Abstract
Acute lung injury (ALI) in mouse lung occurs after distal airway deposition of IgG immune complexes (IgGICs), resulting in a breakdown of the vascular-airway barrier, causing intrapulmonary edema, hemorrhage, and accumulation of neutrophils [polymorphonuclear leukocytes (PMNs)] in the alveolar compartment, these changes being complement (C5a) and C5a receptor (C5aR) dependent. In this ALI model, C5aR expression (protein) was found to occur on upper (bronchial) and lower (alveolar) airway epithelial cells. An adenovirus construct (siRNA) was used to silence mRNA for C5aR in the lung. Under such conditions, C5aR protein was markedly reduced on lung epithelial cells, resulting in much reduced leakage of albumin into the lung, diminished buildup of PMNs, and lower levels of proinflammatory mediators in bronchoalveolar lavage fluids. These studies indicate that bronchial and alveolar epithelial cell C5aR is up-regulated and greatly contributes to inflammation and injury in the lung. The use of siRNA administered into the airways avoids systemic suppression of C5aR, which might compromise innate immunity. It is possible that such an intervention might be employed in humans with ALI or acute respiratory distress syndrome as well as in upper-airway inflammatory diseases, such as chronic obstructive pulmonary disease and asthma, where there is evidence for complement activation and buildup of PMNs.—Sun, L., Guo, R.-F., Gao, H., Sarma, J. V., Zetoune, F. S., Ward, P. A. Attenuation of IgG immune complex-induced acute lung injury by silencing C5aR in lung epithelial cells.
Keywords: complement, siRNA, PMNs, inflammation
The rodent lung injury model triggered by the intrapulmonary deposition of IgG immune complexes (IgGICs) is used to study the roles of cytokines, chemokines, and complement in the induction of acute inflammation (1). In this model, IgGIC deposition triggers complement activation and activation of residential lung macrophages via engagement of FcγRs. One of the complement activation products, C5a, has been demonstrated to be required for the full development of injury and neutrophil accumulation in an IgGIC model of lung injury (2). Together with other cytokines/chemokines and vascular adhesion molecules, C5a is required for recruiting leukocytes from the vascular space into the interstitial and distal airway compartments (3,4,5,6). C5a also directly activates neutrophils and macrophages for chemokine production. Both in vitro and in vivo studies have shown that, in the presence of IgGIC, C5a and MAC (C5b-9, membrane attack complex) cause synergistic intrapulmonary generation of rat CXC (MIP-2 and CINC) and CC (MIP-1α, MIP-1β, and MCP-1) chemokines, resulting in increased neutrophil accumulation as well as intensified lung injury (7, 8). C5a can also activate endothelial and alveolar epithelial cells, resulting in the release of proinflammatory mediators, thereby setting the stage for neutrophil migration. C5a increases CD11b/CD18 (CR3) expression in neutrophils and enhances adhesive interactions of both neutrophils and eosinophils to unstimulated human umbilical vein entotheilial cells (HUVECs) and to human bronchial epithelial cells (9, 10). C5a is also involved in the up-regulation of vascular adhesion molecules such as P- and E-selectin and ICAM-1 in the lung (11, 12).
C5a induces its inflammatory functions by interacting with the C5a receptor (C5aR) that belongs to the rhodopsin family of 7-transmembrane G-protein-coupled receptors (13,14,15). Originally thought to be exclusively expressed in myeloid bone marrow cells (16), neutrophils (17), monocytes (18), basophils (19), and eosinophils (20), recent studies have demonstrated the presence of C5aR in other cell types, such as bronchial and alveolar epithelial cells (21,22,23), kidney tubular epithelial cells (24), astrocytes (25), hepatocyte-derived cell lines (26), and endothelial, smooth muscle, and other parenchymal cells of solid organs, including the liver, kidney, and lung (27,28,29,30,31). Up-regulation of C5aR has been seen under pathological conditions (32, 33). In septic animals, increased expression of C5aR was detected in organs including the heart, liver, lungs, and kidneys (34). Studies from our group have shown that blockade of either C5a or C5aR (using IgG antibodies or a C5aR antagonist) results in a significant improvement in survival rates in a rodent model of sepsis after cecal ligation and puncture (CLP) (35,36,37). The protective effects of C5a blockade appeared to derive from the interception of C5a/C5aR signaling, because blockade of C5aR achieved a similar level of improvement in survival of CLP animals (34). In IgGIC-induced lung injury model, C5aR antagonist treatment markedly reduced the lung permeability index (extravascular leakage of albumin) in mice (37). Consistently, mice deficient in C5aR were protected from IC-induced alveolitis (38, 39).
Due to the detrimental effects of complement activation under pathological conditions, interventions aimed at blocking C5a or C5aR signaling may represent a promising target for therapeutic treatment in inflammatory disorders. In the progression of acute lung injury, it is unclear to what extent C5aR in epithelial cells contributes to the detrimental outcome. Previously, we reported the in vivo knockdown of C5aR gene expression with intrapulmonary infection of siRNA-expressing adenovirus, but where knockdown occurred in the lung was not determined, nor was any functional significance of this knockdown assessed (40). In the current study, we took advantage of adenovirus-targeting infection of epithelial cells in the lung to address the role of C5aR expression in epithelial cells in the lung injury induced by IgGIC deposition.
MATERIALS AND METHODS
All experiments were done with the approval of the University of Michigan University Committee on Use and Care of Animals (UCUCA).
Reagents
Rabbit anti-BSA IgG was purchased from ICN Biomedicals (Irvine, CA, USA). ELISA kits for mouse IL-6, keratinocyte-derived chemokine (KC), and TNF-α were purchased from Biosource International (Camarillo, CA, USA). The ELISA kit for the detection of mouse albumin levels in bronchial alveolar lavage (BAL) fluids was purchased from Bethyl Labs (Montgomery, TX, USA). The detection limit for this ELISA was 7 ng/ml.
A 37-aa peptide spanning the N terminus of the mouse C5aR (MDPIDNSSFEINYDHYGTMDPNIPADGIHLPKRQPGDC) was synthesized using an Applied Biosystems 430A peptide synthesizer (Applied Biosystems, Foster City, CA, USA). The peptide was then coupled to keyhole limpet hemocyanin by the glutaraldehyde method and used for the immunization of rabbits and the production of immunoreactive antisera. The anti-peptide-specific Ab was purified by affinity chromatography using the synthetic peptide coupled to cyanogen bromide-activated Sepharose 4B (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Adenovirus
An adenovirus that expresses the short hairpin RNA of C5aR (adeno-C5aR-shRNA) or luciferase (adeno-luc-shRNA; control virus) was described previously (40). The virus-expressing vector contains a dsRed fluorescent protein cDNA. Viruses were produced by infecting HEK293 cells. Briefly, cells were infected with 5–10 MOI of stock virus and incubated at 37°C for 2–3 d until 80–90% of the cells rounded up and started to float. Cells were spun down and lysed in PBS with 3 consecutive freeze-thaw cycles. Supernatants containing infectious adenoviruses were purified by column (Puresyn, Inc., Malvern, PA, USA) and concentrated by YM-50 centricon (Millipore, Billerica, MA, USA). Titers of the viruses were determined by Adeno-X rapid titer kit (BD Clontech, Mountain View, CA, USA).
