Abstract
Binding of the host complement regulator, factor H (FH), by some pathogenic microbes constitutes an important virulence mechanism, whereby complement is broken down to help microbes survive in the host. Although it has been hypothesized for the past two decades that GBS type III binds FH via sialic acid present on its capsule, neither the binding of FH to GBS has been demonstrated nor the mechanism of interaction identified. We observed that FH bound to both wild-type and capsule or sialic acid-deficient GBS that were used as negative controls. Wild-type and acapsular GBS were incubated with serum or pure FH degraded almost 90% of C3b, suggesting that the GBS-bound FH maintained cofactor activity. In addition, dot-blot analysis showed ∼5–10% of C5 and C9 formation, as compared to an Escherichia coli control, suggesting breakdown at the C3b level. Protease treatment of the bacteria completely abolished binding of FH. Using overlay assays and mass spectroscopic analysis, we identified the FH receptor as the streptococcal histidine triad (SHT) surface protein. The ability of binding FH to SHT was further confirmed by using recombinant SHT. This report describes the identification of the SHT as an FH-binding protein on the surface of GBS type III, revealing a novel mechanism by which the bacterium acquires FH to evade complement opsonization.—Maruvada, R., Prasadarao, N. V., Rubens, C. E. Acquisition of factor H by a novel surface protein on group B Streptococcus promotes complement degradation.
Keywords: streptococcal histidine triad protein, CRASP, protease treatment, LC-MS, pathogenecity islands, membrane attack complex
Group B streptococci type III (GBS) are gram-positive bacteria that cause significant infections in neonates, immunocompromised adults, and individuals with underlying chronic illnesses, resulting in severe morbidity and mortality (1, 2). GBS possess a number of virulence traits, including a well-defined sialylated capsule, CAMP factor, C5a peptidase, and β-hemolysin (for a review, see ref. 3). GBS strains are classified into 9 serotypes based on the type of capsular polysaccharide, of which serotypes Ia, III, and V are responsible for the majority of neonatal infections. In neonates, the major form of defense against GBS requires the deposition of complement C3, with subsequent phagocytotic uptake and killing by PMNs (4, 5). In the absence of capsule-specific antibodies, C3 interacts with bacterial surface components, leading to the activation of the alternative complement pathway. The amount and form of C3 (C3b and its inactive cleaved product, iC3b) deposited on the surface of GBS has been correlated with its survival in the host (6, 7). Marques et al. (6) demonstrated that wild-type GBS (Wt GBS) binds lower levels of active C3b as compared to the capsule mutants and postulated that the lower levels could be due to the binding of factor H (FH) by sialic acid, as has been demonstrated for other microbes, such as Neisseria gonorrhoeae (6, 8).
FH, also known as regulator of complement activation (RCA), controls the amplification of C3 through the alternative pathway in two ways. First, it competes with the binding of factor B to C3b and prevents conversion of additional C3 to C3b (9). Second, it degrades C3b to the inactive form or iC3b by acting as a cofactor for factor I (FI) (10, 11, 12). Structurally, FH is a globular protein with a molecular mass of 150 kDa that is organized into domains called short consensus repeats (SCRs) or complement control proteins (CCPs) (13, 14). SCRs are small bead-like structures made up of 60 aa cross-linked with cysteine bonds. The SCRs of FH can bind different hosts as well as microbial components. The SCRs 1–4, 6–10, and 16–20 bind different domains of C3, and SCRs 7–13, 20 bind heparin and the M6 protein of group A streptococci (GAS), while SCRs 17–20 bind sialic acid (8, 15,16,17).
Pathogenic microbes have been shown to bind FH through a number of specific surface components, such as sialic acid and membrane proteins, generally referred to as complement regulator acquiring surface proteins (CRASPs) (18). Microbes binding FH have demonstrated increased survival in the host due to their capability to inhibit opsonization by C3. Some examples are the pspC-encoded Hic protein of Streptococcus pneumoniae, the streptococcal β protein of GBS type I, CRASP-1 and CRASP-2 of Borrelia burgodorferi, the Por1a protein of Neisseria gonorrhea, and the orthologs and paralogs of the OpsE and BBA68 proteins of spirochetes (19,20,21,22,23,24,25,26,27,28,29). FH binding by B. burgodorferi also accounts for its increased virulence in comparison with Borrelia afzelii, which does not bind FH (30). Of note, GAS has been shown to bind FH through M proteins, such as M6 and M18, to effect complement evasion (21, 31). During our investigation of the binding of FH with Wt GBS and its capsule mutants, we identified a novel surface protein on the bacterium that bound host serum FH. In this study, we describe the identity of this protein and characterize the role of FH binding of GBS on complement breakdown.
MATERIALS AND METHODS
Bacterial strains and culture
Bacterial strains used in these studies are as described in Table 1. Type III GBS strain COH1, referred to as Wt GBS, is an encapsulated virulent strain isolated from an infected infant (32), COH1-13 is an acapsular mutant of COH1, in which cpsE has been inactivated through a transposon insertion (33), while COH1-350 produces an asialo-capsule (capsule lacking sialic acid) due to a mutation in cpsK, a gene that facilitates the transfer of sialic acid to the polysaccharide backbone (34). Escherichia coli strains K1 (35) and Bl21 were cultured in Luria broth, while GBS were grown in Todd Hewitt broth (Oxoid, Lenexa, KS, USA) with the appropriate antibiotic at 37°C to either log or stationary phase and used in the experiments described below.
TABLE 1.
GBS strains, plasmids, and primers used in this study
| Strain or plasmid | Genotype/phenotype | Reference |
|---|---|---|
| COH1 | cpsIII, Tetr/wild type | 32 |
| COH1-13 | COH1, Tn916 E cpsE/CPS Tetr Emr | 33 |
| COH1-350 | COH1; cpsK Tetr Cmr | 34 |
| E. coli K1 BL21 | F− ompT hsdS (r− m−) gal dcm | |
| Plasmid vectors | ||
| pET-32a(+) | Promoter: T7 lac; tags: Trx His; selection: Amp | |
| Primers | ||
| Forward sht (shtfor) | GGAGGACCATGGTGAAGAAAACATATGGTTA | |
| Reverse sht (shtrev) | GGAGGACTCGAGAGGGTTTATTTGTTGA |
Depletion of IgG from pooled human serum
To ensure that deposition of complement on GBS is restricted to the alternative pathway of C3 activation, IgG was depleted from pooled human serum collected from healthy volunteers (according to the IRB policies of Children’s Hospital, Seattle, WA, USA) by using a protein A agarose column (Bio-Rad, Hercules, CA, USA) as per manufacturer’s instructions. The flow-through was concentrated with an Ultra-15 centrifugal filter (Amicon, Temecula, CA, USA) to restore the serum back to its starting volume. Removal of IgG from the serum was confirmed by Western blot analysis using goat anti-human IgG-labeled horseradish peroxidase (HRP) conjugate (KP Labs, Washington, DC, USA), while its complement activity was assessed using CH50 assays, which were performed at the Department of Pathology at the University of Washington, Seattle (36, 37). In addition, the relative amounts of FH and complement C3b were compared with the starting serum semiquantitatively by SDS-PAGE and Western blot analysis to ensure there was no loss during the IgG depletion process.
