Abstract
OBJECTIVE
To re-examine the anatomy of the perineal membrane and its anatomical relationships in whole-pelvis and histological serial section as well as gross anatomical dissection.
METHODS
Serial trichrome-stained histologic sections of 5 female pelvic specimens (0 to 37 years old) were examined. Specimens included the urethra, perineal membrane, vagina and surrounding structures. Macroscopic whole pelvis sections of 3 adults 28 to 56 years in axial, sagittal and coronal sections were also studied. Dissections of 6 female cadavers 48 to 90 years were also performed.
RESULTS
The perineal membrane is composed of 2 regions, one dorsal and one ventral. The dorsal portion consists of bilateral transverse fibrous sheets that attach the lateral wall of the vagina and perineal body to the ischiopubic ramus. This portion is devoid of striated muscle. The ventral portion is part of a solid 3-dimensional tissue mass in which several structures are embedded. It is intimately associated with the compressor urethrae and the urethrovaginal sphincter muscle of the distal urethra with the urethra and its surrounding connective. In this region the perineal membrane is continuous with the insertion of the arcus tendineus fascia pelvis. The levator ani muscles are connected with the cranial surface of the perineal membrane. The vestibular bulb and clitoral crus are fused with the membrane's caudal surface.
CONCLUSION
The structure of the perineal membrane is a complex 3-dimensional structure with two distinctly different dorsal and ventral regions; not a simple trilaminar sheet with perforating viscera.
Keywords: perineal membrane, urogenital diaphragm, pelvic floor dysfunction, pelvic organ prolapse, anatomy, female
Introduction
Pelvic floor dysfunction is a common problem that leads to significant suffering among women.1 Its origin during vaginal birth and surgical correction later in life unite the two primary elements of our specialty; obstetrics and gynecology. Understanding the nature of pelvic floor dysfunction must begin with an accurate anatomical appreciation of the structures that comprise the pelvic floor. The perineal membrane (formerly called the urogenital diaphragm) is often discussed in both the delivery room and descriptions of gynecologic operations. In the process of examining pelvic organ supports and levator ani muscles,2,3,4 it became evident that existing descriptions of this region were at variance with the anatomy seen in human cadavers.
In his defining paper on the striated urogenital sphincter muscle in women,5 Dr. Thomas Oelrich provides a detailed history of articles on the urogenital sphincter complex, the adjacent “urogenital diaphragm”, and the deep perineal space from the mid-1800s to 1960. In this paper he disproved the concept of a urogenital diaphragm that consists of a layer of striated muscle with superior and inferior fascial coverings. He introduced the term “perineal membrane” that is now the accepted anatomical term by Terminologia Anatomica.6 A recent review article summarizes articles on the same structures from 1960 to the present.7 Although some of the recent research reassesses the descriptions of the perineal membrane and/or the urogenital diaphragm, current knowledge of the structure is based primarily on historical descriptions and conceptual diagrams copied and re-copied in anatomical and surgical texts.
The traditional description of the perineal membrane describes a trilaminar musculo-fascial structure that spans the ischiopubic rami, connects to the perineal body, through which the urethra and vagina perforate.8 More recent descriptions,7, 9, 10 although variable, do not agree with this 3-layered model, clearly stating that the urogenital sphincter musculature is not sandwiched between superior and inferior fascial sheets. However, these more recent descriptions describe the inferior fascial layer of this “historical structure” as the perineal membrane (or triangular ligament): 1) extending between ischiopubic rami, 2) creating a specific boundary between superficial and deep perineal spaces, and 3) with the urethra and vagina piercing this fascial sheet. 7, 9, 10 This does not correlate well with the anatomy seen in cadaver dissection, cross-sectional anatomical specimens or imaging studies of living women. Our objective was to directly examine the anatomy of this area in serial cadaver sections and dissection to see whether the concept of a sheet of fibromuscular tissue pierced by the urethra and vagina holds true or whether the perineal membrane is part of a complex of several interconnected structures.