Intratracheal administration of adenovirus
Adenoviral suspensions (50 μl) with a dosage of 5 × 108 plaque-forming units was injected intratracheally into mouse lungs, using a Hamilton syringe with a sterile 30-gauge needle. Four days later, mice were used for IgGIC-induced lung injury.
IgGIC-induced lung injury
Eight- to 10-wk-old specific-pathogen-free male C57BL/6 mice were anesthetized with ketamine-HCl (150 mg/kg, i.p.). The trachea was surgically exposed by a midline incision, and 100 μg of rabbit anti-BSA IgG in 40 μl of PBS was administered intratracheally with a 30-gauge needle. The incision was closed by two surgical clips, and 1 mg of BSA in 200 μl of PBS was injected intravenously immediately thereafter. Control mice received anti-BSA intratracheally in the absence of an intravenous infusion of BSA.
Animals were sacrificed 4 h after IgG-IC-induced alveolitis. This interval selected for sacrifice represents the time for peak lung injury as determined in previous experiments (1). Four hours after initiation of the acute lung injury, the thorax was opened and 0.8 ml of ice-cold, sterile PBS was instilled into the lung via a tracheal incision. The recovered BAL fluid was centrifuged at 450 g for 6 min, and the cell-free supernatants were stored at −20°C. Lung tissues were harvested, snap-frozen in liquid nitrogen, and stored at −80°C.
Lung pathology
Four days after virus injection, the lungs were inflated with 0.8 ml of tissue-embedding solution (Tissue-Tek OCT compound; Fisher Scientific, Pittsburgh, PA, USA) before being frozen to prevent alveolar collapse. Glass slides with tissue sections of 4–5 μm thickness were examined under fluorescence microscope (lissamine-rhodamine channel) for virus distribution in the lung.
Quantification of C5aR mRNA by Sybr green real-time PCR analysis
Lung total RNA was isolated with the Trizol method. Reverse transcription was performed with 1 μg RNA using the high-capacity cDNA reverse-transcription kit (Applied Biosystems). Sybr green real-time PCR was then performed with primers for C5aR: forward primer, 5′-GAAGCGGCAACCTGGGGATGT-3′ and reverse primer, 5′-CGTCTGGCTCGAAGGCTGTCAC-3′. The Taqman assay was used for the quantification of endogenous control (GAPDH; Applied Biosystems). Results were normalized to GAPDH expression and presented as fold increase in mRNA expression compared with the level detected in control mice. Real-time PCR assay was performed on an Eppendorf Realplex cycler (Eppendorf North America, Westbury, NY, USA).
Permeability index
For permeability index measurements, BSA was labeled with 125I by the chloramine T method. A trace amount of 125I-BSA (specific activity 5 μCi/μg) was added to unlabeled BSA (5 mg/ml in PBS), and 200 μl of this solution was injected intravenously to induce the IgGIC lung injury, as described above. Four hours later, mice were euthanized, and blood was collected from the inferior vena cava. The thorax was opened, the left atrium was incised, and the lung was perfused in situ with 3 ml PBS via the pulmonary artery. The flushed lungs were removed, and the permeability index (indicating the extent of pulmonary leakage) was determined by using a γ counter and expressed as a ratio of counts per minute in the whole lung vs. radioactivity in 100 μl of blood.
Myeloperoxidase (MPO) activity
Animals were sacrificed, and lungs were perfused via the right ventricle using PBS until pulmonary vessels were grossly clear. Tissues were weighed and homogenized in a homogenate buffer: 0.5% hexadecyltrimethylammonium bromide and 5 mM EDTA in 50 mM potassium phosphate buffer, pH 6.0. The samples were sonicated for 1 min and then centrifuged at 20,000 g for 15 min. Ten microliters of each sample was added to a 96-well plate, followed by addition of 250 μl assay buffer: 0.005% H2O2 and 0.5 mM o-dianisidine dihydrochloride in 100 mM potassium phosphate, pH 6.0. The change in optical density (OD) at 460 nm was measured over a period of 6 min at 15-s intervals, using the kinetics mode in a spectrophotometer (Molecular Devices). The slope of the change in OD was calculated to reflect the rate of change in units per gram of lung per minute. All samples were diluted 1:5 to guarantee a linear response.
BAL and in vitro IgGIC treatment
Four days after virus injection, BAL fluids were collected using repetitive (3 times) instillation and withdrawal of 1 ml saline via an intratracheal cannula. BAL samples were centrifuged at 1500 rpm for 10 min, and cell pellets were resuspended in RPMI + 10% fetal calf serum and plated in 96-well plates. Alveolar macrophages were allowed to adhere at 37°C for 1 h, and nonadhering cells were removed. To induce IC formation in vitro, 100 μg of BSA in 100 μl of PBS buffer was incubated with a 5-fold excess of anti-BSA IgG at room temperature for 30 min and then was added to cells. At the 2 and 18 h time points, the supernatants were removed from culture and measured for TNF-α production.
Immunohistochemical staining for C5aR
Mice were injected with adenoviruses as described above. Four days later, mice were used for IgGIC-induced lung injury. Four hours after initiation of injury, the lungs were inflated with OCT, and glass slides with tissue sections of 4–5 μm thickness were processed for immunohistochemical staining. The slides were first washed with PBS for 10 s and then fixed in methanol for 6 min. Next, the tissue sections were washed for 2 min with PBS before incubation with anti-C5aR serum at a dilution of 1:100 for 2 h. Thereafter, the tissue sections were washed for 2 min with PBS before incubation with a 1:500 diluted peroxidase-conjugated goat anti-rabbit IgG for 30 min (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). After being washed with PBS for 2 min, the sections were stained using the AEC vector staining kit (Vector Laboratories, Burlingame, CA, USA). Counterstaining was achieved with hematoxylin for 30 s. Tissue sections were fixed, and the coverslides were mounted with Permount medium (Fisher Scientific). Staining was documented using light microscopy and digital imaging.
Pulmonary leukocyte isolation
Animals were euthanized by approved protocols, and individual mice were used to collect cells for flow cytometry. First, BAL was performed as described above, and then lungs were perfused with PBS via the right heart until pulmonary vessels were grossly clear. The lungs were bluntly dissected free from the chest cavity and minced in complete medium (RPMI+10% fetal calf serum) containing collagenase (15 mg) and DNase I (250 Kunitz units). The suspension was incubated on a rocker for 30 min at 37°C. The cells were dispersed by repetitive suction through a 10-ml syringe and then spun down at 1100 rpm for 10 min. After the supernatant was decanted, each pellet was briefly resuspended with 1 ml sterile ddH2O to lyse red blood cells and then recentrifuged. Cell pellets were resuspended in 5 ml of complete medium and passed through a 70-μm cell strainer.
