Abstract
The molecular mechanisms underlying the development and progression of prostate cancer are poorly understood. AMP-activated protein kinase (AMPK) is a serine-threonine kinase that is activated in response to the hypoxic conditions found in human prostate cancers. In response to energy depletion, AMPK activation promotes metabolic changes to maintain cell proliferation and survival. Here, we report prevalent activation of AMPK in human prostate cancers and provide evidence that inhibition or depletion of AMPK leads to decreased cell proliferation and increased cell death. AMPK was highly activated in 40% of human prostate cancer specimens examined. Endogenous AMPK was active in both the androgen-sensitive LNCap cells and the androgen-independent CWR22Rv1 human prostate cancer cells. Depletion of AMPK catalytic subunits by siRNA or inhibition of AMPK activity with a small molecule AMPK inhibitor (compound C) suppresses human prostate cancer cell proliferation. Apoptotic cell death was induced in LNCap and CWR22Rv1 cells at compound C concentrations that inhibited AMPK activity. The evidence provided here is the first report that the activated AMPK pathway is involved in the growth and survival of human prostate cancer and offers novel potential targets for chemoprevention of human prostate cancer.
Keywords: Prostate cancer, AMP-activated protein kinase, tissue microarray, hypoxia, proliferation, apoptosis, chemoprevention, small molecule inhibitor, drug discovery, stress signaling pathway, glycolysis, Warburg effect
Introduction
Prostate cancer (PC) is the most common non-skin malignancy and the second leading cause of cancer death in American men (1). It is believed that PC, like other malignancies, develops and progresses through an accumulation of genomic/proteomic alterations (2). Molecular studies have identified several candidate genes that are consistent with important aspects of biological features of PC and likely to be important in PC pathogenesis and progression (3). A number of these genes have important roles in regulating cellular metabolism, including androgen receptor (AR), phosphatase and tensin homolog deleted on chromosome 10 (PTEN), p53 and alpha-methylacyl-CoA racemase (AMACR), suggesting that metabolic changes may contribute to the development of PC.
The PTEN tumor suppressor gene is one of the most frequently deleted genes in prostate cancer. Loss of PTEN phosphatase causes the accumulation of phosphatidyl-inositol,3,4,5 triphosphate (PIP3) and the activation of downstream effectors such as AKT. Persistent AKT activation promotes metabolic changes that allow for prostate tumorigenesis including up-regulation of biosynthetic pathways including glycolysis, protein synthesis and fatty acid synthesis. Activation of AKT induces aerobic glycolysis (the Warburg effect) by affecting multiple proteins that are directly involved in glycolysis. In addition, activated AKT phosphorylates ATP citrate lyase (4), which cleaves citrate to form oxaloacetate and acetyl-coenzyme A promoting fatty acid synthesis. p53 can inhibit glycolysis by inhibition of phosphoglycerate mutase (5). Loss of p53 activity is required for Akt-dependent tumorigenesis (6). In addition, decreased expression of p53 targets such as TP53-induced glycolysis and apoptosis regulator (TIGAR) and synthesis of cytochrome oxidase 2 (SCO2) also drives glycolysis by decreasing the capacity of cells to employ oxidative phosphorylation (7).
Hypoxia and metabolic stress are common characteristics of solid tumors (8). They result from an imbalance between tumor blood supply and tumor consumption. It has been suggested that hypoxia may lead to genetic instability, tumorigenesis and disease progression (8). Similar to cervix and head and neck cancer, areas of relatively poor blood flow and hypoxia are found in localized PC (9-12). Immunohistochemical analysis of hypoxia markers (13,14) and molecular imaging (15) support these findings. Importantly, tumor hypoxia has been shown to be an independent prognostic factor in PC progression (11,16).
AMP-activated protein kinase (AMPK) is a serine-threonine kinase that is activated in response to the hypoxic conditions found in human PC. AMP-activated protein kinase (AMPK) is an ubiquitous multi-subunit serine/threonine protein kinase that forms hetero-trimers composed of a catalytic subunit (α1 or α2) and two regulatory subunits (beta and gamma) (17). In response to metabolic stress, AMP levels are elevated and AMP binds to the gamma subunit. Allosteric changes promote phosphorylation of the catalytic subunit's activation loop via upstream kinases (17-20). In response to energy depletion, AMPK activation promotes metabolic changes to maintain cell proliferation and survival by directly phosphorylating rate-limiting enzymes in metabolic pathways, modifying signal transduction cascades and gene expression (17, 21). AMPK has also been shown to stimulate glycolysis through direct phosphorylation and activation of 6-phosphofructo-2-kinase (PFK-2) (17, 22). PFK-2 is the enzyme responsible for the synthesis of fructo 2, 6-bisphosphate, a potent stimulator of glycolysis. In addition, AMPK activation mediates the recruitment of glucose transporters to the cell membrane (17, 23).
We hypothesized that AMPK is activated in PC and may act as a metabolic survival factor. AMPK activity in human prostate cancer samples was investigated by immunohistochemical analysis of the phosphorylation status of the AMPK substrate acetyl-CoA carboxylase (ACC) (24). To specifically block AMPK activity in human prostate cancer cells, we developed siRNA that specifically target the catalytic subunits of AMPK. We demonstrate that AMPK is activated inhuman prostate cancer specimens and cell lines and blocking AMPK activity slows proliferation and induces apoptosis of human prostate cancer cells.