Protease treatment of GBS
Approximately 109 cfu of COH1, COH1-13, and COH1-350 were either untreated or treated with 0.025% trypsin (Sigma, St. Louis, MO, USA), or 0.05% of Proteinase K (Sigma) at 37°C for 1 h. The protease-treated bacteria were washed 4 times with PBS containing 2 mM PMSF to inactivate bound enzyme and then incubated with 200 μl of 75 μg/ml FH in PBS at RT for 1 h. Bound FH was eluted with 20 mM glycine (pH 2.0) and analyzed by Western blotting using an anti-FH antibody (Calbiochem, San Diego, CA, USA).
Analysis of FH binding to GBS
COH1, COH1-13, and COH1-350 were grown to OD600 = 0.6 and washed twice with 10 mM PBS. COH1 (1×108 cfu) was incubated with 0–75 μg/ml of purified FH (Calbiochem) for 1 h at RT. COH1-13 and COH1-350 were similarly incubated with 25 μg/ml FH and served as negative controls (as it was understood that the mutants would not bind FH). The bacteria were washed 3 times with PBS, and bound FH was eluted with 20 mM glycine (pH 2.0) and analyzed by Western blot, as described below. In addition to purified FH, IgG-depleted pooled normal human serum diluted to 40% in gelatin veronal buffer-EDTA (GVB-EDTA) was used to study binding of FH to GBS.
SDS-PAGE, Western blot, overlay assays, and dot-blot analysis of complement bound to GBS
FH and the complement proteins (C3, C5, and C9) deposited onto equal colony-forming units of GBS were analyzed by SDS-PAGE and Western blot (FH and C3) or by dot-blot analysis (C3, C5, and C9). FH and C3 bound to the bacteria were analyzed by boiling in SDS-PAGE sample buffer and resolving by electrophoresis, then transferred to nitrocellulose membranes. For dot-blot analysis, equal numbers of bacteria previously incubated in serum were directly spotted onto nitrocellulose membranes, blocked with 5% milk, and then incubated with specific anti-FH, C3, C5, or C9 antibodies (Calbiochem), followed by either anti-rabbit (Bio-Rad, Hercules CA, USA) or anti-goat secondary antibody (Sigma) conjugated to HRP. Bound enzyme was detected using a chemiluminescent substrate (Super Signal West Pico; Pierce, Rockford, IL, USA), and the luminescence was captured on light-sensitive film. Spotting efficiency of Wt GBS onto nitrocellulose was assessed by probing bound COH1 strains with a GBS type III capsule antibody (Statens Serum Institute, Copenhagen, Denmark) at a concentration of 1:5000, followed by an anti-rabbit HRP-labeled antibody (Bio-Rad) at a concentration of 1:10,000.
For overlay assays, nitrocellulose membranes bearing the bacterial proteins were first incubated either in pooled IgG-depleted normal human serum or FH at appropriate concentrations in GVB-EDTA.
Analysis of C3b breakdown by FH bound to GBS
The cofactor activity of FH to inactivate C3 when bound to GBS was analyzed by examining the breakdown of C3b in solution in the presence of FI. Briefly, COH1 or COH1-13 was cultured and split into 4 separate sets, each containing ∼5 × 108 cfu of bacteria. Two sets of each strain were incubated with 500 μl of 20 μg/ml of purified FH. The FH-incubated GBS were then washed 4 times with PBS and incubated with 1 μg of purified C3b and FI (Calbiochem) in 50 μl of GVB at 37°C for 1 h. The bacteria were pelleted by centrifugation, and supernatants were subjected to Western blot analysis using specific anti-C3 antibody that recognizes both the α and the β chains of C3b (Calbiochem).
Extraction and purification of the FH-binding GBS surface protein (FHBP)
Broth cultures (50 ml) of COH1, COH1-13, and COH1-350 grown overnight were washed 3 times in PBS and incubated in 2 ml of 20 mM glycine, pH 11, for 20 h at 60°C (22). The bacteria were centrifuged at 10,000 rpm, and 50 μl of the supernatants was subjected to SDS-PAGE. Western blot analysis using serum overlays was performed to assess FH binding to bacterial surface proteins.
To purify the FHBP, surface proteins obtained by alkaline extraction of COH1 were applied to a DEAE-Sepharose anion exchange column (Amersham Biosciences, Piscataway, NJ, USA) with a packed length of 7 cm in sample buffer containing 20 mM Tris, pH 8.2. Proteins bound to the column were eluted in a linear gradient of increasing salt concentration from 0–2 M NaCl. Fractions, collected in 1.5-ml volumes, were analyzed for the presence of FHBP by resolving the fractions by SDS-PAGE and Western blot analysis using FH overlay.
Protein estimation and identification of the FHBP in the DEAE fractions by FH capture
Fractions eluted from the DEAE column were quantified for protein by the Micro-BCA protein assay (Pierce; sensitivity of protein detection of 50 μg/ml). The FHBP-containing DEAE fraction was identified by incubating 50 μl of each fraction in a 96-well microtiter plate (Costar, Lowell, MA, USA) containing 150 μl of sodium bicarbonate buffer for 1 h at 37°C. Plates were washed twice and further incubated with 200 μl of 40% pooled human serum per well. The plates were washed again, and FH bound to the bacterial proteins in the microtiter wells was detected using 1:5000 anti-FH antibody followed by 1:10,000 rabbit anti-goat HRP-conjugated antibody. Color developed by the addition of the TMB substrate (KP Laboratories, Gaithersburg, MD, USA) was measured at 450 nm.
LC-MS analysis of the FHBP
LC-MS analysis (Proteomics Division of the Fred Hutchinson Cancer Research Center, Seattle, WA, USA) was conducted on FHBP, which was purified by affinity chromatography, resolved on a 10% SDS-PAGE gel, and excised from the gel. Briefly, the protein-containing gel slice was digested in 0.025% trypsin in ammonium bicarbonate buffer for 45 min on ice followed by overnight at 37°C (38). The digested sample was desalted using a microC18 ZipTip (Millipore, Temecula, CA, USA), vacuum-dried, resuspended in 5 μl of 0.1% TFA, and analyzed by LC/ESI MS/MS with an LCQ mass spectrometer (ThermoElectron, Pittsburgh, PA, USA), as described previously (39). Results were collected in a data-dependent mode in which an MS scan was followed by MS/MS scans of the three most abundant ions from the preceding MS scan. MS peptide data were used to search the Streptococcus agalactiae NEM316 protein database downloaded from the Institute for Genome Research (TIGR; http://www.tigr.org) using the software search algorithm COMET (Institute for Systems Biology, Seattle, WA, USA). Peptide identification was considered valid if ≥2 peptides were identified for a specific protein and if the peptide matches had raw scores >200 for +1 ions, 300 for +2 ions, and 300 for +3 ions, Z scores greater than 4, and % ions greater than 15%. A blank portion of the gel was included as a negative control.
