Abstract
The signal transducer and activator of transcription 1 (STAT1) signaling pathway mediates the biological functions of IFN-γ. We have previously shown that the STAT1 pathway is indispensable for host resistance against Leishmania major infection. In this study, we examined the role of STAT1 in lymphocytes and specifically CD4+ and CD8+ T cells in mediating immunity against L. major by transferring T cells from wild-type (WT) and STAT1−/− C57BL/6 mice into Rag2−/− C57BL/6 mice. Rag2−/− mice reconstituted with unfractionated STAT1−/− splenocytes (B cells and T cells) failed to mount an efficient Th1 response after L. major infection, produced more IL-4, and developed large lesions full of parasites. In contrast, Rag2−/− mice reconstituted with WT (STAT1+/+) splenocytes mounted a Th1 response and developed self-resolving lesions. Studies using Rag2−/− recipients that received a combination of purified CD4+ and CD8+ T cells from WT or STAT1−/− mice revealed that STAT1 deficiency in CD4+ T cells, but not in CD8+ T cells, leads to development of chronic, nonhealing lesions and systemic dissemination of parasites into the spleen after L. major infection. Further studies using Rag2−/− recipients of WT Thy1.1+ and STAT1−/− Thy1.2+ T cells showed that STAT1 in CD4+ T cells was not required for Th1 differentiation during L. major infection. However, it was critical for up-regulation of CXCR3 on CD4+ T cells and their migration to the regional lymph node and the cutaneous site of infection. Together, these studies indicate that the STAT1 pathway in CD4+ T cells plays a critical role in immunity against L. major by controlling the migration of Th1 cells to the site of infection rather than their generation. Further, they reveal an essential role for CD4+ T cell STAT1 in preventing systemic dissemination of L. major infection.—Barbi, J., Snider, H. M., Bhardwaj, N., Lezama-Dávila, C. M., Durbin, J. E., Satoskar, A. R. Signal transducer and activator of transcription 1 in T cells plays an indispensable role in immunity to Leishmania major by mediating Th1 cell homing to the site of infection.
Keywords: CXCR3, protozoa
Leishmania are obligate intracellular parasites that infect host phagocytes and cause a wide range of diseases, such as cutaneous, mucocutaneous, and visceral leishmaniasis (1). Clinically, in immunocompetent hosts, cutaneous leishmaniasis (CL) caused by Leishmania major manifests as localized, self-resolving skin lesions that respond well to antileishmanial drug treatments (1, 2). Most inbred mice, including the strain C57BL/6, are resistant to cutaneous L. major infection and develop small self-healing lesions associated with the development of IL-12-induced Th1 responses and IFN-γ production (2,3,4,5,6). The protective role of IFN-γ in CL has been attributed to its ability to promote Th1 development and induce macrophage microbicidal activity (2,3,4,5,6,7).
The signal transducer and activator of transcription 1 (STAT1) signaling pathway is present in a variety of cells of the immune system, including dendritic cells, macrophages, and T cells (8,9,10). The majority of IFN-γ signaling occurs via STAT1-dependent pathways (10). In addition to IFN-γ, other cytokines, such as IFN-αβ and IL-27, can also activate STAT1 (10, 11). Studies using STAT1−/− mice have demonstrated that STAT1 deficiency alters the functions of both CD4+ and CD8+ T cells. A lack of STAT1 prevents efficient induction of T-bet in CD4+ T cells after activation and reduces IFN-γ production (12). In addition, STAT1 is required for IL-27-induced expression of T-bet, IL-12 receptor β, granzyme B, and perforin in CD8+ T cells (13) and CD8+ T cells isolated from Toxoplasma gondii-infected STAT1−/− mice show significant impairments in IFN-γ production (14).
We and others have shown that STAT1−/− mice are highly susceptible to cutaneous L. major infection (15, 16). Susceptibility in STAT1−/− mice is associated with impaired Th1 development and low levels of IFN-γ production during infection (15, 16). Johnson and Scott (15) found that both CD3+ T cells and enriched CD4+ T cells from the spleens of STAT1−/− mice were able to confer resistance against L. major when transferred into Rag2−/− mice, suggesting that STAT1 in T cells is not necessary for immunity against L. major when non-T-cell STAT1 expression is intact. Although CD4+ T cells are essential for immunity against L. major (2), several studies have shown that CD8+ T cells also play a role in resolution of primary L. major infection (17). We therefore compared the roles of STAT1 in both CD4+ and CD8+ T cells during the development of protective immunity against cutaneous leishmaniasis caused by L. major. Our findings demonstrate that STAT1 signaling in CD4+ T cells, but not in CD8+ T cells, is essential for the development of protective immunity against L. major. In addition, they show that while STAT1 signaling in CD4+ T cells is not required for the generation of IFN-γ-producing CD4+ T cells, it is critical in the homing of CD4+ T cells to the site of infection. In addition, we found that STAT1 in CD4+ T cells was essential for preventing systemic dissemination of infection.
