Abstract
The mechanism of floral organ specification is principally conserved in angiosperms, as demonstrated by the ABC model. By contrast, mechanisms that regulate the development of organs or structures specific to a group of species remain unclear. Grasses have unique inflorescence units, comprising spikelets and florets. In the genus Oryza (rice), the single spikelet consists of a fertile floret subtended by a lemma and a palea, two sterile lemmas, and rudimentary glumes. Each sterile lemma is a tiny glume-like organ with a smooth surface. Here, we have examined a long sterile lemma1 (g1) mutant, in which the sterile lemma is enlarged like the lemma. Detailed phenotypic analysis reveals that the large sterile lemma in the g1 mutant appears to be caused by homeotic transformation of the sterile lemma into a lemma, suggesting that G1 is involved in the repression of lemma identity to specify the sterile lemma. Gene isolation reveals that G1 is a member of a plant-specific gene family that encodes proteins with a previously uncharacterized domain, named here ALOG (Arabidopsis LSH1 and Oryza G1). G1 mRNA is expressed in sterile lemma primordia throughout their development, and G1 protein is localized in the nucleus. A trans-activation assay using the yeast GAL4 system suggests that G1 is involved in transcriptional regulation. Repression of lemma identity by G1 is consistent with a hypothesis proposed to explain the morphological evolution of rice spikelets. We also show that a wild rice species, Oryza grandiglumis, that forms large sterile lemmas has serious mutations in the G1 gene.
Keywords: flower, grass, Oryza, ALOG, morphological evolution
Although angiosperms produce diverse forms of flowers, from beautiful and entomophilous to inconspicuous and anemophilous, the genetic programs directing floral development are fundamentally conserved. Floral organ specification is explained by the ABC model, and genes constituting this model are likely to be functionally conserved in a wide range of flowering plants (1–3). In contrast to our deep understanding of floral organs common to many angiosperms, genes that regulate the development of floral organs and/or flower-associated structures distinctive to a group of plant species remain poorly elucidated.
Grass species, such as Oryza sativa (rice) and Zea mays (maize), bear a unique inflorescence consisting of spikelets and florets (4, 5). Each spikelet produces a defined number of florets depending on species, and is subtended by a pair of glumes. The floret comprises the flower proper (carpels, stamens, and lodicules) and a pair of additional structures (a palea and a lemma) that subtend the flower. The lodicule, an organ homologous to the petal in ordinary flowers, is small and semitransparent, and acts to open the palea and lemma for anthesis. Molecular genetic studies in rice and maize have revealed that the function of B class MADS-box genes is conserved in grasses: these genes specify the identities of the lodicule and stamen similar to the way in which B class genes specify the identities of the petal and stamen in eudicots (4, 6–9). In addition to the crucial role of C class MADS-box genes in carpel specification in eudicots, the identity of the carpel is determined by the YABBY gene DROOPING LEAF (DL) in rice (10). Spatial expression patterns of DL-related genes from maize and other grasses suggest the conserved function of these genes in grasses (11). Stamen identity and floral meristem determinacy, which are controlled by a single C class gene, AGAMOUS, in Arabidopsis, are regulated separately by two or more C class genes that were duplicated before divergence of the grass species (12, 13). The palea and lemma are organs specific to grasses, and enclose inner floral organs (14–16). The LEAFY HULL STERILE1 (LHS1) gene is involved in partially specifying the identity of the lemma and palea in rice, in addition to regulating spikelet meristem determinacy (17, 18). Although LHS1 orthologs are expressed in the palea and lemma in other grasses, the expression patterns of these genes suggest that they are also involved in various aspects of the development and morphological diversification of spikelets in grasses (19). The relationship of the lemma/palea to floral structures in other monocots and eudicots is not yet fully understood, although comparative morphological studies suggest that there is a correlation between the lemma/palea and the outer tepals of a typical monocot flower (20, 21).
Inflorescence architecture and spikelet organization varies from species to species within the grass family. For example, the number of florets varies from one to 40 depending on the species (22). Determinacy of the spikelet meristem, which is closely associated with floret number, is regulated by genes encoding APETALA2 (AP2)-like transcription factors both in rice and maize (23–25). Rice produces a single fertile floret within a spikelet. The floret is flanked by a pair of glume-like organs, which are not usually observed in the spikelets of other grasses such as maize and wheat. A hypothesis has been proposed that these glume-like organs are derived from the lemmas of two sterile florets that have been reduced during the evolution of Oryza (26). According to this hypothesis, these organs should be called sterile lemmas rather than empty glumes, although confusingly both terminologies have been used in the rice literature. However, the size and identity of the sterile lemmas differ greatly from those of lemmas. Despite this attractive hypothesis based on morphological changes during evolution, to our knowledge there have been no studies on the gene responsible for development of the sterile lemma and its evolutionary origin.