Cloning and expression of the streptococcal histidine triad (SHT) protein
The sht gene was amplified by PCR using Wt GBS DNA as template and the forward primer—shtFor (see Table 1 for sequence)—containing an NcoI restriction site and the reverse primer shtRev containing an XhoI site (generated at Integrated DNA Technologies, Coralville, IA, USA). PCR was performed for 30 cycles using Bio-X-ACT short mix (Bioline, Taunton, MA, USA) at an annealing temperature of 54°C, after which the amplified product was ligated into the pET32a cloning vector (Novagen, San Diego, CA, USA). The resulting clone was then electroporated into BL21 cells, and transformed colonies bearing the sht gene were identified by colony PCR using the gene-specific primers (40). Plasmid DNA from clones positive for sht by colony PCR was isolated and analyzed for the orientation and size of the inserted amplicon by digestion with NcoI and XhoI and sequenced at the Seattle Biomedical Research Institute (Seattle, WA, USA), using the shtFor and shtRev primers. Sequence analysis was done using DNAlign software (available at http://genome. cs.mtu.edu/align). The sequence was then compared with the sht sequence of the S. agalactiae NEM316 database present in the TIGR database.
To express the SHT fusion protein, positive BL21 colonies were grown to log phase and induced with 10 mM IPTG (Mediatech, Manassas, VA, USA). Recombinant His-tagged SHT protein was then purified from crude bacterial lysates on Ni-NTA agarose beads (Sigma) and assayed for FH binding by Western blot using serum overlay assays, as described previously. Lysates from transformed uninduced BL21, as well as those containing the pET32a vector alone, were used as negative controls.
RESULTS
GBS binds FH in a dose-dependent manner
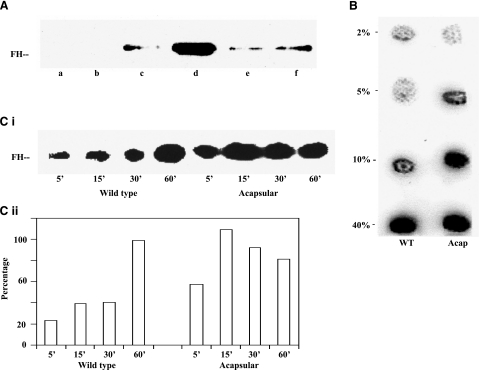
It has been hypothesized that GBS binds FH via the sialic acid moiety present on capsular polysaccharide and that bound FH plays an important role in the evasion of host complement by the microbe (7). To test this hypothesis, Wt GBS, the isogenic acapsular (COH1-13) and the asialo-capsular mutants (COH1-350) were incubated with increasing concentrations of pure FH (25 to 75 μg/ml) and the relative binding of FH among the strains was compared. Analysis of eluted FH from bound bacteria by Western blot analysis demonstrated that the acquisition of FH by the bacteria was dependant on the concentration of FH in solution (Fig. 1A). Wt GBS demonstrated FH binding at concentrations of 50 μg/ml or more. Surprisingly, the acapsular and the asialo strains of GBS (included as negative controls for binding FH), bound FH at lower concentrations (25 μg/ml), suggesting they bind FH more avidly than the wild-type encapsulated GBS. Similar results were obtained when the wild-type and acapsular bacteria were incubated in various concentrations of human serum (2, 5, 10, and 40%) and were analyzed by immunodot blotting (Fig. 1B, lanes a and b). To rule out the possibility that Wt GBS bound less to the nitrocellulose membrane, bacteria incubated in serum, spotted onto membrane, and examined with a capsule-specific antibody showed strong binding, suggesting that the differences observed in FH binding were not due to loading and/or variable binding of bacteria on the membrane (data not shown).
Figure 1.
Binding of FH to Type III GBS. A) Wt GBS (lanes a–d) was incubated with 0, 25, 50, and 75 μg/ml FH, while the asialo (lane e) and acapsular mutants of GBS (lane f) were incubated with 25 μg/ml FH. Bound protein was then released and subjected to Western blot analysis using anti-FH antibody. B) Wild-type (lane a) or acapsular GBS (lane b) incubated with varying concentrations of pooled human serum for 15 min, washed, spotted onto nitrocellulose membranes, and then assayed for bound FH using anti-FH antibody. C) i) Wild-type and acapsular GBS were incubated in 40% NHS for time points ranging from 5 to 60 min. Bacteria were boiled in SDS-PAGE sample buffer, resolved by electrophoresis, and analyzed for the deposition of FH by Western blotting. ii) Amounts of FH bound were estimated by densitometry, using the NIH ImageJ software, and plotted with time of incubation.
As both the wild-type and acapsular GBS bound FH, the kinetics of FH binding to GBS over time was investigated. 108 cfu of Wt GBS and of the acapsular mutant were incubated in 40% normal human serum for 5 to 60 min. Analysis of bound FH by Wt GBS, achieved by boiling the bacteria and resolving the supernatants by SDS-PAGE and Western blotting, showed increased binding of FH over time. However, acapsular GBS demonstrated higher and more rapid binding of FH than Wt GBS. Densitometric analysis showed acapsular GBS to approach saturation levels for binding FH within 15 min, as compared to the 60 min observed for Wt GBS (Fig. 1Ci, ii).
FH bound to GBS maintains cofactor activity and degrades C3b
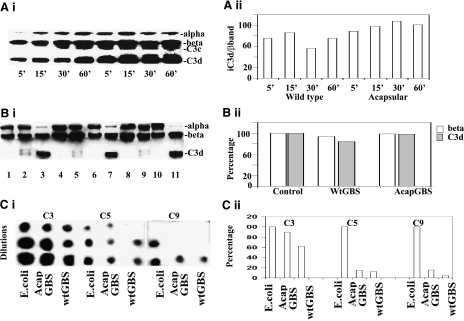
GBS is known to activate the third component of complement through the alternative pathway in which FH acts as a cofactor to FI in degrading C3b (5, 6, 7). These studies suggested that deposition of complement C3b on Wt GBS was regulated by the capsule, leading to a hypothesis that the capsule binds FH to break down or inactivate C3b into various smaller chains, such as C3c (iC3b) and C3d. However, knowledge about deposition and breakdown of complement in Wt GBS as compared to acapsular GBS is still lacking. Therefore, to better understand the role of bacterial-bound FH in C3b inactivation, wild-type and acapsular GBS, incubated in serum for various time points, were boiled in SDS-PAGE sample buffer, and the amounts of C3b and iC3b were assessed by Western blot analysis using a specific C3b antibody that recognized both the α and β chains of C3b. Initially, Wt GBS showed little or no active C3b (represented by the α chain) with most of the bound complement being quickly degraded to the inactive C3d form, suggesting rapid inactivation due to brisk cofactor activity of FH (Fig. 2Ai). However, on prolonged incubation in serum, a small amount of active C3b could be detected, with the larger proportion being converted to either the C3d or the C3c forms (both forms together constitute the inactive or iC3b). The acapsular GBS bound 20–30% more C3b than wild type, as reported earlier (6). However, ∼50–60% more of C3c and C3d could also be observed over time on the mutant strain, as shown by densitometric analysis of the C3d band with respect to the β band (taken as a measure of total complement) (Fig. 2Aii). This suggested that although the deposition of complement was higher on the acapsular strain than on the Wt GBS, degradation was also greater, indicating that higher levels of FH were present on the acapsular strain, as noted in the earlier experiment.