MATERIALS AND METHODS
Mice
Six- to 8-wk-old STAT-1−/− C57BL/6 mice were generated as described previously (16). Wild-type (WT) C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Rag2−/− mice were purchased from Taconic Farms, Inc. (Hudson, NY, USA). All mice were kept in specific pathogen-free facilities at Ohio State University according to institutional guidelines.
Parasites
L. major (LV39) was maintained by serial passage of amastigotes into the footpads of BALB/c mice. Amastigotes isolated from infected lesions were grown in M199 medium (Gibco, Grand Island, NY, USA) supplemented with 20% heat-inactivated FCS (HyClone Laboratories, Logan, UT, USA), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine (all Gibco) at 26°C to stationary phase promastigotes as described previously (16).
Infection protocol and quantification of parasite loads
Animal infection was performed by inoculating 2 × 106 L. major stationary phase promastigotes (obtained after peanut agglutinin selection into the hind footpad). Lesion growth was assessed using a dial-gauge micrometer (Mitutoyo, Kanagawa, Japan) at weekly intervals. The thickness of the infected footpad was compared with that of the uninfected contralateral hind footpad. Parasite loads were determined by limiting dilution assay, as reported previously (16).
Adoptive cell transfers
Spleens were removed aseptically from WT and STAT1−/− mice, and single-cell suspensions were prepared by gentle teasing of the tissue in cold, complete RPMI 1640 (Gibco), supplemented with 10% heat inactivated FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine. After washing to remove tissue debris, red blood cells were lysed using Boyle’s solution, and viable cells were counted by trypan blue exclusion. For lymphocyte transfer studies, Rag2−/− mice were injected i.p. with 2 × 107 WT or STAT1−/− splenocytes. These mice were rested for 3 wk after reconstitution before infection with parasites. For CD4+ and CD8+ T-cell adoptive transfer experiments, cell suspensions were prepared from the spleens of WT and STAT1−/− as described above and passed through Nylon Wool Fiber Columns (Polysciences, Inc., Warrington, PA, USA) to remove B cells. CD4+ T cells and CD8+ T cells were then purified from the T-cell suspensions by immunomagnetic separation and negative selection (MiniMACS; Miltenyi Biotec Inc., Auburn, CA, USA). The purity of CD4+ T cells and CD8+ T cells was 94–95%, as assessed by flow cytometry. Viable cells were counted by trypan blue exclusion, and each T-cell population was adjusted to a concentration of 2 × 106 cells/ml. Four sets of T-cell populations were prepared containing 2 × 106 cells of each subset [WT CD4+/WT CD8+, knockout (KO) CD4+/KO CD8+, WT CD4+/KO CD8+, and KO CD4+/WT CD8+] and transferred i.p. into four groups of Rag2−/− recipients. The mice were rested for 3 wk before infection with L. major as described above.
Antibody ELISA
Peripheral blood was collected at 3-wk intervals from L. major-infected Rag2−/− adoptive transfer recipients receiving WT or STAT1−/− spleen cells. Serum was analyzed for Leishmania-specific Th1-associated IgG2a and for Th2-associated IgG1. Levels of these Abs were measured using ELISA as described previously (16).
T-cell proliferation and cytokine ELISA
Draining lymph nodes and spleens were removed under sterile conditions from the reconstituted Rag2−/− mice 8 wk after infection with L. major. Single-cell suspensions were prepared by gentle teasing in complete RPMI 1640 (Gibco). Viable cells were counted using trypan blue exclusion and adjusted to 3 × 106 cells/ml in the same medium; 3 × 105 cells were plated into each well of 96-well flat-bottom plates (Costar, Cambridge, MA, USA) and stimulated with 20 μg/ml of L. major antigen (LmAg) for 72 h at 37°C. Supernatants were collected from these cultures, and the levels of IL-12, IFN-γ, IL-4, and IL-10 were measured by ELISA. All reagents for cytokine ELISA were purchased from BioLegend Inc. (San Diego, CA, USA).
Cotransfer experiments and flow cytometry
For T-cell tracking studies, Thy1.1+ WT and Thy1.2+ STAT1−/− C57BL/6 mice were used as cell donors. T cells were enriched from lymph nodes and spleens and adoptively transferred to Rag2−/− mice before challenge with L. major as described above. Four to 5 wk after footpad infection, the mice were euthanized, and cell suspensions of the draining lymph node and spleen were obtained as mentioned previously. Lesion-infiltrating cells were isolated by mechanical tissue disruption followed by Percoll (GE Healthcare, Piscataway, NJ, USA) gradient centrifugation. For all samples, concentrations were normalized, and surface marker staining was performed using Alexa Fluor 646-labeled anti-Thy1.1 and anti-Thy1.2, phycoerythrin (PE)-Cy5-labeled anti-T-cell receptor, and PE-labeled anti-CXCR3 (Biolegend). Intracellular cytokine staining (ICS) was performed using FITC-conjugated anti-mouse IFN-γ Abs and a kit (purchased from BD Pharmingen, San Diego, CA, USA) as per the manufacturer’s instructions. All analysis was performed on a BD FACS Calibur system using CellQuest Pro software (BD Pharmingen).
