Abstract
ALK-1 is a transforming growth factor β (TGF-β) superfamily receptor that is predominantly expressed in endothelial cells and is essential for angiogenesis, as demonstrated by the embryonic lethal phentoype when targeted for deletion in mice and its mutation in the human disease hereditary hemorrhagic telangiectasia. Although ALK-1 and the endothelial-specific TGF-β superfamily coreceptor, endoglin, form a heteromeric complex and bind similar TGF-β superfamily ligands, their signaling mechanisms remain poorly characterized. Here we report the identification of CK2β, the regulatory subunit of protein kinase CK2, as a novel enhancer of ALK-1 signaling. The cytoplasmic domain of ALK-1 specifically binds to CK2β in vitro and in vivo. NAAIRS mutagenesis studies define amino acid sequences 181–199 of CK2β and 207–212 of ALK-1 as the interaction domains, respectively. The ALK-1/CK2β interaction specifically enhanced Smad1/5/8 phosphorylation and ALK-1-mediated reporter activation in response to TGF-β1 and BMP-9 treatment. In a reciprocal manner, siRNA-mediated silencing of endogenous CK2β inhibited TGF-β1 and BMP-9-stimulated Smad1/5/8 phosphorylation and ALK-1-mediated reporter activation. Functionally, CK2β enhanced the ability of activated or ligand-stimulated ALK-1 to inhibit endothelial cell migration. Similarly, ALK-1 and CK2β antagonized endothelial tubule formation in Matrigel. These studies support CK2β as an important regulator of ALK-1 signaling and ALK-1-mediated functions in endothelial cells.—Lee, N. Y., Haney, J. C., Sogani, J., Blobe, G. C. Casein kinase 2β as a novel enhancer of activin-like receptor-1 signaling.
Keywords: TGF-β superfamily signaling, endothelial cell biology, angiogenesis, bone morphogenetic protein, migration
The transforming growth factor β (TGF-β) superfamily is a structurally related but functionally diverse family of dimeric polypeptide growth factors, including TGF-β and bone morphogenetic proteins (BMPs), that regulate cellular proliferation, differentiation, and cell survival as well as the processes of embryonic development, wound healing, and angiogenesis in a cell- and context-specific manner (1,2,3). In most tissues, TGF-β superfamily ligands signal through a family of related type I and type II TGF-β superfamily receptors, as well as through the ubiquitously expressed coreceptor, the type III TGF-β receptor (or betaglycan) (1, 3). In endothelial cells, TGF-β superfamily ligands also signal through 2 TGF-β superfamily receptors preferentially expressed in cells derived from the hemangioblast (i.e., endothelial and hematopoietic cells); the type I TGF-β superfamily receptor, activin-like kinase-1 (ALK-1); and the TGF-β superfamily coreceptor, endoglin. Both ALK-1 and endoglin, as well as the type I (TβRI/ALK-5) and the II TGF-β receptor (TβRII), have essential roles in endothelial cell biology and angiogenesis, as demonstrated by germline mutations in endoglin and ALK-1, resulting in the human vascular diseases hereditary hemorrhagic telangiectasia type I (HHT1) (4) and HHT2 (5), respectively. In addition, ALK-1 (6, 7), endoglin (8,9,10), ALK-5 (11), and TβRII (12) -null mice all exhibit an embryonic lethal phenotype due to vascular defects.
TGF-β superfamily receptors signal by phosphorylating Smad transcription factors. Upon phosphorylation, the receptor-activated Smads (1, 2, 3, 5, and 8) form a complex with the co-Smad, Smad 4, translocate to the nucleus, and regulate the expression of specific target genes (13,14,15,16,17). Although TGF-β ligands have traditionally been thought to signal through Smads2/3, whereas BMPs signal through Smads1/5/8, recent studies support a role for TGF-β ligands signaling through Smads1/5/8 as well (18, 19). In endothelial cells, TGF-β ligands are able to stimulate both Smad pathways, with Smad 1/5/8 phosphorylation downstream of ALK-1 and Smad 2/3 phosphorylation downstream of ALK-5 (17, 20). In addition to binding and signaling downstream of TGF-β ligands, both ALK-1 and endoglin have been demonstrated to bind and respond to BMP-9/10 (21, 22), which suggests that these might be physiologically relevant ligands for these endothelial-specific receptors as well. Both BMP-9-stimulated ALK-1 and constitutively active ALK-1 have been reported to inhibit endothelial cell proliferation, migration, and angiogenesis (21, 22). In contrast, TGF-β-stimulated ALK-1 activation has been reported to stimulate endothelial cell proliferation and migration, whereas TGF-β-stimulated ALK-5 activation has been reported to inhibit endothelial cell proliferation and migration (20, 23). Crosstalk between these pathways has also been reported, with the requirement of ALK-5 for TGF-β-stimulated ALK-1 signaling, and TGF-β-stimulated ALK-1 signaling inhibiting ALK-5 signaling (23). However, investigation of the role of ALK-5 and ALK-1 in murine and zebrafish models employing endothelial-specific deletion of these receptors has yielded conflicting results. Studies employing Tie1 promoter-driven Cre expression yielded vascular phenotypes with endothelial-specific deletion of ALK-5 (24). In contrast, studies employing ALK-1 promoter-driven Cre expression yielded no phenotype with endothelial specific deletion of ALK-5 or TβRII gene, whereas deletion of ALK-1 resulted in vascular malformations similar to those found in HHT patients (25).
Protein kinase CK2 (casein kinase II) is a highly conserved, ubiquitously expressed serine/threonine kinase that regulates a number of cellular processes, including the cell cycle, DNA repair, and cell viability (26). CK2 is a tetramer of 2 catalytic alpha subunits (CK2α) and 2 regulatory beta subunits (CK2β). In normal cells, the level of CK2 appears to be tightly controlled, with CK2β serving to regulate the activity and substrate availability for CK2α (26). In addition, CK2β has also been demonstrated to have CK2α-independent binding partners, interacting with and modulating the function of other serine/threonine kinases, including A-Raf (27), Chk1 (28), Chk2 (29), c-Mos (30, 31), PKC-ζ (32), and p90rsk (33). Ck2β interaction may augment kinase function (27) or may inhibit its downstream signaling (30). Here we report the identification of CK2β as a novel ALK-1-interacting protein and investigate the functional consequences of this interaction.
MATERIALS AND METHODS
Cell culture and reagents
Cell lines were obtained from American Type Culture Collection (Manassas, VA, USA) and maintained in DMEM with 10% FBS. TGF-β1 and BMP-9 were obtained from R&D Systems (Minneapolis, MN, USA). COS-7 cells were maintained in DMEM medium with 10% FCS. Human microvascular endothelial (HMEC-1) cells were grown in MCDB-131 medium (Invitrogen, Carlsbad, CA, USA), supplemented with 10% FBS, 1 μg/ml hydrocortisone (Sigma, St Louis, MO, USA), 10 ng/mL EGF (Sigma), and 2mM l-glutamine (Invitrogen). P19 cells were maintained in α-MEM supplemented with 7.5% FCS and 2.5% FBS. Phospho-Smad1/5/8 and Smad1/5/8 antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). CK2β antibodies 6D5 and FL-215 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Caspase-9 antibody was purchased from Assay Designs (Ann Arbor, MI, USA).
