Abstract
The objective of this study was to determine whether surface-modified nanoparticles enhance permeability across nasal mucosa, while retaining the effectiveness of the payload. The uptake and permeability of polystyrene nanoparticles (PS-NPs; FluoSpheres) was evaluated across the various regions of the bovine nasal epithelia following conjugation with deslorelin and transferrin. Uptake and transport of PS-NPs, deslorelin-PS-NPs, and transferrin-PS-NPs exhibited regional differences in the order: inferior turbinate posterior (ITP) > medium turbinate posterior (MTP) > medium turbinate anterior (MTA). Uptake and transport also exhibited directionality and temperature dependence in these tissues. Further, uptake as well as transport of functionalized nanoparticles could be inhibited by excess free functionalizing ligand. Confocal microscopy indicated the presence of functionalized nanoparticles in respiratory epithelial cells, as well as other cell types of the nasal tissue. We chose the ITP region for further studies with deslorelin or transferrin-conjugated poly-l-lactide-co-glycolide nanoparticles (PLGA-NPs) encapsulating an anti-VEGF intraceptor (Flt23k) plasmid. Transport of the nanoparticles, as well as the plasmid from the nanoparticles, exhibited the following order: transferrin-PLGA-NPs > deslorelin-PLGA-NPs > PLGA-NPs ≫ plasmid. The ability of the nanoparticles transported across the nasal tissue to retain the effectiveness of the Flt23k plasmid was evaluated by measuring transfection efficiency (percentage of cells expressing GFP) and VEGF inhibition in LNCaP and PC-3 prostate cancer cells. Transfection efficiencies and VEGF inhibition in LNCaP and PC-3 cells exhibited the following trend: transferrin-PLGA-NPs ≥ deslorelin-PLGA-NPs > PLGA-NPs ≫ plasmid. Further, functionalized nanoparticles exhibited transfection efficiencies and VEGF inhibition significantly superior compared with the routinely used transfecting agent, lipofectamine. Formulating plasmids into nanoparticulate delivery systems enhances the transnasal delivery and gene therapy at remote target cancer cells, which can be further enhanced by nanoparticle functionalization with deslorelin or transferrin.—Sundaram, S., Roy, S. K., Ambati, B. K., Kompella, U. B. Surface functionalized nanoparticles for targeted gene delivery across nasal respiratory epithelium.
Keywords: deslorelin, transferrin, anti-VEGF intraceptor, prostate cancer, gene therapy, poly-l-lactide-co-glycolide
The nasal route allows noninvasive transepithelial systemic delivery of drugs that suffer from very low oral bioavailability, such as peptides, including luteinizing hormone-releasing hormone (LHRH) agonists (∼1.2 kDa) and calcitonin (∼3.5 kDa). Nasal delivery of therapeutic macromolecules >3.5 kDa, however, is formidable and clinically not yet an option because of enzymatic and permeability barriers in the nose (1). Currently, there is a great need to devise approaches such as nanoparticulate systems, capable of protecting macromolecules while allowing enhanced transport, to better facilitate noninvasive delivery of macromolecules. Previous studies have demonstrated that nasal absorption of small 20-nm nanoparticles in rats was only 3.25% (2), indicating that there are significant barriers to overcome for nanosystems used as carriers for noninvasive systemic delivery of therapeutic agents, especially macromolecules. We hypothesized that functionalization of nanoparticle surfaces with peptides or proteins that are ligands for cell-surface receptors can enhance delivery of nanoparticles and any associated macromolecules. Thus, the objective of this study was to determine whether deslorelin/transferrin functionalization of biodegradable nanoparticles enhances the transport of particles across the nasal epithelium and then to apply these nanoparticle technologies in enhancing the delivery and efficacy of gene therapy.
Deslorelin, an LHRH agonist, is a 9-aa peptide of molecular mass 1285 kDa with the amino acid sequence pGlu-His-Trp-Ser-Tyr-D-Trp-Leu-Arg-ProNHEt (3, 4). Binding of LHRH agonists to the LHRH receptor (LHRH-R) leads to the internalization of the LHRH-R-agonist complex (5) via clathrin-coated vesicles, followed by receptor recycling to the plasma membrane. In addition, our previous studies have demonstrated that some receptor agonist complexes are likely exocytosed at the basolateral surface of respiratory epithelial cells, allowing transepithelial receptor-mediated transport of LHRH agonists (6). We have recently demonstrated that deslorelin exhibits differential binding to the LHRH-R in the various regions of the nasal mucosa (7), which correlates with the differential transport of deslorelin across the nasal mucosa. Further, we have previously shown that deslorelin degradation is minimal even after a 6-h exposure to bovine nasal tissue (8). Thus, functionalization of nanoparticles with an LHRH agonist, such as deslorelin, might enhance their nasal delivery via LHRH-Rs. Because functionalization for receptor-mediated uptake of solutes has been well demonstrated with transferrin receptors (TfRs; refs. 9,10,11,12,13), which are expressed in many tissues, it is likely that nanoparticles functionalized with transferrin might allow enhanced nasal delivery. Therefore, transferrin-functionalized nanoparticles were assessed for comparative purposes in this study.
For our studies, we chose a model plasmid capable of inhibiting the secretion of vascular endothelial growth factor (VEGF) as the macromolecule payload. Specifically, we employed Flt23k anti-VEGF intraceptor plasmid, a recombinant construct of the high-affinity subunits 2 and 3 of VEGF receptor-1 (Flt) with endoplasmic reticulum retention signal sequence (KDEL) capable of sequestering VEGF in the ER and expressing GFP (14). Sequestration of VEGF leads to degradation of VEGF molecules, leading to inhibition of VEGF secretion (14). Further, this inhibits the VEGF autocrine loop as well, leading to inhibition of angiogenesis (14, 15).
To assess influence of surface functionalizations on nanoparticle delivery, we employed 20-nm nondegradable fluorescent particles and assessed their permeability across inferior turbinate posterior (ITP), medium turbinate posterior (MTP), and medium turbinate anterior (MTA) regions of bovine nasal tissues. To provide proof of principle for the suitability of this approach in enhancing delivery of a therapeutic macromolecule, we assessed the transport of functionalized biodegradable poly-l-lactide-co-glycolide nanoparticles (PLGA-NPs) loaded with anti-VEGF intraceptor plasmid across the nasal ITP region. Finally, to assess the efficacy of this approach, we assessed gene expression and effects in LNCaP and PC-3 prostate cancer cells after exposing them to the transported fraction of various nanoparticle treatments. Both LNCaP and PC-3 prostate cancer cells are known to express high levels of both LHRH-R and TfR (16, 17).
MATERIALS AND METHODS
Chemicals
Deslorelin was a gift from Balance Pharmaceuticals, Inc. (Santa Monica, CA, USA). Transferrin, polyvinyl alcohol (PVA), 3-(N-morpholino)-propanesulfonic acid (MOPS), and the chemicals required for the preparation of buffers were obtained from Sigma-Aldrich (St. Louis, MO, USA). Polystyrene nanoparticles (PS-NPs; 20 nm, surface modified with aldehyde sulfate; FluoSpheres) were obtained from Molecular Probes (Carlsbad, CA, USA). Resomer 503H [PLGA 50:50; intrinsic viscosity (IV). 0.44 dl/g] was obtained from Boehringer Ingelheim (Petersburg, VA, USA). Flt23k plasmid was provided by B.K.A.
Preparation of solutions
Transport across excised bovine nasal tissue was conducted using assay buffer (18). The assay buffer (pH 7.4) contained 1.14 mM CaCl2, 1.2 mM MgSO4, 3 mM KCl, 0.4 mM KH2PO4, 25 mM NaHCO3, 10 mM glucose, 122 mM NaCl and 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid). The assay buffer was pre-equilibrated to 37°C for 30 min. The assay buffer adjusted to pH 5 with 1 N HCl was used as acid wash buffer.
Isolation of excised bovine nasal mucosa
Bovine noses were obtained from a local slaughterhouse (J & J Meats, Elkhorn, NE, USA) and were processed for transport within 1 h of slaughter. Using a previously described method (8), bovine nasal tissues were isolated for transport. In all our studies, bovine nasal tissue was procured and isolated within 2 h from time of slaughter, and transport study was performed for 4 h during the time in which nasal tissues retain viability (8, 19). Briefly, the different regions of bovine nasal tissue, including the medium turbinate anterior (MTA)—up to 2.5 in (64 mm) from the frontal tip of the nares; the medium turbinate posterior (MTP)—beyond 2.5 in (64 mm) past the tip of the nares; and the inferior turbinate posterior (ITP)—beyond 2.5 in (64 mm) past the tip of the nares, below the MTP region and facing the floor of the nasal cavity, i.e., the inferior meatus, were isolated and used immediately for the experiments. Care was taken not to handle the tissues to prevent damage, and mucosae were separated from the cartilage using a pair of tweezers.