Statistical analysis
An unpaired Student’s t test was used to determine the statistical significance of differences observed. The significance of differences in Ab end point titers were determined using the Mann-Whitney U′ test. Values of P < 0.05 were considered significant.
RESULTS
Rag2−/− C57BL/6 mice reconstituted with STAT1-deficient lymphocytes, but not immunocompetent lymphocytes, are susceptible to cutaneous L. major infection
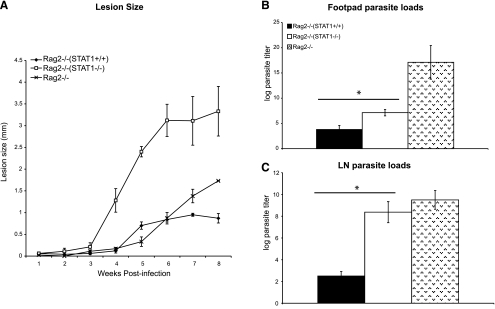
STAT1-deficient mice develop uncontrolled lesion growth and high parasite burdens after infection with L. major (15, 16). This susceptible phenotype is thought to stem from a lack of protective IFN-γ and NO production necessary to kill intracellular parasites (16). To determine whether STAT1 signaling in lymphocytes is required for control of cutaneous leishmaniasis, we compared the course of L. major infection in Rag2−/− mice that were reconstituted with either WT or STAT1-deficient splenocytes. Rag2−/− mice reconstituted with STAT1−/− lymphocytes (Rag2STAT1−/− mice) were highly susceptible to L. major and developed large, nonhealing lesions (Fig. 1A). In contrast, Rag2−/− mice that received WT lymphocytes (Rag2STAT1+/+ mice) developed small lesions after L. major infection, which began healing by wk 8 postinfection (Fig. 1A). At this time, the draining lymph nodes and lesions from Rag2STAT1−/− mice contained significantly more parasites compared with those from Rag2STAT1+/+ mice (Fig. 1B, C). Control Rag2−/− mice developed small lesions, similar to those of Rag2STAT1+/+ mice, but had significantly more parasites in their footpads and draining lymph nodes than the latter (Fig. 1). L. major-infected Rag2STAT1−/− mice displayed lower footpad parasite burdens than unreconstituted Rag2−/− mice, but the lymph node parasite loads were similar in both groups (Fig. 1). Based on these findings, STAT1 signaling in lymphocytes appears to be crucial in the control of L. major infection in C57BL/6 mice.
Figure 1.
Rag2−/− mice reconstituted with STAT1-deficient splenocytes fail to control L. major infection. A) Footpad lesion growth in Rag2−/− mice adoptively transferred with STAT1+/+ or STAT1−/− splenocytes was measured relative to uninfected, contralateral footpads after injection of 2 × 106 L. major promastigotes. B, C) Parasite burdens in the footpad (B) and draining lymph node (C) of infected Rag2−/− mice were determined by a limiting dilution assay and are represented as the log of parasite titer. Unreconstituted Rag2−/− mice were used as controls. All results are expressed as means ± se; n = 6. *P < 0.05.
Rag2−/− C57BL/6 mice reconstituted with STAT1-deficient lymphocytes fail to mount an efficient Th1 response and develop a Th2 response after L. major infection
We and others have previously shown that STAT1−/− C57BL/6 mice fail to mount an efficient Th1 response when infected with L. major (15, 16). However, recent studies have found that STAT1 signaling in T cells is not required for induction of Th1 response during infection with certain intracellular pathogens, including T. gondii and L. major (14, 15). Therefore, we compared serum levels of Leishmania-specific Th1-associated IgG2a and Th2-associated IgG1 antibodies in Rag2STAT1−/− and Rag2STAT+/+ mice after L. major challenge to characterize the T-helper immune response in these mice. In addition, we measured IL-12, IFN-γ, IL-4, and IL-10 production by L. major antigen (LmAg)-stimulated lymph node cells at wk 8 postinfection. At wk 4 and 6 postinfection, the sera of L. major-infected Rag2STAT1+/+ mice contained significantly more LmAg-specific IgG2a compared with that in Rag2STAT1−/− mice, which displayed higher titers of LmAg-specific IgG1 (Fig. 2). After in vitro restimulation with LmAg, the lymph node cells from L. major-infected Rag2STAT1+/+ and Rag2STAT1−/− mice displayed significant and similar degrees of proliferation (data not shown). The lymph node cell culture supernatants from Rag2STAT+/+ mice contained significantly more Th1-associated IFN-γ and IL-12 than those from Rag2STAT1−/− mice (Fig. 2C, D), which produced significantly higher levels of Th2-associated IL-4 and IL-10 (Fig. 2E, F). These results suggest that STAT1 signaling in lymphocytes is important for generating a Th1 response and may be responsible for the resistant phenotype seen in Rag2STAT1+/+ mice infected with L. major.
Figure 2.