Yeast 2-hybrid screen
The yeast 2-hybrid screen was performed with a GAL4-based 2-hybrid system. Briefly, the cytoplasmic domain of ALK-1 was cloned into pDBLeu (Invitrogen) and transformed into AH109 cells and streaked on trp-/his-/3 mM 3-AT to test for self-activation, and grown overnight in SD-Leu medium to test for toxicity. Yeast expressing pDBLeu-bait were then transformed with an endothelial cell library DNA. Transformants were plated on 30 trp-/leu-/his-/3mM 3-AT plates and incubated at 30°C for ∼7–10 d. Primary isolates were restreaked on trp-/leu-/his-/3 mM 3AT plates and grown several days for lacZ assays, which were carried out by a colony lift procedure. LacZ-positive colonies were picked for isolation of DNA and retested by transformation into yeast with the original bait plasmid or with empty vector followed by selection for growth on trp-/leu- plates, followed by trp-/leu-/his-/3 mM 3-AT plates to test for growth.
DNA constructs and protein expression
N-terminally epitope-tagged Alk-1 and CK2β constructs were created by PCR cloning into pCMV-Tag mammalian expression vectors containing inframe HA and FLAG epitopes, respectively (Stratagene, La Jolla, CA, USA). CK2β truncation and Alk-1 deletion mutants were similarly PCR cloned and reinserted into the expression vectors. NAAIRS substitution mutants were created using the QuikChange II site-directed mutagenesis kit (Stratagene) and appropriately designed PCR primers. All constructs were confirmed by direct sequencing. Cos-7 and P-19 cells were transiently transfected with cDNA constructs using Lipofectamine 2000 (Invitrogen). HMEC-1 cells were nucleofected with cDNA constructs using the Amaxa nucleofection system and published protocol.
Coimmunoprecipitation
COS-7 cells expressing FLAG-tagged CK2β and HA-tagged ALK-1 or HMEC-1 cells expressing HA-tagged ALK-1 were lysed on ice with lysis buffer (20 mM HEPES, pH 7.4; 150 mM NaCl; 0.5% Nonidet P-40; 2 mM EDTA; 10 mM NaF; and 10% w/v glycerol) supplemented with protease and phosphatase inhibitors (protease and phosphatase inhibitor cocktails; Sigma). The lysates were precleared by centrifugation and incubated with specified antibodies for 12 h at 4°C. The immunoprecipitates were then collected by centrifugation; the pellets were washed with lysis buffer and stored in 2× sample buffer before Western blot analyses.
Glutathione S-transferase (GST) affinity-binding assay
COS-7 cells expressing FLAG-tagged CK2β, HA-tagged ALK-1, or NAAIRS mutants of FLAG-tagged CK2β and HA-tagged ALK-1 were lysed with lysis buffer and precleared with glutathione agarose beads and equal amounts of cell lysate incubated with GST fusion proteins of the cytoplasmic domain of ALK-1 (GST-ALK-1), CK2β (GST- CK2β), or GST alone, and the beads were harvested by centrifugation and washed with lysis buffer. Binding proteins were analyzed by SDS-PAGE and Western blot analysis with HA or FLAG antibodies.
Orthophosphate labeling
HEK-293 cells were transfected with FLAG-CK2β and with ALK-1 kinase constructs (ALK-1-WT, ALK-1-QD, or ALK-1-KR). Twenty-four hours after transfection, the cells were washed and incubated in phosphate-free DMEM with 100 μCi of [32P]orthophosphate for 1 h and lysed with Nonidet P-40 lysis buffer (20 mM HEPES, ph 7.4; 150 mM NaCl; 0.5% Nonidet P-40; 2 mM EDTA; 10 mM NaF; and 10% (w/v) glycerol supplemented with protease and phosphatase inhibitors). FLAG-CK2β was then immunoprecipitated with anti-FLAG antibody and protein G-sepharose and resolved on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and 32P incorporation was visualized with PhosphoImager analysis.
In vitro kinase assay
HEK-293 cells were transfected with HA-ALK-1-QD or HA-CK2α. Twenty-four hours after transfection, the cells were lysed with Nonidet P-40 lysis buffer, and the kinases were immunoprecipitated with anti-HA antibody. Kinases were then incubated with substrates GST-Smad1 or GST-CK2β in buffer containing [γ32P]ATP (1 μCi; 3000 Ci/mmol). [γ32P]ATP incorporation was then assessed by PhosphoImager analysis.
Luciferase reporter assay
Dual-reporter luciferase assays were performed as described by manufacturer (Promega, Madison, WI, USA). The BMP-responsive vector used as reporter for ALK-1 signaling was XVent2, with Renilla luciferase gene as an internal control. P19 cells were transiently transfected with ALK-1QD, CK2β, or siRNA to CK2β, along with the XVent2 and Renilla reporter constructs, and luciferase assays were performed 24 h post-transfection. In luciferase studies conducted with HMEC-1 cells, wild-type ALK-1, CK2β, and CK2β siRNA were nucleofected with Amaxa Solution R (Amaxa Inc., Gaithersburg, MD, USA). At 24 h postnucleofection, cells were serum deprived (0.5% FBS) in the presence or absence of BMP-9 (5 ng/mL) for an additional 24 h before the luciferase assays. Data are from experiments done in triplicate and are presented as mean ± se fold stimulations of the luciferase induced by control vector or ALK-1 Q-D.
Smad1/5/8 phosphorylation
P19 and HMEC-1 cells were transfected with ALK-1 and either CK2β or siRNA to CK2β and stimulated with either 100 pM of TGF-β1 or 10 ng/mL of BMP-9 for the indicated time intervals. Western blotting was performed using Smad1/5 and phospho-specific Smad1/5/8 antibodies (Cell Signaling Technology). Western blotting for ALK-1 expression was performed using HA-antibody, and CK2β expression was detected with either FLAG-antibody or CK2β-specific antibodies (6D5 or FL-215; Santa Cruz Biotechnology).
Transwell migration assay
Transwells (Costar Corning, Corning, NY, USA; 8-μm polycarbonate membrane, 6.5-mm insert, 24-well plate) were coated with fibronectin before plating. Then 1 × 106 HMEC-1 cells were nucleofected with ALK-1QD, CK2β, or both. Approximately 30,000 cells were plated onto the top of each well of the 24-well membrane and allowed to migrate for 24 h before cell fixation and staining of the nuclei of the migrated cells on the bottom side of the membrane. The transwell membranes containing the stained cells were digitally imaged, then counted using Image J software (U.S. National Institutes of Health, Bethesda, MD, USA) to tabulate cells as they were manually identified.
Endothelial tubule formation
HMEC-1 cells nucleofected with control vector, wild-type ALK-1, and/or CK2β were grown on 0.05% gelatin for 24 h. Subsequently, 1 × 105 cells expressing the appropriate constructs were plated on 24-well plates coated with matrigel matrix (BD Bioscience, San Jose, CA, USA). Sixteen hours after plating, tubules formed were digitally imaged and counted for number of tubes formed.