Figure 2.
Analysis of cofactor activity of FH bound to GBS. A) To analyze the cofactor activity of FH-bound GBS, wild-type and acapsular GBS were incubated in 40% NHS for various time points, boiled, and analyzed for the form by SDS-PAGE and Western blotting (i), and the relative amounts of complement C3 were assessed by densitometry and expressed as a ratio of C3d to the β band, a measure of the amount of deposition and breakdown (ii). B) i) To further test the ability of GBS-bound FH to break down C3b in solution, experiments were done with pure FH, C3b, and FI in solution. Equal colony-forming units of either wild-type or acapsular GBS were incubated with pure FH and then washed and incubated with pure C3b in the presence of FI. Bacteria were then removed by centrifugation, and 25 μl of the supernatants was subjected to Western blot analysis with an anti-C3b antibody. Lanes 1–3 show purified C3b, C3b + FI and C3b +FH + FI, respectively. Lane 3 shows complete degradation of C3b in the presence of FH and FI. Similarly, wild-type (lane 9) and acapsular GBS (lane 11) incubated with pure FH and C3b in the presence of FI show degradation of C3b similar to that of the control lane (lane 3). Other combinations were also included, in which wild-type and acapsular GBS were not incubated either with FI (lanes 8 and 10) or FH (lanes 5 and 7). ii) Amounts of C3d band and β band of lanes 3, 7, and 11 were estimated by densitometry and graphed. C) i) To demonstrate that the complement cascade was inhibited from forming because of the inactivation of C3b, the amounts of C3b, C5, and C9 deposited on the bacteria after incubation in serum were examined by dot-blot analysis using specific antibodies. E. coli K1 served as a positive control for C3b and C5 deposition, as well as C9 formation. ii) Amounts of C3, C5, and C9 in Wt GBS and acapsular GBS (AcapGBS) were estimated by densitometry and plotted in comparison to complement components formed in E. coli.
Next, the cofactor activity of GBS-bound FH using purified components was assessed. Briefly, wild-type or acapsular GBS bound to purified FH were incubated with purified C3 and FI in various combinations (as described in Materials and Methods). The bacteria were removed by centrifugation, and the supernatants were analyzed for the breakdown of C3b by Western blot analysis using a specific anti-C3b antibody (Fig. 2Bi). Appropriate control samples containing C3 but lacking either FH or FI were included (Fig. 2Bi, lanes 1 and 2). Neither the wild-type nor the acapsular GBS could degrade C3b in the presence of FI alone (Fig. 2Bi, lanes 5 and 9). However, ∼90–95% degradation was observed when the bacteria were first incubated with FH and then incubated with C3b and FI (Fig. 2Bi, lanes 7 and 11). The degradation was similar to that observed when using purified C3b and FI along with FH without any bacteria (Fig 2Bi, lane 3). Densitometric analysis showed similar amounts of degraded product on acapsular strain as compared with control, while the wild type showed ∼90% deposition of C3 and ∼75% degradation to the inactive C3d chain (Fig. 2Bii). The above experiments strongly suggested that FH bound to the surface of type III GBS exhibits specific cofactor activity for FI in degrading C3b.
To further prove that C3b was indeed being inactivated, further components in the membrane attack complex downstream of C3b, such as the intermediate component C5 and the terminal component C9 were analyzed, since these components would bear a direct proportion to the amount of bound active or inactive C3b. Hence, the amounts of C3b, C5, and C9 deposited on wild type and acapsular GBS, on incubation with serum, was analyzed by the immunodot blot technique using specific antibodies (Fig. 2Ci). E. coli K1 was included as a control to compare C3b, C5, and C9 deposition as previously demonstrated (41). Densitometric analysis showed ∼90% of C3b deposition on the acapsular strain as compared to E. coli control, while the amount of C3b detected on Wt GBS was considerably lower at ∼60% (Fig. 2Cii). However, the levels of C5 and C9 were only 5–10% on both strains of GBS as compared to that of E. coli. Although this demonstrated that the major portion of deposited C3b was degraded, it also suggested that a small amount of MAC complex does form on gram-positive GBS. However, the small amounts of MAC might not be very effective in killing, especially the encapsulated strains.
Identification and characterization of the FHBP on GBS
As Wt GBS together with the acapsular and asialo-mutant bound FH, attempts were made to identify the receptor since capsule was not the FH receptor. To analyze whether the receptor was a surface protein, the three isogenic strains were treated either with trypsin or proteinase K, washed thoroughly by centrifugation using protease inhibitors, and then incubated with FH. Protease treatment dramatically reduced FH binding to all three strains as compared to controls that were not treated with either enzyme, suggesting that a surface protein of GBS binds FH (Fig. 3A).
Figure 3.
GBS binds FH via 110-kDa surface protein. A) Wild-type, acapsular (Acap), and asialo-capsule mutants of GBS were treated either with trypsin or proteinase K, washed, and then incubated with 200 μl of 75 μg/ml FH for 1 h. GBS not treated with enzymes were included as controls. Bound FH was eluted and subjected to Western blot analysis using an anti-FH antibody. B) Surface proteins of wild-type, acapsular, and asialo GBS extracted by alkaline proteolysis were resolved by electrophoresis, transferred to nitrocellulose membranes, and incubated with IgG-depleted human serum in GVB-EDTA buffer. FH bound by the bacterial proteins was detected using a specific FH antibody (blot A). A similar membrane bearing the surface proteins but not exposed to FH served as a negative control (blot B). C) To purify the putative FHBP, alkaline extracts of Wt GBS were subjected to DEAE-Sepharose column chromatography. Proteins bound to Sepharose were eluted in a linear gradient of sodium chloride and subjected to Western blot analysis using FH overlays to identify the FHBP-containing fractions. Arrows indicate FH-binding surface protein of GBS. D) The sht gene was cloned and ligated into the pET32a expression vector. Ligated gene products were then analyzed by agarose gel electrophoresis. Lane a: undigested pET32a vector. Lane b: linearized pET32a digested with the enzymes. Lane c: pET32a containing the sht gene fragment. Lane d: pET32a containing the sht gene fragment digested with NcoI and XhoI to release the sht fragment. Lane e: 1-kb DNA ladder. E) Crude lysate of BL21 expressing SHT as a fusion protein having a 6x His tag was examined by SDS-PAGE, followed by Coomassie blue staining. The protein was also purified on a Ni-NTA column and compared with crude lysate. F) Recombinant SHT was assessed for FH binding using FH overlay assays. Lane a: lysates from BL21 transformed with the pET32a vector and induced with IPTG. Lane b: lysates from BL21 transformed with pRMsht (pET32a+sht) but not induced with IPTG. Lane c: recombinant SHT protein purified on Ni-NTA agarose beads. Lane d: crude BL21 lysates expressing recombinant SHT protein. Arrows indicate SHT protein.