Flow cytometric analysis for C5aR
Both BAL cells and lung leukocytes were assessed for flow cytometric analysis. BAL cells (50,000 cells) in 100 μl flow assay buffer were first incubated with 0.2 μg of mouse Fc blocker (BD Pharmingen, San Jose, CA, USA) for 15 min and then stained with 10 μl of anti-C5aR IgG for 30 min at room temperature. After being washed, the cells were labeled with FITC-conjugated goat anti-rabbit IgG (1:50; Biosource, Carlsbad, CA, USA) and simultaneously stained with APC-conjugated anti-F4/80 IgG (Biolegend, San Diego, CA, USA). Cells were washed again, resuspended in a fixation buffer (1% paraformaldehyde in PBS with 0.1% sodium azide), and analyzed on a flow cytometer (Accuri C6; Accuri Cytometers Inc., Ann Arbor, MI, USA). To assess both cell surface and intracellular C5aR expression, cells were first stained with APC-conjugated anti-F4/80 IgG and then fixed and permeabilized with 4% paraformaldehyde and 0.1% saponin. The permeabilized cells were reacted with anti-C5aR IgG, followed by staining with FITC-conjugated goat anti-rabbit IgG, and then washed and subjected to flow cytometry analysis. Whole-lung leukocytes were stained using the same procedure as BAL cells, except that 400,000 leukocytes were used in each experimental group. Both nonpermeabilized and permeabilized lung leukocytes were assayed for C5aR expression.
Statistical analysis
All values were expressed as means ± se. Significance was assigned where P < 0.05. Datasets were analyzed using Student’s t test or 1-way ANOVA, with individual group means being compared with the Student-Newman-Keuls multiple comparison test.
RESULTS
Adenovirus-mediated C5aR-siRNA silencing attenuates the up-regulation of C5aR mRNA induced by IgG-IC deposition
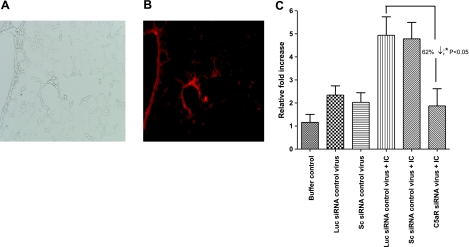
Recently, we demonstrated that in vivo use of the C5aR-siRNA adenovirus construct attenuated C5aR expression in lungs from normal mice and after cecal ligation and puncture-induced sepsis in mice (40). However, the details of what happened in the lung and the functional significance were not evaluated. We have now investigated the effects of in vivo C5aR silencing in acute lung injury induced by distal airway deposition of IgGICs. First, we examined whole-lung C5aR mRNA expression 4 h after the initiation of injury. In all the experiments, we used a dose of 5.0 × 108 plaque-forming units for intratracheal virus injection 4 d before acute lung injury induction. The protocol used in our experiments achieves maximum silencing without triggering an intrapulmonary inflammatory response (40). As shown in Fig. 1A, B, adenoviral infection was displayed as red-colored fluorescence that became prominent 4 d after infection. The viral infection was widely distributed in lung tissue especially in bronchial epithelial cells and lung alveolar epithelial cells (Fig. 1B).
Figure 1.
Sections of mouse lung after intratracheal administration of adenovirus and induction of IgGIC-induced injury. A, B) Assessment of adenovirus distribution in lung after intratracheal administration of dsRed fluorescence protein-expressing adenovirus. Four days after virus injection, lung sections were examined, and dsRed protein was visualized in the lissamine-rhodamine channel under fluorescence microscopy. A) Bright-field image of infected lung (×20). B) Abundant red fluorescence of lung receiving dsRed virus in bronchiolar (left) and alveolar (center) walls (×20). C) Four days after intratracheal injection of either buffer control (PBS), C5aR-siRNA silencing virus (C5aR-siRNA), luciferase control virus (Luc siRNA control virus), or scrambled siRNA control virus (Sc siRNA control virus), mice were subjected to IgGIC-induced lung injury. Lung total RNA was isolated 4 h after injection of IgGIC injury and analyzed for mRNA for C5aR by real-time PCR analysis. Data are expressed as relative fold changes over nonvirus infected, control lungs (buffer control group). Results are means ± se; ≥5 mice/group.
Next, mRNA for C5aR was evaluated by real-time PCR. Mice that received intratracheal injection of PBS buffer served as a negative control (Fig. 1C, 1st vertical bar). Mice receiving luciferase siRNA (Fig. 1C, 2nd vertical bar; Luc siRNA control virus) and scrambled siRNA control virus (Fig. 1C, 3rd vertical bar; Sc siRNA control virus) intratracheally but no IC injury served as controls. C5aR mRNA was up-regulated 4 h after IC deposition (Fig. 1C, 4th and 5th vertical bars). However, in the presence of C5aR-siRNA adenovirus, the injury-induced up-regulation of C5aR in the lung was reduced by 62% when compared with mice that received control viruses. Not surprisingly, control virus infection in the lung (Fig. 1C, 2nd and 3rd vertical bars) caused a modest increase in C5aR mRNA levels. As will be shown, this did not result in a lung inflammatory response. These data indicate that the adenovirus-expressed C5aR siRNA effectively suppresses C5aR expression in vivo in the lung after IgGIC-deposition. As we did not find any difference between luciferase siRNA virus and scrambled siRNA virus in lung C5aR expression in either control mice or IC-challenged mice, luciferase siRNA virus was used as control in the subsequent experiments.
Lung vascular permeability is reduced by in vivo silencing of C5aR-siRNA
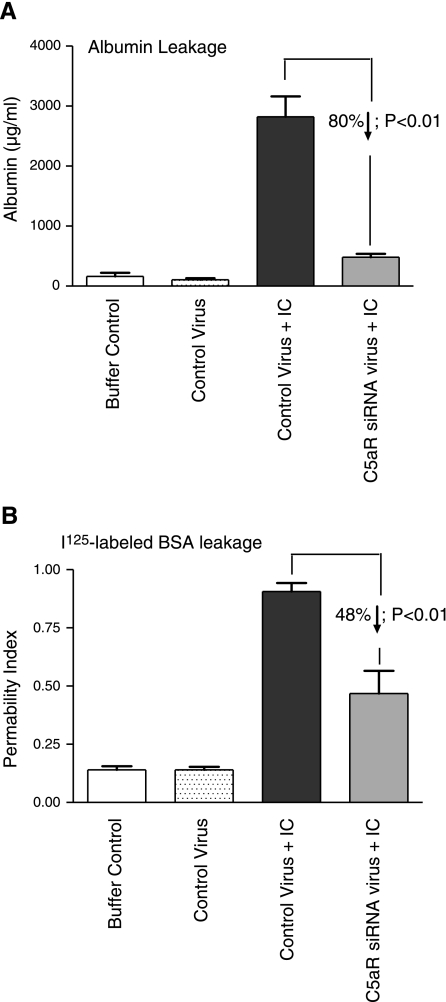
To examine whether siRNA-induced suppression of C5aR affects lung injury after IgGIC deposition, we measured leakage of mouse albumin into the lung. Endogenous mouse albumin levels in BAL fluids were measured by ELISA. In mice that received PBS intratracheally, albumin remained at background levels (Fig. 2A, 1st and 2nd vertical bars). In mice that received intratracheal administration of control virus together with IgGIC deposition, high levels of albumin were detected in the BAL fluids (Fig. 2A, 3rd vertical bar). In contrast, when IC lung injury developed in mice receiving intratracheal injection of C5aR-siRNA virus, the albumin level in the BAL fluid was reduced almost to background levels (Fig. 2A, 4th vertical bar). A similar result was obtained when I125-labeled BSA was employed to measure the leakage of intravenously infused BSA into lung tissue (Fig. 2B). The levels of 125I-BSA fluids of mice receiving PBS or control virus intratracheally were similar (Fig. 2B, 1st and 2nd vertical bars). A significant decrease in the permeability index was found in IC-injured mice that received intratracheal administration of the C5aR-siRNA virus compared with IC-injured mice that received the control virus (Fig. 2B, 3rd and 4th vertical bars).