In this study, we have analyzed a rice mutant, called long sterile lemma1 (G1), that forms a large sterile lemma (LSL) (27–29). Detailed morphological analyses revealed that the LSL in g1 has lemma identity that clearly differs from the identity of the sterile lemma in WT. We isolated the G1 gene by map-based cloning. G1 encodes a protein that has a conserved domain flanked by a nuclear localization signal and is specific to angiosperms. Cellular localization and trans-activation analysis by a yeast system raised the possibility that G1 may be involved in transcriptional regulation. Collectively, these results suggest that G1 is required for the repression of lemma identity in the sterile lemma in rice, and might have been involved in its morphological modification during the evolution of rice.
Results
The LSL in the g1 Mutant has Lemma Identity.
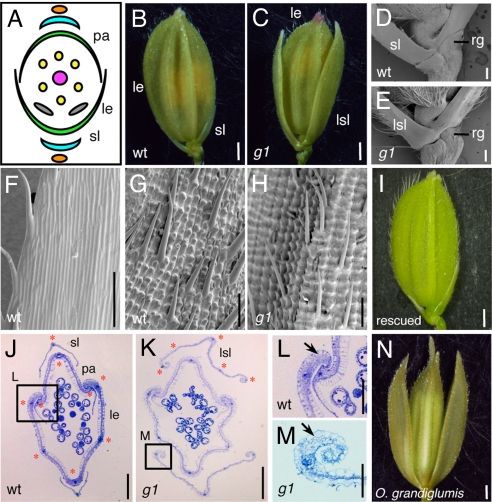
The rice spikelet has one fertile floret in which a pistil, six stamens, and two lodicules develop (Fig. 1A) (4, 14, 26). Glumes are highly reduced in rice, being observed as only vestigial structures at the base of the spikelet, and are thereby called rudimentary glumes (Fig. 1D). A pair of sterile lemmas, which have the appearance of tiny glumes, is formed between the floret and the rudimentary glumes (Fig. 1 B and D).
Fig. 1.
Spikelet phenotypes of the g1 mutant. (A) Schematic representation of the spikelet of WT rice. (B) A WT spikelet. (C) A g1 spikelet showing the LSL. Basal region of the spikelet in WT (D) and g1 (E). Epidermal surface of the sterile lemma (F) and the lemma in WT (G). (H) Epidermal surface of the LSL in g1. (I) A spikelet in a rescued g1 mutant, which was transformed with a genomic fragment encompassing the G1 locus. Cross section of the spikelet in WT (J) and g1 (K). Close-up view of the marginal region of the WT lemma (L) and g1 LSL (M). Red stars indicate vascular bundles (J and K). Arrows indicate the peripheral structure of the lemma and LSL (L and M). (N) A spikelet of O. grandiglumis (W0613). le, lemma; pa, palea; rg, rudimentary glume; sl, sterile lemma. (Scale bars: 1 mm in B, C, I, and N; 200 μm in J and K; 100 μm in D, E, F, G, and H; and 50 μm in L and M.).
In a recessive mutant, g1–1, we observed that the sterile lemma is larger than that of WT (Fig. 1 B and C). Close examination of the base of the spikelet revealed that the LSL in g1 was formed at the expense of the sterile lemma (Fig. 1 D and E). In addition, no abnormality was detected in other spikelet organs such as the paleas and rudimentary glumes, suggesting that the g1 mutation specifically affects the sterile lemma.
To address the effect of the g1 mutation in detail, we examined morphological differences in the epidermal cells between the g1 LSL and the sterile lemma of WT. In WT, the sterile lemma has a smooth surface on the abaxial side and rarely forms trichomes on its surface except for the marginal region (Fig. 1F). By contrast, the abaxial surface of the g1 LSL is very rough (Fig. 1H). Convex structures called tubercles (30) are arranged in parallel and the cell files with a tubercle are very clear. In addition, trichomes are formed on the abaxial surface. This epidermal morphology in the g1 LSL is highly similar to that of the lemma and palea (Fig. 1G), suggesting that epidermal cells in the g1 LSL have an identity similar to those in the lemma/palea. Observations during the developmental process of the spikelet revealed that the epidermal cell identity of the LSL in g1 is determined at an early stage (Fig. S1). The surface morphologies of the g1 LSL and the WT sterile lemma were not distinguishable from each other before initiation of the stamen primordia (Fig. S1 G and P). The surface of the g1 LSL became more similar to that of the lemma than that of the sterile lemma at the stage when the stamen primordia were clearly observed (Fig. S1 E, H, N, and Q). By contrast, the size of the g1 LSL was nearly the same as that of the sterile lemma of a WT flower at this stage (Fig. S1 B and K), suggesting that timing of the specification of cell identity was not coupled to cell elongation in the LSL of the g1 spikelet.
We next observed a cross-section of the spikelet and found that the LSL in g1 seems to resemble the lemma rather than the palea. First, the number of vascular bundles is five in both the g1 LSL and the lemma, whereas it is three in the palea (Fig. 1 J and K). Second, the marginal regions of the g1 LSL curl inward like those of the lemma and differ from those of the palea (Fig. 1 L and M). These phenotypic analyses suggest that the LSL in g1 is not only larger but also has morphological and structural features similar to those of the lemma. Thus, it is likely that G1 is involved in specification of the identity of the sterile lemma.