Materials and Methods
Cell Culture and Reagents
RWPE-1(ATCC, Manassas, VA), a human prostate epithelial cell line immortalized by human papillomavirus 18, was cultured in keratinocyte-serum free medium supplemented with 50 μg/ml bovine pituitary extract and 5 ng/ml human recombinant epidermal growth factor (Invitrogen, Carlsbad, CA). CWR22Rv1, LNCap, LNCap C4−2B, DU145 and PC-3 cells (ATCC, Manassas, VA) were cultured in RPMI 1640 (Mediatech, Herndon, VA) containing 10% fetal bovine serum, 2.5 mM l-glutamine, and penicillin-streptomycin (100 IU/ml and 100 μg/ml, respectively) at 37 °C with 5% CO2. LNCap cells were cultured in the presence of 0.5 nM dihydrotestosterone (5 α -androstan-17β-ol-3-one) (Sigma-Aldrich, St. Louis, MO). Compound C (CC) and 5-aminoimidazole-4-carboxamide-1-ß -D-ribofuranoside (AICAR) were obtained from Calbiochem (Gibbstown, NJ).
Immunohistochemical Detection of P-ACC in Human Prostate Specimens
Paraffin-embedded arrayed prostate cancer specimens (US Biomax, Inc, Rockville, VA) containing normal (38) and malignant (244) prostate tissues were deparaffinized, rehydrated, boiled with citrate buffer (pH 6), treated with 0.3% H2O2, and preincubated in blocking solution (10% normal goat serum). The primary antibody, anti-phospho-ACC (S79P) (Cell Signaling Technology, Inc., Danvers, MA), was incubated with the specimens at a concentration of 1:50 for one hour at room temperature. Antigen-antibody complexes were detected using a horseradish-peroxidase complexed anti-rabbit secondary antibody (Dako Envision-Plus) (Dako North America, Inc., Carpinteria, CA). 3,3’-diaminobenzidine (Dako) was used as chromogen and hematoxylin as counterstain. For negative control, subtype-specific IgG was used. Human pancreas was used as a positive control tissue. Individual prostate samples were scored in a blinded manner (SPC and SS) using semi-quantitative steps of increasing staining intensity, where 0 was undetectable, low immunostaining gave 1+, intermediate immunostaining gave 2+, and high immunostaining gave 3+ as a score. To discriminate among distributions, the Wilcoxon-Mann-Whitney test was performed using StatXact software (Cytel, Cambridge, MA).
Western Blot Analysis
Prostate cancer cells were lysed in the lysis buffer [10 mM Tris-HCl, pH 7.6, 5 mM EDTA, 50 mM sodium chloride, 30 mM sodium pyrophosphate, 50 mM sodium fluoride, 1 mM sodium orthovanadate, 1% Triton X-100, 1x protease inhibitor cocktail (Sigma-Aldrich)]. The protein samples were separated by SDS-PAGE and transferred onto immun-Blot PVDF membranes (Biorad Laboratories, Hercules, CA). The membranes were blocked with 5% nonfat dry milk and probed with the following antibodies: anti-AMPKα, anti-phospho-AMPKα (T172P), anti-ACC, anti-phospho-ACC (S79P), anti-PARP-1 (Cell Signaling Technology), anti-β-Actin (Simgma-Aldrich). Chemiluminescent detection was performed using ECL reagents according to the vendor's instructions (Pierce, Rockford, IL).
Construction of Enhanced Green Fluorescent Protein (EGPF)-TORC2 Expression Plasmid and Transfection
A cDNA clone encoding human TORC2 (GenBank accession number BC05362) was obtained from Open Biosystems( Huntsville, AL). For expression of EGFP-TORC2 fusion protein, the cDNA encoding TORC2 was cloned via PCR into pEGFP-N1 (Clontech, Mountain View, CA). PCR fragments were generated using pairs of a forward primer, GAGGGACTCGAGGCCACCATGGCGACGTCGGGGGCGAAC, and a revere primer, TGAGCAGAATTCGTTGGAGCCGGTCACTGCGGAA to introduce XhoI and EcoRI sites, respectively. After digestion with XhoI and EcoRI, the PCR amplified fragment was cloned into pEGFP-N1. The cloned fragment was sequenced in its entirety to verify that no mutations were introduced via PCR. For transient expression of EGFP-TORC2, CWR22Rv1 and LNCap cells were transfected with EGFP-TORC2 expression plasmid using FuGENE (Roche, Indianapolis, IN) as described by the manufacturer. A single immunoreactive band of appropriate molecular weight was identified on Western blots of EGFP-tagged TORC2-transfected cell extracts (data notshown).
Fluorescence Microscopy
CWR22Rv1 and LNCap cells were grown in 6-well tissue culture chambers to 50% confluency. Cells were transiently transfected with 1 μg of EGFP-TORC2 expression plasmid. After incubation for 24 h, transfected cells were treated with 10 μM Compound C, or 100 μM AICAR for 2h. Cells were washed twice with phosphate-buffered saline (PBS) and fluorescence microscopy was performed. Images were obtained using DP Controller software (Olympus, Center Valley, PA).
siRNA Mediated Knock-Down of AMPK Catalytic Subunits
AMPKα1 (PRKAA1 On-TAplus SMART pool duplex, L-005027) (Dharmacon, Lafayette, CO) and AMPKα2 (PRKAA2 Silencer Validated siRNA, AM51321) (Ambion, Austin, TX) were used for AMPK knock-down. siRNA were transfected into human prostate cancer cells using Lipofectamine RNAiMax following the manufacturer's instructions (Invitrogen, Carisbad,CA). Luciferase (Luc) siRNA (D-001400, Dharmacon) was used as a negative control.
Measurement of Cell Number
Cells were collected after trypsinization, resuspended in serum-containing medium, and counted. Cell number was determined using a Multisizer 3 Coulter Counter (Beckman Coulter, Minneapolis, MN).
Bromodeoxyuridine (BrdU) labeling
Cells were treated with BrdU (10 μg/ml) for 10 min to allow BrdU incorporation into newly synthesized DNA and washed with PBS. They were then harvested after trypsinization and fixed with ice-cold 70% ethanol. The fixed cells were denatured in 4 M HCl for 20 min at room temperature and washed with PBS. The denatured cells were incubated with 1% normal goat serum in PBS for 1h, followed by staining with an Alexa Fluor 488-conjugated anti-BrdU antibody (CALTAG Laboratories) and propidium iodide. The stained cells were analyzed using FACSsort (Becton Dickinson, Franklin Lakes, NJ).