To identify the FHBP, surface proteins from all three strains of GBS were extracted with alkaline glycine, resolved by SDS-PAGE, and subjected to Western blot analysis using a serum overlay (as described in Materials and Methods). Western blot, using an anti-human FH antibody to identify bound FH, showed reactivity to a bacterial surface protein with an approximate molecular mass of 110 kDa in all three strains, suggesting the protein to be an FHBP (Fig. 3B). A control membrane containing bacterial surface proteins not exposed to serum but incubated with the primary and secondary antibodies did not show reactivity, suggesting that binding of FH to the 110-kDa bacterial protein was specific. These results also indicated that acapsular and asialo-strains bound FH through a surface protein similar to that of Wt GBS.
To characterize the 110-kDa FHBP, alkaline extracts of Wt GBS were subjected to partial purification by DEAE sepharose chromatography. Briefly, surface proteins of COH1 obtained by alkaline extraction were applied to a DEAE Sepharose column, and the bound proteins were fractionated in a linear gradient of NaCl. Western blot analysis of the fractions showed the FHBP to elute in 250 mM of sodium chloride gradient (Fig. 3C). LC-MS analysis of the protein revealed a high-sequence homology to at ≥3 surface proteins of GBS Type III when compared to the S. agalactiae NEM316 database. Two of the proteins were the cell-surface-associated protein (cspA) and the GBS C5a peptidase (scpB). The third protein shared homology to the yet uncharacterized SHT protein. The role of cspA and scpB in virulence was earlier characterized (42, 43). However, incubation of either cspA or scpB lacking GBS mutants still showed binding of FH to the bacteria. Hence, the binding of FH to the third protein, SHT, was examined.
To investigate the ability of SHT to bind FH, the sht gene was first expressed in E. coli. Briefly, the sht gene was amplified from COH1 genomic DNA, cloned downstream of the LacZ promoter in the pet32a vector, and the construct expressed in the electrocompetent BL21 E. coli strain. Restriction digest analysis of the recombinant plasmid, pRMsht bearing the sht gene yielded two fragments with molecular masses of 2.5 and 5.9 kB, corresponding to the original sht amplicon and the linear pET32a vector, respectively (Fig. 3D). Sequencing of pRMsht showed proper orientation of the inserted gene in the vector and a complete homology to the sequence of the gbs1306 gene of S. agalactiae (NEM316) in the TIGR database.
Next, the expression of the protein and its ability to bind FH was assessed. Briefly, lysates of IPTG-induced BL21 bearing the pRMsht plasmid were resolved by SDS-PAGE along with those of uninduced BL21 with or without the vector. A fusion protein of ∼116 kDa representing the protein and the vector tags was observed, as predicted according to the molecular mass of the protein (Fig. 3E). This protein was absent in lysates from uninduced BL21 with or without the vector (data not shown). Western blot analysis of the recombinant protein by serum overlays using anti-FH antibody demonstrated binding of FH to the SHT protein in both induced crude lysates, as well as the purified protein, while the negative controls did not react to the FH antibody (Fig. 3F). Taken together, these results provide further evidence that the GBS surface protein SHT binds FH.
Positional characteristics of the sht gene on the GBS genome and prevalence of the gene in closely related serotypes
An analysis of the GBS type III genome (S. agalactiae NEM316) showed the sht gene to be present downstream of the scpB and the lmb genes (Fig. 4A) and upstream of the degenerate transposases gbs1304 and gbs1305. The complete locus lies in one of the several “pathogenecity islands,” a term for closely linked stretches of genetic material known for harboring virulence genes. Similar to the sht gene product, both the lmb and the scpB gene products are known virulence genes encoding surface proteins that interact with the host cell surface.
Figure 4.
Position of the sht gene and its comparison with a similar gene in the FH-binding GAS strain. A) Diagrammatic representation of the sht gene relative to other genes, scpB and lmb, in the pathogenecity island[b]. B) Comparison of the sht-containing locus in various serotypes of GBS type III. C) A similar comparison with that of the emm18 gene of GAS (M18) strain previously documented to bind FH.
The sht gene of COH1 was then compared among various serotypes of S agalactiae, including NEM316, 515, CJB111, H36B, A909, 18RS21, and 2603V/R (Fig. 4B). An analysis of the genetic sequence showed the sht gene to share a homology of ∼80–90% in the gene, as well as the protein sequence. However, in serotype H36B, the gene was truncated by ∼25%. In addition, gene sequences of serotypes 515 and 18RS21 sequences suggested that the gene was capable of bidirectional transcription and probably encodes two different protein products.
The sequence of the sht gene of COH1 was further compared with the M18 FH binding protein from GAS. The sht of COH1 shared a 98% homology with the sht gene of GAS; however, no function has been attributed to it to date, while M18 has been shown to bind FH. Similar to the sht gene in GBS serotypes, the emm18 gene (M18) lies in proximity to the scpB, lmb, and sht genes in GAS, suggesting that a functional relationship might exist between the FHBP-encoding genes and scpB and lmb (Fig. 4C).
An analysis by SMART (Simple Modular Architecture Research Tool; http://smart.embl-heidelberg.de) showed the 822-aa SHT protein to contain 4 putative histidine triad domains and a transmembrane domain. Members of this family of proteins are characterized by having an HxxHxH motif (where H is histidine) that occur multiple times in the protein. The histidine triad-containing protein PhpA of S. pneumoniae has been characterized to cleave C3 in S. pneumoniae. Vaccine studies have shown the PhpA to act as a protective antigen in mice (44). In Pneumococcus sp., the histidine triad protein (PHT) has been identified as a virulence factor of the pathogen and possesses 3 histidine triad domains in comparison to 4 of SHT. Similar to GAS M18, a separate protein, PspC has been shown to bind FH in Pneumococcus sp (29). However, no sequence homology at the gene or protein level was observed between PspC and SHT.
Overall, results in this study unambiguously demonstrate that GBS binds FH through a surface protein to inactivate complement C3b.
DISCUSSION
GBS binds FH to break down complement
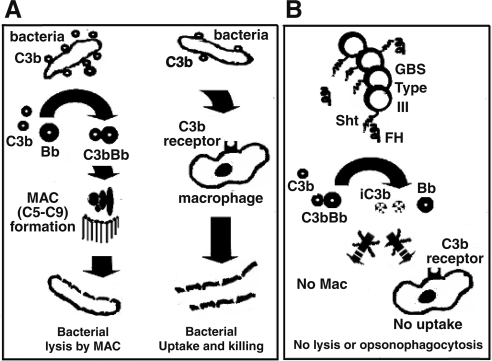
Our present study demonstrated that in addition to Wt GBS, the asialo as well as the acapsular mutants also bind FH. C3b cleavage with both wild-type and acapsular GBS further supported our observation that both strains bound FH and retained cofactor activity. Considerable deposition of both C3b and FH but low levels of C5 and C9, the intermediate and terminal components of the membrane attack complex (MAC), could explain why MAC does not form on some gram-positive bacteria. Binding of FH by pathogenic bacteria helps them evade attack by complement by effecting C3b breakdown (6, 7, 17). We conclude that in addition to the capsule, which hinders the deposition of complement, bound FH cleaves any deposited complement, thus reducing MAC complex and aiding in bacterial survival in the host (Fig. 5).