G1 Encodes a Unique Protein with an Uncharacterized Domain.
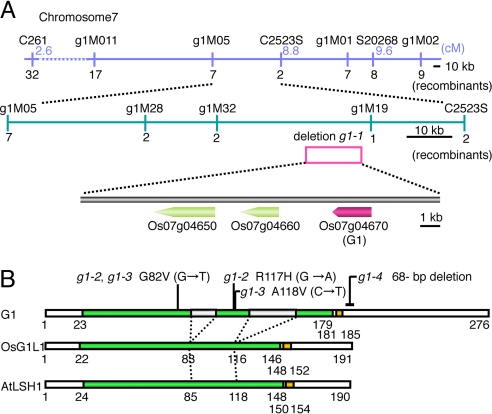
To elucidate its molecular function, we isolated the G1 gene by using a map-based strategy. Using F2 plants from a cross between g1–1 (japonica) and Kasalath (indica), we found that G1 was restricted to a 33.7-kb region between the markers g1M32 and g1M19 on the short arm of chromosome 7 (Fig. 2A). We then found that a segment of approximately 12 kb was deleted between the two markers in g1–1. Within this deleted region, there were three predicted genes [LOC_Os07g04650 (Os07g0139000), LOC_Os07g04660 (Os07g0139100), LOC_Os07g04670 (Os07g0139300)] in the rice database [TIGR release 5 (RAP2 build 4.0)]. Expression analysis by RT-PCR revealed that only one gene, LOC_Os07g04670, was expressed in the young inflorescence in WT (Fig. S2E). Next, we determined the genomic sequence of LOC_Os07g04670 from three putative g1 mutants (g1–2, g1–3, g1–4) that had the LSL (Fig. S2). By a genetic complementation test, g1–4 was shown to be allelic to g1–1. We found missense and nonsense mutations in all three mutants examined (Fig. 2B and Fig. S3). A 3.2-kb genomic fragment containing LOC_Os07g04670 from WT rescued the mutant phenotype of the g1 spikelet (Fig. 1I). Taking these results together, we concluded that LOC_Os07g04670 is the G1 gene.
Fig. 2.
Map-based cloning and protein structure. (A) Physical map of the G1 locus. Numerals under maps indicate the number of recombinants among 1,380 meioses. Pink open box indicates the 12-kb chromosomal deletion found in g1–1. (B) Protein structure of G1 and its related proteins. Green and orange boxes indicate the ALOG domain and nuclear localization signal, respectively. Gray boxes indicate amino acid insertions in the ALOG domain in G1.
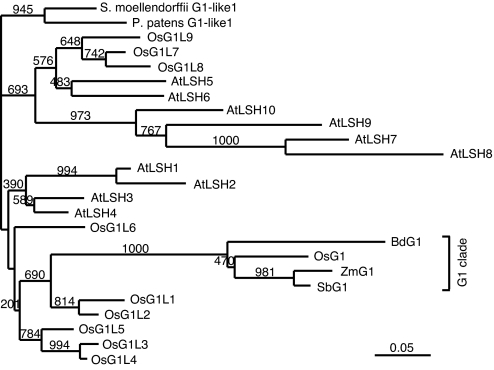
We found that G1 encodes a protein with a conserved domain of unknown function (Fig. 2B and Figs. S3 and S4). In the rice database, this domain is called DUF640 (domain of unknown function 640). A nuclear localization signal flanks the C-terminal region of this domain. There are 10 genes that encode proteins with DUF640 in both rice and Arabidopsis (Fig. 3). The only gene functionally characterized so far in this family is LIGHT-DEPENDENT SHORT HYPOCOTYLS1 (LSH1), which is involved in phytochrome-dependent light signaling in Arabidopsis (31). Here, we named DUF640 the ALOG (Arabidopsis LSH1 and Oryza G1) domain. Genes encoding proteins containing an ALOG domain were also found in the genome of Physcomitrella patens and Selaginella moellendorffii (32, 33), but not in the genome of algae, fungi, or animals, suggesting that ALOG domain genes may be specific to land plants (Fig. 3).
Fig. 3.
Phylogenic tree of proteins containing a ALOG domain. Amino acids of the ALOG domain were compared (Fig. S4) and the tree was constructed by a neighbor-joining method (44). Numbers indicate bootstrap values.
In contrast to other ALOG domains identified, the ALOG domain in G1 is interrupted with two insertions consisting of 9 and 23 aa (Fig. 2B and Figs. S3 and S4). Proteins with an interrupted ALOG domain like G1 are restricted to the grass species: that is, an extensive similarity search did not identify proteins with an interrupted ALOG domain in the genome of other plants, including plants for which the whole genome sequence is available. Missense mutations are present in the ALOG domain of g1–2 and g1–3 (Fig. 2B and Fig. S3). The severity of the spikelet phenotypes of these two alleles is comparable to that of the phenotypes of g1–1 and g1–4, in which G1 is completely defective, suggesting that amino acid substitutions in this domain are critical for protein function.