Figure 2.
Effects of C5aR-siRNA silencing on intensity of IgGIC-induced lung injury. A) Four hours after the onset of IgGIC-induced injury, BAL fluids were harvested, and mouse albumin levels in the BAL fluids were measured by ELISA as an index of vascular leakage; n = 5 mice/group. B) Trace amounts of 125I-labeled BSA were added to the unlabeled BSA and injected intravenously to induce the IgGIC-induced lung injury. Albumin leakage into the lung (permeability index) was determined 4 h after the injury and expressed as the ratio of 125I-BSA in perfused lung to that in 100 μl serum obtained at the time of sacrifice; n = 5 mice/group.
Effects of C5aR-siRNA silencing on MPO content and cytokine/chemokine levels in IgGIC-injured lung
Having found that the degree of lung injury (represented by albumin leakage into the lung) was reduced by in vivo expression of C5aR-siRNA (Fig. 2), we examined MPO content in the lung, which is an indicator of neutrophils present in the lung. Virus administration alone did not cause an increase in MPO activity in the lung (Fig. 3A, 1st and 2nd vertical bars). Four hours after the onset of IC injury, dramatically increased MPO activity was detected (Fig. 3A, 3rd vertical bar) in mice infected with the control virus. In mice infected with C5aR-siRNA virus, there was a significant reduction (Fig. 3A, 4th vertical bar; P<0.01 vs. 3rd bar). The reduced MPO content in the lung correlated with the reduced vascular permeability (Fig. 2). We also evaluated the effects of C5aR-siRNA silencing on levels of proinflammatory cytokines/chemokines in BAL fluids 4 h after IgGIC deposition. Virus infection alone, in the absence of IC injury, did not increase the levels of these proinflammatory mediators (Fig. 3B–D, 1st and 2nd vertical bars). In IgGIC-injured lungs, C5aR-siRNA virus administration led to significantly decreased levels of TNF-α, IL-6, and KC (by 80, 57, and 62% respectively), when compared with mice that were administrated control virus (Fig. 3B–D, 3rd and 4th vertical bars). These data suggest that C5aR silencing can strongly inhibit lung inflammatory responses and neutrophil accumulation after IgGIC-deposition.
Figure 3.
Effects of C5aR-siRNA silencing on lung MPO content and BAL levels of TNF-α, IL-6, and KC. Mice received adenovirus 4 d before induction of acute lung injury. Whole-lung homogenates were analyzed for MPO activity 4 h after the onset of injury. A–D) BAL fluids obtained 4 h after the onset of injury were analyzed for expression levels of MPO (A), TNF-α (B), IL-6 (C), and KC (D) by ELISA. E) Alveolar macrophages were isolated from both control mice and C5aR siRNA virus-challenged mice, and their ability to produce TNF-α when treated with immune complexes in vitro was assessed; n = 6–8 mice/group.
To assess whether in vivo silencing of C5aR affects the functionality of lung alveolar macrophages, we conducted an in vitro IgGIC stimulation experiment. Alveolar macrophages from either control mice or C5aR siRNA virus-challenged mice were isolated and incubated with IgGIC in vitro. In both groups, TNF-α production was strongly induced with the addition of IgGICs. However, no difference was found between control and C5aR siRNA virus groups at both the 2 and 18 h time points examined (Fig. 3E). These data suggest that the capability of alveolar macrophage to release inflammatory mediators was not altered by in vivo delivery of C5aR siRNA virus.
Adenovirus-mediated in vivo silencing of C5aR in lung epithelial cells
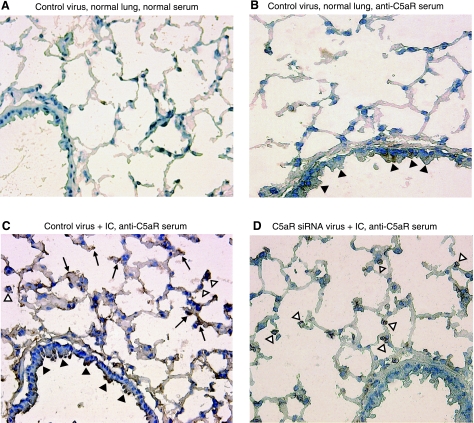
In Fig. 1, we found that C5aR mRNA in whole lung was reduced by in vivo silencing with C5aR-siRNA. To further investigate the specific cell types in the lung that were affected by the intratracheal administration of adenovirus C5aR-siRNA, we examined the expression of C5aR in frozen sections of the lung by immunohistochemistry for C5aR protein. To localize the cells in the lung that were targeted by C5aR-siRNA silencing, we performed immunohistochemical staining in lung frozen sections after 4 d of virus administration (Fig. 4). Lung sections from mice that received control virus but did not undergo IgGIC-induced lung injury served as controls (Fig. 4A, B). With anti-mouse-C5aR staining, low levels of C5aR protein expression were detected in lungs treated with control virus (Fig. 4B). The patterns of staining were mainly in bronchiolar (Fig. 4B; solid arrowheads) and alveolar epithelial cells (Fig. 4B; open arrowheads). With induction of IC-induced injury, the C5aR signal was greatly intensified in mice that received control adenovirus (Fig. 4C). There was intensified staining in bronchial epithelial cells (Fig. 4C; solid arrowheads) and in the alveolar compartment where staining involved both alveolar epithelial cells (Fig. 4C, arrows) and alveolar macrophages (Fig. 4C, open arrowheads). However, in injured mice that received C5aR-siRNA virus, the C5aR signal was attenuated, especially in bronchial and alveolar epithelial cells (Fig. 4D).
Figure 4.
In vivo C5aR-siRNA silencing attenuated C5aR expression in lung epithelial cells. Mice were infected with control virus (A–C) as well as C5aR-siRNA virus (D) 4 d before IgGIC-induced lung injury for 4 h (C, D). Immunohistochemical analysis was performed on mouse lungs stained with control serum (A) or anti-mouse C5aR serum (B–D; ×20). Arrows indicate alveolar epithelial cells; open arrowheads indicate alveolar macrophages; solid arrowheads indicate bronchiolar epithelial cells. Results are means ± se; ≥5 mice/group.
