization with the ER marker calreticulin (Fig. 1). CFTR1-633, which lacks the ER retention signal at the carboxyl terminus, could be detected beyond the ER, although it was predominantly localized into the ER. As expected, WT-CFTR protein was detected predominantly at the plasma membrane (Fig. 1).
Figure 1.
A) Immunolocalization of WT-CFTR, CFTR1-633, and CFTR1-614KKAA. WT-CFTR is expressed at the plasma membrane in HEK293T cells. CFTR1-633 staining is mostly in the ER, and CFTR1-614KKAA colocalizes with the ER marker calreticulin. All results are representative of ≥3 experiments. B) Color profiling of the fluorescence of the CFTR constructs and calreticulin. Images were analyzed with QCapturePro image acquisition and analysis software (QImaging, Tucson, AZ, USA).
Rescue of the processing of ΔF508-CFTR and Δ2-79-CFTR by a CFTR fragment containing an ER retention signal
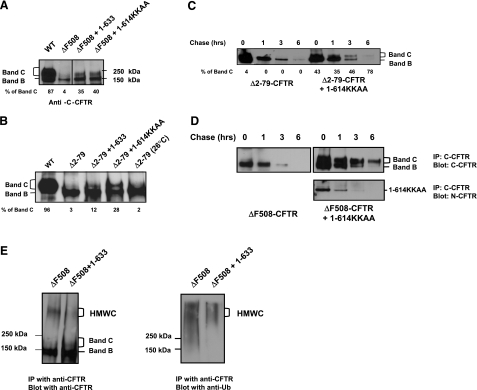
We next analyzed the expression of band C, corresponding to fully glycosylated CFTR, after transient transfection in HEK293T cells. This analysis showed that the CFTR1-614KKAA fragment (containing an ER retention signal at the carboxyl terminus) could rescue processing of ΔF508-CFTR (Fig. 2A). The efficacy of ΔF508-CFTR rescue by CFTR1-614KKAA was similar to that achieved by CFTR1-633. Both CFTR1-633 and CFTR1-614KKAA could induce a mature CFTR band C when coexpressed with a processing mutant of CFTR lacking the amino-terminal tail (Δ2-79-CFTR: deletion of aa 2 to 79). Unlike ΔF508-CFTR, the Δ2-79-CFTR is not a temperature-permissive mutant, and low temperature does not rescue its processing (Fig. 2B) (23). Therefore, correction of ΔF508-CFTR by fragment association works by a different mechanism than temperature, since Δ2-79-CFTR is not correctable by temperature. To further investigate the rescue of the Δ2-79-CFTR mutant by the CFTR1-614KKAA fragment, we evaluated the time course of disappearance of the mature (band C form) of Δ2-79-CFTR. Three hours following cell incubation with cycloheximide to inhibit the protein synthesis, we could still detect mature Δ2-79-CFTR. We did not observe any difference between the magnitude of immature Δ2-79-CFTR expressed in the absence or in the presence of CFTR1-614KKAA (Fig. 2C). Similar results were obtained with ΔF508-CFTR (Fig. 2D). Figure 2D (bottom panel) shows that CFTR1-614KKAA was coimmunoprecipitated with ΔF508-CFTR, which suggests an interaction between these two polypeptides. These results demonstrate that a CFTR fragment containing an ER retention signal can rescue the processing of ΔF508-CFTR and Δ2-79-CFTR proteins and suggest an interaction with CFTR molecules within the ER. Interestingly, a high-molecular-weight complex containing ubiquitin was observed after immunoprecipitation of CFTR using a monoclonal CFTR antibody (Fig. 2E). This high-molecular-weight complex was absent mostly from cells transfected with ΔF508-CFTR and CFTR1-633 or CFTR1-614KKAA (Fig. 2E and data not shown). This latter result suggests that CFTR fragments prevent the ubiquitination and most likely degradation of ΔF508-CFTR.
Figure 2.
Rescue of the trafficking of ΔF508-CFTR and Δ2-79-CFTR by a CFTR fragment containing an ER retention signal. A, B) Rescue of the processing of ΔF508-CFTR (A) and of CFTR mutant lacking the amino-terminal tail of CFTR (Δ2-79-CFTR) (B) by a CFTR fragment containing an ER retention signal (1-614KKAA). C) Time course of disappearance of rescued Δ2-79-CFTR by CFTR1-614KKAA. D) Time course of disappearance of rescued ΔF508-CFTR by CFTR1-614KKAA. CFTR1-614KKAA was coimmunoprecipitated with ΔF508-CFTR using a C-CFTR antibody. Cycloheximide was added at the beginning of the experiment to inhibit protein synthesis. Blots were analyzed by densitometry using Bio-Rad Quantity One program. In each case, the band C signal was normalized to the total band B plus band C signal and expressed as percentage of band C. E) Detection of a high-molecular-weight complex (HMWC) in HEK293T cells transfected with the indicated CFTR constructs. The HMWC was detected after immunoprecipitation using a C-CFTR monoclonal antibody and was probed with a C-CFTR antibody and ubiquitin antibody. All results are representative of 3 to 5 experiments.