The Oryza genus has 23 species including two domesticated species. Among them, Oryza grandiglumis, which has the allotetraploid genome CCDD, forms an LSL similar to that of the g1 mutant of O. sativa (Fig. 1N); indeed, the name of this species is derived from this characteristic (34, 35). The number of vascular bundles and the morphology of the peripheral region of the LSL in O. grandiglumis were similar to those of the LSL in the g1 mutant (Fig. S2 F-H). To know whether the LSL in O. grandiglumis is associated with the function of G1, we sequenced G1 orthologs from this species. We found substitutions of an invariant amino acid in the ALOG domain or nonsense mutations in both genomes of this allotetraploid species (Fig. S5). These results indicate that the LSL in O. grandiglumis is probably caused by loss of function of G1.
Spatial Expression Patterns of G1 mRNA and Molecular Features of G1 Protein.
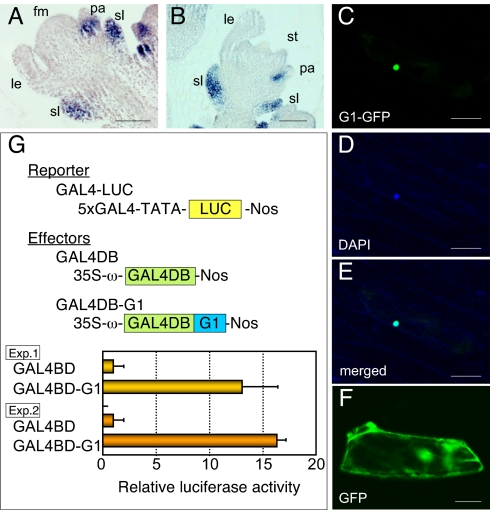
To address the molecular function of G1, we first performed in situ hybridization analysis (Fig. 4 A and B). G1 was expressed in the primordia of the sterile lemma from an early stage of spikelet development. G1 expression in the sterile lemma primordia persisted throughout the development. No expression was detected in the lemma, although G1 signals were detected in the basal region of the palea. Continuous expression of G1 in the sterile lemma primordia is consistent with the mutant phenotype of g1.
Fig. 4.
Spatial expression pattern of G1 mRNA and function of G1 protein. (A and B) in situ localization of G1 mRNA. fm, floret meristem; le, lemma; pa, palea; sl, sterile lemma; st, stamen. (C) Subcellular localization of the G1-GFP fusion protein. (D) DAPI staining of the nucleus. (E) Merged image of C and D. (F) Subcellular localization of GFP. (G) Trans-activation analysis of G1 by a yeast GAL4 system. GAL4BD, DNA-binding domain of GAL4; 5× GAL4, 5 copies of GAL4-upstream activating sequence. (Scale bars: 50 μm.)
Next, we examined the subcellular localization of G1 protein. Constructs producing a fusion protein of G1 and GFP were introduced into onion epidermal cells by particle bombardment. The results clearly showed that the G1–GFP fusion protein was localized in the nucleus (Fig. 4 C–E). Similar results were obtained in an experiment using a GFP-G1 fusion protein (Fig. S6).
Last, we addressed the function of G1 protein by using a trans-activation assay system based on yeast GAL4 (36). The protein-coding region of G1 cDNA was fused in-frame to a sequence encoding the GAL4 DNA-binding domain (GAL4 DB) and placed under control of the cauliflower mosaic virus promoter (i.e., effector). As a reporter, the luciferase gene under the control of five copies of binding sites for GAL4 was used. Both effector and reporter plasmids were delivered to Arabidopsis leaves by particle bombardment. We found that the fusion protein of GAL4BD and G1 enhanced transcriptional activity in Arabidopsis leaves compared with GAL4BD alone (Fig. 4G). This observation suggests that G1 is involved in transcriptional regulation.
Discussion
G1 Is Involved in the Specification of Sterile Lemma Identity.
Rice bears a single fertile floret within a spikelet, in which the glumes are highly degenerated and visible only as two diminutive outgrowths (called rudimentary glumes). Sterile lemmas are produced between the fertile floret and the rudimentary glumes (4, 14, 26). The sterile lemma has not been genetically characterized and is often mistaken for a glume. The present study showed that the G1 gene is specifically involved in specification of the sterile lemma. In the g1 mutant, the LSL has structural features resembling those of the lemma. The size, shape, and arrangement of the abaxial epidermal cells in the LSL in g1 were indistinguishable from those in the lemma. Trichomes were formed on the abaxial surface of the g1 LSL as they are in the lemma. In addition, the number of vascular bundles and the marginal structure were shared by the LSL and the lemma. Thus, the LSL develops at the expense of the sterile lemma, suggesting that the sterile lemma is homeotically transformed into the lemma in the g1 mutant. Because no such clear transformation has previously been reported, G1 seems to be a unique homeotic gene that regulates the development of spikelet organs in grasses.