Transport and uptake studies with FluoSphere nanoparticles
Preparation of deslorelin-conjugated FluoSpheres (deslorelin-PS-NPs) and transferrin-conjugated FluoSpheres (transferrin-PS-NPs)
Deslorelin- or transferrin-conjugated aldehyde sulfate-modified FluoSpheres were prepared using a 1-step mix and wash technique (20). Aldehyde sulfate FluoSpheres (20-nm sulfate PS-NPs surface modified with aldehyde groups) were obtained from Molecular Probes (Invitrogen, Carlsbad, CA, USA). Briefly, 10 ml of deslorelin or transferrin solution (100 μg/μl) was added to 10 ml of aldehyde sulfate nanoparticle suspension (2 mg/ml). The reaction was allowed to proceed overnight at room temperature. The conjugation occurs because of the formation of Schiff’s base between the aliphatic aldehyde surface groups and the lysine ε-amine groups of the peptide/protein. The product was purified by centrifuging the nanoparticles at ∼34,000 g for 50 min. The particles were resuspended in distilled water (20 ml) and pelletized twice more to separate the unbound ligand. Following the final pelletization, the particles were resuspended in distilled water and lyophilized to obtain the final conjugated particles.
Characterization of nanoparticles
Nanoparticle size was measured using a Zeta Plus Analyzer (Brookhaven Instruments Ltd, Redditch, UK). Particle size was analyzed before and after ligand conjugation in deionized filtered water. Further, the particles were visualized using a transmission electron microscope (TEM). Briefly, carbon-coated grids were floated on a suspension droplet of plain or functionalized nanoparticles on a flexible plastic film (Parafilm; Pechiney Plastic Packaging, Neenah, WI, USA) to allow adsorption of nanoparticles onto the grid. After air drying the particles, uranyl acetate was added to the carbon grid as a negative stain and allowed to react with the particles. This procedure made the particles electron dense, allowing their visualization using an electron microscope. The particles were photographed using an EM410 Philips electron microscope (Philips, Eindhoven, The Netherlands) set at 60 kV and ×153,000 for PS-NPs and transferrin-PS-NPs. Deslorelin-PS-NPs were photographed at ×112,000 owing to their larger size. The ligand content on the surface of the FluoSpheres was determined using the BCA (bicinchoninic acid) protein assay kit (Pierce Protein Research Products, Rockford, IL, USA) following the manufacturer’s protocol. The lyophilized product was resuspended in deionized water, and protein content on the surface of the nanoparticles was determined after appropriate dilutions. Standard curves were prepared for deslorelin and transferrin by serial dilution of a 2 mg/ml solution.
Nanoparticle transport studies
Modified Ussing’s chambers (Navicyte, Reno, NV, USA) with an aperture area of 1.78 cm2 were used in all transport studies in this project. The fluid volumes in donor and receiver chambers were 5 ml in the entire study. For PS-NP transport studies, freshly excised bovine MTA, MTP, and ITP regions were mounted in Ussing chambers. The tissues were exposed to 5 ml of assay buffer with PS-NPs, deslorelin-PS-NPs, or transferrin-PS-NPs on the donor side (mucosal or serosal surface). Assay buffer (5 ml) without particles was exposed to the receiver side (serosal or mucosal surface). Buffers used were pre-equilibrated to 37°C or 4°C. The donor nanoparticle concentration was 20 or 100 μg/ml. The chambers were maintained at a constant temperature (37°C or 4°C) with an external circulating water bath. Samples were collected from the receiver chamber at various intervals up to 4 h and replaced with assay buffer. Donor samples were collected at the beginning (replenished with particle stock suspension) and at the end of the experiment. Samples were collected in polypropylene tubes, capped, and stored at 4°C until analysis.
Nanoparticle uptake
At the end of 4 h in the above transport studies, tissues exposed (1.78 cm2) to the nanoparticle suspension were cut and washed 3 times using 1 ml of assay buffer at pH 5. After washes, the tissues were weighed and placed in 1 ml of 2% Triton-X 100 and homogenized until a tissue suspension was formed, using the Tissue Tearor (BioSpec, Bartlesville, OK, USA). Tissue debris was separated by centrifugation at 3000 rpm (Marathon Micro A; Fisher Scientific, Pittsburgh, PA, USA) for 10 min. The supernatant was collected to analyze the amount of nanoparticles taken up by the tissue. Standard curves were prepared by homogenizing serial dilution of the stock solution with ∼100–150 mg of nasal tissue. The homogenates were then centrifuged, and the supernatant was analyzed.
Competition with free ligand
Similar to the transport and uptake studies mentioned above, the competition study was carried out using the modified Ussing’s chambers. Tissues were exposed to 20 μg/ml of nanoparticles conjugated with deslorelin or transferrin in the presence of 10× (200 μg/ml) plain deslorelin or transferrin in the donor chamber. Receiver samples were obtained at various intervals up to 4 h and replaced with prewarmed assay buffer. At the end of 4 h, the tissue region exposed to the nanoparticle preparation was cut and washed in assay buffer at pH 5. The tissues were then homogenized and centrifuged. The supernatants were analyzed to determine nanoparticle uptake.
Confocal laser scanning microscopy and flow cytometry
At the end of 4 h in the above transport studies, the tissues exposed to the drug were cut and washed 3 times in 1 ml of acid buffer. The tissues were then fixed in 4% paraformaldehyde for 15 min, followed by permeabilization for 30 min at room temperature with 0.1% Triton-X solution in PBS. The tissues were finally stained with 1 μg/ml propidium iodide for 5 min. At the end of each step, the tissues were washed with PBS (pH 7.4; 3 times). Images of the particles taken up by the tissues were obtained at ×40 with a confocal laser microscope (Zeiss Confocal LSM410; Carl Zeiss MicroImaging, Thornwood, NY, USA). Following confocal microscopy of the tissues, the epithelial cells on the mucosal surface were isolated. Images of the particle uptake in epithelial cells were obtained at ×100 under an oil-immersion lens. Blank controls with plain assay buffer in the donor chamber served as negative controls and were treated the same way as the experimental samples.
Flow cytometry was performed on single-cell suspensions isolated from the tissues after 4 h of transport of nanoparticles. Briefly, the tissues exposed to nanoparticles were isolated and washed 3 times in 1 ml of acid buffer followed by fixing in 4% paraformaldehyde for 15 min at room temperature. The fixed tissues were placed in a test tube, washed 3 times in PBS at pH 7.4, and exposed to trypsin-EDTA for 5 min at 37°C to loosen the epithelial cells. At the end of 5 min, the tubes were vigorously vortexed, and the trypsin-EDTA was collected. The tissues were also scraped at the mucosal surface, and the cells were collected and treated again with trypsin-EDTA. The two trypsin-EDTA fractions were combined and neutralized with 10% FBS containing RPMI 1640 medium to avoid cell lysis by trypsin-EDTA. The tissue scraping, trypsin-EDTA exposure, and FBS neutralization were repeated 2 more times. The suspension was then centrifuged at 600 g for 5 min. The cell pellet obtained was resuspended in 1 ml of distilled water and analyzed using flow cytometry.
Nanoparticle quantification
The samples obtained from the transport studies, washes, and supernatants of tissue homogenates were analyzed for nanoparticle content using a spectrofluorometer (Cary Eclipse; Varian, Palo Alto, CA, USA). The excitation wavelength of 505 nm and an emission wavelength of 515 nm were used. Bandwidths of 3 or 5 nm were used for the sample analysis after dilution as required. The procedure used for extraction of the nanoparticles was performed as described above in Nanoparticle Uptake. A similar approach was used for the preparation of standards in tissues. Briefly, similar amounts of tissues (weighed to match weight of tissues obtained from the transport experiment) were spiked with serially diluted stock nanoparticle suspensions (0.3–100 μg/ml) prepared in 2% Triton-X 100. The tissues were homogenized and centrifuged, and the supernatant obtained was used for standards. Standard curves were also prepared in assay buffer and acid wash buffer.
Transport and efficacy studies with Flt23k plasmid-loaded PLGA-NPs
Preparation and conjugation of Flt23k plasmid-loaded PLGA-NPs
Resomer 503H (PLGA 50:50; IV 0.44 dl/g; Boehringer Ingelheim) (100 mg) COOH-modified and Flt23k plasmid (500 μl of 10 mg/ml) were used for the preparation of particles. Briefly, plasmid in TE buffer (500 μl) was poured into the organic phase (PLGA dissolved in 1 ml dichloromethane containing 0.25 mg/ml of Nile red) and sonicated using a probe sonicator at 10 W for 1 min to form the primary emulsion. Following sonication, the emulsion was poured into 8 ml of 2% PVA and sonicated at 30 W for 3 min. Following sonication, the double emulsion was poured into 50 ml of 2% PVA, and solvent was allowed to evaporate at moderate stirring at 7000 rpm in a magnetic stirrer (Fisher Scientific) overnight at room temperature. The suspension was further evaporated using a rotovap (Buchi Rotavapor R200; Buchi Analytical, New Castle, DE, USA) for 2 h at 40°C in a heated water bath (Buchi Heat Bath B490) to remove residual solvent. Following evaporation, the suspension was centrifuged at ∼34,000 g for 50 min at 4°C. The particle pellet was resuspended in double-distilled water (20 ml; repeated twice to remove surface bound PVA and plasmid) and lyophilized. Blank particles without the plasmid were prepared as controls following the above procedure.
Following lyophilization, 20 mg of nanoparticles was resuspended in 10 ml of MOPS buffer (pH 6.5) and incubated at room temperature for 2 h with 4 mg of ethylenediamine carbodiimide. Following incubation, 10 ml of 200 μg/ml protein solutions was added dropwise to the particle suspension and incubated at room temperature for 12 h. The conjugated nanoparticles were harvested by centrifugation at 35000 g for 50 min. The particle pellet was resuspended in double-distilled water (20 ml) and lyophilized. Particle size and ζ-potential analysis were done as described above for Fluospheres. Surface protein content of the PLGA-NPs was characterized as described above for FluoSpheres.