Effect of the CFTR fragment on the activity of ΔF508-CFTR
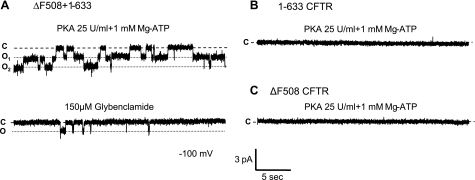
We also assessed whether rescue of ΔF508-CFTR by CFTR fragment would result in detectable chloride-channel activity. For this purpose, microsomes were prepared from HEK293T cells expressing ΔF508-CFTR ± CFTR fragment and fused to planar lipid bilayers. Microsomes represent membrane vesicles and therefore contain proteins present in plasma membrane as well as in the endoplasmic reticulum and the Golgi apparatus. We did not measure activity of channels expressing Δ2-79-CFTR since this mutant lacks the amino-terminal tail, which confers positive regulatory activity and therefore does not exhibit chloride currents (24). CFTR channels were observed only in microsomes of HEK293T cells cotransfected with ΔF508-CFTR and the CFTR1–633 fragment (Fig. 3). Under the same conditions (25 U/ml of PKA), no CFTR channels were detected when microsomes containing ΔF508-CFTR or CFTR1-633 alone were fused to planar lipid bilayers. Concordantly, we previously reported that no chloride-channel activity could be recorded by patch-clamping when the CFTR 1-633 fragment alone was expressed in cells (14). The same amount of CFTR protein was present in different preparations of microsomes as assessed by Western blotting, and therefore protein levels cannot account for differences observed in CFTR function (data not shown). It has been reported that the PKA-dependent phosphorylation rate of activation is much lower for ΔF508-CFTR than for WT-CFTR (25). The results presented here therefore suggest that the fragment can modulate the phosphorylation of ΔF508-CFTR.
Figure 3.
Detection of functional ΔF508-CFTR chloride channels after coexpression of a CFTR fragment. Microsomes, prepared from HEK293T cells transfected with CFTR constructs, were incorporated in planar lipid bilayers. A) CFTR channels could be detected only in cells transfected with ΔF508-CFTR and the CFTR fragment 1-633 using low PKA (25 U/ml). At least 2 channels were present in the lipid bilayer, and addition of 150 μM glybenclamide inhibited these channels. O, open state; C, closed state. Open probabilities (Po) were 0.41 ± 0.06 and 0.06 ± 0.02 after addition of forskolin and glybenclamide, respectively. B, C) No channel could be detected when the cells were transfected only with 1-633 (B) or ΔF508-CFTR alone (C). All results are representative of ≥3 experiments.
Rescue of ΔF508-CFTR in CFBE41o-ΔF epithelial cells
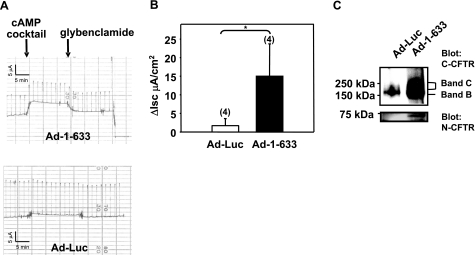
To assess the ability of CFTR fragments to rescue ΔF508-CFTR, the human CFBE41o- cell line (CFBE, homozygous for the ΔF508 mutation) was transduced with an adenoviral vector expressing CFTR1-633 (Ad-CFTR1-633). No increase in short-circuit current was observed in response to a cocktail containing forskolin and genistein that induces CFTR activity (data not shown). In this regard, the levels of endogenous ΔF508-CFTR in CFBE41o- cells were shown previously to be very low and therefore could be insufficient to monitor correction of CFTR processing (26). We therefore took advantage of the CFBE41o-ΔF, a stable cell line overexpressing ΔF508-CFTR (16). When these cells were transduced with Ad-CFTR1-633, we observed an increase in short-circuit current after activation of CFTR channels (Fig. 4A, B). The currents were inhibited partially by the CFTR inhibitor glybenclamide. As previously reported, CFTR inhibitors such as Inh-172 and glybenclamide block CFTR-dependent Isc to a similar magnitude in these cells (16). Conversely, when cells were transduced with an adenovirus encoding luciferase (Ad-Luc), we observed only a negligible increase in activity in response to forskolin and genistein. At the end of the experiment, cells were lysed and CFTR protein was immunoprecipitated using a C-CFTR monoclonal antibody. An increase in CFTR expression could be detected by Western blot in cells transduced with Ad-CFTR1-633 (Fig. 4C). The same experiments were performed with 1-614KKAD, and similar results were obtained (data not shown).