Rag2−/− mice reconstituted with STAT1-deficient splenocytes fail to mount efficient Th1 responses against L. major infection. A, B) Antibody levels of IgG1 (A) or IgG2a (B) in the serum of Rag 2−/− mice transferred with unfractionated splenocytes from STAT1+/+ or STAT1−/− mice. C–F) Cytokine production by LmAg-stimulated lymph node cells of infected Rag2−/− mice reconstituted with STAT1+/+ or STAT1−/− cells was measured. At 8 wk postinfection, lymph node cells of reconstituted Rag2−/− mice were harvested and restimulated ex vivo with 20 μg/ml of LmAg for 72 h. Levels of IFN-γ (C), IL-12 (D), IL-4 (E), and IL-10 (F) were measured by ELISA. Data are expressed as means ± se of 3 independent trials; n = 6–7. *P < 0.05.
STAT1 signaling in CD4+ T cells but not in CD8+ T cells is essential for host resistance against L. major infection
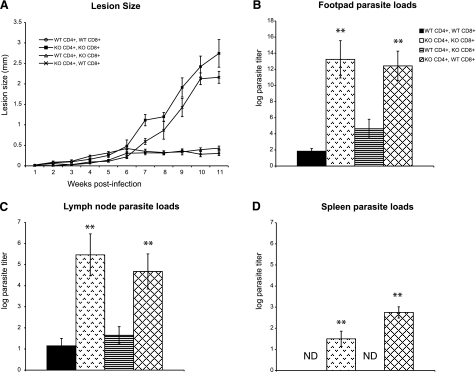
Because we found that STAT1 in lymphocytes was necessary for controlling L. major infection, we examined the specific role of STAT1 in CD4+ vs. CD8+ T cells by using Rag2−/− mice reconstituted with four different combinations of CD4+ and CD8+ T cells from WT or STAT1−/− mice: WT CD4+/WT CD8+, KO CD4+/KO CD8+, WT CD4+/KO CD8+, and KO CD4+/WT CD8+. After footpad inoculation with 2 × 106 L. major, Rag2−/− mice reconstituted with WT CD4+ T cells and CD8+ T cells or WT CD4+ T cells and STAT1−/− CD8+ T cells developed small self-resolving lesions containing significantly fewer parasites than Rag2−/− mice reconstituted with STAT1−/− CD4+ T cells and CD8+ T cells or with STAT1−/− CD4+ T cells and WT CD8+ T cells, which both developed large, nonhealing lesions (Fig. 3A, B). Mice receiving CD4+ T cells with functional STAT1 signaling (WT CD4+/WT CD8+ and WT CD4+/KO CD8+) displayed significantly lower parasite burdens in their lesions and draining lymph nodes compared with mice with STAT1-deficient CD4+ T cells (Fig. 3B, C). Mice receiving STAT1+/+ CD4+ T cells had no detectable parasites in their spleen. In contrast, Rag2−/− mice that received STAT1−/− CD4+ T cells contained significant numbers of parasites in their spleens (Fig. 3D). Interestingly, reconstituting Rag2−/− mice with STAT1−/− CD8+ T cells did not negatively affect the outcome of L. major infection (Fig. 3). As expected, Rag2−/− mice were highly susceptible to infection and developed chronic, nonhealing lesions and contained high number of parasites in their lesions, lymph nodes, and spleen (data not shown). These findings imply that STAT1 signaling in CD4+ cells (but not in CD8+ T cells) is not only important for controlling L. major replication but is also critical for preventing systemic dissemination of parasites.
Figure 3.
Rag2−/− mice reconstituted with STAT1-deficient CD4+ T cells fail to control L. major infection and display systemic spread of infection. Rag2−/− mice received either wild type (WT) or STAT1-deficient (KO) T cells in the following combinations: WT CD4+/WT CD8+, KO CD4+/KO CD8+, WT CD4+/KO CD8+, or KO CD4+/WT CD8+. Several weeks after transfer, these mice were injected in the footpad with 2 × 106 L. major promastigotes. A) Footpad lesion growth was measured relative to the uninfected, contralateral footpad. B–D) Parasite loads in the lesion (B), draining lymph node (C), and spleen (D) of infected Rag2−/− mice were determined by a limiting dilution assay and are represented as the log of parasite titer. All results are expressed as means ± se of 3 independent experiments; n = 4–5/experiment. **P < 0.05. ND, not detected.