Plasmid loading
Lyophilized Flt23k plasmid-loaded PLGA-NPs were weighed (1 mg) into glass Kimble tubes. Methylene chloride (1 ml) was added to each tube, and the tubes were sealed and vortexed at high speed (Vortex Genie at speed setting 10; Scientific Industries, Bohemia, NY, USA). After 1 h of vortexing, 1 ml of water was added to the organic phase with further vortexing for 30 min. At the end of 30 min, the mixture was allowed to settle to separate the organic and aqueous phases. The aqueous phase was collected into the quartz UV cuvette (with appropriate dilution) and analyzed using UV spectrophotometry. Similarly, the standard curves were prepared by extraction of plasmid from known amounts of plasmid and polymer (6 and 14 mg, respectively) followed by serial dilution.
In vitro release of Flt23k plasmid from PLGA-NPs
The in vitro release study was conducted following a previously described method. Briefly, nanoparticles (5 mg) were weighed and dispersed in 1 ml of Tris-EDTA buffer (pH 7.4) containing 0.1% (w/v) sodium azide. The nanoparticles were maintained under constant shaking at 200 rpm at 37°C. At predetermined time intervals, the particle suspension was centrifuged at 45,000 g at 4°C for 30 min. Flt23k plasmid released in the supernatant was quantified using a Pico Green assay. The pellet was redispersed in 1 ml of fresh Tris-EDTA buffer for continuation of the release study. Cumulative release as a percentage of encapsulated plasmid was calculated as a function of time.
Stability of Flt23k plasmid in PLGA-NPs during formulation and release
The stability of the plasmid was tested during various stages of the formulation and following release in the in vitro release medium. The stability of the plasmid was tested following exposure to methylene chloride, sonication, and lyophilization. After sonication and harvesting of the nanoparticles, the plasmid present in the supernatant was assessed for the effects of sonication on plasmid stability. The plasmid was concentrated by chloroform: isopropanol precipitation. To determine the effect of lyophilization of nanoparticles on the stability of plasmid, the plasmids were extracted following the method described above for plasmid loading. The plasmid in the release medium was obtained from the in vitro release study described above. Controls for each stage of plasmid stability, including formation of open circular, linear, and fragmented plasmids, were also prepared. To prepare open circular plasmid, the plasmid solution (100 μl of 1 mg/ml) was heated at 60°C for 12 h. Linearized and fragmented plasmids were obtained by incubation with various endonucleases. The plasmid samples thus obtained were analyzed by agarose gel electrophoresis [0.8% agarose (Sigma-Aldrich) containing 10 μl of 10 mg/ml ethidium bromide] at 110 V for 2 h. The plasmid bands were visualized under UV light using a GelDoc (Bio-Rad, Hercules, CA, USA).
Flt23k plasmid-loaded PLGA-NP transport studies
The ITP region of bovine nasal tissue was used for permeability studies with the Flt23k plasmid-loaded PLGA-NPs (conjugated and unconjugated) following the protocol listed above. The experiment was performed once to quantify particle permeability and repeated to quantify plasmid permeability. In both cases, sampling was followed as described above for PS-NP transport. The donor plasmid and nanoparticle concentration was 100 μg/ml (500 μg dose for plasmid-alone groups in 5 ml donor solution) and 500 μg/ml (∼100 μg dose of plasmid), respectively, in the plasmid-alone and nanoparticle groups. Samples (500 μl) were collected from the receiver chamber at various intervals up to 4 h and replaced with equivalent amount of assay buffer. Donor samples were collected at the end of the experiment. For particle permeability quantification based on Nile red tracking, the entire volume of sample collected was quantified for particles based on standards of Nile red-loaded particles of each type. For plasmid permeability studies, the samples were centrifuged at 6000 g to separate the nanoparticles from the plasmid released during transport. Following centrifugation, the pellet was subjected to an extraction process similar to the plasmid-loading method, as mentioned above. The plasmid was analyzed in the supernatant, as well as the pellet, using a fluorescent PicoGreen assay and following the manufacturer’s protocol. Total plasmid transported was estimated by adding plasmid contents in the supernatant and the pellet.
Cell culture of prostate cancer cell lines
LNCaP and PC-3 cells (kindly provided by Dr. Ming-Fong Lin, University of Nebraska Medical Center) were cultured in RPMI 1640 medium containing 10% FBS, 100 U/ml penicillin, 100 U/ml streptomycin, and 2 mM l-glutamine. The medium was changed every 3 d, and all studies were conducted with 60–70% confluent cells.
Flt23k plasmid-loaded PLGA-NP efficacy studies
The donor and receiver chamber samples (remaining 4.5 ml after removing 0.5 ml for plasmid quantifcation) were collected at the end of the 4-h plasmid transport study. The samples were lyophilized and resuspended in 2 ml serum-free medium. Medium (500 ml) was added to the cells and incubated for 12 h. Afterward, the medium was removed, and fresh serum containing medium was added. Following 24 h of incubation, the medium was collected (for VEGF studies), and the cells were harvested with trypsin-EDTA. The trypsin was neutralized with serum containing medium, followed by centrifugation at 3000 rpm for 10 min. The cells were resuspended in 500 μl of PBS buffer at pH 7.4 and analyzed for GFP expression using flow cytometry, with excitation and emission wavelengths of 488 and 510 nm, respectively.
VEGF inhibition studies in LNCaP and PC-3 cells were performed on the medium collected after 24 h incubation of the cells in the above study. Briefly, the manufacturer’s protocol was followed for VEGF ELISA (Research Diagnostics, Concord, MA, USA). The kit is capable of detecting the secreted human VEGF isoforms VEGF165 and VEGF121. The kit has no cross-reactivity with recombinant human VEGF-B196. Following primary antibody coating, the 96-well plates were blocked with blocking solution. The samples were then loaded onto the plates, followed by incubation with secondary antibody. The binding was detected by poly-HRP streptavidin and biotin reaction using an ELISA plate reader at 450 and 550 nm. Untreated cells and cells treated with plasmid, plasmid complexed with lipofectamine, and blank nanoparticles were included as controls for the efficacy studies.
Statistical analysis
Experiments were carried out with n = 4 in all studies, except where mentioned otherwise. Data in all cases are expressed as means ± sd. Comparison of mean values between different treatments was carried out using 2-way analysis of variance (ANOVA) followed by Tukey’s post hoc analysis with SPSS 8 software (SPSS Inc., Chicago, IL, USA). The level of significance was set at P < 0.05.
RESULTS
Functionalized PS-NP uptake and transport
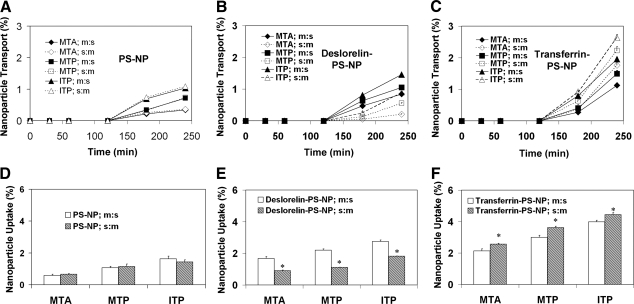
Particle size, ζ potential, and surface ligand content for functionalized and nonfunctionalized PS-NPs are summarized in Table 1, and TEM pictures are shown in Fig. 1. Transport and uptake of nanoparticles across excised bovine nasal tissue exhibited regional differences and directionality. Cumulative 4-h percentage transport and percentage uptake of PS-NPs, deslorelin-PS-NPs, and transferrin-PS-NPs was in the following order: ITP > MTP > MTA in both the mucosal:serosal (m:s) and serosal:mucosal (s:m) directions. Further, cumulative transport and percentage uptake of nanoparticles was in the following order: transferrin-PS-NPs > deslorelin-PS-NPs > PS-NPs in both m:s and s:m directions (Fig. 2). A mass balance analysis in various experiments for the particles used in this study accounted for nearly 100% of initial amount added to the donor chamber (Table 2). There was no significant difference in the transport and uptake of PS-NPs between the two directions (Fig. 2A, D). The mass balance was done using nanoparticle suspensions from the receiver chamber, tissue washes, and remainder of the particles in the donor chamber, and tissue extracts.
TABLE 1.
Physicochemical properties of various PS-NPs used
| Particle type | Diameter (nm) | ζ Potential (mV) | Particle surface protein/peptide [μg/mg (% w/w)] |
|---|---|---|---|
| PS-NP | 79.94 ± 5.48 | −22.36 ± 4.63 | − |
| Deslorelin-PS-NP | 129.64 ± 6.37 | −19.76 ± 5.34 | 32.8 ± 1.9 (3.3 ±0.2) |
| Transferrin-PS-NP | 90.24 ± 4.59 | −17.94 ± 5.24 | 42.4 ± 6.9 (4.2 ±0.6) |
Data are expressed as means ± sd; n = 3.
Figure 1.
Surface morphology of nanoparticles. A–C) TEM pictures of PS-NPs (A), deslorelin-PS-NPs (B), and transferrin-PS-NPs (C). D–F) TEM pictures of PLGA-NPs (D), deslorelin-PLGA-NPs (E), and transferrin-PLGA-NPs (F). TEM pictures of PS-NPs and transferrin-PS-NPs were obtained at ×153,000; all others at ×112,000.
Figure 2.