Adenovirus-mediated in vivo delivery of C5aR siRNA did not silence C5aR expression in BAL and interstitial macrophages
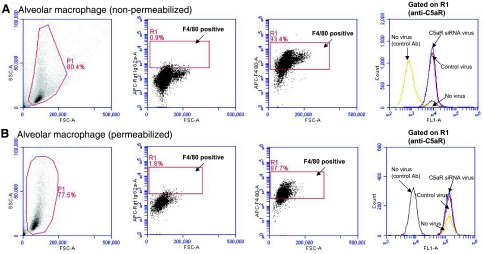
To further quantify C5aR expression in alveolar macrophages (in BAL fluid), we did flow cytometric analysis on BAL cells obtained in BAL fluids under noninjury conditions (Fig. 5A). Initial gates were set based on light-scattering characteristics to eliminate debris, red cells, and cell clusters. Alveolar macrophages were identified with a macrophage marker (F4/80). In BAL fluids, >80% of the cells were macrophages (Fig. 5; F4/80-positive gating). C5aR expression was compared in the gated macrophages. Intratracheal administration of control adenovirus or C5aR-siRNA adenovirus 4 d prior did not change the levels of C5aR expression in macrophages retrieved from otherwise normal lungs (no virus or control virus-challenged mice). As receptor internalization was reported for C5aR (41,42,43), we also examined C5aR expression in permeabilized alveolar macrophages (Fig. 5B). BAL cells were permeabilized by saponin and then stained with anti-C5aR antibody. Similarly, we did not detect any changes of C5aR expression in the permeabilized macrophages retrieved from different groups of mice (Fig. 5B).
Figure 5.
Flow cytometric analysis of in vivo C5aR-siRNA silencing on C5aR expression in alveolar macrophages. BAL fluids from mice infected with control virus or C5aR-siRNA virus were isolated as described in Materials and Methods. Alveolar macrophages, which represent ∼80–90% of cell population in BAL fluids, were identified by positive staining for F4/80. Expression of C5aR on alveolar macrophages was examined under both nonpermeabilized (A) and permeabilized conditions (B). Overlay analysis showed that expression of C5aR on alveolar macrophages was not changed by in vivo C5aR-siRNA silencing. Experimental data represent measurements from ≥4 mice/group.
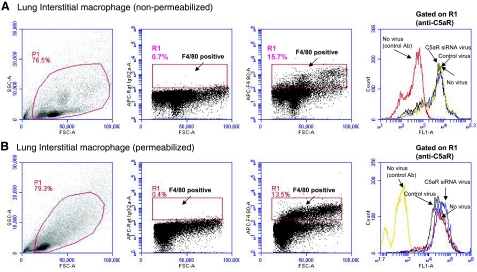
To examine the effect of C5aR siRNA adenovirus on interstitial macrophages, we isolated leukocytes from whole-lung digest and evaluated C5aR expression in the gated interstitial macrophages (Fig. 6). Both nonpermeabilized and permeabilized conditions were performed for the staining of C5aR. As shown in Fig. 6, interstitial macrophages represent 10–15% of lung leukocytes, and C5aR expression in these cells was not altered by C5aR in vivo silencing. We and others (40, 44,45,46) have previously reported that alveolar macrophages were not targeted by adenovirus because of the absence of coxsackievirus and adenovirus receptors (CARs) on the surfaces of macrophages. Thus, it appears that the decreased C5aR expression observed with C5aR-siRNA in vivo silencing was primarily due to the suppressed expression of C5aR on alveolar and bronchial epithelial cells (Fig. 4).
Figure 6.
Flow cytometric analysis of in vivo C5aR-siRNA silencing on C5aR expression in interstitial macrophages. Whole-lung leukocytes were isolated as described in Materials and Methods. As shown by F4/80 gating, interstitial macrophages represent ∼10–15% of whole-lung leukocytes. Lung leukocytes were treated with or without 0.1% saponin to examine C5aR expression in interstitial macrophages under both nonpermeabilized (A) and permeabilized (B) conditions. No change of C5aR expression was found among groups under both conditions. Experimental data represent measurements from ≥4 mice/group.
DISCUSSION
RNA interference (RNAi) is an emerging technology that selectively inhibits targeted gene expression in vitro and in vivo. Several viral vectors have been used to induce RNAi silencing in cultured cells and in experimental animals, including lentivirus (47, 48), retrovirus (49), adenovirus (50,51,52,53), and adenovirus-associated viruses (54, 55). Under conditions of lung inflammation (chronic or acute), adenovirus-mediated local gene delivery appears to be a promising tool for the study and the treatment of inflammatory disorders (56,57,58,59). In the current study, we used siRNA-expressing adenovirus to specifically knock down mRNA and protein for C5aR, which has been demonstrated to be a key regulator of IgGIC-induced lung injury (60).
C5aR is present in various cell types, including both myeloid- and nonmyeloid-derived cells. Up-regulation of C5aR occurs under several conditions. For example, during experimental sepsis (cecal ligation and puncture), C5aR is strongly induced in the lung, liver, kidney, and heart (34). In this study, we report for the first time that, in a murine model of acute lung injury induced by intrapulmonary IC deposition, C5aR expression is up-regulated in the lung and that such up-regulation can be linked to the requirement of C5a for the full development of injury. With the administration of C5aR-siRNA-expressing virus into mouse lung, IC-induced C5aR up-regulation in bronchial and epithelial cells, but not in lung macrophages, was abolished. Accordingly, the degree of injury [represented by neutrophil accumulation in the lung (MPO) and vascular permeability index] was attenuated. The results are consistent with the previous study (37) from our laboratory that showed similarly decreased lung injury after C5aR antagonist treatment. Thus, the interaction of C5a/C5aR is crucial for the complement-mediated lung injury.
Both primary alveolar macrophages and alveolar epithelial cells have been studied for the effects of C5a on chemokine production in vitro (7, 8, 22). Macrophages do not express receptors for the coxsackie virus and usually are not targets for adenovirus infection (45, 46). In our studies, we did not observe evidence for down-regulation of C5aR expression in alveolar macrophages and interstitial macrophages isolated from control virus and C5aR-siRNA-virus-infected mice (Figs. 5 and 6). However, a substantially attenuated C5aR signal was detected in the bronchial and alveolar epithelial cells in the lungs of the C5aR-siRNA-silenced mice (Fig. 4C, D). Thus, the decreased cytokines and chemokines production observed in the C5aR-silenced mice might be due to the down-regulation of C5aR in lung epithelial cells. It is known that of the CXC chemokines, MIP-2 and KC are strong chemoattractants for the recruitment of neutrophils into the inflamed lung (6), and it is also known that alveolar epithelial cells via C5aR may contribute significantly to proinflammatory chemokines and chemokines in the lung after IgGIC deposition (22). The reduction of KC expression appeared to account for the decreased MPO values observed in the silenced mice. It is also possible that, in the setting of IgGIC-induced acute lung injury, alveolar epithelial cells interact with alveolar macrophages to bring about optimal production of chemokines and cytokines. In our experiments with C5aR-siRNA silencing, TNF-α expression was decreased, which would be expected to cause down-regulation of these adhesion molecules, thereby inhibiting neutrophil migration.