Selective deficiency of STAT1 in CD4+ T cells impairs recruitment of IFN-γ-producing T cells to the lesions, but not Th1 development during L. major infection
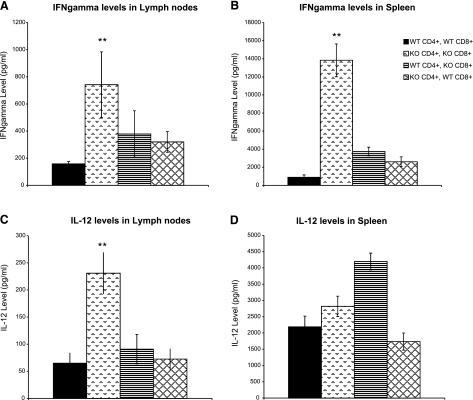
We next investigated the potential mechanism by which STAT1 signaling in CD4+ T cells promotes resistance to L. major in reconstituted Rag2−/− mice. Given the important role played by STAT1 in IFN-γ signaling and antimicrobial NO production (8,9,10), we examined the ability of STAT1-expressing CD4+ and CD8+ T cells to promote a host-protective Th1 response. Interestingly, draining lymph node cells and splenocytes from STAT1−/− CD4+ and CD8+ T-cell-reconstituted Rag2−/− mice (KO CD4+/KO CD8+) produced marginally more IFN-γ than mice receiving WT CD4+ and CD8+ (WT CD4+/WT CD8+) T cells (Fig. 4A, B). Similarly, cells from the draining lymph nodes (Fig. 4C) and spleens (Fig. 4D) of STAT1-deficient T-cell recipients were found to produce higher levels of IL-12 than those from WT T-cell-reconstituted mice. Draining lymph node and splenocyte cultures from mice reconstituted with CD8+ T cells from WT mice and CD4+ T cells from STAT1−/− (KO CD4+/WT CD8+) and their inverse group (WT CD4+/KO CD8+) contained levels of IFN-γ comparable to each other and to those of the wholly WT reconstituted group (Fig. 4A, B). IL-12 production by lymph node cells of these groups followed a similar pattern, but WT CD4+/KO CD8+ spleen cells produced considerably more IL-12 than those of all other groups (Fig. 4D). Cells from the lymph nodes and spleens of all mice examined produced comparatively insignificant levels of IL-4 and IL-10 on ex vivo LmAg stimulation (data not shown). These results suggest that despite being resistant to L. major infection, Rag2−/− mice reconstituted with functional STAT1 signaling in only their CD4+ T cells are not more proficient in Th1 cytokine responses. Furthermore, these results rule out the possibility that STAT1-competent T cells impart resistance to L. major solely on the induction of a Th1 response. Such T-cell STAT1-independent generation of Th1 cells is in agreement with the findings of Johnson and Scott (15), who recently reported the importance of dendritic-cell-specific STAT1 in the induction of Th1 responses to L. major.
Figure 4.
Rag2−/− mice reconstituted with STAT1−/− CD4+ T cells are not impaired in their ability to mount a Th1 response. Th1 cytokine production by LmAg-stimulated lymph node cells (A, C) and splenocytes (B, D) of Rag2−/− mice reconstituted with WT CD4+/WT CD8+, KO CD4+/KO CD8+, WT CD4+/KO CD8+, or KO CD4+/WT CD8+ cells was measured. At 11 wk postinfection, lymph node cells and splenocytes of reconstituted Rag2−/− mice were harvested and restimulated ex vivo with 20 μg/ml of LmAg for 72 h. Levels of IFN-γ (A, B) and IL-12 (C, D) were measured by ELISA. Data are expressed as means ± se of 3 independent trials; n = 4–5/experiment. **P < 0.05.
Because STAT1 and the IFN-γ signaling pathway have been shown to be key for several aspects of CD4+ T-cell trafficking (18, 19), we hypothesized that STAT1 signaling plays a critical role in lesion-homing of Th1 cells and therefore mediates the delivery of protective IFN-γ to the site of infection. To test this hypothesis we coinjected equal numbers of T cells from a WT C57BL/6 mouse expressing the congenic marker Thy1.1 (CD90.1+) along with those from a Thy1.2 (CD90.2+) STAT1−/− donor into a Rag2−/− mouse. Four weeks after footpad challenge with L. major, we analyzed cellular populations from the spleen, draining lymph node, and lesions. Of the T cells successfully recruited to the footpad lesion, WT/Thy1.1+ CD4+ T cells comprised a much larger percentage than STAT1−/−/Thy1.2+ CD4+ T cells (Fig. 5A). Similar results were found in the draining lymph node (Fig. 5B), whereas the difference was not pronounced in the spleen (Fig. 5C). STAT1−/− and STAT1+/+ CD8+ T cells were not markedly different in their distribution (data not shown). These results suggest that effective mobilization of CD4+ T cells to sites of infection during L. major infection is highly dependent on STAT1 signaling in these cells.
Figure 5.
STAT1−/− derived CD4+ T cells fail to migrate to the footpad lesion during L. major infection. T cells from WT (Thy1.1+) and STAT1−/− (Thy1.2+) mice were cotransferred into Rag2−/− mice before infection with L. major. Four weeks later, footpad (A), lymph node (B), and spleen (C) cells were recovered and stained for the congenic markers Thy1.1 or Thy1.2 and other surface markers. Numbers represent the percentage of T cells staining positive for the indicated markers. Results are representative of pooled samples from 3 to 4 mice from one representative experiment of two.