In vitro transport and uptake of nanoparticles across various regions of the bovine nasal epithelium. Time course of transport (A–C) and percentage uptake (D–F) for PS-NPs (A, D), deslorelin-PS-NPs (B, E), and transferrin-PS-NPs (C, F) at the end of 4 h in the mucosal to serosal (m:s) direction and serosal to mucosal (s:m) direction for MTA, MTP, and ITP regions of excised bovine nasal tissue. Nanoparticle transport and uptake exhibit regional variations in the order: MTA < MTP < ITP. Different nanoparticles are transported and taken up in the following order: PS-NPs < deslorelin-PS-NPs < transferrin-PS-NPs, in all of the regions of bovine nasal tissues. Data are means ± sd; n = 4. *P < 0.05 vs. m:s uptake in same region.
TABLE 2.
Mass balance of PS-NPs, deslorelin-PS-NPs, and transferrin-PS-NPs at the end of 4-h nasal transport studies in the m:s direction at 37°C
| Particle type | Region | Tissue uptake | Transport | Washes | Remainder in donor | Total |
|---|---|---|---|---|---|---|
| PS-NP | MTA | 0.58 ± 0.08 | 0.34 ± 0.02 | 0.20 ± 0.05 | 97.70 ± 0.32 | 99.25 ± 0.45 |
| MTP | 1.07 ± 0.09 | 0.71 ± 0.04 | 0.19 ± 0.04 | 96.54 ± 0.55 | 99.14 ± 0.19 | |
| ITP | 1.64 ± 0.16 | 1.05 ± 0.03 | 0.17 ± 0.04 | 95.49 ± 1.40 | 99.87 ± 0.12 | |
| Deslorelin-PS-NP | MTA | 1.68 ± 0.12 | 0.84 ± 0.04 | 0.15 ± 0.02 | 96.30 ± 0.42 | 98.08 ± 0.85 |
| MTP | 2.20 ± 0.08 | 1.06 ± 0.01 | 0.28 ± 0.04 | 95.06 ± 0.28 | 98.24 ± 1.95 | |
| ITP | 2.75 ± 0.09 | 1.45 ± 0.07 | 0.47 ± 0.02 | 94.31 ± 0.24 | 102.74 ± 1.07 | |
| Transferrin-PS-NP | MTA | 2.13 ± 0.13 | 1.13 ± 0.07 | 0.50 ± 0.13 | 93.06 ± 0.99 | 98.77 ± 0.95 |
| MTP | 2.99 ± 0.14 | 1.52 ± 0.11 | 0.60 ± 0.11 | 92.14 ± 2.20 | 98.99 ± 0.52 | |
| ITP | 3.98 ± 0.10 | 1.97 ± 0.05 | 0.75 ± 0.10 | 94.51 ± 1.10 | 98.62 ± 1.13 |
Values indicate mass balance, expressed as percentage of initial dose, for the contents of donor chamber, receiver chamber, tissue, and washes. Data are expressed as means ± sd; n = 4.
Transport and uptake of deslorelin-PS-NPs were higher in the m:s direction as compared to transport in the s:m direction. Deslorelin-PS-NP transport in the s:m direction when compared to m:s direction was 60, 45, and 30% lower in the MTA, MTP, and ITP regions, respectively (Fig. 2B). The corresponding decrease in uptake was 46, 49, and 34%, respectively (Fig. 2E). Transport and uptake of transferrin-PS-NPs were higher in the s:m direction as compared with transport in the m:s direction. Transferrin-PS-NP transport in the s:m direction, when compared to the m:s direction is 16, 17, and 10% greater in the MTA, MTP, and ITP regions, respectively (Fig. 2C). The uptake for transferrin-PS-NPs in m:s direction compared to s:m direction was 40, 29, and 25% lower in the MTA, MTP, and ITP regions, respectively (Fig. 2F).
Particle uptake in tissue and isolated epithelial cells was visualized using a confocal microscope at ×40 and ×100 (Fig. 3). The epithelial cells observed were pseudostratified columnar cells, with nonciliated cells observed in the MTA region and ciliated cells observed in the MTP and ITP regions. The particles (green fluorescence) were seen to be present in the cytoplasm of epithelial cells around the nucleus. No green fluorescence was observed in the negative controls. Similar to the uptake measured using a spectrofluorometer, the uptake visualized using confocal microscopy showed regional differences in the following order: ITP > MTP > MTA and the uptake of transferrin-PS-NPs > deslorelin-PS-NPs > PS-NPs (Fig. 3A–L). Confocal microscopy further revealed that the particles can enter ciliated respiratory epithelial cells of the nose (Fig. 3M, N). Particle uptake in epithelial cells of bovine nasal tissues from all three regions was also quantified using flow cytometry. No particle uptake was observed in the blank control tissues. Epithelial cell uptake in the bovine nasal tissues was in the following order: ITP > MTP > MTA. The uptake of the various nanoparticles was in the following order: transferrin-PS-NPs > deslorelin-PS-NPs > PS-NPs (Fig. 3O).
Figure 3.
Representative confocal photomicrographs and flow cytometric quantifications showing uptake of PS-NPs, deslorelin-PS-NPs, and transferrin-PS-NPs in bovine nasal tissue and epithelial cells. A, E, I) Negative controls for MTA (A), MTP (E), and ITP regions (I). B, F, J) PS-NPs in MTA (B), MTP (F), and ITP regions (J). C, G, K) Deslorelin-PS-NPs in MTA (C), MTP (G), and ITP regions (K). D, H, L) Transferrin-PS-NPs in MTA (D), MTP (H), and ITP regions (L). M, N) Untreated (blank) (M) and transferrin-PS-NPs-treated ITP regions (N). O) Percentage of cell uptake (based on flow cytometry) of nanoparticles in the epithelial cells isolated from the MTA, MTP, and ITP regions of the bovine nasal epithelia. Data are means ± sd; n = 3.
At low temperature (4°C), when compared to 37°C, the transport of deslorelin (Fig. 4A, B) and transferrin (Fig. 4D, E) functionalized nanoparticles was completely inhibited, and the directionality (Fig. 4C, F) was abolished. Uptake of nanoparticles was also lower at 4°C and did not exhibit directionality. Deslorelin-PS-NP uptake in the m:s direction at 4°C was 70, 78, and 82% lower in the MTA, MTP, and ITP regions, respectively, when compared to 37°C. The analogous decrease in uptake in the s:m direction was 43, 57, and 72%, respectively (Fig. 4C). Transferrin-PS-NP uptake in the m:s direction at 4°C decreased by 58, 70, and 77% in the MTA, MTP, and ITP regions, respectively, when compared to the values at 37°C. The analogous decrease in uptake in the s:m direction was 65, 75, 80%, respectively (Fig. 4F).
Figure 4.
Directionality and temperature dependence of in vitro transport and uptake of deslorelin-PS-NPs and transferrin-PS-NPs in various regions of bovine nasal epithelium following mucosal or serosal exposure. A, D) Time course of deslorelin-PS-NP (A) and transferrin-PS-NP (D) transport in m:s direction across MTA, MTP, and ITP regions of bovine nasal tissue. B, E) Time course of deslorelin-PS-NP (B) and transferrin-PS-NP (E) transport in s:m direction. C, F) Percentage uptake of deslorelin-PS-NPs (C) and transferrin-PS-NPs (F) at the end of 4 h. Experiments were conducted at 37 and 4°C in m:s and s:m directions. At 4°C, nanoparticle uptake in both m:s and s:m directions was not significantly different for any of the regions. At 37°C, uptake of functionalized nanoparticles was significantly different in the following order: ITP > MTP > MTA. Data are means ± sd; n = 4. *P < 0.05 vs. m:s uptake in same region at 37°C; †P < 0.05 vs. s:m uptake in same region at 37°C.
Free ligands (deslorelin and transferrin) competed with functionalized nanoparticles for m:s uptake and transport. Cotreatments with excessive free peptides significantly reduced the transport of deslorelin- or transferrin-PS-NPs. Deslorelin cotreatment decreased deslorelin-PS-NP transport by 30, 31, and 28% in the MTA, MTP, and ITP regions, respectively (Fig. 5A). The corresponding decrease in uptake was 39, 33, and 27%, respectively (Fig. 5B). Transferrin cotreatment decreased transferrin-PS-NP transport by 22, 33, and 25%, respectively (Fig. 5C). The corresponding decrease in transferrin-PS-NP uptake was 43, 45, and 44%, respectively (Fig. 5D). However, even in the presence of free peptide/protein, the transport still exhibited regional differences in the following order: ITP > MTP > MTA. The apparent permeability coefficients (Papp; cm/s) of PS-NPs transported across the various regions of the excised bovine mucosa at the end of 4 h of transport experiment are summarized in Table 3.
Figure 5.
Effect of the presence of excess free ligands (deslorelin and transferrin) on in vitro transport and uptake of deslorelin-PS-NPs and transferrin-PS-NPs in various regions of bovine nasal epithelium following mucosal or serosal exposure. A, B) Percentage cumulative transport of deslorelin-NPs (A) and transferrin-NPs (B) in m:s direction in the absence and presence of excess free ligands. C, D) Percentage uptake of deslorelin-NPs (C) and transferrin-NPs (D) at 4 h following exposure to free ligands. Experiments were conducted in the absence and presence of excess free ligand (200 μg/ml). Data are means ± sd; n = 4. *P < 0.05 vs. transport in absence of competition for same region; †P < 0.05 vs. uptake in absence of competition for same region.