Immunofluorescence
HMEC-1 cells were serum starved for 2–4 h, washed with PBS, fixed with 4% paraformaldehyde, permeabilized in 0.1% Triton X-100/PBS for 5 min, and then blocked with 5% bovine serum albumin in PBS containing 0.05% Triton X-100 for 1 h. ALK-1 expression was detected using HA-antibody for 1 h at room temperature, whereas endogenous CK2β distribution was detected using CK2β-specific antibody (FL-215; Santa Cruz Biotechnology). Cells were mounted with Prolong Antifade (Sigma) before immunofluorescence microscopy analyses.
RESULTS
CK2β specifically interacts with ALK-1
As ALK-1 has an essential role in endothelial cell biology, we sought to define novel ALK-1-interacting proteins using the cytoplasmic domain of ALK-1 as bait to screen an endothelial cell library in a GAL4-based 2-hybrid system. This screen identified 10 nearly full-length clones of FKBP12, which has been previously identified as an ALK-1-interacting protein (34), verifying the validity of the screen. In addition to FKBP12, the screen yielded 3 full-length clones of the regulatory subunit of CK2, CK2β. As CK2β has previously identified roles in binding to and regulating the function of other serine/threonine kinases, including CK2α, A-Raf (27), Chk1 (28), Chk2 (29), Mos (30, 31), PKZ-ζ (32), and p90rsk (33), we further investigated the interaction of ALK-1 and CK2β. Association of ALK-1 with CK2β was specific because CK2β was not isolated in screens with ALK-2 or endoglin (data not shown). In addition, the cytoplasmic domain of ALK-1 could interact with CK2β in the yeast 2-hybrid interaction assay, while the cytoplasmic domain of endoglin, and 2 random cytoplasmic proteins, Rin and NCAP, could not (data not shown).
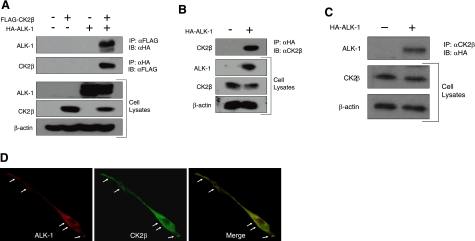
To determine whether ALK-1 interacts with CK2β in a physiological context, we initially coexpressed FLAG-tagged CK2β with HA-tagged ALK-1 in COS-7 cells and performed coimmunoprecipitation studies. As suggested by the yeast 2-hybrid screen, immunoprecipitation of CK2β was able to specifically coprecipitate ALK-1 (Fig. 1A, top panel). In a reciprocal manner, immunoprecipitation of ALK-1 specifically coprecipitated CK2β (Fig. 1A, second panel). Because ALK-1 is preferentially expressed in endothelial cells, we tested the ability of ALK-1 and CK2β to interact in HMEC-1 cells. Although we readily detected endogenous CK2β (Fig. 1B), we were unable to detect endogenous ALK-1 by Western blot (data not shown). Accordingly, we expressed HA-tagged ALK-1 in HMEC-1 cells and assessed for its interaction with endogenous CK2β. The immunoprecipitation of ectopically expressed ALK-1 specifically coprecipitated endogenous CK2β (Fig. 1B). In a reciprocal manner, immunoprecipitation of endogenous CK2β specifically coprecipitated ALK-1 (Fig. 1C). As we were unable to detect endogenous ALK-1 by immunofluorescence microscopy to determine whether the two proteins colocalize in intact cells, we ectopically expressed ALK-1 in HMEC-1 cells and probed for ALK-1 and endogenous CK2β. Consistent with the coimmunoprecipitation data, endogenous CK2β and ectopically expressed ALK-1 colocalized along the cell periphery in HMEC-1 cells (Fig. 1D, arrows).
Figure 1.
ALK-1 specifically interacts with CK2β. A) COS-7 cells were transfected with FLAG-CK2β and HA-ALK-1. After 48 h, cells were lysed in lysis buffer, immunoprecipitated with HA or FLAG antibodies as indicated, resolved by SDS-PAGE, and immunoblotted with HA or FLAG antibodies as indicated. B, C) HMEC-1 cells were nucleofected with HA-ALK-1. After 48 h, cells were lysed, immunoprecipitated with either HA antibody or CK2β-specific antibody, and the immunoprecipitates analyzed on SDS-PAGE followed by immunoblot analysis with HA or CK2β antibody. Portions of the lysates were analyzed by immunoblot for the indicated proteins for expression, and β-actin was assessed as loading control. D) HMEC-1 cells expressing HA-ALK-1 were fixed and probed with HA antibody (left panel) and CK2β-specific antibody (middle panel). Arrows along the cell periphery indicate their colocalization (merged image, right panel). Data are representative of 3 independent experiments.
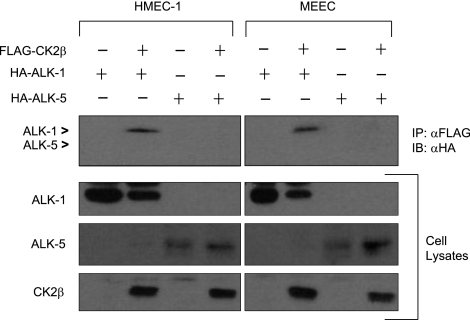
Given the potential crosstalk between ALK-5 and ALK-1 signaling in endothelial cells and to examine the specificity and generality of the ALK-1/CK2β interaction, we explored whether CK2β interacted with ALK-5 or ALK-1 in HMEC-1 cells and mouse embryonic endothelial cells (MEECs). While CK2β was able to coimmunoprecipitate ALK-1 in HMEC-1 cells and MEECs, CK2β could not coimmunoprecipitate ALK-5 in either endothelial cell system (Fig. 2). Taken together, these findings suggest a specific interaction between ALK-1 and CK2β in endothelial cells.
Figure 2.
CK2β preferentially associates with ALK-1. HMEC-1 cells and MEECs were nucleofected with HA-ALK-1, HA-ALK-5, and FLAG-CK2β, as indicated. Lysates were prepared and immunoprecipitated with FLAG antibody and immunoblotted for HA. Coimmunoprecipitations of ALK-1 and ALK-5 are shown in the top panel, indicated by arrowheads. Portions of the lysates were analyzed by immunoblot for the indicated proteins for expression.