Taken together, our data suggest that in the murine model of IgGIC-induced acute lung injury, the complement activation product C5a participates in a regulatory network of cytokine, chemokine, and adhesion molecule expression. By interaction with C5aR, C5a induces strong expression of proinflammatory mediators, which cause neutrophil accumulation and subsequent lung injury. By introducing the silencing C5aR-siRNA into the lung, the interaction of C5a with C5aR was not only interrupted but the whole network of inflammatory response was impaired, thereby ameliorating the harmful effects of C5a and the downstream events. The up-regulation of C5aR expression during the development of lung injury induced by IgG-IC deposition appears to be an essential event in the chain of inflammatory reactions. However, as shown in our recent study (61), the essential role of C5aR for the development of lung inflammation might only be limited to complement activation-driven models, as we did not see a significant role of C5aR in LPS-induced lung injury. Based on the current data, it appears that adenovirus-mediated in vivo silencing of C5aR could serve as a useful tool to study the mechanisms of lung inflammation and might also be an alternative strategy for the treatment of disorders related to complement activation.
Finally, we have shown that the cyclical peptide inhibitor of C5aR (62) attenuates IgGIC-induced lung injury in mice (37). However, this inhibitor has not been commercialized for a variety of reasons. The pharmacokinetics of the compound have not been adequately developed in rodents or humans. Furthermore, phase Ib clinical trials involving patients with rheumatoid arthritis failed to show clinical efficacy (63). Therefore, it is unlikely that this compound will be used in humans, even though in a variety of animal models involving complement-dependent inflammatory diseases in rodents, it was considered efficacious (64, 65). It is possible that “gene therapy” as described in this study might ultimately be tested in humans with pulmonary inflammatory diseases (such as asthma, chronic obstructive pulmonary disease, acute respiratory distress syndrome, and acute lung injury) where there are indications of a C5a presence.
Acknowledgments
This work is supported by the U.S. National Institutes of Health (grants GM-61656 and HL-31963). The authors thank Niels C. Riedemann for his significant assistance with the adenoviral construct and its application to the animal model.
References
- Johnson K J, Ward P A. Acute immunologic pulmonary alveolitis. J Clin Invest. 1974;54:349–357. doi: 10.1172/JCI107770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R F, Ward P A. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- Mulligan M S, Jones M L, Bolanowski M A, Baganoff M P, Deppeler C L, Meyers D M, Ryan U S, Ward P A. Inhibition of lung inflammatory reactions in rats by an anti-human IL-8 antibody. J Immunol. 1993;150:5585–5595. [PubMed] [Google Scholar]
- Mulligan M S, Schmid E, Beck-Schimmer B, Till G O, Friedl H P, Brauer R B, Hugli T E, Miyasaka M, Warner R L, Johnson K J, Ward P A. Requirement and role of C5a in acute lung inflammatory injury in rats. J Clin Invest. 1996;98:503–512. doi: 10.1172/JCI118818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M S, Vaporciyan A A, Warner R L, Jones M L, Foreman K E, Miyasaka M, Todd R F, 3rd, Ward P A. Compartmentalized roles for leukocytic adhesion molecules in lung inflammatory injury. J Immunol. 1995;154:1350–1363. [PubMed] [Google Scholar]
- Shanley T P, Schmal H, Warner R L, Schmid E, Friedl H P, Ward P A. Requirement for C-X-C chemokines (macrophage inflammatory protein-2 and cytokine-induced neutrophil chemoattractant) in IgG immune complex-induced lung injury. J Immunol. 1997;158:3439–3448. [PubMed] [Google Scholar]
- Czermak B J, Lentsch A B, Bless N M, Schmal H, Friedl H P, Ward P A. Synergistic enhancement of chemokine generation and lung injury by C5a or the membrane attack complex of complement. Am J Pathol. 1999;154:1513–1524. doi: 10.1016/S0002-9440(10)65405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czermak B J, Sarma V, Bless N M, Schmal H, Friedl H P, Ward P A. In vitro and in vivo dependency of chemokine generation on C5a and TNF-alpha. J Immunol. 1999;162:2321–2325. [PubMed] [Google Scholar]
- Jagels M A, Daffern P J, Hugli T E. C3a and C5a enhance granulocyte adhesion to endothelial and epithelial cell monolayers: epithelial and endothelial priming is required for C3a-induced eosinophil adhesion. Immunopharmacology. 2000;46:209–222. doi: 10.1016/s0162-3109(99)00178-2. [DOI] [PubMed] [Google Scholar]
- Molad Y, Haines K A, Anderson D C, Buyon J P, Cronstein B N. Immunocomplexes stimulate different signalling events to chemoattractants in the neutrophil and regulate L-selectin and beta 2-integrin expression differently. Biochem J. 1994;299:881–887. doi: 10.1042/bj2990881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czermak B J, Lentsch A B, Bless N M, Schmal H, Friedl H P, Ward P A. Role of complement in in vitro and in vivo lung inflammatory reactions. J Leukoc Biol. 1998;64:40–48. doi: 10.1002/jlb.64.1.40. [DOI] [PubMed] [Google Scholar]
- Mulligan M S, Schmid E, Till G O, Hugli T E, Friedl H P, Roth R A, Ward P A. C5a-dependent up-regulation in vivo of lung vascular P-selectin. J Immunol. 1997;158:1857–1861. [PubMed] [Google Scholar]
- Gerard N P, Gerard C. The chemotactic receptor for human C5a anaphylatoxin. Nature. 1991;349:614–617. doi: 10.1038/349614a0. [DOI] [PubMed] [Google Scholar]
- Amatruda T T, 3rd, Gerard N P, Gerard C, Simon M I. Specific interactions of chemoattractant factor receptors with G-proteins. J Biol Chem. 1993;268:10139–10144. [PubMed] [Google Scholar]
- Siciliano S J, Rollins T E, Springer M S. Interaction between the C5a receptor and Gi in both the membrane-bound and detergent-solubilized states. J Biol Chem. 1990;265:19568–19574. [PubMed] [Google Scholar]
- Chenoweth D E, Goodman M G, Weigle W O. Demonstration of a specific receptor for human C5a anaphylatoxin on murine macrophages. J Exp Med. 1982;156:68–78. doi: 10.1084/jem.156.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth D E, Hugli T E. Demonstration of specific C5a receptor on intact human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1978;75:3943–3947. doi: 10.1073/pnas.75.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werfel T, Oppermann M, Schulze M, Krieger G, Weber M, Gotze O. Binding of fluorescein-labeled anaphylatoxin C5a to human peripheral blood, spleen, and bone marrow leukocytes. Blood. 1992;79:152–160. [PubMed] [Google Scholar]