Figure 4.
Rescue of ΔF508-CFTR in CFBE41o-ΔF cells, using a CFTR fragment. The CFTR fragment 1–633 was delivered to the CFBE41o-ΔF cells using an adenovirus vector. A) CyclicAMP-activated currents in CFBE41o-ΔF cells transduced with Ad-1-633 (top panel) or Ad-Luc (bottom panel). B) Histogram representation of peak currents assessed by Ussing chambers. C) Expression of ΔF508-CFTR and CFTR1-633 fragment in CFBE41o-ΔF cells transduced with Ad-Luc or Ad-1–633. Same number of cells was used per immunoprecipitation. CFTR1-633 fragment coimmunoprecipitated with ΔF508-CFTR was detected using an N-CFTR monoclonal antibody (Chemicon). n = number of experiments. *P < 0.05.
Correction of chloride transport in ΔF508-homozygous CF mice
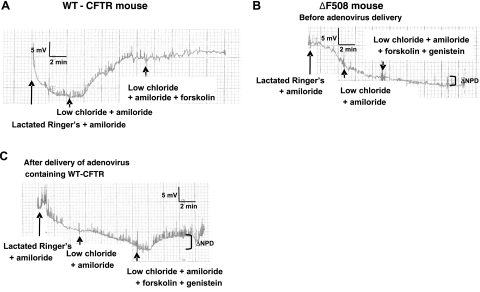
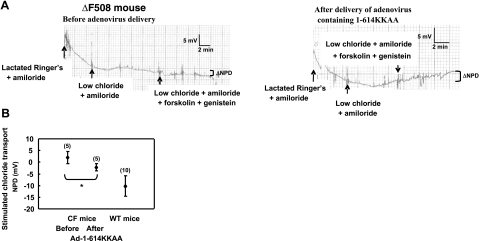
To determine whether a CFTR fragment could correct chloride transport in ΔF508-homozygous CF mice (Cftrtm1Kth C57BL/6J mice), we measured the NPD. As expected, a significant hyperpolarization of potential difference was observed in WT mice (harboring WT-CFTR) following a switch to low-chloride solution (Fig. 5A). We performed these experiments with forskolin alone, as genistein does not increase chloride transport further in WT mice (data not shown and ref. 27). We next measured the NPD in ΔF508/ΔF508 CF mice (expressing endogenous levels of mutated CFTR) and found no evidence of a chloride-diffusion potential even after addition of forskolin and genistein in the perfusion solution (Fig. 5B). Figure 5B is representative of 19 mice (ΔF508/ΔF508) and shows no indication of hyperpolarization in response to forskolin and genistein. To establish the time required to express CFTR after nasal delivery using an adenoviral vector, CF mice received 10 μl containing 109 PFU/nostril of an adenovirus expressing WT-CFTR 2×/wk. We recorded the NPD every 3 to 4 d and detected a hyperpolarization of potential difference after a 2-wk cumulative treatment on addition of forskolin and genistein (Fig. 5C). We then delivered Ad-CFTR1-614KKAA to the nasal airways of CF mice (ΔF508/ΔF508) and observed a hyperpolarization representing chloride transport at 2 wk (Fig. 6A, B). The difference in NPD measurements before and after Ad-CFTR1–614KKAA treatment was 4.2 ± 1.5 mV, with values of 2.0 ± 2.6 and −2.2 ± 1.6 mV, respectively. WT mice exhibited an NPD of −10.2 ± 4.4 mV. To confirm that the chloride secretion was not due to the fragment alone, we performed NPD measurements in CFTR-knockout mice. The NPD measurements of CFTR-knockout mice before and after receiving the Ad-CFTR1-614KKAA did not differ significantly (−0.5±2.8 mV). This latter result confirms specificity of the rescue and indicates that the CFTR fragment alone does not confer chloride transport. In summary, the results presented here demonstrate that expression of a CFTR fragment corrects the ΔF508-CFTR folding defect in mice in vivo and leads to a functional CFTR that can transport chloride.
Figure 5.