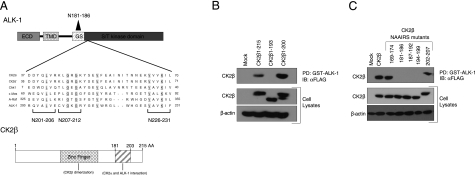
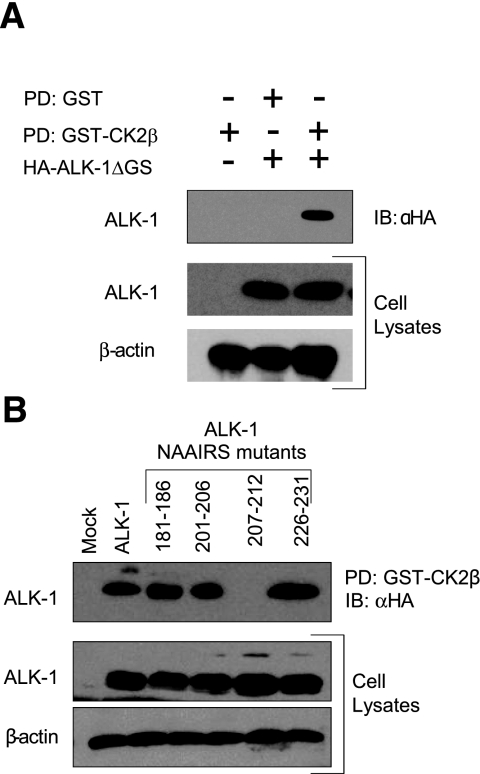
To define the regions mediating the interaction between ALK-1 and CK2β, we examined the previously reported interactions between CK2β and other serine/threonine kinases, including CK2α, A-Raf (27), Chk1 (28), Chk2 (29), Mos (30, 31), PKZ-ζ (32), and p90rsk (33). In cases where the site mediating interaction of CK2β with these kinases was mapped, the CK2β interaction domain was determined to be the C-terminal region of CK2β (aa 178-205; Fig. 3A, bottom) and in the serine/threonine kinase domain of the kinases (35). Indeed, alignment of the ALK-1 sequence with several other CK2β binding partners revealed a region of homology in ALK-1 from aa 201 to 231, which suggests this as a potential interaction domain (Fig. 3A, top). To establish whether these regions were responsible for mediating the interaction of CK2β with ALK-1, we initially created truncation mutants of CK2β and assessed their ability to interact with a GST-fusion protein of the cytoplasmic domain of ALK-1 (GST-ALK-1). While full-length CK2β (CK2β 1-215) and a truncation mutant of CK2β lacking the last 15 aa (CK2β 1-200) were pulled down by GST-ALK-1 (Fig. 3B), GST-ALK-1 was unable to pull down a truncation mutant of CK2β lacking the last 23 aa (CK2β 1-193, Fig. 3B). Although these data suggest that the region between aa 193 and 200 might be necessary for the interaction (Fig. 3B), the truncation approach may alter the structural properties of the expressed protein. To overcome these difficulties we employed a NAAIRS substitution mutagenesis strategy, where 6 aa from the C-terminal region of CK2β were sequentially replaced with the sequence NAAIRS. This sequence has the unique ability to adopt multiple structural conformations including both α-helical and β-sheet secondary structures (36) and should thus have minimal effect on the overall conformation of the protein. Although all the NAAIRS mutants expressed, the NAAIRS substitution mutants between aa 181 and 199 were not pulled down by GST-ALK-1, whereas NAAIRS substitution mutants on either side of this region were precipitated (Fig. 3C). These studies establish aa 181-199 on CK2β as the ALK-1-interacting domain. To define the CK2β-interacting domain on ALK-1, we initially created truncation mutants of ALK-1 and assessed their ability to interact with a GST-fusion protein of CK2β (GST-CK2β). While full-length ALK-1 and a truncation mutant of ALK-1 lacking the glycine/serine (GS) domain were pulled down by GST-CK2β (Fig. 4A), GST-CK2β was unable to pull down a truncation mutant of ALK-1 lacking the kinase domain (data not shown). These data support the kinase domain of ALK-1 as the CK2β interaction domain, as suggested by the sequence alignment of other CK2β-interacting serine/threonine kinases (Fig. 3A, top). Based on the ability of ALK-1ΔGS to interact (Fig. 4A) and the alignment data, we constructed ALK-1 NAAIRS mutants in the GS domain and in the region of ALK-1 homology with other CK2β-interacting proteins. All of these NAAIRS mutants expressed (Fig. 4B), but the NAAIRS substitution mutant between aa 207 and 212 was specifically not pulled down by GST-CK2β (Fig. 4B). This region contains 2 glycine residues conserved in all known CK2β-interacting serine/threonine kinases (Fig. 3A), supporting aa 207-212 on ALK-1 as the CK2β-interacting domain.
Figure 3.
ALK-1 interacts with the carboxy-terminal domain of CK2β. A) Top panel: structure of full-length ALK-1, including the extracellular domain (ECD), transmembrane domain (TMD), glycine/serine-rich (GS) domain, and S/T kinase domains. Middle panel: region of homology with other CK2β-interacting proteins. Conserved amino acids are underscored; position of NAAIRS mutants is indicated. Bottom panel: structure of full-length CK2β. B) A GST fusion protein of the cytoplasmic domain of ALK-1 was incubated with equal amounts of lysate from COS-7 cells expressing FLAG-tagged CK2β, FLAG-tagged CK2β 1–193, or FLAG-tagged CK2β 1–200, as indicated. GST-fusion proteins were pulled down by glutathione beads, and proteins pulled down were analyzed on SDS-PAGE followed by immunoblot analysis with FLAG antibody. C) A GST fusion protein of the cytoplasmic domain of ALK-1 was incubated with equal amounts of lysate from COS-7 cells expressing FLAG-tagged CK2β or the indicated NAAIRS mutants of FLAG-tagged CK2β. GST-fusion proteins were pulled down by glutathione beads, and proteins pulled down were analyzed on SDS-PAGE, followed by immunoblot analysis with FLAG antibody. Portions of the lysates were analyzed by immunoblot for the indicated proteins for expression, and β-actin was assessed as a loading control. Data are representative of 3 independent experiments.
Figure 4.
CK2β interacts with a conserved motif within the kinase domain of ALK-1. A) Equal amounts of lysate from COS-7 cells expressing HA-tagged ALK-1 with the GS domain deleted were incubated with GST alone or with GST fusion protein of CK2β. GST-fusion proteins were pulled down by glutathione beads, and proteins pulled down were analyzed on SDS-PAGE, followed by immunoblot analysis with αHA antibody. B) A GST fusion protein of CK2β was incubated with equal amounts of lysate from COS-7 cells expressing HA-tagged ALK-1 or the indicated NAAIRS mutants of HA-tagged ALK-1 as indicated. GST-fusion proteins were pulled down by glutathione beads, and proteins pulled down were analyzed on SDS-PAGE, followed by immunoblot analysis with αHA antibody. Portions of the lysates were analyzed by immunoblot for the indicated proteins for expression, and β-actin was assessed as a loading control. Data are representative of 3 independent experiments.
CK2β enhances ALK-1 signaling
CK2β specifically interacts with ALK-1, and CK2β has been reported to be phosphorylated by other interacting serine/threonine kinases, including CK2α (37) and Chk1 (38). In addition, the interaction of CK2β with ALK-1 has the potential to result in the phosphorylation of ALK-1 by other CK2β-associated serine/threonine kinases. Accordingly, we investigated whether ligand (TGF-β1 or BMP-9) stimulated or constitutively active ALK-1 phosphorylated CK2β, or whether CK2β altered ALK-1 phosphorylation by expressing both proteins in orthophosphate-labeled HEK-293 cells. Although CK2β could be detected as a phosphoprotein, constitutively active ALK-1, ALK-1-QD, did not increase CK2β phosphorylation relative to kinase dead ALK-1 (ALK-1-KR) or wild-type ALK-1 (Supplemental Fig. 1A). In addition, although ALK-1-QD could efficiently phosphorylate a GST fusion protein of Smad1, and CK2α could phosphorylate a GST fusion protein of CK2β (GST- CK2β) in vitro (Supplemental Fig. 1B), ALK-1-QD could not phosphorylate GST-CK2β (Supplemental Fig. 1B). In a similar manner, when coexpressed in orthophosphate-labeled HEK-293 cells, CK2β did not alter the level of ALK-1 phosphorylation (data not shown). Thus, although CK2β and ALK-1 interact, they do not appear to alter their respective phosphorylation status.