- Kurimoto Y, de Weck A L, Dahinden C A. Interleukin 3-dependent mediator release in basophils triggered by C5a. J Exp Med. 1989;170:467–479. doi: 10.1084/jem.170.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard N P, Hodges M K, Drazen J M, Weller P F, Gerard C. Characterization of a receptor for C5a anaphylatoxin on human eosinophils. J Biol Chem. 1989;264:1760–1766. [PubMed] [Google Scholar]
- Drouin S M, Kildsgaard J, Haviland J, Zabner J, Jia H P, McCray P B, Jr, Tack B F, Wetsel R A. Expression of the complement anaphylatoxin C3a and C5a receptors on bronchial epithelial and smooth muscle cells in models of sepsis and asthma. J Immunol. 2001;166:2025–2032. doi: 10.4049/jimmunol.166.3.2025. [DOI] [PubMed] [Google Scholar]
- Riedemann N C, Guo R F, Sarma V J, Laudes I J, Huber-Lang M, Warner R L, Albrecht E A, Speyer C L, Ward P A. Expression and function of the C5a receptor in rat alveolar epithelial cells. J Immunol. 2002;168:1919–1925. doi: 10.4049/jimmunol.168.4.1919. [DOI] [PubMed] [Google Scholar]
- Strunk R C, Eidlen D M, Mason R J. Pulmonary alveolar type II epithelial cells synthesize and secrete proteins of the classical and alternative complement pathways. J Clin Invest. 1988;81:1419–1426. doi: 10.1172/JCI113472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayyazi A, Scheel O, Werfel T, Schweyer S, Oppermann M, Gotze O, Radzun H J, Zwirner J. The C5a receptor is expressed in normal renal proximal tubular but not in normal pulmonary or hepatic epithelial cells. Immunology. 2000;99:38–45. doi: 10.1046/j.1365-2567.2000.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasque P, Chan P, Fontaine M, Ischenko A, Lamacz M, Gotze O, Morgan B P. Identification and characterization of the complement C5a anaphylatoxin receptor on human astrocytes. J Immunol. 1995;155:4882–4889. [PubMed] [Google Scholar]
- Buchner R R, Hugli T E, Ember J A, Morgan E L. Expression of functional receptors for human C5a anaphylatoxin (CD88) on the human hepatocellular carcinoma cell line HepG2. Stimulation of acute-phase protein-specific mRNA and protein synthesis by human C5a anaphylatoxin. J Immunol. 1995;155:308–315. [PubMed] [Google Scholar]
- Haviland D L, McCoy R L, Whitehead W T, Akama H, Molmenti E P, Brown A, Haviland J C, Parks W C, Perlmutter D H, Wetsel R A. Cellular expression of the C5a anaphylatoxin receptor (C5aR): demonstration of C5aR on nonmyeloid cells of the liver and lung. J Immunol. 1995;154:1861–1869. [PubMed] [Google Scholar]
- Schieferdecker H L, Rothermel E, Timmermann A, Gotze O, Jungermann K. Anaphylatoxin C5a receptor mRNA is strongly expressed in Kupffer and stellate cells and weakly in sinusoidal endothelial cells but not in hepatocytes of normal rat liver. FEBS Lett. 1997;406:305–309. doi: 10.1016/s0014-5793(97)00292-5. [DOI] [PubMed] [Google Scholar]
- Schlaf G, Schieferdecker H L, Rothermel E, Jungermann K, Gotze O. Differential expression of the C5a receptor on the main cell types of rat liver as demonstrated with a novel monoclonal antibody and by C5a anaphylatoxin-induced Ca2+ release. Lab Invest. 1999;79:1287–1297. [PubMed] [Google Scholar]
- Gasque P, Singhrao S K, Neal J W, Gotze O, Morgan B P. Expression of the receptor for complement C5a (CD88) is up-regulated on reactive astrocytes, microglia, and endothelial cells in the inflamed human central nervous system. Am J Pathol. 1997;150:31–41. [PMC free article] [PubMed] [Google Scholar]
- Wetsel R A. Expression of the complement C5a anaphylatoxin receptor (C5aR) on non-myeloid cells. Immunol Lett. 1995;44:183–187. doi: 10.1016/0165-2478(94)00212-a. [DOI] [PubMed] [Google Scholar]
- Koleva M, Schlaf G, Landmann R, Gotze O, Jungermann K, Schieferdecker H L. Induction of anaphylatoxin C5a receptors in rat hepatocytes by lipopolysaccharide in vivo: mediation by interleukin-6 from Kupffer cells. Gastroenterology. 2002;122:697–708. doi: 10.1053/gast.2002.31883. [DOI] [PubMed] [Google Scholar]
- Zahedi R, Braun M, Wetsel R A, Ault B H, Khan A, Welch T R, Frenzke M, Davis A E. The C5a receptor is expressed by human renal proximal tubular epithelial cells. Clin Exp Immunol. 2000;121:226–233. doi: 10.1046/j.1365-2249.2000.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedemann N C, Guo R F, Neff T A, Laudes I J, Keller K A, Sarma V J, Markiewski M M, Mastellos D, Strey C W, Pierson C L, Lambris J D, Zetoune F S, Ward P A. Increased C5a receptor expression in sepsis. J Clin Invest. 2002;110:101–108. doi: 10.1172/JCI15409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czermak B J, Sarma V, Pierson C L, Warner R L, Huber-Lang M, Bless N M, Schmal H, Friedl H P, Ward P A. Protective effects of C5a blockade in sepsis. Nat Med. 1999;5:788–792. doi: 10.1038/10512. [DOI] [PubMed] [Google Scholar]
- Huber-Lang M, Sarma V J, Lu K T, McGuire S R, Padgaonkar V A, Guo R F, Younkin E M, Kunkel R G, Ding J, Erickson R, Curnutte J T, Ward P A. Role of C5a in multiorgan failure during sepsis. J Immunol. 2001;166:1193–1199. doi: 10.4049/jimmunol.166.2.1193. [DOI] [PubMed] [Google Scholar]
- Huber-Lang M S, Riedeman N C, Sarma J V, Younkin E M, McGuire S R, Laudes I J, Lu K T, Guo R F, Neff T A, Padgaonkar V A, Lambris J D, Spruce L, Mastellos D, Zetoune F S, Ward P A. Protection of innate immunity by C5aR antagonist in septic mice. FASEB J. 2002;16:1567–1574. doi: 10.1096/fj.02-0209com. [DOI] [PubMed] [Google Scholar]
- Bozic C R, Lu B, Hopken U E, Gerard C, Gerard N P. Neurogenic amplification of immune complex inflammation. Science. 1996;273:1722–1725. doi: 10.1126/science.273.5282.1722. [DOI] [PubMed] [Google Scholar]
- Shushakova N, Skokowa J, Schulman J, Baumann U, Zwirner J, Schmidt R E, Gessner J E. C5a anaphylatoxin is a major regulator of activating versus inhibitory FcgammaRs in immune complex-induced lung disease. J Clin Invest. 2002;110:1823–1830. doi: 10.1172/JCI200216577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Gao H, Sarma V J, Guo R F, Ward P A. Adenovirus-mediated in vivo silencing of anaphylatoxin receptor C5aR. J Biomed Biotechnol. 2006;2006:28945. doi: 10.1155/JBB/2006/28945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert T L, Bennett T A, Maestas D C, Cimino D F, Prossnitz E R. Internalization of the human N-formyl peptide and C5a chemoattractant receptors occurs via clathrin-independent mechanisms. Biochemistry. 2001;40:3467–3475. doi: 10.1021/bi001320y. [DOI] [PubMed] [Google Scholar]