Restoration of chloride transport in CF mice after delivery of WT-CFTR. A) Measure of NPD in a mouse expressing WT-CFTR. Trace is representative of 10 WT mice. B) NPD measurements from a mouse expressing ΔF508-CFTR. Trace is representative of 19 CF mice (ΔF508/ΔF508). C) NPD measurements in ΔF508-CFTR mouse after delivery of WT-CFTR. Trace is representative of 3 CF mice (ΔF508/ΔF508). Brackets represent changes in NPD after stimulation of CFTR.
Figure 6.
Restoration of chloride transport in CF mice after expression of the CFTR fragment 1-614KKAA. A) Representative NPD measurements of CF mice expressing ΔF508-CFTR before (left panel) and after (right panel) delivery of the CFTR fragment 1-614KKAA. Traces represent 5 separate experiments. B) NPD changes on activation of the CFTR chloride channel in WT mice and in CF mice (ΔF508/ΔF508) before and after delivery of an adenovirus vector bearing CFTR1-614KKAA fragment. Brackets indicate changes in NPD after stimulation of CFTR. n = number of experiments. *P < 0.05.
DISCUSSION
The ΔF508-CFTR mutant protein responsible for many cases of cystic fibrosis exhibits a folding defect that affects its processing and impairs chloride-channel function. Like other conformational diseases due to impaired protein folding, no therapeutic strategy currently exists that specifically corrects this abnormality in vivo. Here we provide evidence that expression of complementary protein fragments of WT-CFTR rescues endogenous ΔF508-CFTR and restores chloride transport in human CF airway epithelial cells and in CF mice.
Previous attempts to correct the functional defect associated with ΔF508-CFTR have demonstrated the need to act at two levels, including both folding and subsequent trafficking of CFTR, as well as its stability and activity at the plasma membrane (28,29,30). In this regard, we and others have shown that incubation of cells at low temperature (i.e., 26°C) helps process some ΔF508-CFTR to the cell membrane (6,7,8). However, the ΔF508-CFTR that reaches the membrane under these conditions is not properly phosphorylated (9) and is less stable than WT-CFTR (6, 10). Here we show that a CFTR fragment comprising nonmutated residues 1-633 can rescue the trafficking and the functional activity of the endogenous ΔF508-CFTR protein. Future studies will investigate the stability of rescued ΔF508-CFTR at the plasma membrane. It was also important to determine the subcellular sites where ΔF508-CFTR rescue occurs, as others have proposed that a fraction of ΔF508-CFTR protein reaches the plasma membrane (21). One of the goals of the study was to investigate whether the CFTR fragment was stabilizing escaped ΔF508-CFTR that reached the plasma membrane, or stabilizing ΔF508-CFTR in the ER. Using the CFTR1-614KKAA fragment containing an ER retention signal at the carboxyl terminus, we could show that the ER is the primary site at which rescue of ΔF508-CFTR trafficking occurs. These findings suggest that a transcomplementation strategy with fragments of WT-CFTR could restore endogenous ΔF508-CFTR by conferring a properly folded state in the ER and thus prevent its flagging by ubiquitin for subsequent degradation. In this regard, we observed a high-molecular-weight complex in HEK293T cells transfected with ΔF508-CFTR. Such a complex has been reported previously and contains ubiquitinated CFTR and probably chaperones involved in the degradation of CFTR (31, 32). Interestingly, this high-molecular-weight complex decreased when ΔF508-CFTR was coexpressed with a rescuing CFTR fragment (see Fig. 2E), suggesting that the CFTR fragment prevents such complex formation.
The PKA-dependent phosphorylation rate of activation of ΔF508-CFTR was reported to be much lower than for WT-CFTR (25). Our results show that at low PKA, ΔF508-CFTR channels could be detected only when CFTR1-633 was present. At this stage, we can speculate that the fragment could act on phosphorylation and/or dephosphorylation. Studies of WT-CFTR and CFTR mutants of NBDs indicate that NBD1 and NBD2 do interact and that disruption of their interaction affects CFTR gating (33,34,35). Moreover, NBD1 also associates with the regulatory (R) domain, and phosphorylation of this latter domain by PKA facilitates NBD1-NBD2 interaction (36). In this regard, ΔF508-CFTR has a gating defect due to the mutation located in NBD1 and characterized by a slow rate of opening. Therefore, one could hypothesize that the presence of the CFTR fragment, which does not harbor a mutation, might facilitate CFTR domain interactions and ultimately ΔF508-CFTR activation.