We found that G1 is a member of an uncharacterized gene family that is shared by land plants. Furthermore, subcellular localization and trans-activation analysis by a yeast GAL4 system suggest that G1 is involved in transcriptional regulation. G1 is expressed in the primordia of the sterile lemma throughout its development. It is therefore likely that G1 specifies the identity of the sterile lemma by repressing lemma identity via the regulation of downstream target genes.
Evolution of the Spikelet Architecture and the Role of G1 in Rice Spikelets.
The number of florets in a spikelet is a key feature of spikelet architecture in grasses. Two florets are formed in a maize spikelet, although the lower floret is degenerated in female spikelets (4, 5, 23). Interestingly, the number of florets per spikelet is associated with ploidy level in wheat (37).
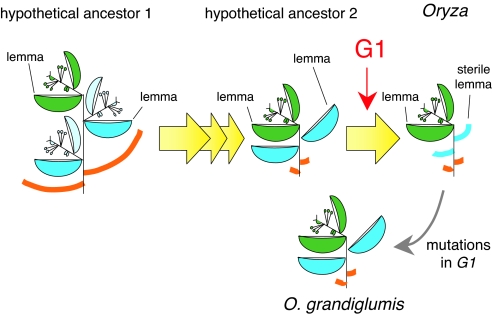
All extant rice species in the genus Oryza have a single fertile floret in the spikelet. From comparative morphological studies, it has been hypothesized that a putative ancestor of Oryza had three florets and that, during evolution, the two lateral florets degenerated, leaving only the lemma (26). The sterile lemma in the extant Oryza species seems to be derived from morphological modification of the remaining lemma. In line with this hypothesis, the default state of the sterile lemma would seem to be the lemma. Consistent with this idea, we revealed that the sterile lemma has the potential to transform into the lemma if G1 activity is lost. Thus, it seems an attractive idea that G1 may have been recruited to specify the sterile lemma by repressing the identity of the lateral remaining lemma during the evolution of Oryza (Fig. 5).
Fig. 5.
Model of hypothesized changes in spikelet architecture during Oryza evolution (26) and putative function of G1.
O. grandiglumis has LSLs similar to those of the g1 mutant (34, 35). It is possible that the spikelet of O. grandiglumis corresponds to an intermediate type of spikelet with an LSL before evolution of the repression of lemma identity, and that other rice species have lost the lemma identity of the sterile lemma in independent lineages during rice evolution. Alternatively, it is also possible that the spikelet phenotype in O. grandiglumis is caused by mutations that occurred in the G1 locus after structural features of the sterile lemma were established. Sequencing of the G1 gene in two accessions of O. grandiglumis clearly supported the latter idea. The two accessions shared a substitution of an invariant amino acid, which is completely conserved in all ALOG domain proteins, in one genome. In the other genome, each accession had a nonsense mutation at a different position. Thus, the G1 gene might have been compromised sequentially during the evolution of this tetraploid species, although we need to confirm that the amino acid substitution in the ALOG domain results in loss of G1 function. In addition, we are interested in the process of how the tetraploid species O. grandiglumis accumulated the mutation in G1 in both genomes. It is possible that close relatives of O. grandiglumis with a CCDD genome, O. alta and O. latifolia (38), have a mutation in G1 in one of the genomes, and the combination of two genomes with the mutation may be associated only with the lineage of the O. grandiglumis.
Analyses of O. grandiglumis support the idea that G1 is a key regulator of sterile lemma development. Based on the hypothesis of Arber (26), the spikelets of both O. grandiglumis and the g1 mutant of O. sativa appear to be a reversal. If so, it is surprising that the sterile lemma of both O. sativa and ancestral species of O. grandiglumis has maintained the potential to express genes responsible for lemma identity for a long evolutionary period. The genus Leersia, which, like Oryza, belongs to Oryzeae, lacks the sterile lemma (16). It would be interesting to know whether G1 activity is involved in developmental arrest of the sterile lemma in this genus. Sterile lemmas are widely distributed in the grass family, which suggests that the sterile lemma either evolved in several independent lineages or was lost multiple times after its establishment near the base of the grass clade. It is possible that G1 orthologs may be involved in morphological modification of the sterile lemma in lineages other than those of Oryza evolution. Alternatively, G1 function may be restricted to the sterile lemma of Oryza, because the epidermal convex structures (i.e., tubercles), which are suppressed by G1 in the sterile lemma, are a synapomorphy of the Oryza genus (16, 30) and/or G1-related proteins may have diverse functions as a result of the insertion of amino acids, which are highly variable among grasses.
Characteristics of G1 Protein.
G1 has a unique conserved domain, named ALOG, that is flanked by a nuclear localization signal. The 125-residue ALOG domain is rich in basic amino acids, especially arginine, and is highly conserved among land plants: 53 of 125 aa were found to be completely conserved in 25 proteins from seven species including rice, Arabidopsis, and Physcomitrella. G1 is unique in that its ALOG domain is interrupted by two insertions. This interruption is observed only in proteins closely related to rice G1 in grasses (i.e., G1 clade). Despite these insertions, G1 seems to be functional in rice, as shown here by genetic and molecular biological studies. Conservation of amino acids in the ALOG domain among proteins in the G1 clade also supports this idea, because mutations might have accumulated if such G1-related proteins had no function. The restricted distribution of this interrupted ALOG domain raises the possibility that ALOG domain proteins in the G1 clade are associated with developmental regulation specific to grasses. The role of the ALOG domain is unknown; thus, it would be interesting to elucidate the biochemical function of this domain, together with the effect of the insertions in the G1 clade, in future studies.