TABLE 3.
Apparent permeability coefficients (Papp; cm/s) of PS-NPs, deslorelin-PS-NPs, and transferrin-PS-NPs across the MTA, MTP, and ITP regions of excised bovine nasal tissue, based on a 4-h transport study
| Particle type | Region | m:s; 37°C | s:m; 37°C | m:s; 4°C | s:m; 4°C | Competition |
|---|---|---|---|---|---|---|
| PS-NP | MTA | 6.56E-07 ± 3.02E-08 | 6.11E-07 ± 1.87E-07 | |||
| MTP | 1.08E-06 ± 6.72E-08 | 1.19E-06 ± 2.53E-07 | ||||
| ITP | 1.49E-06 ± 2.23E-07 | 1.97E-06 ± 1.78E-07 | ||||
| Deslorelin-PS-NP | MTA | 2.32E-06 ± 3.73E-07 | 1.23E-06 ± 1.88E-07 | 0.00E+0.0 | 0.00E+0.0 | 1.82E-06 ± 1.01E-07 |
| MTP | 4.02E-06 ± 1.06E-07 | 3.30E-06 ± 1.94E-07 | 0.00E+0.0 | 0.00E+0.0 | 2.28E-06 ± 6.19E-07 | |
| ITP | 5.66E-06 ± 1.55E-08 | 4.70E-06 ± 1.76E-07 | 0.00E+0.0 | 0.00E+0.0 | 4.25E-06 ± 2.21E-07 | |
| Transferrin-PS-NP | MTA | 4.31E-06 ± 6.64E-08 | 7.19E-06 ± 1.35E-06 | 0.00E+0.0 | 0.00E+0.0 | 1.30E-06 ± 3.43E-07 |
| MTP | 5.83E-06 ± 1.17E-07 | 9.83E-06 ± 1.05E-06 | 0.00E+0.0 | 0.00E+0.0 | 1.77E-06 ± 5.76E-07 | |
| ITP | 7.58E-06 ± 1.11E-07 | 1.17E-05 ± 5.89E-07 | 0.00E+0.0 | 0.00E+0.0 | 5.29E-06 ± 2.74E-07 |
Permeability was measured at 37 and 4°C. Permeability was measured in both m:s and s:m directions. Deslorelin-PS-NPs and transferrin-PS-NPs did not exhibit any quantifiable transport at 4°C. PS-NP transport at lower temperatures was not performed. Data are expressed as means ± sd; n = 4.
Functionalized Flt23k plasmid-loaded PLGA-NP transport and efficacy studies
Particle size, ζ potential, and surface ligand content for PLGA-NPs loaded with plasmid and Nile red are summarized in Table 4. Further, TEM images of all three particles, including PLGA-NPs, deslorelin-PLGA-NPs, and transferrin-PLGA-NPs, are shown in Fig. 1D–F. PLGA-NPs were loaded with a plasmid content of 20.9 μg/mg particles (2.1% w/w).
TABLE 4.
Physicochemical properties of various PLGA-NPs used
| Particle type | Diameter (nm) | ζ Potential (mV) | Particle surface protein/peptide [μg/mg (% w/w)] |
|---|---|---|---|
| PLGA-NP | 217.98 ± 0.78 | −13.89 ± 0.99 | − |
| Deslorelin-PLGA-NP | 203.89 ± 0.76 | −14.87 ± 0.78 | 38 ± 11.5 (3.8 ± 1.2) |
| Transferrin-PLGA-NP | 207.45 ± 0.91 | −13.87 ± 0.67 | 45.5 ± 8 (4.6 ± 0.8) |
Data are expressed as means ± sd; n = 3.
Stability of Flt23k plasmid encapsulated in the nanoparticles was observed following exposure to dichloromethane, sonication, lyophilization, and in vitro release. Plasmids under the various formulation conditions, including exposure to dichloromethane, sonication, lyophilization, and in vitro release, exhibited bands similar to the original stock of plasmid obtained during amplification and isolation from E. coli (Fig. 6A). The in vitro plasmid release, as shown in Fig. 6B, indicated biphasic plasmid release from PLGA-NPs, including a rapid-release phase and a slow-release phase. At the end of d 30 of the study, the cumulative percentage of plasmid released accounted for 17% of the total loaded plasmid. Further, stability of the plasmid was also studied using the plasmids in the release medium at d 15. As shown in Fig. 6A, the plasmid structure in the release medium is similar to the original stock plasmid solution. Thus, the plasmid maintained its stability during in vitro release.
Figure 6.
Flt23k plasmid in vitro stability and release from PLGA-NPs. A) Stability of the plasmid during formulation and following release. B) In vitro release profile of plasmid from PLGA-NPs. Data are means± sd; n = 3.
In plasmid permeability studies, in the receiver at the end of 4-h transport study, ∼64, 62, and 52% of the plasmid was in the pellet for the transferrin-PLGA-NPs, deslorelin-PLGA-NPs, and PLGA-NPs, respectively. The remaining plasmid was present in the supernatant. The plasmid concentrations in the pellet and supernatant were added in order to determine the total amount of plasmid transported. The cumulative percentage transport of Flt23k plasmid loaded PLGA-NPs (with an encapsulation of 20 μg of plasmid/mg of particles) across the ITP region of the bovine nasal epithelium was in the following order: transferrin-PLGA-NPs > deslorelin-PLGA-NPs > PLGA-NPs ≫ plasmid. The percentage transport of plasmid material estimated using PicoGreen assay was also in the same order, with the plasmid transport being ∼7.2-, 6.2-, and 4.3-fold higher with transferrin-PLGA-NPs, deslorelin-PLGA-NPs, and PLGA-NPs, respectively, when compared to plasmid alone (Fig. 7A, B).
Figure 7.
In vitro transport and efficacy PLGA-NPs and Flt23k plasmid. Following transport of nanoparticles across the ITP region of bovine nasal epithelium, the efficacy of the transported particles and plasmid in the receiver chamber was assessed in LNCaP and PC-3 prostate cancer cell lines. A) Time course of nanoparticle transport in m:s direction of ITP region of excised bovine nasal tissue, based on Nile red tracking. B) Time course of transport of total plasmid (free and encapsulated). C) Transfection efficiency (GFP expression) of ITP-transported Flt23k-loaded PLGA-NPs in LNCaP and PC-3 prostate cancer cells. D) VEGF-inhibitory efficacy (reduction in VEGF levels in culture supernatant) of ITP-transported Flt23k-loaded PLGA-NPs in LNCaP and PC-3 prostate cancer cells. Data are means ± sd; n = 3 (transport studies), n = 4 (efficacy studies).
Nanoparticles transported across the nasal ITP region, as well as those on the source or donor side of tissue, were assessed for their efficacy in prostate cancer cells. Because anti-VEGF intraceptor construct had GFP as a marker protein, the transfection efficiency was measured using GFP expression in the LNCaP and PC-3 prostate cancer cell lines. The percentage of cells expressing GFP with the receiver chamber samples in both target cells was in the following order: transferrin-PLGA-NPs > deslorelin-PLGA-NPs > PLGA-NPs ≫ plain plasmid alone. These differences were statistically significant in both cell types (P<0.05). Similar trends were observed in the donor chamber samples as well (Fig. 7C), with the differences being less marked between functionalized and nonfunctionalized nanoparticles. As expected, the donor chamber samples with greater nanoparticle dose exhibited higher GFP expression when compared with the receiver chamber samples in all the treatment groups.
Plasmid-loaded deslorelin-PLGA-NPs from the receiver chamber exhibited gene expression that was ∼55, 98, and 95% higher in LNCaP cells and 32, 90, and 68% higher in PC-3 cells, as compared with plasmid complexed with lipofectamine, plasmid alone control, and PLGA-NPs from the receiver chamber samples, respectively. Transferrin-PLGA-NPs from the receiver chamber exhibited GFP expression ∼64, 99, 96, and 19% higher in LNCaP cells and 42, 92, 73, and 14% higher in PC-3 cells, as compared with plasmid complexed with lipofectamine, plasmid alone in controls, PLGA-NPs from the receiver, and deslorelin-PLGA-NPs from the receiver, respectively. In receiver as well as donor samples, there was no significant difference between deslorelin-PLGA-NPs and transferrin-PLGA-NPs in either the LNCaP or PC-3 cells (Fig. 7C).
VEGF inhibition was also measured in LNCaP and PC-3 prostate cancer cell lines. The VEGF inhibition in the two cells with the receiver chamber samples was in the following order: transferrin-PLGA-NPs ≥ deslorelin-PLGA-NPs > PLGA-NPs ≫ plasmid alone. The donor chamber samples exhibited higher VEGF inhibition as compared with the receiver chamber samples in all the treatment groups. Deslorelin-PLGA-NPs from the receiver chamber exhibited VEGF inhibition ∼74 and 48% higher in LNCaP cells and 95 and 68% higher in PC-3 cells, as compared with plasmid complexed with lipofectamine and PLGA-NPs from the receiver chamber, respectively. Transferrin-PLGA-NPs from the receiver chamber exhibited VEGF inhibition ∼56, 36, and 19% higher in LNCaP cells and 97, 80, and 40% higher in PC-3 cells, as compared with plasmid complexed with lipofectamine, PLGA-NPs from the receiver chamber, and deslorelin-PLGA-NPs from the receiver chamber, respectively. All nanoparticles inhibited VEGF expression to a greater extent compared to plain plasmid. There was no significant difference between donor chamber samples of PLGA-NPs, deslorelin-PLGA-NPs, and transferrin-PLGA-NPs in both the LNCaP and PC-3 cells (Fig. 7D).