The specificity of our transcomplementation strategy represents a major advantage over other strategies that correct protein trafficking. For example, chemicals such as DMSO, 4-phenyl-butyrate, or CPX can rescue processing of ΔF508-CFTR in vitro (11, 12). These molecules act as cellular chaperones and therefore affect other proteins in the ER. In contrast, the transcomplementation strategy described here appears to involve direct interactions between CFTR fragments and endogenous ΔF508-CFTR in the ER, and therefore should not be expected to affect the expression of other proteins. As previously reported, the rescue was specific to mutations located in the amino-terminal part of CFTR (N-tail and NBD1), as the CFTR fragment 1-633 did not rescue a CFTR mutant harboring a mutation located close to the carboxyl tail of CFTR, H1085R-CFTR (14). Similarly, introduction of the ΔF508 mutation in rescuing CFTR fragments prevented the rescue of the trafficking and chloride-transport properties of ΔF508-CFTR (14, 15, 37). Therefore, the CFTR fragments used in the present study act by a different mechanism than Δ264-CFTR that increases CFTR trafficking by associating with key components involved in the degradation of ΔF508-CFTR (38). We speculate that the introduction of the ΔF508 mutation to the CFTR protein results in a conformation where ER retention signal, or hydrophobic patches are exposed. Coexpression of the CFTR fragment will cover those sites by interacting with the ΔF508-CFTR protein. Therefore, we can speculate that the presence of the CFTR fragment, which does not harbor a mutation, might facilitate intermolecular interactions. Our findings are also in agreement with the current view that NBD1 participates in domain-domain assembly that is key to the folding of full-length CFTR protein (39, 40). More specifically, recent reports indicated that NBD1 interacts with cytosolic loop 4 of the CFTR molecule and that introduction of the ΔF508 mutation disrupts these interactions (41). The present research provides means by which further study could explore the relationship between transcomplementation and disruption at ΔF508-NBD/cytosolic loop interactions.
CFTR is a transmembrane protein, and currently no efficient method exists for delivery of transmembrane proteins into target cell membranes. We used an adenovirus to deliver a CFTR fragment, since this method offers the advantage of infecting both quiescent and proliferating cells. This property facilitates transduction of polarized epithelial cells on filter supports as well as intact airway epithelium in vivo. The adenovirus vector was used as a proof of concept to determine the ability of genetic transfer of a CFTR fragment to restore chloride transport in vivo. However, because of the possibility of mounting immune responses against viral vectors, liposomes or nanoparticles would certainly serve as better delivery systems for such treatment in humans. As the size of the cftr gene is an additional problem for gene therapy, transcomplementation offers the advantage of introducing a smaller cftr gene that allows the inclusion of an active promoter. Therefore, delivery of CFTR fragments as a therapeutic approach depends on the success of viral or nonviral delivery systems in vivo. Previous studies have shown adenovirus efficient in transferring WT-CFTR cDNA to airway epithelial cells in vivo (42). In addition, reports have indicated that adenovirus-mediated gene transfer to airway epithelia in vitro requires prolonged incubation times (43).
CF mouse nose represents a useful model to study the rescue of ΔF508-CFTR, as the mice exhibit a clear defect attributable to the ΔF508 mutation in nasal airways in vivo. CF mice express a subtle CF-like phenotype involving nasal epithelial goblet cell hyperplasia (44). This manuscript shows for the first time that 2 wk of cumulative administration of Ad-CFTR1–614KKAA, corresponding to 4 administrations, was sufficient to confer some chloride secretion in a CFTR-specific fashion in CF mice receiving CFTR1-614KKAA. The magnitude of correction of endogenous ΔF508-CFTR in vivo remains to be determined. However, our findings are consistent with a previous report that very little CFTR is needed in epithelial cells to correct chloride transport to near normal levels (45).
Most cases of CF are caused by mutations such as ΔF508 that cause misfolding of the CFTR protein. Such alteration of protein folding is unfortunately not unique to CF, as it occurs in many other genetic disorders, including, α1-antitrypsin deficiency, Alzheimer’s disease, nephrogenic diabetes insipidus, and numerous other conditions. The findings presented here establish that in vivo transcomplementation by a fragment of intact protein can help develop new therapeutic approaches against CF and possibly other important diseases of protein folding.
Acknowledgments
This work was supported in part by a research grant and a pilot and feasibility grant awarded by the Cystic Fibrosis Foundation (to E.C.-B.), and U.S. National Institutes of Health grant DK37206 (to D.J.B.).