Cell cycle Analysis
Cells were harvested and fixed in 70% ethanol. The fixed cells were then stained with propidium iodide (50 μg/ml) after treatment with RNase (5 μg/ml). The stained cells were analyzed for DNA content using FACSsort (Becton Dickinson). Cell cycle fractions were quantified with Cell Quest (Becton Dickinson) or ModFit LT (Verity Software House).
Results
AMPK Is Frequently Activated in Human Prostate Cancer
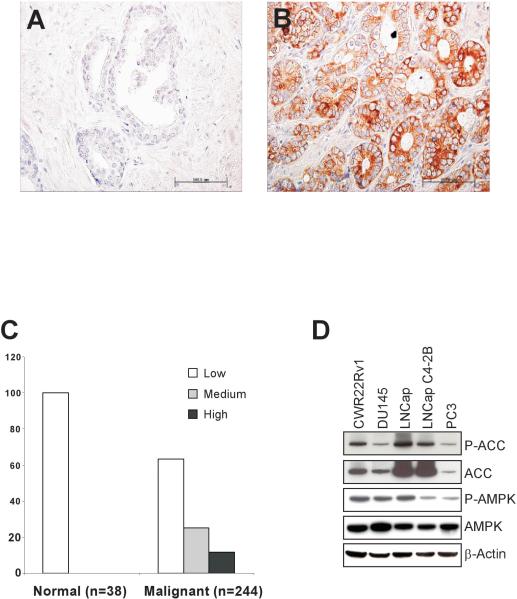
We examined AMPK activity in normal and malignant human prostate tissues by immunohistocytochemistry. Since AMPK is the sole kinase that phosphorylates ACC on serine 79 (24), Ser79 phosphorylation was used as a measure of AMPK activation. The phospho-ACC (Ser 79) antibody detects ACC (25) only when it is phosphorylated at serine 79. This antibody detected a single band of appropriate molecular weight on Western blot and its specificity was verified by phosphorylation inhibition studies. Immunostained sections were quantitated with a semi-quantitative scoring method (0−3+) based on the intensity of staining. Normal prostate epithelia were either completely negative or showed only weak staining (intensity score of 0 to 1+) for p-ACC (see a representative sample in Fig. 1A). Phosphorylated ACC (p-ACC) was found only in the cytoplasm of primary human cancerous specimens (see a representative sample in Fig. 1B). Figure 1C gives the percent distribution of p-ACC staining (0−3) and tissue status (normal vs. malignant). The distribution of p-ACC staining was statistically different between the normal and cancer tissues (P < 0.0001). Approximately 40% of the cancerous specimens exhibited medium to high staining (intensity score of 2 to 3+) for p-ACC. The prevalence and intensity of p-ACC staining did not correlate with the samples’ Gleason grade (data not shown). In summary, activated AMPK is expressed frequently in primary human PC specimens of various Gleason grades.
Figure 1. AMPK is activated in human prostate cancer.
Phosphorylated-ACC expression in normal human prostate (A) and primary human prostate cancers (B) was analyzed by immunohistochemical staining using a polyclonal anti-phospho-ACC antibody in paraffin-embedded tissue sections. C, Staining intensity of phosphorylated ACC in normal and malignant prostate tissues. D, Western blot analyses of extracts from various human prostate cancer cell lines. Cell lysates were analyzed by immunoblotting with anti-phospho-AMPK, anti-AMPK, anti-phospho-ACC or anti-ACC antibody. Blots were probed with anti-β-actin antibody to normalize for protein loading.
AMPK Is Activated in Human Prostate Cancer Cell Lines
We next examined AMPK activity in various human PC cell lines, including the androgen-sensitive LNCap cells and the androgen-independent LNCap C4−2B, CWR22Rv1, DU-145 and PC-3 cells. To evaluate AMPK activation, we assessed the phosphorylation of AMPK's activation loop (threonine 172) and its direct substrate ACC. Western blot analyses reveal that high levels of p-ACC were found in all the examined cell lines (Fig. 1D). The phosphorylation levels of the AMPK's catalytic loop were also highly elevated. We interpret these data as support for the hypothesis that AMPK is active in human PC cells.
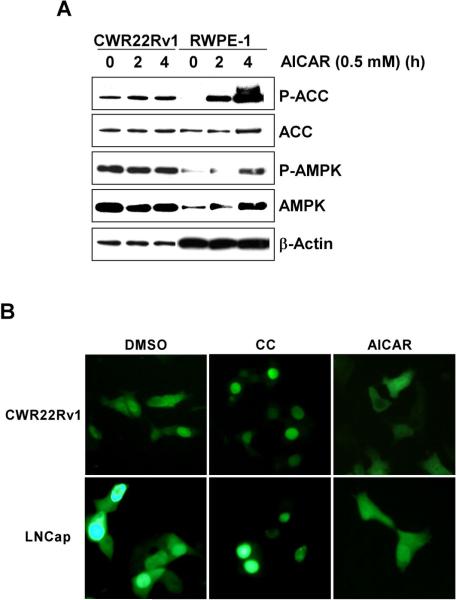
AMPK activity is low in normal human cells grown under standard tissue culture conditions. Mammalian cells convert 5-aminoimidazole-4-carboxamide-1-ß -D-ribofuranoside (AICAR) to 5-aminoimidaz-ole-4-carboxamide ribonucleoside (ZMP), an AMP mimetic which is a known activator of AMPK (26, 27). To determine if AMPK activity could be induced in human prostate cells, non-tumorigenic prostate epithelial cells (RWPE-1) and CWR22Rv1 cells were treated with or without AICAR (0.5 mM) for various periods of time (Fig. 2A). Western blot analysis of untreated RWPE-1 cells revealed low basal levels of AMPK and ACC phosphorylation. AICAR induced the phosphorylation of AMPK and ACC in a time-dependent manner. In cancerous CWR22Rv1 cells, AICAR treatment did not induce a further increase in the phosphorylation of the AMPK activation loop. Only a minimal increase in the phosphorylation of serine 79 of ACC was observed in AICAR-treated cells. Increasing the length of treatment (Fig. 2A) or the AICAR concentration (data not shown) failed to increase the modest induction of ACC phosphorylation. Similar results were seen in the other human PC cell lines (data not shown). These results support the idea that AMPK is highly activated in human PC cells and this activation is minimally enhanced by AMP.