Figure 5.
Diagrammatic representation of the role of SHT in the evasion of complement attack by GBS. A) Deposition and activation of C3 on bacteria through the alternative complement pathway. Active C3b binds serum factor B to form C3bBb that helps activate C5. Active C5b binds complement proteins C6 to C9 to form the MAC. MAC makes pores in the bacterial membrane, leading to its lysis. Furthermore, bacterial-bound C3b can also take part in opsonophagocytosis by binding to the C3b receptor present on macrophages or neutrophils. B) Depiction of how SHT of GBS type III binds FH of serum to dissociate any C3b–factor B complexes and inactivate C3b to iC3b. Such inactivation helps bacteria survive because of the inability of the inactivated complement to form MACs. In addition, the inactivated C3b also fails to bind macrophages, leading to lower levels of bacterial uptake by killer cells.
However, small amounts of active C3b still persisted in spite of degradation by FH on both the wild-type and acapsular GBS. This active C3b probably leads to the formation of small amounts of MAC complex, as seen by the presence of C5 and C9 in our studies. Although it has been argued that gram-positive bacteria, such as GBS, are resistant to serum killing, probably various factors, such as the amount of MAC and the presence of capsule, play significant roles. Formation of MAC complex has been reported recently on another gram-positive bacterium, Staphylococcus aureus (45). In addition, we showed earlier that GBS does succumb to fresh serum when present at low cfu highlighting the role of complement in killing GBS (41).
On the basis of the above observations, we postulate that the MAC complex might be unable to form on GBS type III because of the binding of FH and subsequent inactivation of C3b. Taken together, our studies suggest that both binding of FH to the surface of GBS, as well as deposition of lower amounts of complement help GBS survive in serum.
A novel CRASP on GBS type III
Proteins binding to complement regulators such as FH or C4BP are usually referred to as CRASPs. CRASPs have been envisaged as potential vaccine candidates against invading pathogens. They have been identified in GAS (the M6 and M18 proteins), in Borrelia sp., and in Neisseria sp. (17, 19, 20), to name a few. In GBS type I, Areschoug et al. (22) showed that the streptococcal β protein (Bac) binds FH. However, no CRASP has been identified in GBS type III to date, although it has been hypothesized that the sialic acid of the GBS capsule binds FH (6, 46, 47, 48).
The fact that protease-treated GBS lost the ability to bind FH suggested the involvement of a surface protein in the binding of FH from serum, in opposition to the hypothesis touting capsule as the FH receptor. Analysis of the alkali-extracted FHBP by mass spectrometry indicated that proteins encoded by either sht, cspA, or scpB genes could be involved in binding to FH. However, as the isogenic mutant strains lacking cspA and scpB still bound FH, the third yet uncharacterized sht gene was the focus of our attention (42, 43). Despite several attempts to generate sht−/− GBS, we were unsuccessful. To circumvent, SHT was expressed as a fusion protein, and the ability of recombinant SHT to bind FH was determined. Overall, our data demonstrated that SHT, an alkali cleavable protein off the surface of GBS, binds FH and facilitates the major mechanism for acquisition of FH by GBS.
The sht gene, documented in the TIGR database of S. agalactiae NEM316 (GBS database, type III) as gbs1306, is 2.48 kB in length and is localized in a pathogenecity island, a unique segment of DNA that encodes multiple virulence factors and lies in close association with two other virulence genes, scpB and lmb (49).
Significance of our study
This is the first study that demonstrates the role of the sht gene in the survival and virulence of GBS type III. The presence of sht in a pathogenicity island and its positional proximity to the scpB and lmb virulence genes reveals a potentially interesting relationship with FH regulation in the host. The scpA/scpB complex inactivates C5a, which, in turn, up-regulates FH in rat Kupffer cells (50, 51). Laminin, present on host cells with which the lmb protein of GBS interacts, has been shown to be highly up-regulated in the glomeruli of FH-knockout mice (52). Additionally, we show in this study that the SHT protein binds FH to inactivate C3. Although we do not have a clear theory yet as to how the translated products of three genes regulate or affect each other in the host, their positional proximity might suggest a functional relationship with FH binding and/or regulation. Further studies are needed to identify the exact relationship of these three proteins in the host. Although not much is known about the SHT protein with respect to virulence, the PHT homolog has been reported to contribute to virulence in Pneumococcus sp., and has been pursued as a vaccine candidate against pneumococcal induced sepsis (43). Recently, the PHT protein has also been documented to bind FH and break down complement (53). Thus, identifying the role SHT plays in virulence of GBS may also lead to novel therapies for GBS infection.
FH is a multifunctional multidomain protein that might play a role in pathogenesis other than the inactivation of complement. Pathogenic microorganisms have been reported to utilize bound FH or FHL (a truncated form of FH) to adhere to host cells through specific receptors (54, 55) and assist in invasion of host cells by the microbe (27). Earlier studies from our laboratory suggested that binding of FH to GBS not only affects the inactivation of complement aiding the bacteria in evasion of phagocytic clearance but might also influence other activities, such as adhesion to and invasion of host cells, such as brain endothelial cells to cause bacterial meningitis (46).
It has been a challenge to create vaccines for GBS, especially due to the “inertness” of the capsule in evoking antibody responses in the host. The identification of specific targets that would help in mounting a response against the GBS challenge has been much sought after. We believe that the SHT should serve as a potential vaccine candidate against GBS infections in future. In addition, the two genes scpB and cspA, which occupy the same locus as sht, have been extensively characterized, while the sht gene is yet uncharacterized. We believe further study of the sht gene would throw more light on the pathogenesis of the bacterium.
Acknowledgments
This work was supported by NIH grants AI22498 to C.E.R. and AI40567 to N.V.P. We thank Dr. Phil Gafken (Fred Hutchinson Cancer Research Institute, Seattle, WA, USA) for mass spectroscopy studies, and Dr. Hal Soucier (University of Southern California. Los Angeles, CA, USA) for flow cytometry studies. We thank Dr. Ravin Seepersaud and Donald Chaffin for technical advice. We thank Dr. Kellie Howard for careful review of the manuscript.