References
- Denning G M, Ostedgaard L S, Cheng S H, Smith A E, Welsh M J. Localization of cystic fibrosis transmembrane conductance regulator in chloride secretory epithelia. J Clin Invest. 1992;89:339–349. doi: 10.1172/JCI115582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopito R R. Biosynthesis and degradation of CFTR. Physiol Rev. 1999;79:S167–173. doi: 10.1152/physrev.1999.79.1.S167. [DOI] [PubMed] [Google Scholar]
- Tsui L C. The spectrum of cystic fibrosis mutations. Trends Genet. 1992;8:392–398. doi: 10.1016/0168-9525(92)90301-j. [DOI] [PubMed] [Google Scholar]
- Jensen T J, Loo M A, Pind S, Williams D B, Goldberg A L, Riordan J R. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell. 1995;83:129–135. doi: 10.1016/0092-8674(95)90241-4. [DOI] [PubMed] [Google Scholar]
- Gelman M S, Kannegaard E S, Kopito R R. A principal role for the proteasome in endoplasmic reticulum-associated degradation of misfolded intracellular cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2002;277:11709–11714. doi: 10.1074/jbc.M111958200. [DOI] [PubMed] [Google Scholar]
- Rennolds J, Boyaka P N, Bellis S L, Cormet-Boyaka E. Low temperature induces the delivery of mature and immature CFTR to the plasma membrane. Biochem Biophys Res Commun. 2008;366:1025–1029. doi: 10.1016/j.bbrc.2007.12.065. [DOI] [PubMed] [Google Scholar]
- Heda G D, Marino C R. Surface expression of the cystic fibrosis transmembrane conductance regulator mutant DeltaF508 is markedly upregulated by combination treatment with sodium butyrate and low temperature. Biochem Biophys Res Commun. 2000;271:659–664. doi: 10.1006/bbrc.2000.2684. [DOI] [PubMed] [Google Scholar]
- Sharma M, Benharouga M, Hu W, Lukacs G L. Conformational and temperature-sensitive stability defects of the delta F508 cystic fibrosis transmembrane conductance regulator in post-endoplasmic reticulum compartments. J Biol Chem. 2001;276:8942–8950. doi: 10.1074/jbc.M009172200. [DOI] [PubMed] [Google Scholar]
- Dalemans W, Barbry P, Champigny G, Jallat S, Dott K, Dreyer D, Crystal R G, Pavirani A, Lecocq J P, Lazdunski M. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature. 1991;354:526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- Lukacs G L, Chang X B, Bear C, Kartner N, Mohamed A, Riordan J R, Grinstein S. The delta F508 mutation decreases the stability of cystic fibrosis transmembrane conductance regulator in the plasma membrane. Determination of functional half-lives on transfected cells. J Biol Chem. 1993;268:21592–21598. [PubMed] [Google Scholar]
- Brown C R, Hong-Brown L Q, Biwersi J, Verkman A S, Welch W J. Chemical chaperones correct the mutant phenotype of the delta F508 cystic fibrosis transmembrane conductance regulator protein. Cell Stress Chaperones. 1996;1:117–125. doi: 10.1379/1466-1268(1996)001<0117:ccctmp>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Ward C L, Krouse M E, Wine J J, Kopito R R. Glycerol reverses the misfolding phenotype of the most common cystic fibrosis mutation. J Biol Chem. 1996;271:635–638. doi: 10.1074/jbc.271.2.635. [DOI] [PubMed] [Google Scholar]
- Flotte T R, Laube B L. Gene therapy in cystic fibrosis. Chest. 2001;120:124S–131S. doi: 10.1378/chest.120.3_suppl.124s. [DOI] [PubMed] [Google Scholar]
- Cormet-Boyaka E, Jablonsky M, Naren A P, Jackson P L, Muccio D D, Kirk K L. Rescuing cystic fibrosis transmembrane conductance regulator (CFTR)-processing mutants by transcomplementation. Proc Natl Acad Sci U S A. 2004;101:8221–8226. doi: 10.1073/pnas.0400459101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsianik G, Cao L, Nilius B. Rescue of functional DeltaF508-CFTR channels by co-expression with truncated CFTR constructs in COS-1 cells. FEBS Lett. 2003;554:173–178. doi: 10.1016/s0014-5793(03)01162-1. [DOI] [PubMed] [Google Scholar]
- Bebok Z, Collawn J F, Wakefield J, Parker W, Li Y, Varga K, Sorscher E J, Clancy J P. Failure of cAMP agonists to activate rescued deltaF508 CFTR in CFBE41o- airway epithelial monolayers. J Physiol. 2005;569:601–615. doi: 10.1113/jphysiol.2005.096669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketner G, Boyer J. Isolation, growth, and purification of defective adenovirus deletion mutants. Wold W S M, Tollefson A, editors. Totowa, NJ, USA: Humana Press; 2006:19–28. doi: 10.1385/1-59745-166-5:19. [DOI] [PubMed] [Google Scholar]