Figure 2. AMPK is highly activated in human prostate cancer cells.
A, Time course of AMPK activation by AICAR in RWPE-1 and CWR22Rv1 cells. Cells were treated for the indicated hours with AICAR (0.5 mM). Cell lysates were resolved by 4−12% SDS-PAGE and probed with anti-phospho-AMPK, anti-AMPK, anti-phospho-ACC or anti-ACC antibody. Blots were probed with anti-β-actin antibody to normalize for protein loading. B, Inhibition of AMPK activity promotes nuclear localization of TORC2 in human prostate cancer cells. LNCap or CWR22Rv1 cells transiently expressing EGFP-TORC2 were exposed to either vehicle control, CC (10 μM) or AICAR (100 μM) for 2 hours. EGFP-TORC2 was visualized by fluorescence microscopy.
Since AMPK activation has not been documented under non-stressed tissue culture conditions, we assessed the activation status of AMPK in vitro. Transducer of regulated CREB-binding protein 2 (TORC2) is phosphorylated by AMPK at serine 171 within an optimal consensus sequence for AMPK (28). Phosphorylation at this site by active AMPK restricts TORC2 to the cytoplasm. TORC2 was chosen as a good substrate for analyzing AMPK activity since Ser171 is phosphorylated specifically by AMPK family members (29, 30). To monitor TORC2 localization in vitro, we constructed a carboxy-terminal EGFP-tagged TORC2 mammalian expression vector. As shown in Fig. 2B, TORC2 was localized in the cytoplasm and the nucleus of sub-confluent proliferating LNCap and CWR22Rv1 cells. When these cells were treated with AICAR, there was a modest shift to the cytoplasm. Consistent with constitutive activity of AMPK in prostate cancer cells, treatment of cells with compound C (CC), a small molecule ATP competitive inhibitor of AMPK (31), promoted TORC2 nuclear translocation in both cell lines. Taken together, these data strongly supported the activated status of AMPK in human PC cells.
Down regulation of AMPK Catalytic Subunits by Small Interfering RNA (siRNA) Inhibits Cell Proliferation in Human Prostate Cancer Cells
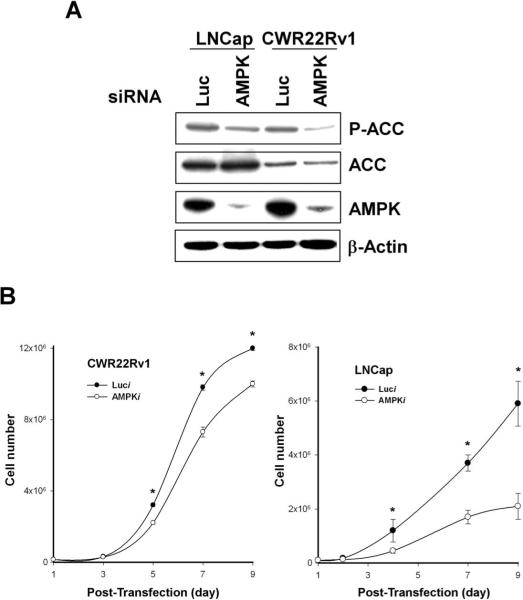
To determine whether AMPK activity promotes growth in human prostate cancer cells, we developed AMPK-siRNA that reduced the protein levels of AMPK catalytic subunits (α-1 and α-2) to less than 10% of control by 72 hours (Fig. 3A). Western blot analyses using the p-ACC antibody verified that treatment of cells with AMPK-siRNA inhibits AMPK activity in PC cell lines (Fig. 3A) as indicated by p-ACC level.
Figure 3. Downregulation of AMPK's catalytic subunits by siRNA decreases cellular proliferation in human prostate cancer cells.
LNCap or CWR22Rv1 cells were transfected with a mixture of siRNA targeting the two AMPK catalytic subunits (AMPK) or luciferase (Luc). A, Western blot analysis showing 72 hours after transfection of LNCap or CWR22Rv1 with siRNA targeting the AMPK catalytic subunits or Luc. Cell lysates, prepared from cancer cell lines, were resolved by 4−12% SDS-PAGE and probed with anti-AMPK, anti-phospho-ACC or anti-ACC antibody. B, At the indicated number of days post-transfection, cells were collected and counted. Results are representative of three independent experiments. *, Significantly different (P < 0.05) from control cells.
Subconfluent cultures of human PC cells were transfected with siRNA targeting the AMPK catalytic subunits or control siRNA. As shown in Figure 3B, silencing of the AMPK catalytic subunit genes caused a reduction in the number of cells whereas control cells continued proliferating normally (Fig. 3B). Similar results were obtained in the androgen-dependent LNCap and androgen-independent CWR22Rv1 cells. Further support was provided by Bromodeoxyuridine (BrdU) incorporation experiments which revealed that AMPK-siRNA significantly decreased S-phase entry in both cell lines (data not shown).