- Guo R F, Riedemann N C, Bernacki K D, Sarma V J, Laudes I J, Reuben J S, Younkin E M, Neff T A, Paulauskis J D, Zetoune F S, Ward P A. Neutrophil C5a receptor and the outcome in a rat model of sepsis. FASEB J. 2003;17:1889–1891. doi: 10.1096/fj.03-0009fje. [DOI] [PubMed] [Google Scholar]
- Naik N, Giannini E, Brouchon L, Boulay F. Internalization and recycling of the C5a anaphylatoxin receptor: evidence that the agonist-mediated internalization is modulated by phosphorylation of the C-terminal domain. J Cell Sci. 1997;110:2381–2390. doi: 10.1242/jcs.110.19.2381. [DOI] [PubMed] [Google Scholar]
- Greber U F, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- Huang S, Endo R I, Nemerow G R. Upregulation of integrins alpha v beta 3 and alpha v beta 5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J Virol. 1995;69:2257–2263. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaner R J, Worgall S, Leopold P L, Stolze E, Milano E, Hidaka C, Ramalingam R, Hackett N R, Singh R, Bergelson J, Finberg R, Falck-Pedersen E, Crystal R G. Modification of the genetic program of human alveolar macrophages by adenovirus vectors in vitro is feasible but inefficient, limited in part by the low level of expression of the coxsackie/adenovirus receptor. Am J Respir Cell Mol Biol. 1999;20:361–370. doi: 10.1165/ajrcmb.20.3.3398. [DOI] [PubMed] [Google Scholar]
- Hwang J I, Fraser I D, Choi S, Qin X F, Simon M I. Analysis of C5a-mediated chemotaxis by lentiviral delivery of small interfering RNA. Proc Natl Acad Sci U S A. 2004;101:488–493. doi: 10.1073/pnas.0307549100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomber T, Kalberer C P, Wodnar-Filipowicz A, Skoda R C. Gene silencing by lentivirus-mediated delivery of siRNA in human CD34+ cells. Blood. 2004;103:4511–4513. doi: 10.1182/blood-2003-07-2397. [DOI] [PubMed] [Google Scholar]
- Brummelkamp T R, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002;2:243–247. doi: 10.1016/s1535-6108(02)00122-8. [DOI] [PubMed] [Google Scholar]
- Arts G J, Langemeijer E, Tissingh R, Ma L, Pavliska H, Dokic K, Dooijes R, Mesic E, Clasen R, Michiels F, van der Schueren J, Lambrecht M, Herman S, Brys R, Thys K, Hoffmann M, Tomme P, van Es H. Adenoviral vectors expressing siRNAs for discovery and validation of gene function. Genome Res. 2003;13:2325–2332. doi: 10.1101/gr.1332603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden D, Pusch O, Lee F, Tucker L, Ramratnam B. Efficient gene transfer of HIV-1-specific short hairpin RNA into human lymphocytic cells using recombinant adeno-associated virus vectors. Mol Ther. 2004;9:396–402. doi: 10.1016/j.ymthe.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Xia H, Mao Q, Paulson H L, Davidson B L. siRNA-mediated gene silencing in vitro and in vivo. Nat Biotechnol. 2002;20:1006–1010. doi: 10.1038/nbt739. [DOI] [PubMed] [Google Scholar]
- Zhao L J, Jian H, Zhu H. Specific gene inhibition by adenovirus-mediated expression of small interfering RNA. Gene. 2003;316:137–141. doi: 10.1016/s0378-1119(03)00750-9. [DOI] [PubMed] [Google Scholar]
- Kay M A, Nakai H. Looking into the safety of AAV vectors. Nature. 2003;424:251. doi: 10.1038/424251b. [DOI] [PubMed] [Google Scholar]
- Thomas C E, Ehrhardt A, Kay M A. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- Bonniaud P, Margetts P J, Kolb M, Haberberger T, Kelly M, Robertson J, Gauldie J. Adenoviral gene transfer of connective tissue growth factor in the lung induces transient fibrosis. Am J Respir Crit Care Med. 2003;168:770–778. doi: 10.1164/rccm.200210-1254OC. [DOI] [PubMed] [Google Scholar]
- Gao H, Hoesel L M, Guo R F, Rancilio N J, Sarma J V, Ward P A. Adenoviral-mediated overexpression of SOCS3 enhances IgG immune complex-induced acute lung injury. J Immunol. 2006;177:612–620. doi: 10.4049/jimmunol.177.1.612. [DOI] [PubMed] [Google Scholar]
- Suda T, Tagawa T, Kanaan S A, Kozower B D, Daddi N, Mohanakumar T, Patterson G A. Adenovirus encoding soluble tumor necrosis factor alpha receptor immunoglobulin prolongs gene expression of a cotransfected reporter gene in rat lung. J Thorac Cardiovasc Surg. 2003;126:1155–1161. doi: 10.1016/s0022-5223(03)00939-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Manevich Y, Feinstein S I, Fisher A B. Adenovirus-mediated transfer of the 1-cys peroxiredoxin gene to mouse lung protects against hyperoxic injury. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1188–L1193. doi: 10.1152/ajplung.00288.2003. [DOI] [PubMed] [Google Scholar]
- Ward P A. Rous-Whipple Award Lecture. Role of complement in lung inflammatory injury. Am J Pathol. 1996;149:1081–1086. [PMC free article] [PubMed] [Google Scholar]
- Rittirsch D, Flierl M A, Day D E, Nadeau B A, McGuire S R, Hoesel L M, Ipaktchi K, Zetoune F S, Sarma J V, Leng L, Huber-Lang M S, Neff T A, Bucala R, Ward P A. Acute lung injury induced by lipopolysaccharide is independent of complement activation. J Immunol. 2008;180:7664–7672. doi: 10.4049/jimmunol.180.11.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch A M, Wong A K, Paczkowski N J, Wadi S K, Craik D J, Fairlie D P, Taylor S M. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J Med Chem. 1999;42:1965–1974. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- Vergunst C E, Gerlag D M, Dinant H, Schulz L, Vinkenoog M, Smeets T J, Sanders M E, Reedquist K A, Tak P P. Blocking the receptor for C5a in patients with rheumatoid arthritis does not reduce synovial inflammation. Rheumatology (Oxford) 2007;46:1773–1778. doi: 10.1093/rheumatology/kem222. [DOI] [PubMed] [Google Scholar]
- Arumugam T V, Woodruff T M, Stocks S Z, Proctor L M, Pollitt S, Shiels I A, Reid R C, Fairlie D P, Taylor S M. Protective effect of a human C5a receptor antagonist against hepatic ischaemia-reperfusion injury in rats. J Hepatol. 2004;40:934–941. doi: 10.1016/j.jhep.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Woodruff T M, Costantini K J, Taylor S M, Noakes P G. Role of complement in motor neuron disease: animal models and therapeutic potential of complement inhibitors. Adv Exp Med Biol. 2008;632:143–158. [PubMed] [Google Scholar]