Materials and Methods
Serial trichrome-stained histologic sections of 5 female pelvic specimens (0 to 37 years old) were examined. Specimens included the urethra, vagina, perineal erectile tissue and muscles, and surrounding structures from Dr. TM Oelrich described in previous work of the senior author,2 as well as in Dr. Oelrich's original paper.5 The histologic sections were used to define tissue types (smooth muscle, connective tissue, striated muscle), specific fiber direction and connection as well as the detailed structural spatial relationships. In addition, serial macroscopic whole-pelvis cross sections of three adult females (28 to 56 years old) were studied. The pelves were fixed by flotation to avoid embalming artifact, and then prepared by freezing the lower body and upper thighs in dry ice and cutting then on a band saw at 5 mm intervals in axial, sagittal and coronal planes.4 In addition, to correlate cross-sectional observations, gross dissection of the perineal region was conducted on a convenience sample of six Caucasian female cadavers ranging in age from 48 to 90 years old. These observations were supplemented with the senior author's experience in dissecting approximately 100 unembalmed cadavers while teaching pelvic anatomy to gynecological surgeons.
Results
The perineal membrane and its interconnected structures form a complex apparatus that attach many structures (Figure 1). It is a 3-dimensional mass of tissue with several attachments. There are has two distinct regions; a dorsal portion lateral to the perineal body and a ventral portion lateral to the urethra. The dorsal portion consists of bilateral transverse fibrous bands of connective tissue that attach the perineal body and lateral wall of the vagina to the ischiopubic rami. This portion is bounded above by ischiorectal fossa fat and below by the structures of the perineum including the vestibular bulb, clitoral crus and their investing muscles the bulbospongiosus and ischiocavernosus muscles. In the ventral region, the membrane is part of a solid 3-dimensional tissue mass in which several structures are embedded. It is continuous with the paraurethral and paravaginal connective tissues and contains the compressor urethrae and urethrovaginal sphincter muscles of the distal urethra. The ventral margin of this mass is continuous with the insertion of the arcus tendineus fascia pelvis into the pubic bone. The levator ani muscles are attached to the cranial surface of the perineal membrane complex, while the vestibular bulbs and clitoral crus are fused with the caudal surface. Medially, these structures fuse with the walls of the urethra and vagina.
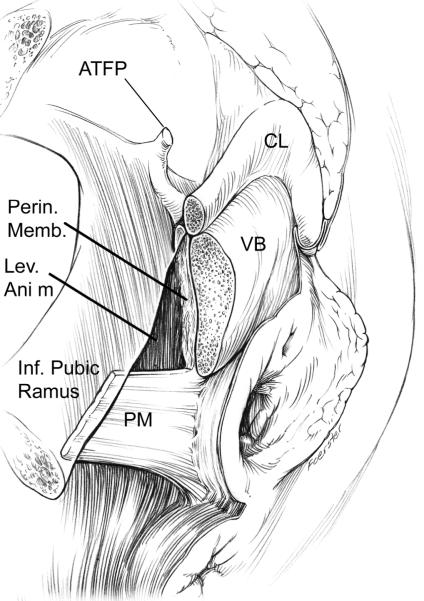
Figure 1.