AMPK Inhibition by a Small Molecule Decreases Cell Proliferation and Promotes Apoptosis in Human Prostate Cancer Cells
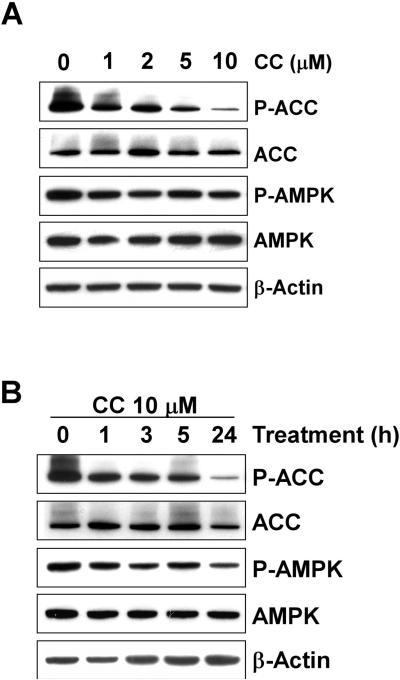
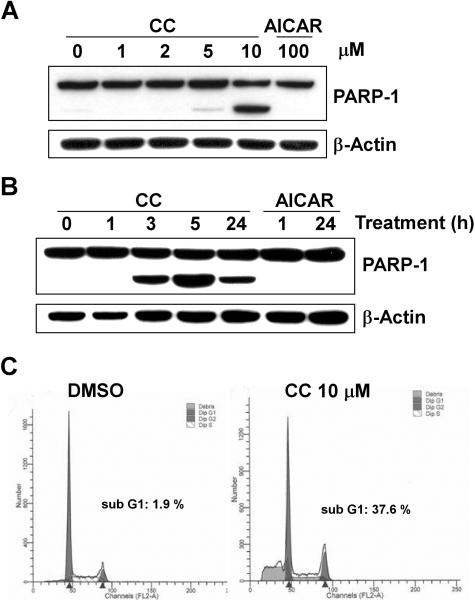
To support the findings obtained with siRNA, an alternative method of AMPK inhibition was employed. Compound C (CC) was previously identified in a chemical library screen as a potent and specific ATP-mimetic competitive inhibitor of AMPK (31). Western blot experiments using the phospho-specific antibody to ACC verified that treatment of human PC cells with CC inhibits AMPK activity in a dose-dependent (Fig. 4A) and time-dependent manner (Fig. 4B). As expected, CC had no effect on the phosphorylation of AMPK's catalytic loop.
Figure 4. Dose-dependent and time-dependent inhibition of AMPK activity by Compound C.
CWR22Rv1 cells were treated with various concentrations of CC for 24 hours (A) or with CC (10 μM) for various times as indicated (B). Samples were collected and analyzed by Western blots using antibodies for phospho-ACC, ACC, phospho-AMPK and AMPK. Blots were probed with anti-beta-actin antibody to normalize for protein loading.
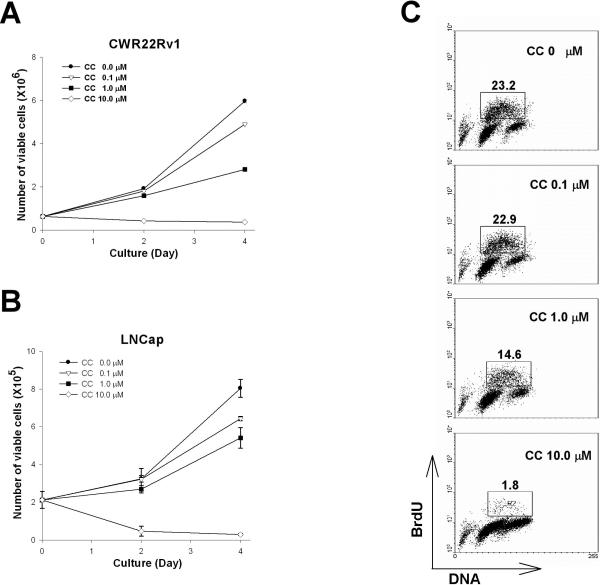
To explore the effect of CC on cell proliferation, cells collected at different time points after treatment with various concentrations of CC were counted. Treatment of CWR22Rv1 (Fig. 5A) or LNCap cells (Fig. 5B) with CC resulted in a dose-dependent decrease in the total number of viable cells. The decrease in proliferation correlated closely with the level of AMPK inhibition (Fig. 4A and 5A, B). Dose-dependent inhibition of proliferation was confirmed by BrdU incorporation studies (Fig. 5C). BrdU is incorporated as a thymidine analogue into newly synthesized DNA and allows assessment of the proportion of cells cycling through S phase. BrdU incorporation was reduced from ∼23% of cells to ∼2% with CC (10 μM) treatment for 24 hours (Fig. 5C).
Figure 5. Inhibition of AMPK activity by CC decreases cellular proliferation in human prostate cancer cells.
CWR22Rv1 (A) or LNCap (B) cells were treated with various concentration of CC as indicated. At the indicated number of days post-initiation of treatment, cells were collected and counted. C, CWR22Rv1 cells treated with various concentrations of CC for 24 hours were assayed for BrdU incorporation. The number in each panel indicates percentage of BrdU positive cells.
Microscopic assessment of the effect of CC on PC cell viability revealed morphologicalchanges consistent with apoptotic cell death including cell rounding, detachment, shrinkage and blebbing (data not shown). To test whether CC–treated cancer cells die through induction of apoptosis, PARP-1 cleavage was analyzed. Western blot experiments using the anti-PARP-1 antibody verified that treatment with CC induced PARP-1 cleavage in a dose-dependent (Fig. 6A) and time-dependent manner (Fig. 6B). The concentration of CC required for cell killing corresponded closely with the dose of CC required for AMPK inhibition (Fig. 4A and Fig. 6A). AICAR treatment with concentrations that activate AMPK (32) did not promote PARP-1 cleavage (Fig. 6A and 6B). Apoptotic cell death of prostate cancer cells treated with a concentration of CC that inhibits AMPK was also verified by cell cycle analysis. As shown in Figure 6C, extensive DNA fragmentation (hypodiploid cell fragments) was detected in CC treated cells. The percentages of cellular DNA in sub-G1 were ∼2% in control cells and ∼38% in CC-treated cells.