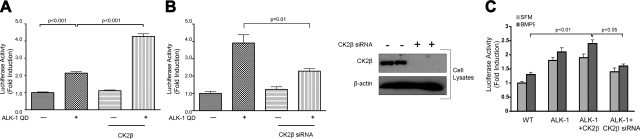
CK2β has been demonstrated to alter the kinase activity of other interacting serine/threonine kinases, including inhibiting Mos kinase activity (31) and enhancing Chk1 (28) and A-raf kinase activity (39), whereas ALK-1 exerts its biological effects via phosphorylating the Smad transcription factors to activate TGF-β superfamily responsive genes, including XVent2 (40). Given the known functions of CK2β, we assessed the ability of CK2β to enhance ALK-1QD-mediated XVent2 promoter-driven luciferase activity in P19 embryonal carcinoma cells. The expression of the constitutively active kinase form of ALK-1 (ALK-1QD) yielded an ∼2-fold induction (Fig. 5A, lanes 1 vs. 2), whereas the expression of CK2β on its own had no effect (Fig. 5A, lane 3). In contrast, the coexpression of ALK-1QD and CK2β markedly enhanced the XVent2 luciferase activity (Fig. 5A, lane 4). In a reciprocal experiment, siRNA-mediated silencing of endogenous CK2β led to a substantial reduction in ALK-1QD-mediated induction of luciferase activity (Fig. 5B, lane 4). We also assessed the ability of CK2β to regulate ligand-stimulated ALK-1 signaling at the transcriptional level in endothelial cells (Fig. 5C). Ectopic expression of ALK-1 enhanced basal and BMP-9 stimulated transcriptional responses (Fig. 5C), whereas coexpression of CK2β resulted in a modest enhancement in ALK-1-mediated transcriptional responses (Fig. 5C). However, silencing of endogenous CK2β expression significantly decreased ALK-1-mediated, basal, and BMP-9 stimulated transcriptional responses (Fig. 5C), supporting CK2β as an enhancer of ALK-1 mediated signaling.
Figure 5.
CK2β specifically activates ALK-1 signaling. A, B) P19 cells were transfected with 100 ng of ALK-1QD, 100 ng of XVent2, and 100 ng of CK2β expression vector (A), or siRNA to CK2β (B) as indicated. Forty-eight hours after transfection, cells were lysed, and the lysates were assayed for luciferase activity. C) MEECs were nucleofected with 3GC2 luciferase construct along with control vector, wild-type ALK-1, and/or CK2β or CK2β siRNA, as indicated. Cells were treated with BMP-9 for 24 h before luciferase assays. In parallel, lysates were assessed for the ability of siRNA to CK2β to knock down CK2β expression, with β-actin as a loading control (B, right panel). Data are from 3 experiments done in triplicate and are presented as mean ± se fold-induction of the luciferase induced by constitutively active ALK-1 relative to mock transfected (vector-only) samples.
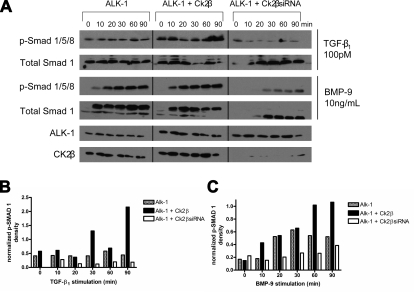
ALK-1 has been demonstrated to signal in response to TGF-β isoforms (41) and to BMP-9/10 (22, 42). To explore the direct effects of CK2β on ligand-stimulated ALK-1 function, we assessed the effects of increasing or silencing CK2β expression on TGF-β1- and BMP-9-stimulated Smad1/5/8 phosphorylation. Both TGF-β1 and BMP-9 stimulated Smad1/5 phosphorylation in a time-dependent manner (Fig. 6A, first and third panels). While a more robust effect on Smad1/5/8 phosphorylation was observed in response to BMP-9, increasing CK2β expression further enhanced both TGF-β1- and BMP-9-induced Smad1/5/8 phosphorylation, both at early and late time points (Fig. 6A, first and third panels). In contrast, silencing CK2β expression dramatically reduced TGF-β1- and BMP-9-stimulated Smad1/5/8 phosphorylation (Fig. 6A, first and third panels). In parallel experiments we examined whether CK2β influences Smad2 signaling by monitoring ligand-stimulated Smad2 phosphorylation in HMEC-1 cells. As BMP-9 did not stimulate Smad2 phosphorylation (data not shown), we focused on TGF-β1-stimulated Smad2 phosphorylation. In contrast to the results with Smad1, increasing or decreasing CK2β expression did not alter TGF-β1-stimulated Smad2 phosphorylation (Supplemental Fig. 2). Taken together, these results suggest an important role for CK2β in specifically augmenting ALK-1-mediated Smad1/5/8 signaling.
Figure 6.
CK2β enhances TGFβ1- and BMP-9 stimulated Smad1/5/8 phosphorylation. A) P19 cells were transfected with ALK-1, CK2β expression vector, or siRNA to CK2β, as indicated. Twenty-four hours after transfection, cells were serum starved for 12 h and then stimulated with the indicated amounts of TGFβ1 or BMP-9 for the indicated periods of times, the cells were lysed, and the lysates were assayed for pSmad1 and total Smad1 expression by immunoblot analysis. Portions of the lysates were analyzed by immunoblot for the indicated proteins to confirm expression and/or knockdown of CK2β expression. Data are representative of 3 independent experiments. B, C) Quantification of Smad-1 phosphorylation was performed with the assistance of the ImageJ analysis program. Integrated density measurements for each variable were taken, and background was subtracted. A ratio of phosphorylated/total Smad-1 was then calculated for every condition, and this was then normalized against the ALK-1 signal obtained for that condition.
CK2β cooperates with ALK-1 to inhibit endothelial cell migration and tubule formation
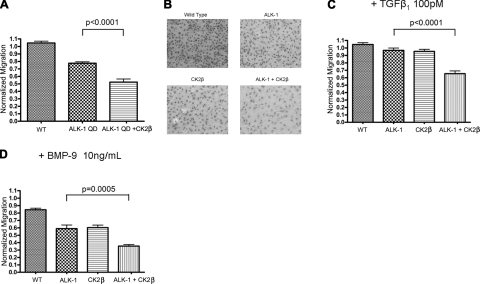
Both constitutively active ALK-1 and BMP-9 stimulated ALK-1 and have been reported to inhibit endothelial cell migration (21, 22). Accordingly, to investigate the effect of CK2β on ALK-1 function in endothelial cells, we first assessed the effects on endothelial cell migration. As previously reported, constitutively active ALK-1 inhibited the migration of HMEC-1 cells (Fig. 7A). Increasing CK2β expression significantly enhanced ALK-1 function in inhibiting endothelial cell migration (Fig. 7A). In addition, in the presence of TGF-β1 and more potently in the presence of BMP-9, both ALK-1 and CK2β were able to inhibit endothelial cell migration, and expression of ALK-1 and CK2β further inhibited endothelial cell migration (Fig. 7B–D), supporting a role for CK2β in enhancing ALK-1 signaling to inhibit endothelial cell migration.