References
- Schuchat A. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev. 1998;11:497–513. doi: 10.1128/cmr.11.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C J, Edwards M S. Group B streptococcal infections. Remington J S, Klein J O, editors. Philadelphia: W. B. Saunders; Infectious Diseases of the Fetus and Newborn Infant. 1995:980–1054. [Google Scholar]
- Nizet V, Ferrieri P, Rubens C. Molecular pathogenesis of group B streptococcal disease in newborns. Stevens D L, Kaplan E L, editors. New York: Oxford University Press; Streptococcal InfectionsClinical Aspects, Microbiology and Molecular Pathogenesis. 2000:180–221. [Google Scholar]
- Antal J M, Cunningham J V, Goodrum K J. Opsonin-independent phagocytosis of group B streptococci: role of complement receptor type three. Infect Immun. 1992;60:1114–1121. doi: 10.1128/iai.60.3.1114-1121.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M S, Wessels M R, Baker C J. Capsular polysaccharide regulates neutrophil complement receptor interactions with type III group B streptococci. Infect Immun. 1993;61:2866–2871. doi: 10.1128/iai.61.7.2866-2871.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques M B, Kasper D L, Pangburn M K, Wessels M R. Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of Type III group B streptococci. Infect Immun. 1992;60:3986–3993. doi: 10.1128/iai.60.10.3986-3993.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M S, Nicholson-Weller A, Baker C J, Kasper D L. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med. 1980;151:1275–1287. doi: 10.1084/jem.151.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram S, Sharma A K, Simpson S D, Gulati S, McQuillen D P, Pangburn M K, Rice P A. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med. 1988;187:743–752. doi: 10.1084/jem.187.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vik D P, Munoz-Canoves P, Chaplin D D, Tack B F. Factor H. Curr Top Microbiol Immunol. 1990;153:147–162. doi: 10.1007/978-3-642-74977-3_8. [DOI] [PubMed] [Google Scholar]
- Whaley K, Ruddy S. Modulation of the alternative complement pathway by β1H globulin. J Exp Med. 1976;144:1147–1163. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler J M, Daha M R, Austen K F, Fearon D T. Control of the amplification convertase of complement by the plasma protein β1H. Proc Natl Acad Sci U S A. 1976;73:3268–3272. doi: 10.1073/pnas.73.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim E, Wood A B, Hsiung L M, Sim R B. Pattern of degradation of human complement fragment C3b. FEBS Lett. 1981;132:55–60. doi: 10.1016/0014-5793(81)80426-7. [DOI] [PubMed] [Google Scholar]
- Ripoche J, Day A J, Harris T J R, Sim R B. The complete amino acid sequence of human complement factor H. Biochem J. 1988;249:593–602. doi: 10.1042/bj2490593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen T, Tack B F. Murine factor H is comprised of 20 repeating units, 61 amino acids in length. Proc Natl Acad Sci. 1986;83:3963–3967. doi: 10.1073/pnas.83.11.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M D, DiScipio R G, Cooper N R, Nemerow G R. Hydrodynamic, electron microscopic and ligand binding analysis of the Epstein-Barr virus/C3dg receptor (CR2) J Biol Chem. 1989;264:20,576–20,582. [PubMed] [Google Scholar]
- Zipfel P F, Hellwage J, Friese M A, Hegasy G, Jokiranta S T, Meri S. Factor H and disease: a complement regulator affects vital body functions. Mol Immunol. 1999;36:241–248. doi: 10.1016/s0161-5890(99)00038-3. [DOI] [PubMed] [Google Scholar]
- Blackmore T K, Fischetti V A, Sadlon T A, Ward H M, Gordon D L. M protein of the group A Streptococcus binds to the seventh short consensus repeat of human complement factor H. Infect Immun. 1988;66:1427–1431. doi: 10.1128/iai.66.4.1427-1431.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraiczy P, Skerka C, Zipfel P F, Brade V. Complement regulator-acquiring surface proteins of Borrelia burgdorferi: a new protein family involved in complement resistance. Wien Klin Wochenschr. 2002;114:568–573. [PubMed] [Google Scholar]
- Kraiczy P, Skerka C, Kirschfink M, Brade V, Zipfel P F. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and factor H. Eur J Immunol. 2001;31:1674–1684. doi: 10.1002/1521-4141(200106)31:6<1674::aid-immu1674>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Ram S, Mackinnon F G, Gulati S, McQuillen D P, Vogel U, Frosch M, Elkins C, Guttormsen H K, Wetzler L M, Oppermann M. The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B Neisseria meningitides. Mol Immunol. 1999;36:915–928. doi: 10.1016/s0161-5890(99)00114-5. [DOI] [PubMed] [Google Scholar]
- Horstmann R D, Sievertsen H J, Knobloch J, Fischetti V A. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc Natl Acad Sci U S A. 1988;85:1657–1661. doi: 10.1073/pnas.85.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Areschoug T, Stalhammar-Carlemalm M, Karlsson I, Lindahl G. Streptococcal beta protein has separate binding sites for human factor H and IgA-Fc. J Biol Chem. 2002;277:12,642–12,648. doi: 10.1074/jbc.M112072200. [DOI] [PubMed] [Google Scholar]
- Kotarsky H, Hellwage J, Johnsson E, Skerka C, Svensson H G, Lindahl G, Sjöbring U, Zipfel P F. Identification of a domain in human factor H and factor H-like protein-1 required for the interaction with streptococcal M proteins. J Immunol. 1988;160:3349–3354. [PubMed] [Google Scholar]
- Johnsson E, Berggard K, Kotarsky H, Hellwage J, Zipfel P F, Sjöbring U, Lindahl G. Role of the hypervariable region in streptococcal M proteins: binding of a human complement inhibitor. J Immunol. 1998;161:4894–4901. [PubMed] [Google Scholar]
- Kotarsky H, Gustafsson M, Svensson H G, Zipfel P F, Truedsson L, Sjöbring U. Group A streptococcal phagocytosis resistance is independent of complement factor H and factor H-like protein 1 binding. Mol Microbiol. 2001;41:817–826. doi: 10.1046/j.1365-2958.2001.02496.x. [DOI] [PubMed] [Google Scholar]
- Pandiripally V, Gregory E, Cue D. Acquisition of regulators of complement activation by Streptococcus pyogenes serotype M1. Infect Immun. 2002;70:6206–6214. doi: 10.1128/IAI.70.11.6206-6214.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiripally V, Wei L, Skerka C, Zipfel P F, Cue D. Recruitment of complement factor H-like protein 1 promotes intracellular invasion by group A streptococci. Infect Immun. 2003;71:7119–7128. doi: 10.1128/IAI.71.12.7119-7128.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janulczyk R, Lannelli F, Sjoholm A G, Pozzi G, Bjorck L. Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J Biol Chem. 2000;275:37,257–37,263. doi: 10.1074/jbc.M004572200. [DOI] [PubMed] [Google Scholar]