Figure 6. Inhibition of AMPK activity by CC induces apoptotic cell death in human prostate cancer cells.
Western blot analyses of PARP-1 cleavage in CWR22Rv1 cells after exposure to various concentrations of CC for 24 hours (A) or 10 μM of CC for the indicated times (B). Samples were collected and analyzed using an antibody to PARP-1. Blots were probed with anti-beta-actin antibody to normalize for protein loading. C, CC induced apoptosis in CWR22Rv1 cells as determined by flow cytometry. Cellular apoptosis were detected as an increased hypodiploid fraction (sub G1) after treatment with DMSO or CC (10 μM) for 48 hours.
Discussion
Under stressed conditions, such as those found in PC, activated AMPK plays a major role in maintaining energy homeostasis by inducing ATP-producing catabolic pathways (33). In this study, we compared the level of AMPK activity in normal and malignant prostate specimens by examining the phosphorylation status of the well characterized AMPK substrate ACC. As expected, low levels of P-ACC were detected in non-stressed normal human prostate tissues. To our knowledge, this is the first report that demonstrates prevalent AMPK activity in human prostate cancer specimens. This finding suggests that prostate cancer cells are energetically stressed due to their environment and the demands of continuous cell proliferation. The degree of AMPK activity varied among the cancerous specimens. This suggests that human prostate cancers vary in their levels of metabolic stress. Of note, we did not see a correlation between AMPK activity and tumor Gleason grade. This finding is consistent with microelectrode studies, reported by others, showing no relationship between pO2 levels and Gleason grade (10). Alternatively, low AMPK activity in some of the cancerous specimens could be secondary to deficiencies in upstream kinases as seen in other cancer types (34). The selective activation of AMPK in prostate cancer specimens raises the question of a possible connection between AMPK activation and prognosis which is currently being explored in our laboratory.
Surprisingly, we found that AMPK was highly activated not only in human PC specimens but also in human PC cells growing under standard tissue culture conditions. We hypothesize that AMPK activation is an important downstream effector of an unknown genomic and /or proteomic change found in transformed prostate epithelial cells (2). Transformed cells demonstrate altered metabolism when compared to normal cells. One of the most fundamental metabolic alterations occurring with malignant transformation is the up-regulation of aerobic glycolysis, a phenomenon known as the Warburg effect (35, 36). Currently, the molecular mechanisms leading to constitutive upregulation of aerobic glycolysis are poorly understood (37). Activated AMPK, a possible contributor to the Warburg effect, has been shown to promote glycolysis by enhancing glucose uptake (17,22) and activating PFK-2 (17,23). In addition, AMPK is known to induce numerous glycolytic genes (38). Further studies are required to determine the relationship between transformation, aerobic glycolysis and AMPK activity in human PC.
The mechanism by which AMPK is activated in human prostate cancer cells is currently unclear. Under normal physiologic conditions, AMPK is activated under conditions that deplete cellular ATP such as glucose deprivation, heat shock, hypoxia, and ischemia (17). However, AMPK activity may also be elevated under non-stressed conditions. For example, AMPK is activated by hormones like leptin, adiponectin and interleukin-6 (39). These adipokines have been implicated in the development and progression of human prostate cancer (40). LKB1 and CAMKKβ are central candidates for AMPK activation in prostate cancer since these enzymes are responsible for AMPK activation in non-cancerous tissues (17-20). While LKB1 is a known tumor suppressor (41), CAMKKβ activation has not been tied to prostate cancer progression. Alternatively, an unidentified kinase(s) could be acting as an AMP-activated kinase kinase in prostate cancer.
In normal cell physiology, AMPK activation has been proposed to protect cells from injurydue to hypoxia and other metabolic stressors (42) by slowing cell growth and proliferation. The target-of-rapamycin (TOR) stimulates the initiation step of protein synthesis which is required for cell growth via phosphorylation of multiple targets (43). TOR is activated by Akt phosphorylation of its binding partner Raptor and an upstream pathway involving tuberous sclerosis complex 2 (TSC2) (44). AMPK inhibit TOR regulate protein synthesis via phosphorylation of Raptor (45) and the TSC1-TSC2 complex (46). To delay progression through the cell cycle, AMPK activation decreases expression of important cell cycle regulators (47) and induces stabilization of p53 and cyclin-dependent kinase inhibitors (48, 49). In cancer cells, AMPK has been demonstrated to bestow tolerance to nutrient deprivation (50) and hypoxia (33) without restricting cell growth and proliferation. It has been proposed that AMPK promotes cancer cell survival by providing energy for essential cellular functions through processes such as fatty acid beta-oxidation and/or autophagy (50, 51). The opposing effect of AMPK activation in normal and transformed cells could be due to differential deletion of downstream tumor suppressors allowing AMPK activation, while mitigating the growth limiting effect of the enzyme. For example, some cancers harbor TSC2 and/or p53 mutations, allowing AMPK activation without inhibition of protein synthesis or cell cycle arrest (52).
During the course of these experiments, reports suggesting that AICAR-induced AMPK activation inhibits proliferation, induces senescence and promotes apoptosis in various cancer cells were published (53, 54). It was proposed that this was secondary to AMPK inhibition of anabolic processes such as protein synthesis (45, 46) or activation of p53 (48). While AICAR is the best characterized pharmacologic activator of AMPK and most of its effects have been prescribed to AMPK activation, AMPK-independent effects have been documented (26). In an attempt to compare their findings with ours, we treated human prostate cancer cells with AICAR. In our hands, millimolar concentrations of AICAR induce S-phase arrest and senescence independent of AMPK activation (data not shown). These data suggest that decreased proliferation in response to AICAR is not secondary to AMPK activation but a non-specific effect of AICAR on nucleotide metabolism (26). New selective AMPK activators (55) may help to further define the role of AMPK in cancer therapy.