Materials and Methods
Plant Materials.
Taichung 65 was used as the WT strain for standard spikelet and for in situ analysis. Strains HO680 (g1–1), H-138 (g1–2), and 7578-RD-3 (g1–3) were provided by A. Yoshimura (Fukuoka, Japan), I. Takamure (Sapporo, Japan), and H. Kitano (Nagoya, Japan), respectively (28, 29). g1–4 (ND4295) was identified in the TOS17 mutant panel and provided by H. Hirochika and A. Miyao (39) (Tsukuba, Japan).
Isolation of G1.
The primers used for PCR amplification are shown in Table S2. The G1 locus was mapped by using F2 plants from a cross between g1–1 and Kasalath (ssp. indica). First, the G1 locus was mapped to a region between CAPS markers C261 and S20268 on the distal end of the short arm of chromosome 7 by using 690 g1 homozygotes. Next, the locus was narrowed to a 33.7-kb region between two closely linked CAPS markers, g1M32 and g1M19, which were designed by comparing the genomic sequences of japonica and indica (Table S2). A chromosomal deletion was found in this region, the extent of which was restricted to 12 kb. Three putative genes were located in this 12-kb region. For complementation analysis, a 3.7-kb DNA fragment encompassing the G1 locus was isolated from BAC clone OSJNBa0088E18 (Clemson University Genome Institute) and cloned into a gateway vector (Invitrogen). By an LR recombination reaction, the genomic fragment was transferred to a binary vector, pBI-Hm12-GW, which was constructed by introducing a Gateway rfC cassette into pBI-Hm12 in this study. The resulting chimeric plasmid carrying the G1 gene was used for transformation of g1–4 by the Agrobacterium-mediated method (40). G1 cDNA was amplified by using a cDNA pool synthesized from total RNA isolated from the young panicles. After sequencing of the RT-PCR product, the ORF was predicted.
Cytological and Molecular Analyses.
For in situ hybridization, a 552-bp fragment in the 3′ region of G1 cDNA was amplified (Table S2) and cloned into a T-vector by TA cloning (Novagen). Probe synthesis, preparation of sections, in situ hybridization, and microscopic observation were performed as described previously (41, 42). For analysis of subcellular localization, a DNA fragment corresponding to the coding region of G1 was amplified and inserted into a Gateway binary vector, pGWB5 or pGWB6 (43), by recombination to produce a G1–GFP or GFP–G1 fusion protein (Invitrogen). The resulting construct was bombarded into onion epidermal cells by a particle-mediated DNA delivery system (PDS1000/He; Bio-Rad). After overnight incubation at 22 °C in the dark, the florescence of GFP was detected by a confocal micro-scanning laser microscope (Nikon). Last, cells were stained with DAPI to visualize the nucleus. The vectors used for the GAL4 trans-activation system were kindly provided by M. Ohme-Takagi (36) (Tsukuba, Japan). A DNA fragment amplified from G1 cDNA (Table S2) was digested with EcoRV and SalI and inserted into a vector containing the cauliflower mosaic virus CaMV35S promoter and a sequence encoding the for GAL4 DNA binding domain (GAL4DB) to make a G1-GAL4BD fusion protein (i.e., effector). The resulting plasmid was introduced into Arabidopsis leaves by particle bombardment as described earlier. In co-transfection assays, we used 1.6 μg of reporter construct and 1.2 μg of effector construct for each bombardment. As an internal control, an RLUC reporter gene from Renilla (0.4 μg) under the control of 35S promoter (Promega) was co-bombarded with both reporter and effector plasmids. After bombardment, the leaves were put on agar medium containing Murashige and Skoog medium (pH 5.7) and incubated at 22 °C for 12 h. Luciferase assays were performed with the Dual-Luciferase Reporter Assay System according to the instruction manual (Promega). After measurement of LUC and RLUC activity, the activity of LUC relative to RLUC was estimated.
Supplementary Material
Acknowledgments.
We thank Drs. A. Yoshimura, H. Kitano, and I. Takamure for their kind distribution of g1 alleles; Drs. Y. Takagi and T. Nakagawa for providing vectors for GAL4 activation analysis and gateway binary vectors, respectively; Drs. Y. Fujimoto, H. Takanashi, and N. Tsutsumi for technical support; and Ms. K. Ohsawa for technical assistance. This work was supported in part by Grants-in-Aid for Scientific Research from MEXT (20380005, 20061006) and the Program of Basic Research Activities for Innovative Biosciences (PROBRAIN) (to H.-Y.H.), the Global COE Program (Integrative Life Science Based on the Study of Biosignaling Mechanisms) from the Ministry of Education, Culture, Sports, Science and Technology (to A.Y. and W.T.), and a Sasakawa Scientific Research Grant from The Japan Science Society (to A.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the DNA Data Bank of Japan. For a list of accession numbers, see Table S1.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907896106/DCSupplemental.