DISCUSSION
Functionalized nanoparticles loaded with macromolecules can potentially enable the noninvasive systemic delivery of macromolecules that cannot be delivered by current methods. Our studies were aimed at designing such functionalized nanoparticulate delivery systems with enhanced uptake and transport across the nasal mucosa in order to facilitate noninvasive delivery of large molecules via the nose. On the basis of our studies using an anti-VEGF plasmid as a model drug, we report the following key findings: 1) deslorelin and transferrin functionalizations improve transnasal delivery of nanoparticles as compared with nonfunctionalized nanoparticles; 2) nanoparticles are taken up by various cell types, including respiratory epithelial cells, leading to their enhanced permeability across the nasal mucosa; 3) encapsulation or loading in nanoparticulate delivery systems maintains stability and efficacy of the plasmid payload; and 4) functionalization of nanoparticles encapsulating a therapeutic plasmid improves the transport and subsequent transfection efficiency, as compared with plain plasmid, nonfunctionalized nanoparticles, and lipofectamine, a common transfection agent.
Our initial studies with deslorelin- and transferrin-functionalized polystyrene FluoSpheres were aimed at characterizing whether surface functionalization enhances transnasal delivery of nanoparticles. Our studies demonstrated higher uptake and transport for deslorelin-PS-NPs, as compared to PS-NPs. Further, uptake and transport of deslorelin-PS-NPs also exhibited regional variation in the order: ITP > MTP > MTA. We previously reported similar regional variations in the nose for the expression of LHRH-R (7) and deslorelin transport (8). On the basis of these studies, it is likely that the deslorelin-PS-NP uptake and transport are facilitated by deslorelin binding to the LHRH-R, and the ability of the LHRH-R to transcytose bound ligands. Transferrin-PS-NPs also exhibited higher uptake and transport, as compared with PS-NPs, suggesting the value of this approach in enhancing transnasal delivery. Further, when compared with deslorelin-PS-NPs, transferrin-PS-NPs exhibited higher uptake and transport. This could be due to one or more of the following reasons: 1) deslorelin-PS-NPs exhibited higher sizes (∼130 nm), as compared with transferrin-PS-NPs (∼90 nm) (Table 1); 2) the short length of deslorelin compared to transferrin would restrict the mobility and accessibility of the smaller ligand to the LHRH-R, leading to lower uptake and transport of deslorelin-PS-NPs; 3) the potential differences in the rates of internalization and exocytosis of the two receptor-ligand complexes; and 4) the 29% greater mass of ligand on the surface of transferrin-functionalized nanoparticles compared to deslorelin-functionalized nanoparticles (Table 1). For both deslorelin- and transferrin-functionalized nanoparticles at the end of the 4-h transport study, the tissue uptake of nanoparticles was greater compared to the cumulative percentage transported (Fig. 2), suggesting enhanced uptake as the principal mechanism contributing to the observed increase in nanoparticle transport. Thus, both deslorelin and transferrin functionalizations enhance nasal uptake and transport of nanoparticles.
Solutes transported by membrane transporters or carriers are well known to exhibit vectorial transport. That is, the transport of solutes from the mucosal (outside) to the serosal (inside) is expected to differ from their transport in the opposite (s:m) direction. This is because receptor or carrier proteins are usually expressed in a polarized manner in the epithelial cell layers. In this study, both deslorelin-PS-NPs and transferrin-PS-NPs exhibited directionality, that is, vectorial transport. Deslorelin-PS-NPs exhibited greater uptake and transport in the m:s direction, while transferrin-PS-NPs exhibited greater uptake and transport in the s:m direction (Fig. 2). This could be attributed to the polarization or relative abundance of the respective receptors in the nasal tissue. Indeed, the LHRH-R expression in respiratory cells was suggested to be polarized, with greater expression in the apical surface compared to the basolateral surface (21). TfR expression in nasal tissue was also reported to be polarized, with greater receptor expression in the serosal surface compared to the mucosal surface (19). These opposite polarizations for LHRH-Rs and TfRs are consistent with the functions of these receptors in the nasal tissue. The LHRH-R is involved in the modulation of the chemosensory reception of externally presented pheromones, which factors in mate selection, maternal behavior, and sexual arousal via the autocrine loop within the chemosensory system (22). The TfR on the other hand is involved in the uptake of iron in the transferrin-iron complex from the bloodstream into the cells via endocytosis, followed by exocytosis or recycling of transferrin out of the cells (9).
In addition to vectorial transport, solute transport mediated by receptors is expected to be sensitive to temperature and competition by other ligands for the receptors. In this study, we observed that nasal uptake and transport of functionalized nanoparticles exhibits temperature dependence, as well as sensitivity to competing ligands. For both deslorelin-PS-NPs and transferrin-PS-NPs, nasal uptake was significantly reduced at 4°C, transnasal transport was completely inhibited, and the directionality of transport was abolished (Fig. 4). These findings confirm that there are active or cellular energy-dependent processes involved in the uptake and transport of deslorelin-PS-NPs and transferrin-PS-NPs. Further, in the presence of competing agents (excess free deslorelin/transferrin), both uptake and transport were reduced for functionalized nanoparticles, indicating receptor involvement in the nasal uptake and transport of functionalized nanoparticles (Fig. 5). Interestingly, although nonfunctionalized PS-NPss exhibited regional variations in nasal delivery in the same order as the functionalized nanoparticles, they did not exhibit directionality (Fig. 2). One explanation for this observation is that these nonfunctionalized nanoparticles are transported by a nonspecific endocytotic process that does not exhibit polarization. Another explanation for this observation is that the nonfunctionalized particles are transported predominantly via a paracellular pathway and not the transcellular pathway (23) followed by the functionalized nanoparticles. Indeed, our confocal microscopy studies (Fig. 3) provided visual evidence for regional differences in nanoparticles uptake, superiority of functionalized nanoparticles when compared to nonfunctionalized nanoparticles in entering nasal tissue, and for the ability of functionalized nanoparticles to enter cell body of nasal respiratory epithelial and other cell types via the transcellular pathway (Fig. 3N). Flow cytometry of isolated nasal cells also indicated that the ability of the particles to enter nasal cells is in the following order: transferrin-PS-NPs > deslorelin-PS-NPs > PS-NPs in all three regions of the nose.
Although TfRs are expressed ubiquitously, they are up-regulated in a differential manner in disease states. For instance, elevated transferrin binding was observed in the breast tumors in the range of 11–35% bound transferrin, compared to ∼2% bound transferrin in non-neoplastic tissues (24). Further, colorectal cancer patients exhibit 1.83 ± 0.32 nmol/g TfR compared to 0.85 ± 0.18 nmol/g in control (25). In addition, cancerous epithelial cells of human prostate, but not the surrounding benign stromal cells, express TfR (26). Similar to TfRs, LHRH-Rs are up-regulated in some cancers, including cancers of the prostate (17), breast (27), and ovaries (27). Thus, after crossing the nasal tissue and entering the bloodstream, nanoparticles functionalized with deslorelin/transferrin are expected to target the cancer tissues overexpressing LHRH-R/TfR better than normal tissues. Once at the target site, functionalized nanoparticles are expected to be internalized by the target cells to a greater extent compared to nonfunctionalized nanoparticles. Because the receptors are overexpressed at the target cancer cells, as opposed to the normal nasal tissue, the effects of functionalized nanoparticles might be much superior at these secondary targets compared to nonfunctionalized nanoparticles. To determine whether deslorelin/transferrin functionalization indeed enhances noninvasive nasal macromolecule delivery and subsequent uptake by target cancer cells, we conducted additional investigations. Specifically, we prepared anti-VEGF intraceptor (Flt3k) plasmid and Nile red-loaded, functionalized PLGA-NPs and assessed plasmid stability, permeability, and release; particle permeability; and efficacy of the transported plasmid in terms of transfection efficiency and anti-VEGF activity in LNCaP and PC-3 prostate cancer cells.
A critical attribute of any delivery system, especially that of a macromolecule, is that it must maintain the integrity of the payload. Our studies demonstrated that the formulation of plasmid into nanoparticles using the described sonication method does not degrade the plasmid significantly (Fig. 6A). Further, these studies also prove that the plasmids released in vitro in the release medium maintain their integrity. Nanoparticles are capable of sustaining drug delivery over long periods of time (28). Indeed, our studies suggest that the plasmid-loaded nanoparticles sustain delivery over 30 d following an initial burst release of plasmid (Fig. 6B). The initial burst release is likely due to the release of surface-bound plasmids, unlike the slow-release phase, wherein the plasmids present within the core of the particles are likely released by diffusion, as well as particle degradation (29). Alternatively, two populations of plasmid, one fraction loosely bound and another tightly bound, may have contributed to the observed results. We prepared the plasmid-loaded nanoparticles using a water/oil/water emulsion-solvent evaporation method. The nanoparticles were then harvested by centrifugation at 34,000 g for 50 min at 4°C and washed by resuspending the particle pellet in double-distilled water, followed by pelletization with repeat centrifugation. The wash procedure was repeated twice. The lyophilized particles obtained following the washes were used to assess plasmid loading in the particles. We observed ∼2% plasmid loading in these particles. The plasmids are highly water soluble and hence, we believe that they can be washed off by repeated resuspension and centrifugation steps, as described above. However, it is likely that the surface-bound plasmid, as well as any free plasmid that settled with the particles and did not get washed off, will contribute to the plasmid loading and initial burst release observed. Although the release profile with nanoparticles is not ideal in terms of its burst phase, a compromise has to be made since enhanced penetration requires smaller particle size, which inevitably leads to greater surface area and hence burst release. Possibly because of their ability to protect plasmid from degradation, even nonfunctionalized PLGA-NPs increased the plasmid permeability across bovine ITP region by 27-fold (Table 5).