Protein kinase signaling pathways important in maintaining cell proliferation and survival under stressed conditions may provide the critical growth signals for premalignant lesions to progress to clinical prostate cancer. We show that AMPK is activated in primary prostate cancers and may promote prostate cancer proliferation and survival. The prevalent detection of activated AMPK in primary human prostate cancer specimens indicates that AMPK is a potential candidate molecular target for chemoprevention of prostate cancer.
Acknowledgements
Shared Resources of Cancer Center are partially supported by National Institute of Health Grant CA56036-08 (Cancer Center Support Grant, to Lombardi Comprehensive Cancer Center). This work was supported by American Cancer Society (IRG 97-152-13).
Abbreviations
- AR
androgen receptor
- AMACR
alpha-methylacyl-CoA racemase
- AMPK
5’-AMP-activated protein kinase
- ACC
Acetyl-CoA carboxylase
- AICAR
5-aminoimidazole-4-carboxamide-1-ß -D-ribofuranoside
- BrdU
Bromodeoxyuridine
- CC
Compound C
- DHT
dihydrotestosterone
- EGFP
enhanced green fluorescent protein
- FACS
fluorescence-activated cell sorting
- PARP-1
poly(ADP-ribose)polymerase-1
- PIP3
phosphatidyl-inositol,3,4,5 triphosphate
- PC
prostate cancer
- siRNA
small interfering RNA
- TIGAR
TP53-induced glycolysis and apoptosis regulator
- TMA
tissue microarray
- TOR
target-of-rapamycin
- TORC2
transducer of regulated CREB-binding protein 2
- TSC2
tuberous sclerosis complex 2
- ZMP
5-aminoimidaz-ole-4-carboxamide ribonucleoside
Footnotes
H. U. Park and S. Suy contributed equally to this work
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Tomlins SA, Rubin MA, Chinnaiyan AM. Integrative biology of prostate cancer progression. Annu Rev Pathol. 2006;1:243–71. doi: 10.1146/annurev.pathol.1.110304.100047. [DOI] [PubMed] [Google Scholar]
- 3.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 4.Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 2005;24:6314–6322. doi: 10.1038/sj.onc.1208773. [DOI] [PubMed] [Google Scholar]
- 5.Corcoran CA, Huang Y, Sheikh MS. The regulation of energy generating metabolic pathways by p53. Cancer Biol Ther. 2006;5:1610–1613. doi: 10.4161/cbt.5.12.3617. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, Cordon-Cardo C, Pandolfi PP. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green DR, Chipuk JE. p53 and metabolism: Inside the TIGAR. Cell. 2006;126:30–32. doi: 10.1016/j.cell.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 8.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–16. [PubMed] [Google Scholar]
- 9.Movsas B, Chapman JD, Horwitz EM, et al. Hypoxic regions exist in human prostate carcinoma. Urology. 1999;53:11–8. doi: 10.1016/s0090-4295(98)00500-7. [DOI] [PubMed] [Google Scholar]
- 10.Movsas B, Chapman JD, Greenberg RE, et al. Increasing levels of hypoxia in prostate carcinoma correlate significantly with increasing clinical stage and patient age: an Eppendorf pO(2) study. Cancer. 2000;89:2018–24. doi: 10.1002/1097-0142(20001101)89:9<2018::aid-cncr19>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 11.Movsas B, Chapman JD, Hanlon AL, et al. Hypoxic prostate/muscle pO2 ratio predicts for biochemical failure in patients with prostate cancer: preliminary findings. Urology. 2002;60:634–9. doi: 10.1016/s0090-4295(02)01858-7. [DOI] [PubMed] [Google Scholar]
- 12.Parker C, Milosevic M, Toi A, et al. Polarographic electrode study of tumor oxygenation in clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58:750–7. doi: 10.1016/S0360-3016(03)01621-3. [DOI] [PubMed] [Google Scholar]
- 13.Zhong H, Agani F, Baccala AA, et al. Increased expression of hypoxia inducible factor-1alpha in rat and human prostate cancer. Cancer Res. 1998;58:5280–4. [PubMed] [Google Scholar]
- 14.Cvetkovic D, Movsas B, Dicker AP, et al. Increased hypoxia correlates with increased expression of the angiogenesis marker vascular endothelial growth factor in human prostate cancer. Urology. 2001;57:821–5. doi: 10.1016/s0090-4295(00)01044-x. [DOI] [PubMed] [Google Scholar]
- 15.Hoskin PJ, Carnell DM, Taylor NJ, et al. Hypoxia in prostate cancer: correlation of BOLD-MRI with pimonidazole immunohistochemistry-initial observations. Int J Radiat Oncol Biol Phys. 2007;68:1065–71. doi: 10.1016/j.ijrobp.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Vergis R, Corbishley CM, Norman AR, et al. Intrinsic markers of tumour hypoxia and angiogenesis in localised prostate cancer and outcome of radical treatment: a retrospective analysis of two randomised radiotherapy trials and one surgical cohort study. Lancet Oncol. 2008;9:342–51. doi: 10.1016/S1470-2045(08)70076-7. [DOI] [PubMed] [Google Scholar]
- 17.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–85. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 18.Woods A, Johnstone SR, Dickerson K, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–8. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 19.Hawley SA, Pan DA, Mustard KJ, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 52:9–19. doi: 10.1016/j.cmet.2005.05.009. 200. [DOI] [PubMed] [Google Scholar]
- 20.Woods A, Dickerson K, Heath R, et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 21.McGee SL, Hargreaves M. AMPK and transcriptional regulation. Front Biosci. 2008;13:3022–33. doi: 10.2741/2907. [DOI] [PubMed] [Google Scholar]