Figure 7.
CK2β increases ALK-1-mediated and TGFβ1-and BMP-9-stimulated inhibition of endothelial cell migration. HMEC-1 cells were nucleofected with ALK-QD (A), ALK-1 (B–D), and CK2β (B–D), as indicated. Twenty-four hours later, cells were trypsinized and counted. Equal numbers of cells were plated on transwells coated with fibronectin and treated with the indicated amounts of TGFβ1 (B) or BMP-9 (C, D) and assessed for migration 12 h later. Cells on the underside of the transwell filter were fixed, stained for their nuclei, and counted (A, C, D). Numbers of migrated cells were counted using Image J software, normalized to HMEC-1 cells, and graphed. Panel B shows sample images of HMEC-1 cells that migrated under the indicated conditions in the presence of BMP-9. Data are means of 3 independent experiments; error bars = sd.
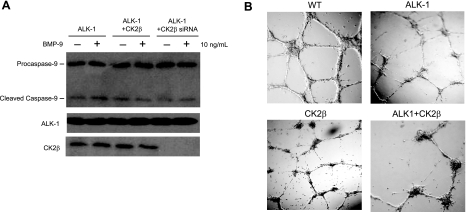
BMP-9 has been demonstrated to stimulate apoptosis in prostate cancer cells (43). To address whether the BMP-mediated inhibition of migration in HMEC-1 is due in part to apoptosis, we examined the apoptotic response to BMP-9 in the context of our ALK-1/CK2β interaction studies by assessing the level of caspase-9 cleavage in HMEC-1 cells. Although a basal level of caspase-9 cleavage was present irrespective of ALK-1 and/or CK2β expression (Fig. 8A), BMP-9 treatment did not have a significant effect on caspase-9 cleavage (Fig. 8A), which suggests that BMP-9 does not stimulate apoptosis in HMEC-1 cells.
Figure 8.
CK2β/ALK-1 interaction does not mediate BMP-9-induced apoptosis but regulates endothelial cell tubule formation. A) HMEC-1 cells expressing ALK-1, ALK-1 with CK2β, or siRNA to CK2β were serum starved for 2–4 h before treatment with BMP-9 for 2 h. Cells were harvested, resolved on SDS-PAGE, and immunoblotted with caspase-9 antibody to detect procaspase-9 and cleaved form, as indicated. B) HMEC-1 cells expressing ALK-1, CK2β, or ALK-1 and CK2β were plated on Matrigel matrix in triplicates. Sixteen hours later, individual endothelial tubules were counted and normalized to the vector control. Representative images are shown.
To further explore the biological relevance of ALK-1/CK2β interaction, we investigated the effects of their interaction on endothelial cell tubule formation. The ectopic expression of ALK-1 or CK2β in HMEC-1 cells did not alter the overall length of individual tubules. Instead, ALK-1 and CK2β appeared to significantly disrupt the capacity for HMEC-1 cells to form an efficient network of tubules when compared to the wild-type vector control (Fig. 8B). Relative to the control, transient expression of ALK-1 or CK2β each yielded a modest reduction (15 to 25±5%) in the number of tubules formed. However, the coexpression of ALK-1 and CK2β synergistically antagonized tubule formation (70 to 75±10%) (Fig. 8B). Taken together, these findings support a physiologically relevant role for CK2β in enhancing ALK-1 signaling to inhibit endothelial cell migration and tubule formation.
DISCUSSION
ALK-1 and endoglin play critical roles during embryonic cardiovascular development. Mutations in either receptor result in the human disease hereditary hemorrhagic telangiectasia. Yet the molecular mechanisms by which these receptors modulate signaling through various TGF-β superfamily ligands to affect endothelial cell function and vascular biology remain poorly understood. Although both endoglin and ALK-1 were initially characterized as TGF-β receptors, endoglin was demonstrated to interact with other TGF-β superfamily ligands, including BMPs and activin, in the context of their respective ligand-binding receptors (44), and both ALK-1 and endoglin have been demonstrated to directly bind BMP-9/10 (22, 42). As endoglin and ALK-1 both bind TGF-β superfamily ligands and form a heteromeric complex, these receptors are thought to transduce signals essential for endothelial cell function.
Given the pivotal roles both ALK-1 and endoglin have in endothelial cell biology and human vascular disease, the mechanisms regulating their signaling and effects on endothelial cell function all have been the subject of intense investigation. We have previously reported that endoglin directly binds the scaffolding protein β-arrestin2, resulting in their cointernalization in endocytic vesicles. The association between endoglin and β-arrestin2 appears to antagonize TGF-β-mediated ERK signaling, alter the subcellular distribution of activated ERK, and inhibit endothelial cell migration (45). We also recently demonstrated that endoglin binds to the scaffolding protein GIPC (GAIP-interacting protein, C terminus), which stabilizes endoglin on the cell surface, and that this interaction enhances TGF-β1-induced Smad 1/5/8 phosphorylation and inhibits endothelial cell migration (46). Endoglin has also been demonstrated to interact with the focal adhesion proteins zyxin (47) and ZRP-1 (48) to deplete these proteins from focal adhesions to regulate the actin cytoskeleton and inhibit cell migration. All these recent findings describe new functions for endoglin, but they do not address whether ALK-1 is involved in many of the characterized signaling pathways and biological outputs. Indeed, as the main signaling partner for endoglin, ALK-1 has also been the focus of extensive efforts with the aim of identifying new ALK-1-interacting signal transducers. We had previously established a novel interaction of ALK-1 with the nuclear receptor LXRβ (49). In this case constitutively active ALK-1 was able to specifically phosphorylate LXRβ, whereas LXRβ inhibited ALK-1-mediated XVent2 promoter-driven luciferase activity (49). In addition, ALK-1 has been demonstrated to interact with human retroviral gag- and gag-pol-like proteins (50) and with FKBP12 (34), which also function to inhibit ALK-1 signaling.
In the present work, we define another ALK-1-interacting protein, CK2β, which serves to enhance ALK-1 functions, including reporter activation, ligand-stimulated Smad 1/5/8 phosphorylation, and inhibition of endothelial cell migration and tubule formation. These functions of CK2β are consistent with its regulation of other serine/threonine kinases, including Mos (31), Chk1 (28), and A-raf (39). The interaction of ALK-1 with numerous proteins that regulate its kinase activity and function reflect the physiological importance of fine-tuning ALK-1 signaling. Precisely how LXRβ, FKBP12, and CK2β coordinate to regulate ALK-1 function is currently under investigation.