TABLE 5.
Apparent permeability coefficient of plasmid-loaded PLGA-NPs, deslorelin-PLGA-NPs, and transferrin-PLGA-NPs across the ITP region of excised bovine tissue in the m:s direction, based on a 4-h transport study
| Permeability | Plasmid alone | PLGA-NPs | Deslorelin-PLGA-NP | Transferrin-PLGA-NP |
|---|---|---|---|---|
| Particles (cm/s) | − | 6.34E-06 ± 4.98E-07 | 8.76E-06 ± 1.02E-06* | 1.02E-05 ± 4.04E-07*,† |
| Plasmid (cm/s) | 2.00E-08 ± 2.01E-09 | 5.36E-07 ± 3.47E-08* | 6.87E-07 ± 2.29E-08*,† | 7.82E-07 ± 1.94E-08*,†,‡ |
Permeability of particles was assessed based on Nile red tracking. Data are expressed as means ± sd; n = 3.
P < 0.05 vs. PLGA-NPs for particle permeability and plasmid-alone group for plasmid permeability;
P < 0.05 vs. deslorelin-PLGA-NPs for particle permeability and PLGA-NPs for plasmid permeability;
P < 0.05 vs. deslorelin-PLGA-NPs for plasmid permeability.
Permeability of various PLGA-NPs loaded with plasmid was determined across bovine nasal ITP region, which exhibited highest uptake and transport for functionalized PS-NPs. Since Nile red is well retained in PLGA particles (30), we quantified PLGA-NP movement across nasal tissue using standards of PLGA particles based on Nile red fluorescence. These studies with the PLGA-NPs reaffirmed that functionalization of nanoparticles with the cell-surface receptor ligands deslorelin or transferrin leads to an enhanced delivery across the bovine nasal mucosa in the following order: transferrin-PLGA-NPs (3.2%) > deslorelin-PLGA-NPs (2.7%) > PLGA-NPs (1.7%) (Fig. 7A). In addition to the higher particle transport achieved by functionalization, plasmid transport was also increased (Fig. 7B). We observed that PLGA-NPs, deslorelin-PLGA-NPs, and transferrin-PLGA-NPs elevate plasmid permeability coefficient by 27-, 34-, and 39-fold respectively, suggesting the superiority of the nanoparticle formulations (Table 5). However, the actual plasmid transported by the nanoparticles systems in 4 h was <1%. The transport of nanoparticles was carried out using a small area of the nasal tissue mounted in Ussing chambers. Specifically, permeability was measured across a tissue area of 1.78 cm2. However, the nasal epithelial tissue in human nose occupies ∼150 cm2 (23). Thus, the extent of delivery can be somewhat better in the whole animal. Further, given the potent ability of the permeating molecules in inhibiting VEGF, this approach will likely be viable in the whole animal.
The transport achieved by PLGA-NPs (Fig. 7A) is greater compared to transport achieved by the polystyrene particles (Fig. 4). PLGA-NPs are ∼2-fold greater in size compared to the measured size of polystyrene particles, and PLGA-NPs are expected to exhibit lower transport based on size. However, the opposite is true in this case. One possible explanation is that conjugation efficiency likely plays a role in the differences observed in transport and uptake. However, this is unlikely, as we observed very similar ligand loading for polystyrene and PLGA-NPs (Tables 1and 4). A second possible explanation could be the method of conjugation used for the two types of particles. Although we conjugated the ligands using carbodiimide reaction in case of PLGA particles, we used the ability of aldehyde surface groups of polystyrene particles to attach the ligands via Schiff’s base formation, which could have resulted in a less favorable orientation or binding of ligands and/or reduced ligand stability with polystyrene particles. Another possible explanation for the observation is the relative binding and retention of nanoparticles in the tissue. If the aldehyde groups on polystyrene particles react with surface proteins of the tissue, the particles may be bound and retained on the surface to a greater extent compared to PLGA particles. That is, the extent of internalization may be reduced. These differences may have contributed, in part, to the observed differences in the permeability of various nanoparticles.
Once transported across the nasal tissue, it is critical to ensure that the plasmid containing nanoparticles retain their functional abilities and enhance efficacy of the plasmid in target cells. Following transport across the ITP region, we assessed the in vitro pharmacological efficacy of the transported plasmid containing nanoparticles in LNCaP and PC-3 prostate cancer cells (Fig. 7C, D). Gene therapy is an important strategy for the therapy of various diseases, including cancers (31). We chose to use the LNCaP and PC-3 cells as a model of disease owing to the overexpression of both LHRH-R, as well as TfR on these cells (16, 17). The Flt23k plasmid is capable of expressing GFP marker proteins, which was used as a measure of the transfection efficiency. We observed that the transfection efficiencies were in the following order: transferrin-PLGA-NPs ≥ deslorelin-PLGA-NPs > PLGA-NPs ≫ plasmid alone. The higher efficiencies observed with the functionalized nanoparticles is likely due to the better transport of these particles across nasal tissue, as well as better uptake of these particles by the cancer cells. We used lipofectamine, a commonly used transfecting agent, to complex with Flt23k plasmid, as a control. Interestingly, deslorelin- and transferrin-PLGA-NPs exhibited transfection efficiencies far superior to the lipofectamine complexes. Surprisingly, we did not see any significant difference in the transfection efficiencies of deslorelin- and transferrin-PLGA-NPs. This might be an outcome of greater expression of LHRH-Rs on the prostate cancer cells compared to TfRs. Alternatively, better retention of receptor binding properties by deslorelin-functionalized nanoparticles as opposed transferrin-functionalized nanoparticles after nasal transport might explain these results. As expected, the donor chamber samples achieved greater transfection efficiencies when compared with the receiver chamber samples, owing to the higher concentrations of particles in the donor chamber. Another measure to determine the pharmacological efficacy of the anti-VEGF intraceptor plasmid-encapsulated nanoparticles is the percentage of VEGF inhibition. We observed that VEGF inhibition was significantly higher in the cells treated with the transnasally delivered functionalized nanoparticles compared to both plasmid alone, as well as PLGA-NPs alone. VEGF inhibition with particles from receiver chamber exhibited the trend: transferrin-PLGA-NPs ≥ deslorelin-PLGA-NPs > PLGA-NPs ≫ plasmid. Similar to the GFP expression, based on the VEGF inhibition measured the nanoparticles were significantly more effective compared with lipofectamine complexes of Flt23k. Again, owing to higher concentrations in the donor chamber, as expected, the groups treated with the nanoparticles from donor chamber exhibited greater VEGF inhibition as compared with the receiver chamber samples. Furthermore, plasmid alone did not exhibit any significant VEGF inhibition in either receiver or donor chamber samples. Compared to the fold-enhancement in functionalized nanoparticle transport across ITP region when compared to nonfunctionalized nanoparticles, the differences were higher between functionalized and nonfunctionalized nanoparticles in terms of GFP expression, as well as VEGF inhibition. This might be due to a second level of advantage offered by functionalized nanoparticles after nasal transport, that is, enhanced uptake by target cancer cells. The fold-difference in VEGF expression between functionalized and nonfunctionalized nanoparticles is not as high as that observed for GFP expression. Both GFP and Flt3k plasmid were presented in the same plasmid; this might be due to the fact that the dose of the Flt3k plasmid presented by all three nanoparticles might be near its maximum response. With donor chamber samples, VEGF inhibition by various nanoparticles (Fig. 7D) could not be distinguished, and the inhibition was nearly complete, consistent with a high dose of delivery by all particles for maximal effects.
CONCLUSIONS
Functionalization of nanoparticles with ligands to cell-surface receptors were prepared (Fig. 8A, B) and shown to enhance the nasal delivery of nanoparticles, as well as the encapsulated macromolecular payload (Fig. 8C). Delivery to the back of the nose, targeting the inferior turbinate region, in particular, will provide significant delivery of macromolecules to the systemic circulation. Nanoparticles maintain the integrity of the macromolecules during the delivery process across the nasal tissues, as evidenced by their efficacy in cancer cells following nasal transport. Surface functionalization improves the uptake of these nanoparticles at the secondary, cancer target (Fig. 8D), resulting in much enhanced efficacy compared to functionalized nanoparticles. Although transferrin-functionalized particles enhanced nasal transport of particles and plasmid better than deslorelin-functionalized nanoparticles, in target prostate cancer cells, they exerted similar efficacy, which is much greater than nonfunctionalized nanoparticles. Thus, this study advances surface-functionalized nanoparticles as novel drug delivery systems for noninvasive transnasal delivery of macromolecules to target cancer cells.
Figure 8.
Functionalized nanoparticles for enhanced nasal and transnasal gene delivery. A, B) Approaches for functionalization of PS-NPs (A) and PLGA-NPs (B) via conjugation of deslorelin and transferrin on particle surfaces. C) Potential pathways for nasal epithelial cell uptake and transport of functionalized nanoparticles. D) Mechanisms of action of the Flt23k plasmid-loaded nanoparticles in inhibiting VEGF secretion.