- 22.Marsin AS, Bertrand L, Rider MH, et al. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 2000;10:1247–55. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 23.Chavez JA, Roach WG, Keller SR, Lane WS, Lienhard GE. Inhibition of GLUT4 translocation by Tbc1d1, a Rab GTPase activating protein abundant in skeletal muscle, is partially relieved by AMPK activation. J Biol Chem. 2008;283:9187–95. doi: 10.1074/jbc.M708934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ha J, Daniel S, Broyles SS, Kim KH. Critical phosphorylation sites for acetyl-CoA carboxylase activity. J Biol Chem. 1994;269:22162–8. [PubMed] [Google Scholar]
- 25.Brownsey RW, Boone AN, Elliott JE, Kulpa JE, Lee WM. Regulation of acetyl-CoA carboxylase. Biochem Soc Trans. 2006;34:223–7. doi: 10.1042/BST20060223. [DOI] [PubMed] [Google Scholar]
- 26.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–65. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 27.Swinnen JV, Beckers A, Brusselmans K, et al. Mimicry of a cellular low energy status blocks tumor cell anabolism and suppresses the malignant phenotype. Cancer Res. 2005;65:2441–8. doi: 10.1158/0008-5472.CAN-04-3025. [DOI] [PubMed] [Google Scholar]
- 28.Scott JW, Norman DG, Hawley SA, Kontogiannis L, Hardie DG. Protein kinase substrate recognition studied using the recombinant catalytic domain of AMP-activated protein kinase and a model substrate. J Mol Biol. 2002;317:309–23. doi: 10.1006/jmbi.2001.5316. [DOI] [PubMed] [Google Scholar]
- 29.Koo SH, Flechner L, Qi L, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–11. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 30.Screaton RA, Conkright MD, Katoh Y, et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Zhou G, Myers R, Li Y, Chen Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiang X, Saha AK, Wen R, Ruderman NB, Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun. 2004;321:161–7. doi: 10.1016/j.bbrc.2004.06.133. [DOI] [PubMed] [Google Scholar]
- 33.Laderoute KR, Amin K, Calaoagan JM, et al. 5'-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26:5336–47. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carretero J, Medina PP, Blanco R, et al. Dysfunctional AMPK activity, signalling through mTOR and survival in response to energetic stress in LKB1-deficient lung cancer. Oncogene. 2007;26:1616–25. doi: 10.1038/sj.onc.1209951. [DOI] [PubMed] [Google Scholar]
- 35.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 36.Ashrafian H. Cancer's sweet tooth: the Janus effect of glucose metabolism in tumorigenesis. Lancet. 2006;367:618–21. doi: 10.1016/S0140-6736(06)68228-7. [DOI] [PubMed] [Google Scholar]
- 37.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–9. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 38.Nilsson EC, Long YC, Martinsson S, et al. Opposite transcriptional regulation in skeletal muscle of AMP-activated protein kinase gamma3 R225Q transgenic versus knock-out mice. J Biol Chem. 2006;281:7244–52. doi: 10.1074/jbc.M510461200. [DOI] [PubMed] [Google Scholar]
- 39.Kola B, Boscaro M, Rutter GA, Grossman AB, Korbonits M. Expanding role of AMPK in endocrinology. Trends Endocrinol Metab. 2006;17:205–15. doi: 10.1016/j.tem.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Mistry T, Digby JE, Desai KM, Randeva HS. Obesity and prostate cancer: a role for adipokines. Eur Urol. 2007;52:46–53. doi: 10.1016/j.eururo.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 41.Hemminki A, Markie D, Tomlinson I, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–7. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 42.Young LH, Li J, Baron SJ, Russell RR. AMP-activated protein kinase: a key stress signaling pathway in the heart. Trends Cardiovasc Med. 2005;15:110–8. doi: 10.1016/j.tcm.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Arsham AM, Neufeld TP. Thinking globally and acting locally with TOR. Curr Opin Cell Biol. 2006;18:589–97. doi: 10.1016/j.ceb.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Hardie DG. AMPK and Raptor: matching cell growth to energy supply. Mol Cell. 2008;30(9):263–5. doi: 10.1016/j.molcel.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 47.Wang W, Fan J, Yang X, et al. AMP-activated kinase regulates cytoplasmic HuR. Mol Cell Biol. 2002;22:3425–36. doi: 10.1128/MCB.22.10.3425-3436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones RG, Plas DR, Kubek S, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–93. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 49.Liang J, Shao SH, Xu ZX, et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–24. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 50.Kato K, Ogura T, Kishimoto A, et al. Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene. 2002;21:6082–90. doi: 10.1038/sj.onc.1205737. [DOI] [PubMed] [Google Scholar]
- 51.Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–52. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 52.Kaper F, Dornhoefer N, Giaccia AJ. Mutations in the PI3K/PTEN/TSC2 pathway contribute to mammalian target of rapamycin activity and increased translation under hypoxic conditions. Cancer Res. 2006;66:1561–9. doi: 10.1158/0008-5472.CAN-05-3375. [DOI] [PubMed] [Google Scholar]
- 53.Rattan R, Giri S, Singh AK, Singh I. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J Biol Chem. 2005;280:39582–93. doi: 10.1074/jbc.M507443200. [DOI] [PubMed] [Google Scholar]
- 54.Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H. Cell cycle regulation via p53 phosphorylation by a 5'-AMP activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun. 2001;287:562–7. doi: 10.1006/bbrc.2001.5627. [DOI] [PubMed] [Google Scholar]
- 55.Cool B, Zinker B, Chiou W, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–16. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]