Next, we assessed the cytokine-producing capabilities of these cotransferred cells. Cells were recovered from lesions, lymph nodes, and spleens and restimulated ex vivo. ICS revealed that a greater proportion of footpad-infiltrating WT (Thy1.1+) T cells produced IFN-γ than their STAT1−/− (Thy1.2+) counterparts in the lesion (Fig. 6A). Furthermore, more WT T cells were found in the draining lymph nodes of infected mice, with a significantly higher percent of WT CD4+ T cells stained positive for IFN-γ in these tissues compared with STAT1−/− cells (Fig. 6B). In the spleen, T cells from STAT1−/− mice showed no observable difference in IFN-γ expression compared with WT cells (Fig. 6C). This observation may explain the elevated levels of Th1-associated cytokines mentioned earlier (Fig. 4). These results support the notion that despite mounting a robust Th1 response, Rag2−/− mice reconstituted with STAT1−/− CD4+ T cells are susceptible L. major as a result of impairments in CD4+ T-cell trafficking to the site of infection. Supporting this possibility, WT (Thy1.1+) T cells recovered from the lesion and draining lymph node expressed higher levels of the Th1-associated chemokine receptor CXCR3 than STAT1-deficient T cells (Thy1.2+) (Fig. 7A, B). However, the frequencies of WT (Thy1.1+) and STAT1−/− (Thy1.2+) CD4+ T cells expressing basal levels of CXCR3 were comparable in the spleen (Fig. 7C). This chemokine receptor and its ligands have been shown to be important for Th1-cell trafficking (18, 19). Similar differences in CXCR3 expression were also noted on T cells isolated from WT and STAT1−/− C57BL/6 mice infected with L. major (data not shown).
Figure 6.
Lesions and the lymph nodes but not spleens of Rag2−/− mice reconstituted with STAT1+/+ (Thy1.1+) and STAT1−/− (Thy1.2+) T cells contain significantly higher numbers of IFN-γ-producing STAT1+/+ CD4+ T cells than STAT1-deficient cells. Cells were recovered from the footpad (A), lymph node (B), and spleen (C) of Rag2−/− mice reconstituted with STAT1+/+ (Thy1.1+) and STAT1−/− (Thy1.2+) T cells. Cells were restimulated ex vivo for staining of intracellular IFN-γ, and the percentage of T cells staining positive for this cytokine and either Thy1.1 or Thy1.2 was measured. Results are means ± seof 3 independent trials. **P < 0.05.
Figure 7.
STAT1-deficient CD4+ T cells express significantly lower levels of CXCR3 than STAT1+/+ CD4+ T cells during L. major infection. Cells were recovered from the footpad (A), lymph node (B), and spleen (C) of Rag2−/− mice reconstituted with STAT1+/+ (Thy1.1+) and STAT1−/− (Thy1.2+) T cells. Cells were stained for surface Thy1.1 or Thy1.2 and CXCR3. Numbers represent the percentage of T cells staining positive for the indicated markers. Results are representative of pooled samples from 3 to 4 mice from one representative experiment of two.
DISCUSSION
STAT1 is a key participant in the signaling pathways of several cytokines including IFN-γ (8,9,10). Although this cytokine and the Th1-biased immune responses associated with its production have been shown to contribute to an effective immune response against L. major (16), reports concerning the requirement of STAT1 in the induction of Th1 responses during intracellular infections such as leishmaniasis and toxoplasmosis have generated conflicting results (14, 15). For example, L. major infections do not result in Th1 cellular immune responses in STAT1−/− mice, whereas STAT1−/− mice infected with T. gondii do mount Th1 responses (14, 15). In addition, investigations into the exact role of STAT1 during protective immune responses against L. major have yielded seemingly conflicting results (15, 16).
Here we report that STAT1 signaling, specifically in CD4+ T cells, is required for host resistance to L. major infection. Our results differ somewhat from the findings by Johnson and Scott (15). In that work, the authors found that STAT1 expression in dendritic cells but not in T cells is required for Th1 development and immunity against L. major (15). The disparity between our data and the report of Johnson and Scott is possibly the result of several differences in experimental design. Our studies use a more virulent strain of L. major (LV39). It is possible that a less virulent parasite could be overcome in the absence of CD4+ T-cell STAT1 signaling. In addition, differences such as the route of adoptive transfer (i.p. vs. i.v.) and the time allowed after the transfer before challenge with L. major may account for the apparent discrepancy. Setting aside the differences between this and the previous study, it should be noted that our observation of a T-cell-STAT1-independent Th1 response to L. major is in agreement with the findings of Johnson and Scott (15). Similar to our results, these authors found that considerable amounts of IFN-γ were made by the lymph node cells of Rag2−/− mice reconstituted with STAT1−/− T cells after L. major infection (15). Also, it should be noted that our findings do not exclude an additional contribution of antigen-presenting cell-specific STAT1 signaling toward host resistance.