- Wang W, Oliva C, Li G, Holmgren A, Lillig C H, Kirk K L. Reversible silencing of CFTR chloride channels by glutathionylation. J Gen Physiol. 2005;125:127–141. doi: 10.1085/jgp.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennolds J, Tower C, Musgrove L, Fan L, Maloney K, Clancy J P, Kirk K L, Sztul E, Cormet-Boyaka E. Cystic fibrosis transmembrane conductance regulator trafficking is mediated by the COPI coat in epithelial cells. J Biol Chem. 2008;283:833–839. doi: 10.1074/jbc.M706504200. [DOI] [PubMed] [Google Scholar]
- Zsembery A, Fortenberry J A, Liang L, Bebok Z, Tucker T A, Boyce A T, Braunstein G M, Welty E, Bell P D, Sorscher E J, Clancy J P, Schwiebert E M. Extracellular zinc and ATP restore chloride secretion across cystic fibrosis airway epithelia by triggering calcium entry. J Biol Chem. 2004;279:10720–10729. doi: 10.1074/jbc.M313391200. [DOI] [PubMed] [Google Scholar]
- Kalin N, Claass A, Sommer M, Puchelle E, Tummler B. DeltaF508 CFTR protein expression in tissues from patients with cystic fibrosis. J Clin Invest. 1999;103:1379–1389. doi: 10.1172/JCI5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson H, Kappeler F, Hauri H P. Protein targeting to endoplasmic reticulum by dilysine signals involves direct retention in addition to retrieval. J Biol Chem. 1999;274:15080–15084. doi: 10.1074/jbc.274.21.15080. [DOI] [PubMed] [Google Scholar]
- Prince L S, Peter K, Hatton S R, Zaliauskiene L, Cotlin L F, Clancy J P, Marchase R B, Collawn J F. Efficient endocytosis of the cystic fibrosis transmembrane conductance regulator requires a tyrosine-based signal. J Biol Chem. 1999;274:3602–3609. doi: 10.1074/jbc.274.6.3602. [DOI] [PubMed] [Google Scholar]
- Naren A P, Cormet-Boyaka E, Fu J, Villain M, Blalock J E, Quick M W, Kirk K L. CFTR chloride channel regulation by an interdomain interaction. Science. 1999;286:544–548. doi: 10.1126/science.286.5439.544. [DOI] [PubMed] [Google Scholar]
- Wang F, Zeltwanger S, Hu S, Hwang T C. Deletion of phenylalanine 508 causes attenuated phosphorylation-dependent activation of CFTR chloride channels. J Physiol. 2000;524:637–648. doi: 10.1111/j.1469-7793.2000.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiatecka-Urban A, Brown A, Moreau-Marquis S, Renuka J, Coutermarsh B, Barnaby R, Karlson K H, Flotte T R, Fukuda M, Langford G M, Stanton B A. The short apical membrane half-life of rescued ΔF508-cystic fibrosis transmembrane conductance regulator (CFTR) results from accelerated endocytosis of ΔF508-CFTR in polarized human airway epithelial cells. J Biol Chem. 2005;280:36762–36772. doi: 10.1074/jbc.M508944200. [DOI] [PubMed] [Google Scholar]
- Salinas D B, Pedemonte N, Muanprasat C, Finkbeiner W F, Nielson D W, Verkman A S. CFTR involvement in nasal potential differences in mice and pigs studied using a thiazolidinone CFTR inhibitor. Am J Physiol Lung Cell Mol Physiol. 2004;287:L936–L943. doi: 10.1152/ajplung.00354.2003. [DOI] [PubMed] [Google Scholar]
- Mazzei M, Nieddu E, Folli C, Caci E, Galietta L V. 2-(dialkylamino)-4H-1-benzopyran-4-one derivatives modify chloride conductance in CFTR expressing cells. Farmaco. 2003;58:961–970. doi: 10.1016/S0014-827X(03)00155-1. [DOI] [PubMed] [Google Scholar]
- Ma T, Vetrivel L, Yang H, Pedemonte N, Zegarra-Moran O, Galietta L J, Verkman A S. High-affinity activators of cystic fibrosis transmembrane conductance regulator (CFTR) chloride conductance identified by high-throughput screening. J Biol Chem. 2002;277:37235–37241. doi: 10.1074/jbc.M205932200. [DOI] [PubMed] [Google Scholar]
- Pedemonte N, Lukacs G L, Du K, Caci E, Zegarra-Moran O, Galietta L J, Verkman A S. Small-molecule correctors of defective DeltaF508-CFTR cellular processing identified by high-throughput screening. J Clin Investig. 2005;115:2564–2571. doi: 10.1172/JCI24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C L, Omura S, Kopito R R. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell. 1995;83:121–127. doi: 10.1016/0092-8674(95)90240-6. [DOI] [PubMed] [Google Scholar]