Drawing of dissection revealing the perineal membrane (PM) showing its lateral attachment to the inferior pubic ramus. A window in the perineal membrane has been cut to reveal the attachment of the levator ani muscle (LA) and its fusion with the vestibular bulb (VB). Extension to the arcus tendineus fascia pelvic is also shown (ATFP) which is shown inside the pubic bone attaching to its inner surface; clitoris (CL). (© DeLancey 2007)
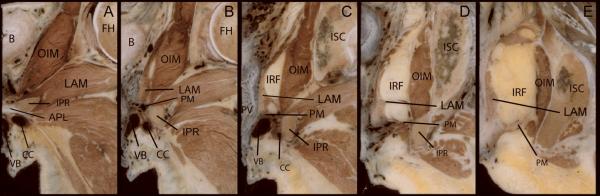
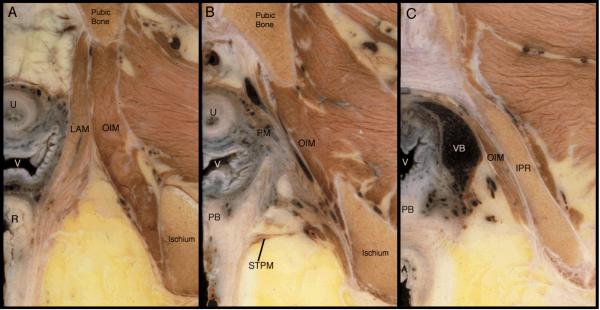
Figure 2 demonstrates the changing nature of the perineal membrane in coronal section. Panel A shows the arcuate pubic ligament that spans the distance between the ischiopubic rami ventral to the first appearance of the perineal membrane complex seen in Panel B. Here, the ventral portion of the perineal membrane complex is defined by the location of its surrounding structures. It lies cephalad to the vestibular bulb and the clitoral crus, and caudal to the levator ani muscles. Medially a portion of the wall of the urethra can be seen as well as the paraurethral portion of the vagina. The fact that the ventral portion of the perineal membrane is one component of this an integral part of these inter-related structures –and not a freestanding structure– is evident here.
Figure 2.
Contiguous coronal sections of a 33-year-old female cadaver demonstrating the changing nature of the perineal membrane from its ventral margin (B) to its dorsal-most extent (E). The left side of each image is the midline portion of the specimen. Abbreviations: Arcuate pubic ligament (APL); bladder (B); clitoral crus (CC); femoral head (FH); ischiopubic ramus (IPR); ischio-rectal fossa (IRF); Ischium (ISC); levator ani muscles (LAM); obturator internus muscle (OIM); perineal body (PB); perineal membrane (PM); posterior vaginal wall (PV); vagina (V); vestibular bulb (VB). (© DeLancey 2007)
Panel C lies at the level of the vaginal lumen where the transition from the ventral to the dorsal portion of the perineal membrane complex occurs. At this point the structure becomes a distinct layer of connective tissue between the lateral vagina and the ischiopubic ramus though still maintaining its close relationship to the levator ani muscle. Like the traditional paradigm, this dorsal portion is distinct from its surrounding structures creating a “deep space”; i.e., ischio-anal fossa. The ischio-anal fossa defines the upper margin of this dorsal portion, and the erectile structures (VB and CC) its caudal margin.
Panels D and E show the dorsal portion of the perineal membrane complex. The fibromuscular layer of the posterior vaginal wall (D) and the perineal body (E) are attached to the ischiopubic ramus via this fibrous dorsal portion of the perineal membrane. The distance between the midline viscera and the lateral ischiopubic rami has widened in this region compared with panels A – C so that the perineal membrane is a distinct structure with less interconnectedness between surrounding structures.
Reviewing the Panels from B to E reveals the constant anatomic relationships. The viscera are medial; the ischiopubic rami are lateral; the erectile structures are caudal; and the levator ani muscles cranial in the ventral sections and the ischio-anal fossa cranial in the dorsal sections.
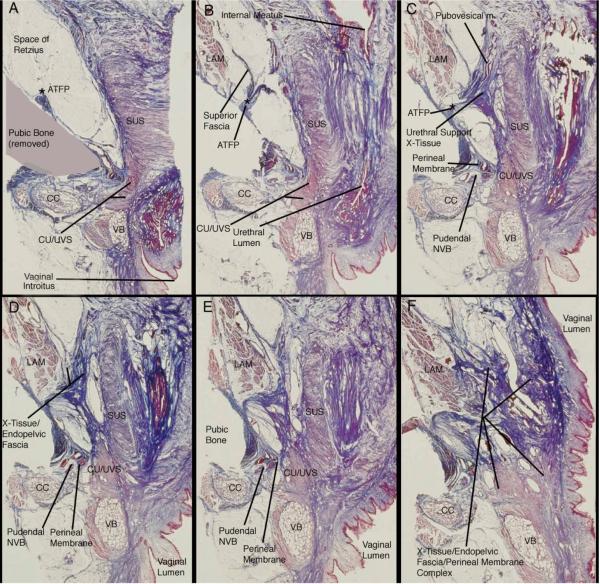
Figure 3 demonstrates the histologic detail in coronal section of the ventral portion of the perineal membrane. Panel A shows the ventral-most portion of the striated urethral sphincter muscle and the compressor urethrae/urethrovaginal sphincter muscle complex. Panel A indicates, via shading, the location of the pubic bone that was removed to facilitate cutting the histological sections. Note the attachment of the arcus tendineus fascia pelvis to the pubic bone indicated by the asterisk. There is no evidence of any perineal membrane structures in this section.