We also found that Rag2−/− mice reconstituted with unfractionated STAT1−/− splenocytes mounted poor Th1 responses on L. major challenge, whereas recipients of purified STAT1-deficient T cells generated strong, albeit lymphoid tissue-confined, Th1 cytokine responses. This observation could be due to some unknown Th1-inhibiting action mediated by STAT1-deficient splenocytes other than T cells (i.e., dendritic cells, B cells, or others). This is perhaps not surprising because previous studies have shown that antibodies and B cells play a disease-exacerbating role in CL (20, 21). Interestingly, we also found that lesion sizes in Rag2−/− mice did not correlate with the parasite numbers in their skin. Rag2−/− mice displayed smaller lesion sizes than Rag2−/− recipients receiving STAT1−/− spleen cells, but both groups contained comparable and high number of parasites in their lesions. Smaller lesions in L. major-infected Rag2−/− mice are most probably due to the inability of these mice to mount an optimal inflammatory response in the absence of lymphocytes. Further, we also found that Rag2−/− mice reconstituted with STAT1-deficient CD4+ and CD8+ T cells as well as STAT1-deficient CD4+ and WT CD8+ T cells failed to restrict parasite replication in spleen and the lymph nodes despite producing significant amounts of IFN-γ in both these organs. It is likely that high IFN-γ levels in these mice are due to increased IFN-γ production by non-T cells, such as NK cells, dendritic cells, and macrophages, owing to high parasite loads. Indeed, previous studies have shown that antigen-presenting cells including dendritic cells and macrophages produce large amounts of IFN-γ during infections caused by intracellular pathogens such as Listeria (22,23,24). These findings suggest that STAT1 in CD4+ T cells is required to control parasite growth in the spleen and lymph node via an IFN-γ independent mechanism.
Previous studies have shown that STAT1 plays a critical role in regulation trafficking of macrophages (25) and CD4+ T cells (18, 19) during inflammation and infection. In the present study, we also found that STAT1-deficient CD4+ T cells produced significant amounts of IFN-γ after L. major infection, but their recruitment to the infected skin and draining lymph nodes was significantly impaired compared with that for WT CD4+ T cells. This defect in migration of STAT1-deficient CD4+ T cells was similar to that we had reported previously in CXCR3−/− mice infected with L. major (26). CXCR3−/− mice, like STAT1−/− CD4+ T-cell-reconstituted Rag2−/− mice, mount a Th1 response, but recruit fewer IFN-γ-producing T cells into their lesions and fail to overcome L. major infection (26). We had proposed that this was the result of a Th1 cell “bottleneck” effect resulting from impaired migration of IFN-γ-producing T cells to the lesion and their accumulation in the lymph node (26). In the present study, we found that adoptively transferred STAT1-deficient CD4+ T cells express significantly less CXCR3 compared with WT CD4+ T cells. Therefore, it is likely that an inability of STAT1-deficient T cells to up-regulate CXCR3 results in their poor in vivo migration after L. major infection. This finding is perhaps not surprising because previous investigations into CXCR3 regulation have shown that STAT1 is required for CXCR3 induction by CD4+ T cells (27).
CONCLUSIONS
Our findings show that STAT1 signaling in CD4+ T cells may be expendable for the generation of an early Th1 immune response to L. major but remains critical for the proper mobilization of IFN-γ-producing CD4+ T cells to the lesion site and ultimately for the control of parasite growth as well as for preventing systemic spread of infection. Furthermore, they suggest that STAT1 regulates recruitment of CD4+ T cells to the lesion by up-regulating expression of the chemokines receptor CXCR3.
Acknowledgments
N.B. was an M.Sc. student at Ohio State University; C.M.L.D. is an international scholar supported by Fundación Pablo Garcia of the government of Campeche. This work received financial aid from a U.S. National Institutes of Health/National Institute of Allergy and Infectious Diseases grant (R56 AI 068829) to A.R.S. and a National Institute of Dental and Craniofacial Research fellowship (F30 DE020001-0109) to H.M.S. The authors declare no conflicts of interest.
References
- Murray H W, Berman J D, Davies C R, Saravia N G. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- Reiner S L, Locksley R M. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- Heinzel F P, Sadick M D, Mutha S S, Locksley R M. Production of interferon γ, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci U S A. 1991;88:7011–7015. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F P, Ahmed F, Hujer A M, Rerko R M. Immunoregulation of murine leishmaniasis by interleukin-12. Res Immunol. 1995;146:575–581. doi: 10.1016/0923-2494(96)83034-3. [DOI] [PubMed] [Google Scholar]
- Locksley R M, Heinzel F P, Holaday B J, Mutha S S, Reiner S L, Sadick M D. Induction of Th1 and Th2 CD4+ subsets during murine Leishmania major infection. Res Immunol. 1991;142:28–32. doi: 10.1016/0923-2494(91)90007-6. [DOI] [PubMed] [Google Scholar]