- Alberti S, Bohse K, Arndt V, Schmitz A, Hohfeld J. The cochaperone HspBP1 inhibits the CHIP ubiquitin ligase and stimulates the maturation of the cystic fibrosis transmembrane conductance regulator. Mol Biol Cell. 2004;15:4003–4010. doi: 10.1091/mbc.E04-04-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense M, Vergani P, White D M, Altberg G, Nairn A C, Gadsby D C. In vivo phosphorylation of CFTR promotes formation of a nucleotide-binding domain heterodimer. EMBO J. 2006;25:4728–4739. doi: 10.1038/sj.emboj.7601373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D N, Rich D P, Ostedgaard L S, Gregory R J, Smith A E, Welsh M J. Mutations in CFTR associated with mild-disease-form Cl- channels with altered pore properties. Nature. 1993;362:160–164. doi: 10.1038/362160a0. [DOI] [PubMed] [Google Scholar]
- Powe A C, Jr, Al-Nakkash L, Li M, Hwang T C. Mutation of Walker-A lysine 464 in cystic fibrosis transmembrane conductance regulator reveals functional interaction between its nucleotide-binding domains. J Physiol. 2002;539:333–346. doi: 10.1113/jphysiol.2001.013162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J M, Hudson R P, Kanelis V, Choy W Y, Thibodeau P H, Thomas P J, Forman-Kay J D. CFTR regulatory region interacts with NBD1 predominantly via multiple transient helices. Nat Struct Mol Biol. 2007;14:738–745. doi: 10.1038/nsmb1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L L, Gawenis L R, Hwang T C, Walker N M, Gruis D B, Price E M. A domain mimic increases DeltaF508 CFTR trafficking and restores cAMP-stimulated anion secretion in cystic fibrosis epithelia. Am J Physiol Cell Physiol. 2004;287:C192–C199. doi: 10.1152/ajpcell.00337.2003. [DOI] [PubMed] [Google Scholar]
- Cebotaru L, Vij N, Ciobanu I, Wright J, Flotte T, Guggino W B. Cystic fibrosis transmembrane regulator missing the first four transmembrane segments increases wild type and DeltaF508 processing. J Biol Chem. 2008;283:21926–21933. doi: 10.1074/jbc.M709156200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Aleksandrov L, Chang X B, Hou Y X, He L, Hegedus T, Gentzsch M, Aleksandrov A, Balch W E, Riordan J R. Domain interdependence in the biosynthetic assembly of CFTR. J Mol Biol. 2007;365:981–994. doi: 10.1016/j.jmb.2006.10.086. [DOI] [PubMed] [Google Scholar]
- Du K, Sharma M, Lukacs G L. The DeltaF508 cystic fibrosis mutation impairs domain-domain interactions and arrests post-translational folding of CFTR. Nat Struct Mol Biol. 2005;12:17–25. doi: 10.1038/nsmb882. [DOI] [PubMed] [Google Scholar]
- Serohijos A W, Hegedus T, Aleksandrov A A, He L, Cui L, Dokholyan N V, Riordan J R. Phenylalanine-508 mediates a cytoplasmic-membrane domain contact in the CFTR 3D structure crucial to assembly and channel function. Proc Natl Acad Sci U S A. 2008;105:3256–3261. doi: 10.1073/pnas.0800254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaria A, St. George J A, Jiang C, Kaplan J M, Wadsworth S C, Gregory R J. Adenovirus-mediated persistent cystic fibrosis transmembrane conductance regulator expression in mouse airway epithelium. J Virol. 1998;72:7302–7309. doi: 10.1128/jvi.72.9.7302-7309.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabner J, Zeiher B G, Friedman E, Welsh M J. Adenovirus-mediated gene transfer to ciliated airway epithelia requires prolonged incubation time. J Virol. 1996;70:6994–7003. doi: 10.1128/jvi.70.10.6994-7003.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarran R, Grubb B R, Parsons D, Picher M, Hirsh A J, Davis C W, Boucher R C. The CF salt controversy: in vivo observations and therapeutic approaches. Mol Cell. 2001;8:149–158. doi: 10.1016/s1097-2765(01)00286-6. [DOI] [PubMed] [Google Scholar]
- Dorin J R, Farley R, Webb S, Smith S N, Farini E, Delaney S J, Wainwright B J, Alton E W, Porteous D J. A demonstration using mouse models that successful gene therapy for cystic fibrosis requires only partial gene correction. Gene Ther. 1996;3:797–801. [PubMed] [Google Scholar]