References
- 1.Coen ES, Meyerowitz EM. The war of the whorls: genetic interactions controlling flower development. Nature. 1991;353:31–37. doi: 10.1038/353031a0. [DOI] [PubMed] [Google Scholar]
- 2.Theissen G, et al. A short history of MADS-box genes in plants. Plant Mol Biol. 2000;42:115–149. [PubMed] [Google Scholar]
- 3.Lohmann JU, Weigel D. Building beauty: The genetic control of floral patterning. Dev Cell. 2002;2:135–142. doi: 10.1016/s1534-5807(02)00122-3. [DOI] [PubMed] [Google Scholar]
- 4.Bommert P, Satoh-Nagasawa N, Jackson D, Hirano H-Y. Genetics and evolution of inflorescence and flower development in grasses. Plant Cell Physiol. 2005;46:69–78. doi: 10.1093/pcp/pci504. [DOI] [PubMed] [Google Scholar]
- 5.Zanis MJ. Grass spikelet genetics and duplicate gene comparisons. Int J Plant Sci. 2007;168:93–110. [Google Scholar]
- 6.Ambrose BA, et al. Molecular and genetic analyses of the silky1 gene reveal conservation in floral organ specification between eudicots and monocots. Mol Cell. 2000;5:569–579. doi: 10.1016/s1097-2765(00)80450-5. [DOI] [PubMed] [Google Scholar]
- 7.Nagasawa N, et al. SUPERWOMAN 1 and DROOPING LEAF genes control floral organ identity in rice. Development. 2003;130:705–718. doi: 10.1242/dev.00294. [DOI] [PubMed] [Google Scholar]
- 8.Whipple CJ, et al. Conservation of B-class floral homeotic gene function between maize and Arabidopsis. Development. 2004;131:6083–6091. doi: 10.1242/dev.01523. [DOI] [PubMed] [Google Scholar]
- 9.Thompson BE, Hake S. Translational biology: from Arabidopsis flowers to grass inflorescence architecture. Plant Physiol. 2009;149:38–45. doi: 10.1104/pp.108.129619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi T, et al. The YABBY gene DROOPING LEAF regulates carpel specification and midrib development in Oryza sativa. Plant Cell. 2004;16:500–509. doi: 10.1105/tpc.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishikawa M, et al. The spatial expression patterns of DROOPING LEAF orthologs suggest a conserved function in grasses. Genes Genet Syst. 2009;84:137–146. doi: 10.1266/ggs.84.137. [DOI] [PubMed] [Google Scholar]
- 12.Mena M, et al. Diversification of C-function activity in maize flower development. Science. 1996;274:1537–1540. doi: 10.1126/science.274.5292.1537. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi T, et al. Functional diversification of the two C-class genes, OSMADS3 and OSMADS58, in Oryza sativa. Plant Cell. 2006;18:15–28. doi: 10.1105/tpc.105.037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang T-T, Bardenas EA. Los Banos, Philippines: IRRI; 1965. The morphology and varietal characteristics of the rice plant. [Google Scholar]
- 15.Kellogg EA. Evolutionary history of the grasses. Plant Physiol. 2001;125:1198–1205. doi: 10.1104/pp.125.3.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellogg EA. The evolutionary history of Ehrhartoideae, Oryzeae, and Oryza. Rice. 2009;2:1–14. [Google Scholar]
- 17.Jeon JS, et al. leafy hull sterile1 is a homeotic mutation in a rice MADS box gene affecting rice flower development. Plant Cell. 2000;12:871–884. doi: 10.1105/tpc.12.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasad K, Parameswaran S, Vijayraghavan U. OsMADS1, a rice MADS-box factor, controls differentiation of specific cell types in the lemma and palea and is an early-acting regulator of inner floral organs. Plant J. 2005;43:915–928. doi: 10.1111/j.1365-313X.2005.02504.x. [DOI] [PubMed] [Google Scholar]
- 19.Malcomber ST, Kellogg EA. Heterogeneous expression patterns and separate roles of the SEPALLATA gene LEAFY HULL STERILE1 in grasses. Plant Cell. 2004;16:1692–1706. doi: 10.1105/tpc.021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sajo MG, Longhi-Wagner HM, Rudall PJ. Reproductive morphology of the early-divergent grass Streptochaeta and its bearing on the homologies of the grass spikelet. Plant Syst Evol. 2008;275:245–255. [Google Scholar]
- 21.Preston JC, Christensen A, Malcomber ST, Kellogg EA. MADS-box gene expression and implications for developmental origins of the grass spikelet. Am J Bot. 2009;96:1419–1429. doi: 10.3732/ajb.0900062. [DOI] [PubMed] [Google Scholar]
- 22.Clifford HT, Soderstrom TR, Hilu KW, Campbell CS, Barkworth ME. Spikelet and floral morphology. In: Soderstrom TR, Hilu KW, Campbell CS, Barkworth ME, editors. Grass Systematics and Evolution. Washington, DC: Smithsonian Institution Press; 1987. pp. 21–30. [Google Scholar]