- Sher A, Sacks D L, Scott P A. Host and parasite factors influencing the expression of cutaneous leishmaniasis. Ciba Found Symp. 1983;99:174–189. doi: 10.1002/9780470720806.ch10. [DOI] [PubMed] [Google Scholar]
- Liew F Y, O'Donnell C A. Immunology of leishmaniasis. Adv Parasitol. 1993;32:161–259. doi: 10.1016/s0065-308x(08)60208-0. [DOI] [PubMed] [Google Scholar]
- O'Shea J J, Gadina M, Schreiber R D. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109:S121–S131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- Ramana C V, Chatterjee-Kishore M, Nguyen H, Stark G R. Complex roles of Stat1 in regulating gene expression. Oncogene. 2000;19:2619–2627. doi: 10.1038/sj.onc.1203525. [DOI] [PubMed] [Google Scholar]
- Ramana C V, Gil M P, Schreiber R D, Stark G R. Stat1-dependent and -independent pathways in IFN-γ-dependent signaling. Trends Immunol. 2002;23:96–101. doi: 10.1016/s1471-4906(01)02118-4. [DOI] [PubMed] [Google Scholar]
- Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak T W, Yoshimura A, Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- Afkarian M, Sedy J R, Yang J, Jacobson N G, Cereb N, Yang S Y, Murphy T L, Murphy K M. T-bet is a STAT1-induced regulator of IL-12R expression in naïve CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- Morishima N, Owaki T, Asakawa M, Kamiya S, Mizuguchi J, Yoshimoto T. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J Immunol. 2005;175:1686–1693. doi: 10.4049/jimmunol.175.3.1686. [DOI] [PubMed] [Google Scholar]
- Lieberman L A, Banica M, Reiner S L, Hunter C A. STAT1 plays a critical role in the regulation of antimicrobial effector mechanisms, but not in the development of Th1-type responses during toxoplasmosis. J Immunol. 2004;172:457–463. doi: 10.4049/jimmunol.172.1.457. [DOI] [PubMed] [Google Scholar]
- Johnson L M, Scott P. STAT1 expression in dendritic cells, but not T cells, is required for immunity to Leishmania major. J Immunol. 2007;178:7259–7266. doi: 10.4049/jimmunol.178.11.7259. [DOI] [PubMed] [Google Scholar]
- Rosas L E, Keiser T, Pyles R, Durbin J, Satoskar A R. Development of protective immunity against cutaneous leishmaniasis is dependent on STAT1-mediated IFN signaling pathway. Eur J Immunol. 2003;33:1799–1805. doi: 10.1002/eji.200323163. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Von Stebut E, Mendez S, Lira R, Caler E, Bertholet S, Udey M C, Sacks D. CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. J Immunol. 2002;168:3992–4000. doi: 10.4049/jimmunol.168.8.3992. [DOI] [PubMed] [Google Scholar]
- Mikhak Z, Fleming C M, Medoff B D, Thomas S Y, Tager A M, Campanella G S, Luster A D. STAT1 in peripheral tissue differentially regulates homing of antigen-specific Th1 and Th2 cells. J Immunol. 2006;176:4959–4967. doi: 10.4049/jimmunol.176.8.4959. [DOI] [PubMed] [Google Scholar]
- Lord G M, Rao R M, Choe H, Sullivan B M, Lichtman A H, Luscinskas F W, Glimcher L H. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood. 2005;106:3432–3439. doi: 10.1182/blood-2005-04-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane M M, Mosser D M. The role of IL-10 promoting disease progression in leishmaniasis. J Immunol. 2001;166:1141–1147. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]
- Hoerauf A, Solbach W, Lohoff M, Rollinghoff M. The Xid defect determines an improved clinical course of murine leishmaniasis in susceptible mice. Int Immunol. 1994;6:1117–1124. doi: 10.1093/intimm/6.8.1117. [DOI] [PubMed] [Google Scholar]
- Suzue K, Asai T, Takeuchi T, Koyasu S. In vivo role of IFN-γ produced by antigen-presenting cells in early host defense against intracellular pathogens. Eur J Immunol. 2003;33:2666–2675. doi: 10.1002/eji.200323292. [DOI] [PubMed] [Google Scholar]
- Sober D, Schirmbeck R, Reimann J. IL-12/IL-18-dependent IFN-γ release by murine dendritic cells. J Immunol. 2001;167:957–965. doi: 10.4049/jimmunol.167.2.957. [DOI] [PubMed] [Google Scholar]
- Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon γ upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med. 1998;179:1731–1736. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas L E, Snider H M, Barbi J, Satoskar A A, Lugo-Villarino G, Keiser T, Papenfuss T, Durbin J E, Radzioch D, Glimcher L H, Satosakr A R. STAT1 and T-bet play distinct roles in determining outcome of visceral leishmaniasis caused by Leishmania donovani. J Immunol. 2006;177:22–25. doi: 10.4049/jimmunol.177.1.22. [DOI] [PubMed] [Google Scholar]
- Rosas L E, Barbi J, Lu B, Fujiwara Y, Gerard C, Sanders V M, Satoskar A R. CXCR3−/− mice mount an efficient Th1 response but fail to control Leishmania major infection. Eur J Immunol. 2005;35:515–523. doi: 10.1002/eji.200425422. [DOI] [PubMed] [Google Scholar]
- Barbi J, Oghumu S, Lezama-Davila C M, Satoskar A R. IFN-γ and STAT1 are required for efficient induction of CXC chemokine receptor 3 (CXCR3) on CD4+ but not CD8+ T cells. Blood. 2007;110:2215–2216. doi: 10.1182/blood-2007-03-081307. [DOI] [PMC free article] [PubMed] [Google Scholar]