- Dave S, Brooks-Walter A, Pangburn M K, McDaniel L S. PspC, a pneumococcal surface protein, binds human factor H. Infect Immun. 2001;69:3435–3437. doi: 10.1128/IAI.69.5.3435-3437.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell J V, Wolfgang J, Tran E, Metts M S, Hamilton D, Marconi R T. Comprehensive analysis of the factor H binding capabilities of Borrelia species associated with Lyme disease: delineation of two distinct classes of factor H binding proteins. Infect Immun. 2003;71:3597–3602. doi: 10.1128/IAI.71.6.3597-3602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Caballero D, García-Laorden I, Cortés G, Wessels M R, de Córdoba S R, Albertí S. Interaction between complement regulators and Streptococcus pyogenes: binding of C4b-binding protein and factor H/factor H-like protein 1 to M18 strains involves two different cell surface molecules. J Immunol. 2004;173:6899–6904. doi: 10.4049/jimmunol.173.11.6899. [DOI] [PubMed] [Google Scholar]
- Martin T R, Rubens C E, Wilson C B. Lung antibacterial defense mechanisms in infant and adult rats: implications for the pathogenesis of group B streptococcal infections in neonatal lung. J Infect Dis. 1988;157:91–100. doi: 10.1093/infdis/157.1.91. [DOI] [PubMed] [Google Scholar]
- Rubens C E, Heggen L M, Haft R F, Wessels M R. Identification of cpsD, a gene essential for type III capsule expression in group B streptococci. Mol Microbiol. 1993;8:843–855. doi: 10.1111/j.1365-2958.1993.tb01631.x. [DOI] [PubMed] [Google Scholar]
- Wessels M R, Haft R F, Heggen L M, Rubens C E. Identification of a genetic locus essential for capsule sialylation in type III group B streptococci. Infect Immun. 1992;60:392–400. doi: 10.1128/iai.60.2.392-400.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen E, Bonacorsi S, Brahimi N, Denamur E, Elion J. Virulence patterns of Escherichia coli K1 strains associated with neonatal meningitis. J Clin Microbiol. 1997;35:2981–2982. doi: 10.1128/jcm.35.11.2981-2982.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbal M, Tkaczuk J, Praud C, Msayeh F, Ohayon E. Computer-assisted kinetic assay for quantitation of total complement activity. Complement Inflamm. 1991;8:92–103. doi: 10.1159/000463185. [DOI] [PubMed] [Google Scholar]
- Ahmed A E E, Peter J B. Clinical utility of complement assessment. Clin Diagn Lab Immunol. 1995;2:509–517. doi: 10.1128/cdli.2.5.509-517.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Gatlin C L, Kleemann G R, Hays, Link A J, Yate J R., III Protein identification at the low femtomole level from silver-stained gels using a new fritless electrospray interface for liquid chromatography-microspray and nanospray mass spectrometry. Anal Biochem. 1998;263:93–101. doi: 10.1006/abio.1998.2809. [DOI] [PubMed] [Google Scholar]
- Zon L I, Dorfman D M, Orkin S H. The polymerase chain reaction colony miniprep. BioTechniques. 1989;7:696–698. [PubMed] [Google Scholar]
- Maruvada R, Blom A M, Prasadarao N V. Effects of complement ,regulators bound to Escherichia coli K1 and group B Streptococcus on the interaction with host cells. Immunology. 2008;124:265–276. doi: 10.1111/j.1365-2567.2007.02764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T O, Shelver D W, Bohnsack J F, Rubens C E. A novel streptococcal surface protease promotes virulence, resistance to opsonophagocytosis, and cleavage of human fibrinogen. J Clin Invest. 2003;111:61–70. doi: 10.1172/JCI16270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C, Waggoner J D, Harris T O, Tamura G S, Rubens C E. Identification of novel adhesins from group B streptococci by use of phage display reveals that C5a peptidase mediates fibronectin binding. Infect Immun. 2002;70:2869–2876. doi: 10.1128/IAI.70.6.2869-2876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamou J E, Heinrichs J H, Erwin A L, Walsh W, Gayle T, Dormitzer M, Dagan R, Brewah Y A, Barren P, Lathigra R, Langermann S, Koenig S, Johnson S. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect Immun. 2001;69:949–958. doi: 10.1128/IAI.69.2.949-958.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooijakkers S H M, Ruyken M, Roos A, Daha M R, Presanis J S, Sim R B, van Wamel W J, van Kessel K P, van Strijp J A. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat Immunol. 2005;6:920–927. doi: 10.1038/ni1235. [DOI] [PubMed] [Google Scholar]
- Campbell J R, Baker C J, Edwards M S. Influence of serotype of group B streptococci on C3 degradation. Infect Immun. 1992;60:4558–4562. doi: 10.1128/iai.60.11.4558-4562.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarva H T, Jokiranta S, Wurzner R, Meri S. Complement resistance mechanisms of streptococci. Mol Immunol. 2003;40:95–107. doi: 10.1016/s0161-5890(03)00108-1. [DOI] [PubMed] [Google Scholar]
- Vogel U, Claus H, Heinze G, Frosch M. Role of lipopolysaccharide sialylation in serum resistance of serogroup B and C meningococcal disease isolates. Infect Immun. 1999;67:954–957. doi: 10.1128/iai.67.2.954-957.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J, Bender L, Ott M, Wingender J, Lund B, Marre R, Goebel W. Deletions of chromosomal regions coding for fimbriae and hemolysins occur in vitro and in vivo in various extraintestinal Escherichia coli isolates. Microb Pathog. 1990;8:2213–2225. doi: 10.1016/0882-4010(90)90048-u. [DOI] [PubMed] [Google Scholar]
- Wexler D E, Chenoweth D, Cleary P P. Mechanism of action of the group A streptococcal C5a inactivator. Proc Natl Acad Sci U S A. 1985;82:8144–8148. doi: 10.1073/pnas.82.23.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaf G, Nitzki F, Heine I, Hardeland R, Schieferdecker H L, Gotze O. C5a anaphylatoxin as a product of complement activation up-regulates the complement inhibitory factor H in rat Kupffer cells. Eur J Immunol. 2004;34:3257–3266. doi: 10.1002/eji.200324806. [DOI] [PubMed] [Google Scholar]
- Alexander J J, Pickering M C, Haas M, Osawe I, Quigg R J. Complement factor H limits immune complex deposition and prevents inflammation and scarring in glomeruli of mice with chronic serum sickness. J Am Soc Nephrol. 2005;1:52–57. doi: 10.1681/ASN.2004090778. [DOI] [PubMed] [Google Scholar]
- Ogunniyi A D, Grabowicz M, Mahdi L K, Cook J, Gordon D L, Sadlon T A, Paton J C. Pneumococcal histidine triad proteins are regulated by the Zn2+-dependent repressor AdcR and inhibit complement deposition through the recruitment of complement factor H. FASEB J. 2009;23:731–738. doi: 10.1096/fj.08-119537. [DOI] [PubMed] [Google Scholar]
- Avery V M, Gordon D L. Characterization of factor H binding to human polymorphonuclear leukocytes. J Immunol. 1993;15:5545. [PubMed] [Google Scholar]
- Albanyan E A, Vallejo J G, Smith C W, Edwards M S. Nonopsonic binding of type III group B streptococci to human neutrophils induces interleukin-8 release mediated by the p38 mitogen-activated protein kinase pathway. Infect Immun. 2000;68:2053–2060. doi: 10.1128/iai.68.4.2053-2060.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]