Acknowledgments
This work was supported in part by U.S. National Institutes of Health grants DK064172 and EY013842. S.S. was supported by a Unviersity of Nebraska Medical Center (UNMC) Bukey Fellowship and a Predoctoral Fellowship from the American Heart Association. We thank Prof. Ming-Fong Lin (Department of Biochemistry and Molecular Biology, UNMC) for kindly providing us with the prostate cancer cell lines used in this study. We thank Janice Taylor of the Confocal Laser Scanning Microscopy Core Facility at UNMC, which is supported by the Nebraska Research Initiative, for providing assistance with confocal microscopy. We thank Tom Bargar of the Electron Microscopy Facility for his assistance in obtaining nanoparticle images. We thank the Flow Cytometry Core Facility for assistance in FACS analysis for nanoparticle uptake.
References
- Relf M, LeJeune S, Scott P A, Fox S, Smith K, Leek R, Moghaddam A, Whitehouse R, Bicknell R, Harris A L. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–969. [PubMed] [Google Scholar]
- Brooking J, Davis S S, Illum L. Transport of nanoparticles across the rat nasal mucosa. J Drug Target. 2001;9:267–279. doi: 10.3109/10611860108997935. [DOI] [PubMed] [Google Scholar]
- Millar R P. GnRHs and GnRH receptors. Anim Reprod Sci. 2005;88:5–28. doi: 10.1016/j.anireprosci.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Sundaram S, Bontha S, Kompella U B. Respiratory delivery of deslorelin, a peptide drug. Am Pharm Rev. 2004;7:130–139. [Google Scholar]
- Hazum E, Cuatrecasas P, Marian J, Conn P M. Receptor-mediated internalization of fluorescent gonadotropin-releasing hormone by pituitary gonadotropes. Proc Natl Acad Sci U S A. 1980;77:6692–6695. doi: 10.1073/pnas.77.11.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H, Heinrich N, Albrecht E, Kertscher U, Oehlke J, Bienert M, Schafer H, Baeger I, Mehlis B. Gonadotropin-releasing hormone (GnRH) analogs: relationship between their structure, proteolytic inactivation and pharmacokinetics in rats. Regul Pept. 1991;33:299–311. doi: 10.1016/0167-0115(91)90232-6. [DOI] [PubMed] [Google Scholar]
- Sundaram S, Roy S K, Kompella U B. Differential expression of LHRH-receptor in bovine nasal tissue and its role in deslorelin delivery. Peptides. 2009;30:351–358. doi: 10.1016/j.peptides.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Koushik K N, Kompella U B. Transport of deslorelin, an LHRH agonist, is vectorial and exhibits regional variation in excised bovine nasal tissue. J Pharm Pharmacol. 2004;56:861–868. doi: 10.1211/0022357023646. [DOI] [PubMed] [Google Scholar]
- Li H, Qian Z M. Transferrin/transferrin receptor-mediated drug delivery. Med Res Rev. 2002;22:225–250. doi: 10.1002/med.10008. [DOI] [PubMed] [Google Scholar]
- Li H, Sun H, Qian Z M. The role of the transferrin-transferrin-receptor system in drug delivery and targeting. Trends Pharmacol Sci. 2002;23:206–209. doi: 10.1016/s0165-6147(02)01989-2. [DOI] [PubMed] [Google Scholar]
- Qian Z M, Li H, Sun H, Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev. 2002;54:561–587. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- Widera A, Kim K J, Crandall E D, Shen W C. Transcytosis of GCSF-transferrin across rat alveolar epithelial cell monolayers. Pharm Res. 2003;20:1231–1238. doi: 10.1023/a:1025005232421. [DOI] [PubMed] [Google Scholar]
- Widera A, Norouziyan F, Shen W C. Mechanisms of TfR-mediated transcytosis and sorting in epithelial cells and applications toward drug delivery. Adv Drug Deliv Rev. 2003;55:1439–1466. doi: 10.1016/j.addr.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Singh N, Amin S, Richter E, Rashid S, Scoglietti V, Jani P D, Wang J, Kaur R, Ambati J, Dong Z, Ambati B K. Flt-1 intraceptors inhibit hypoxia-induced VEGF expression in vitro and corneal neovascularization in vivo. Invest Ophthalmol Vis Sci. 2005;46:1647–1652. doi: 10.1167/iovs.04-1172. [DOI] [PubMed] [Google Scholar]
- Yla-Herttuala S. Vascular gene transfer. Curr Opin Lipidol. 1997;8:72–76. doi: 10.1097/00041433-199704000-00004. [DOI] [PubMed] [Google Scholar]
- Straub B, Muller M, Krause H, Schrader M, Goessl C, Heicappell R, Miller K. Increased incidence of luteinizing hormone-releasing hormone receptor gene messenger RNA expression in hormone-refractory human prostate cancers. Clin Cancer Res. 2001;7:2340–2343. [PubMed] [Google Scholar]
- Halmos G, Arencibia J M, Schally A V, Davis R, Bostwick D G. High incidence of receptors for luteinizing hormone-releasing hormone (LHRH) and LHRH receptor gene expression in human prostate cancers. J Urol. 2000;163:623–629. [PubMed] [Google Scholar]
- Bandi N, Kompella U B. Budesonide reduces multidrug resistance-associated protein 1 expression in an airway epithelial cell line (Calu-1) Eur J Pharmacol. 2002;437:9–17. doi: 10.1016/s0014-2999(02)01267-0. [DOI] [PubMed] [Google Scholar]
- Schmidt M C, Simmen D, Hilbe M, Boderke P, Ditzinger G, Sandow J, Lang S, Rubas W, Merkle H P. Validation of excised bovine nasal mucosa as in vitro model to study drug transport and metabolic pathways in nasal epithelium. J Pharm Sci. 2000;89:396–407. doi: 10.1002/(SICI)1520-6017(200003)89:3<396::AID-JPS10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Molecular Probes/Invitrogen Detection Technologies Carlsbad, CA, USA: Molecular Probes; Working with FluoSpheres fluorescent microspheres. 2004:1–5. [Google Scholar]
- Koushik K, Bandi N, Sundaram S, Kompella U B. Evidence for LHRH-receptor expression in human airway epithelial (Calu-3) cells and its role in the transport of an LHRH agonist. Pharm Res. 2004;21:1034–1046. doi: 10.1023/b:pham.0000029294.70707.74. [DOI] [PubMed] [Google Scholar]
- Wirsig-Wiechmann C R. Function of gonadotropin-releasing hormone in olfaction. Keio J Med. 2001;50:81–85. doi: 10.2302/kjm.50.81. [DOI] [PubMed] [Google Scholar]
- Illum L. Nasal drug delivery–possibilities, problems and solutions. J Control Release. 2003;87:187–198. doi: 10.1016/s0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- Shindelman J E, Ortmeyer A E, Sussman H H. Demonstration of the transferrin receptor in human breast cancer tissue. Potential marker for identifying dividing cells. Int J Cancer. 1981;27:329–334. doi: 10.1002/ijc.2910270311. [DOI] [PubMed] [Google Scholar]
- Prost A C, Menegaux F, Langlois P, Vidal J M, Koulibaly M, Jost J L, Duron J J, Chigot J P, Vayre P, Aurengo A, Legrand J C, Rosselin G, Gespach C. Differential transferrin receptor density in human colorectal cancer: A potential probe for diagnosis and therapy. Int J Oncol. 1998;13:871–875. doi: 10.3892/ijo.13.4.871. [DOI] [PubMed] [Google Scholar]
- Keer H N, Kozlowski J M, Tsai Y C, Lee C, McEwan R N, Grayhack J T. Elevated transferrin receptor content in human prostate cancer cell lines assessed in vitro and in vivo. J Urol. 1990;143:381–385. doi: 10.1016/s0022-5347(17)39970-6. [DOI] [PubMed] [Google Scholar]
- Grundker C, Gunthert A R, Westphalen S, Emons G. Biology of the gonadotropin-releasing hormone system in gynecological cancers. Eur J Endocrinol. 2002;146:1–14. doi: 10.1530/eje.0.1460001. [DOI] [PubMed] [Google Scholar]
- Kang S W, Lim H W, Seo S W, Jeon O, Lee M, Kim B S. Nanosphere-mediated delivery of vascular endothelial growth factor gene for therapeutic angiogenesis in mouse ischemic limbs. Biomaterials. 2008;29:1109–1117. doi: 10.1016/j.biomaterials.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Mo Y, Barnett M E, Takemoto D, Davidson H, Kompella U B. Human serum albumin nanoparticles for efficient delivery of Cu, Zn superoxide dismutase gene. Mol Vis. 2007;13:746–757. [PMC free article] [PubMed] [Google Scholar]
- Singh S R, Grossniklaus H E, Kang S J, Edelhauser H F, Ambati B K, Kompella U B. Intravenous transferrin, RGD peptide and dual-targeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther. 2009;16:645–659. doi: 10.1038/gt.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel A, Groth A, Schlesinger S, Bruns H, Schemmer P, Buchler M W, Herr I. Suitability of human mesenchymal stem cells for gene therapy depends on the expansion medium. Exp Cell Res. 2009;315:498–507. doi: 10.1016/j.yexcr.2008.11.013. [DOI] [PubMed] [Google Scholar]