- 23.Chuck G, Meeley RB, Hake S. The control of maize spikelet meristem fate by the APETALA2-like gene indeterminate spikelet1. Genes Dev. 1998;12:1145–1154. doi: 10.1101/gad.12.8.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee DY, Lee J, Moon S, Park SY, An G. The rice heterochronic gene SUPERNUMERARY BRACT regulates the transition from spikelet meristem to floral meristem. Plant J. 2007;49:64–78. doi: 10.1111/j.1365-313X.2006.02941.x. [DOI] [PubMed] [Google Scholar]
- 25.Chuck G, Meeley R, Hake S. Floral meristem initiation and meristem cell fate are regulated by the maize AP2 genes ids1 and sid1. Development. 2008;135:3013–3019. doi: 10.1242/dev.024273. [DOI] [PubMed] [Google Scholar]
- 26.Arber A. The Gramineae: a study of cereal, bamboo, and grasses. Cambridge, UK: Cambridge Univ Press; 1934. [Google Scholar]
- 27.Kinoshita T. Gene analysis and linkage map. In: Tsunoda S, Takahashi N, editors. Biology of Rice. Tokyo/Amsterdam: Japan Sci Soc Press/Elsevier; 1984. [Google Scholar]
- 28.Takamure I, Kinoshita T. Modification of glume characters due to genic interactions and environmental conditions. Rice Genet Newslett. 1992;9:85–88. [Google Scholar]
- 29.Nagato Y, Yoshimura A. Report of the committee on gene symbolization, nomenclature and linkage groups. Rice Genet Newslett. 1998;15:13–74. [Google Scholar]
- 30.Terrell EE, Peterson PM, Wergin WP. Epidermal features and spikelet micromorphology in Oryza and related genera (Poaceae: Oryzeae) Smithsonian Contr Bot. 2001;91:1–50. [Google Scholar]
- 31.Zhao L, et al. Overexpression of LSH1, a member of an uncharacterised gene family, causes enhanced light regulation of seedling development. Plant J. 2004;37:694–706. doi: 10.1111/j.1365-313x.2003.01993.x. [DOI] [PubMed] [Google Scholar]
- 32.Rensing SA, et al. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science. 2008;319:64–69. doi: 10.1126/science.1150646. [DOI] [PubMed] [Google Scholar]
- 33.Banks JA. Selaginella and 400 million years of separation. Annu Rev Plant Biol. 2009;60:223–238. doi: 10.1146/annurev.arplant.59.032607.092851. [DOI] [PubMed] [Google Scholar]
- 34.Vaughan DA. The wild relatives of rice: a genetic resources hand book. Los Banos, Philippines: IRRI; 1994. [Google Scholar]
- 35.Zamora A, Barboza C, Lobo J, Espinoza AM. Diversity of native rice (Oryza poaceae) species of Costa Rica. Genet Res Crop Evol. 2003;50:855–870. [Google Scholar]
- 36.Ohta M, Ohme-Takagi M, Shinshi H. Three ethylene-responsive transcription factors in tobacco with distinct transactivation functions. Plant J. 2000;22:29–38. doi: 10.1046/j.1365-313x.2000.00709.x. [DOI] [PubMed] [Google Scholar]
- 37.Shitsukawa N, Kinjo H, Takumi S, Murai K. Heterochronic development of the floret meristem determines grain number per spikelet in diploid, tetraploid and hexaploid wheats. Ann Bot. 2009;104:1–9. doi: 10.1093/aob/mcp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bao Y, Ge S. Origin and phylogeny of Oryza species with the CD genome based on multiple-gene sequence data. Plant Syst Evol. 2004;249:55–66. [Google Scholar]
- 39.Miyao A, et al. A large-scale collection of phenotypic data describing an insertional mutant population to facilitate functional analysis of rice genes. Plant Mol Biol. 2007;63:625–635. doi: 10.1007/s11103-006-9118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hiei Y, Ohta S, Komari T, Kumashiro T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994;6:271–282. doi: 10.1046/j.1365-313x.1994.6020271.x. [DOI] [PubMed] [Google Scholar]
- 41.Suzaki T, et al. The gene FLORAL ORGAN NUMBER1 regulates floral meristem size in rice and encodes a leucine-rich repeat receptor kinase orthologous to Arabidopsis CLAVATA1. Development. 2004;131:5649–5657. doi: 10.1242/dev.01441. [DOI] [PubMed] [Google Scholar]
- 42.Suzaki T, Yoshida A, Hirano H-Y. Functional diversification of CLAVATA3-related CLE proteins in meristem maintenance in rice. Plant Cell. 2008;20:2049–2058. doi: 10.1105/tpc.107.057257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakagawa T, et al. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng. 2007;104:34–41. doi: 10.1263/jbb.104.34. [DOI] [PubMed] [Google Scholar]
- 44.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.