How does CK2β function to increase ALK-1 signaling? The presented data demonstrate that the presence of CK2β augments both constitutively active and ligand-stimulated ALK-1. Reciprocally, silencing CK2β expression is sufficient to markedly attenuate either TGF-β1- or BMP-9-stimulated Smad1/5/8 but not Smad2 signaling. Taken together, these observations suggest that CK2β functions downstream of ligand stimulation to specifically enhance ALK-1-mediated Smad1/5/8 signaling. CK2β may function by altering the ability of ALK-1 to access substrates, including the Smad proteins. Alternatively, CK2β might alter the localization of ALK-1. Because ALK-1 is a member of a family of related serine/threonine kinases that share homology in the identified CK2β-interacting motif, CK2β might regulate the activity of other ALK family receptors as well. Indeed, we have obtained preliminary data supporting an interaction of CK2β with ALK-3 and ALK-6 (data not shown), although it does not interact with ALK-5 (Fig. 2). The mechanisms by which CK2β regulates ALK-1 signaling and other ALK family receptors are currently under investigation.
HHT is an autosomal dominant disease in which primary vascular dysplasias result in telangiectasia and arteriovenous malformations. Clinically, these arteriovenous malformations result in skin telangectases, epistaxis, gastrointestinal bleeding, shunting phenomena, and neurological sequela (51). Although endoglin and ALK-1 are responsible for most cases of HHT, there have been cases of HHT not linked to either of the loci. Although CK2β is unlikely to be specifically mutated in HHT, as CK2β is involved in a host of cellular processes, and loss of CK2β results in early embryonic death (52), alterations in CK2β levels or localization could have implications for vascular physiology and pathophysiology, including in HHT. The role of CK2β and other ALK-1-interacting proteins in vascular diseases merits further exploration.
Supplementary Material
Acknowledgments
These studies were supported by National Institute of Health/National Cancer Institute grants R01-CA105255 (to G.C.B.), F32-CA124139 (to N.Y.L), and T32-CA093245 (to J.C.H.). The GST-CK2β expression vector was generously supplied by Dr. Jonathan Cooper (Fred Hutchinson Cancer Center, Seattle, WA, USA). Special thanks to Dr. Chandra Tucker and the Yeast Model Systems Genomics Group at Duke University for their assistance with the yeast 2-hybrid screen, and Dr. Kevin Peters (Procter & Gamble Pharmaceuticals) for the endothelial cell library.
References
- Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang Y E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Elliott R L, Blobe G C. Role of transforming growth factor beta in human cancer J. Clin Oncol. 2005;23:2078–2093. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- McAllister K A, Grogg K M, Johnson D W, Gallione C J, Baldwin M A, Jackson C E, Helmbold E A, Markel D S, McKinnon W C, Murrell J, McCormick M K, Pericak-Vance M A, Heutink P, Oostra B A, Haitjema T, Westerman C J, Porteous M E, Guttmacher A E, Letarte M, Marchuk D A. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8:345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- Johnson D W, Berg J N, Baldwin M A, Gallione C J, Marondel I, Yoon S J, Stenzel T T, Speer M, Pericak-Vance M A, Diamond A, Guttmacher A E, Jackson C E, Attisano L, Kucherlapati R, Porteous M E, Marchuk D A. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet. 1996;13:189–195. doi: 10.1038/ng0696-189. [DOI] [PubMed] [Google Scholar]
- Urness L D, Sorensen L K, Li D Y. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat Genet. 2000;26:328–331. doi: 10.1038/81634. [DOI] [PubMed] [Google Scholar]
- Oh S P, Seki T, Goss K A, Imamura T, Yi Y, Donahoe P K, Li L, Miyazono K, ten Dijke P, Kim S, Li E. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A. 2000;97:2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D Y, Sorensen L K, Brooke B S, Urness L D, Davis E C, Taylor D G, Boak B B, Wendel D P. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- Bourdeau A, Dumont D J, Letarte M. A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest. 1999;104:1343–1351. doi: 10.1172/JCI8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur H M, Ure J, Smith A J, Renforth G, Wilson D I, Torsney E, Charlton R, Parums D V, Jowett T, Marchuk D A, Burn J, Diamond A G. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol. 2000;217:42–53. doi: 10.1006/dbio.1999.9534. [DOI] [PubMed] [Google Scholar]
- Larsson J, Goumans M J, Sjostrand L J, van Rooijen M A, Ward D, Leveen P, Xu X, ten Dijke P, Mummery C L, Karlsson S. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J. 2001;20:1663–1673. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima M, Oshima H, Taketo M M. TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol. 1996;179:297–302. doi: 10.1006/dbio.1996.0259. [DOI] [PubMed] [Google Scholar]
- Blobe G C, Schiemann W P, Lodish H F. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- Massague J, Attisana L, Wrana J L. The TGF-beta family and its composite receptors. Trends Cell Biol. 1994;4:172–178. doi: 10.1016/0962-8924(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Wrana J L, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- Nakao A, Imamura T, Souchelnytskyi S, Kawabata M, Ishisaki A, Oeda E, Tamaki K, Hanai J, Heldin C H, Miyazono K, ten Dijke P. TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J. 1997;16:5353–5362. doi: 10.1093/emboj/16.17.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Dijke P, Goumans M J, Itoh F, Itoh S. Regulation of cell proliferation by Smad proteins. J Cell Physiol. 2002;191:1–16. doi: 10.1002/jcp.10066. [DOI] [PubMed] [Google Scholar]
- Daly A C, Randall R A, Hill C S. Transforming growth factor beta-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth. Mol Cell Biol. 2008;28:6889–6902. doi: 10.1128/MCB.01192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu I M, Schilling S H, Knouse K A, Choy L, Derynck R, Wang X F. TGFbeta-stimulated Smad1/5 phosphorylation requires the ALK5 L45 loop and mediates the pro-migratory TGFbeta switch. EMBO J. 2009;28:88–98. doi: 10.1038/emboj.2008.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goumans M J, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David L, Mallet C, Vailhe B, Lamouille S, Feige J J, Bailly S. Activin receptor-like kinase 1 inhibits human microvascular endothelial cell migration: potential roles for JNK and ERK. J Cell Physiol. 2007;213:484–489. doi: 10.1002/jcp.21126. [DOI] [PubMed] [Google Scholar]
- Scharpfenecker M, van Dinther M, Liu Z, van Bezooijen R L, Zhao Q, Pukac L, Lowik C W, ten Dijke P. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci. 2007;120:964–972. doi: 10.1242/jcs.002949. [DOI] [PubMed] [Google Scholar]
- Goumans M J, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol Cell. 2003;12:817–828. doi: 10.1016/s1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- Carvalho R L, Itoh F, Goumans M J, Lebrin F, Kato M, Takahashi S, Ema M, Itoh S, van Rooijen M, Bertolino P, ten Dijke P, Mummery C L. Compensatory signalling induced in the yolk sac vasculature by deletion of TGFbeta receptors in mice. J Cell Sci. 2007;120:4269–4277. doi: 10.1242/jcs.013169. [DOI] [PubMed] [Google Scholar]
- Park S O, Lee Y J, Seki T, Hong K H, Fliess N, Jiang Z, Park A, Wu X, Kaartinen V, Roman B L, Oh S P. ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2. Blood. 2008;111:633–642. doi: 10.1182/blood-2007-08-107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield D W. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldyreff B, Issinger O G. A-Raf kinase is a new interacting partner of protein kinase CK2 beta subunit. FEBS Lett. 1997;403:197–199. doi: 10.1016/s0014-5793(97)00010-0. [DOI] [PubMed] [Google Scholar]
- Guerra B, Issinger O G, Wang J Y. Modulation of human checkpoint kinase Chk1 by the regulatory beta-subunit of protein kinase CK2. Oncogene. 2003;22:4933–4942. doi: 10.1038/sj.onc.1206721. [DOI] [PubMed] [Google Scholar]
- Bjorling-Poulsen M, Siehler S, Wiesmuller L, Meek D, Niefind K, Issinger O G. The ‘regulatory’ beta-subunit of protein kinase CK2 negatively influences p53-mediated allosteric effects on Chk2 activation. Oncogene. 2005;24:6194–6200. doi: 10.1038/sj.onc.1208762. [DOI] [PubMed] [Google Scholar]
- Chen M, Li D, Krebs E G, Cooper J A. The casein kinase II beta subunit binds to Mos and inhibits Mos activity. Mol Cell Biol. 1997;17:1904–1912. doi: 10.1128/mcb.17.4.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman S L, Ruderman J V. CK2 beta, which inhibits Mos function, binds to a discrete domain in the N-terminus of Mos. Dev Biol. 2004;268:271–279. doi: 10.1016/j.ydbio.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Bren G D, Pennington K N, Paya C V. PKC-zeta-associated CK2 participates in the turnover of free IkappaBalpha. J Mol Biol. 2000;297:1245–1258. doi: 10.1006/jmbi.2000.3630. [DOI] [PubMed] [Google Scholar]
- Kusk M, Ahmed R, Thomsen B, Bendixen C, Issinger O G, Boldyreff B. Interactions of protein kinase CK2beta subunit within the holoenzyme and with other proteins. Mol Cell Biochem. 1999;191:51–58. [PubMed] [Google Scholar]
- Wang T, Donahoe P K, Zervos A S. Specific interaction of type I receptors of the TGF-beta family with the immunophilin FKBP-12. Science. 1994;265:674–676. doi: 10.1126/science.7518616. [DOI] [PubMed] [Google Scholar]
- Bolanos-Garcia V M, Fernandez-Recio J, Allende J E, Blundell T L. Identifying interaction motifs in CK2beta—a ubiquitous kinase regulatory subunit. Trends Biochem Sci. 2006;31:654–661. doi: 10.1016/j.tibs.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Wilson I A, Niman H L, Houghten R A, Cherenson A R, Connolly M L, Lerner R A. The structure of an antigenic determinant in a protein. Cell. 1984;37:767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Litchfield D W, Lozeman F J, Cicirelli M F, Harrylock M, Ericsson L H, Piening C J, Krebs E G. Phosphorylation of the beta subunit of casein kinase II in human A431 cells. Identification of the autophosphorylation site and a site phosphorylated by p34cdc2. J Biol Chem. 1991;266:20380–20389. [PubMed] [Google Scholar]
- Kristensen L P, Larsen M R, Hojrup P, Issinger O G, Guerra B. Phosphorylation of the regulatory beta-subunit of protein kinase CK2 by checkpoint kinase Chk1: identification of the in vitro CK2beta phosphorylation site. FEBS Lett. 2004;569:217–223. doi: 10.1016/j.febslet.2004.05.069. [DOI] [PubMed] [Google Scholar]
- Hagemann C, Kalmes A, Wixler V, Wixler L, Schuster T, Rapp U R. The regulatory subunit of protein kinase CK2 is a specific A-Raf activator. FEBS Lett. 1997;403:200–202. doi: 10.1016/s0014-5793(97)00011-2. [DOI] [PubMed] [Google Scholar]
- Chen Y G, Massague J. Smad1 recognition and activation by the ALK1 group of transforming growth factor-beta family receptors. J Biol Chem. 1999;274:3672–3677. doi: 10.1074/jbc.274.6.3672. [DOI] [PubMed] [Google Scholar]
- Lux A, Attisano L, Marchuk D A. Assignment of transforming growth factor beta1 and beta3 and a third new ligand to the type I receptor ALK-1. J Biol Chem. 1999;274:9984–9992. doi: 10.1074/jbc.274.15.9984. [DOI] [PubMed] [Google Scholar]
- David L, Mallet C, Mazerbourg S, Feige J J, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood. 2007;109:1953–1961. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- Ye L, Kynaston H, Jiang W G. Bone morphogenetic protein-9 induces apoptosis in prostate cancer cells, the role of prostate apoptosis response-4. Mol Cancer Res. 2008;6:1594–1606. doi: 10.1158/1541-7786.MCR-08-0171. [DOI] [PubMed] [Google Scholar]
- Barbara N P, Wrana J L, Letarte M. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily. J Biol Chem. 1999;274:584–594. doi: 10.1074/jbc.274.2.584. [DOI] [PubMed] [Google Scholar]
- Lee N Y, Blobe G C. The interaction of endoglin with beta-arrestin2 regulates transforming growth factor-beta-mediated ERK activation and migration in endothelial cells. J Biol Chem. 2007;282:21507–21517. doi: 10.1074/jbc.M700176200. [DOI] [PubMed] [Google Scholar]
- Lee N Y, Ray B, How T, Blobe G C. Endoglin promotes transforming growth factor beta-mediated Smad 1/5/8 signaling and inhibits endothelial cell migration through its association with GIPC. J Biol Chem. 2008;283:32527–32533. doi: 10.1074/jbc.M803059200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley B A, Koleva R, Smith J D, Kacer D, Zhang D, Bernabeu C, Vary C P. Endoglin controls cell migration and composition of focal adhesions: function of the cytosolic domain. J Biol Chem. 2004;279:27440–27449. doi: 10.1074/jbc.M312561200. [DOI] [PubMed] [Google Scholar]
- Sanz-Rodriguez F, Guerrero-Esteo M, Botella L M, Banville D, Vary C P, Bernabeu C. Endoglin regulates cytoskeletal organization through binding to ZRP-1, a member of the Lim family of proteins. J Biol Chem. 2004;279:32858–32868. doi: 10.1074/jbc.M400843200. [DOI] [PubMed] [Google Scholar]
- Mo J, Fang S J, Chen W, Blobe G C. Regulation of ALK-1 signaling by the nuclear receptor LXRbeta. J Biol Chem. 2002;277:50788–50794. doi: 10.1074/jbc.M210376200. [DOI] [PubMed] [Google Scholar]
- Lux A, Beil C, Majety M, Barron S, Gallione C J, Kuhn H M, Berg J N, Kioschis P, Marchuk D A, Hafner M. Human retroviral gag- and gag-pol-like proteins interact with the transforming growth factor-beta receptor activin receptor-like kinase 1. J Biol Chem. 2005;280:8482–8493. doi: 10.1074/jbc.M409197200. [DOI] [PubMed] [Google Scholar]
- Guttmacher A E, Marchuk D A, White R I., Jr Hereditary hemorrhagic telangiectasia. N Engl J Med. 1995;333:918–924. doi: 10.1056/NEJM199510053331407. [DOI] [PubMed] [Google Scholar]
- Buchou T, Vernet M, Blond O, Jensen H H, Pointu H, Olsen B B, Cochet C, Issinger O G, Boldyreff B. Disruption of the regulatory beta subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol Cell Biol. 2003;23:908–915. doi: 10.1128/MCB.23.3.908-915.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.