Figure 3.
Histologic (trichrome) sections of an adult female cadaver in coronal plane demonstrating the complex, 3-dimensional nature of the ventral portion and its relationship with the superior fascia of the levator ani muscles and the endopelvic fascia of the paraurethral/paravaginal supports. The right of each image is the midline portion of the specimen; cross sections run from ventral (A) to dorsal (F). Abbreviations: arcus tendineus fascia pelvis (ATFP); clitoral crus (CC); compressor urethrae/urethrovaginal sphincter muscles (CU/UVS); levator ani muscles (LAM); neurovascular bundle (NVB); striated urethral sphincter (SUS); vestibular bulb (VB). (© DeLancey 2007)
Panels B and C show the ventral margin of the perineal membrane complex developing. Notice that the superior fascia of the levator ani muscles is continuous with the insertion of the arcus tendineus fascia pelvis (B), which is continuous with the paraurethral connective tissue supports (C), which is in turn continuous with the ventral portion of the perineal membrane complex (D & E). As the distance between the midline viscera and the pubic bone increases (C,D,E) the connective tissue mass of this ventral portion of the perineal membrane increases. Also, notice the pudendal neurovascular bundle embedded in this tissue (C).
Panels D and E show the continued development of the 3-dimensional mass of tissue; the perineal membrane. As seen in previous ventral panels, this mass of tissue is continuous with the superior fascia of the levator ani muscles and with the paraurethral connective tissue or endopelvic fascia. The compressor urethrae and urethrovaginal sphincter muscles are embedded into the medial portion of this tissue mass, while the pudendal neurovascular bundle is embedded into the lateral portion near the periosteum of the pubic bone. Also notice the clitoral crus and the vestibular bulb and their respective muscles fused to the caudal surface of the ventral portion of the perineal membrane complex.
Panel F is a section through the vaginal lumen at which point the transition from the ventral to the dorsal perineal membrane complex occurs. The distance between the midline viscera and the lateral pubic bone has increased, and therefore, the connective tissue mass has increased. It is continuous with the superior fascia of the levator ani muscles, the paravaginal connective tissue, and likely the periosteum of the pubic bone as well. The levator ani muscles are inserting into this connective tissue mass cranially, while the clitoral crura and vestibular bulbs, with their respective muscles, are inserting into or are fused with the tissue mass caudally.
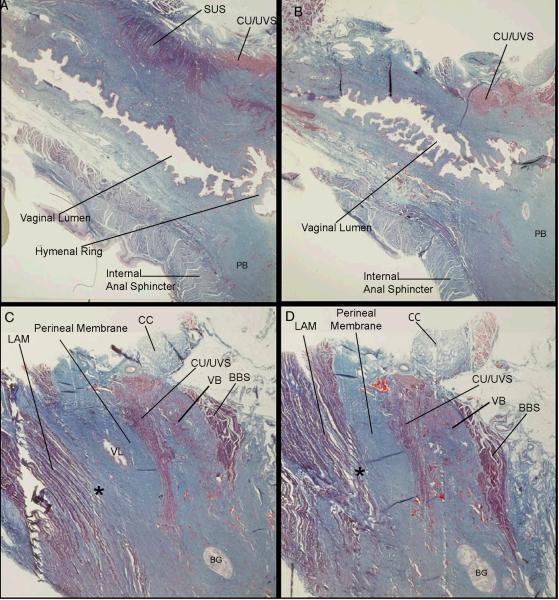
Figure 4 demonstrates in sagittal section the insertion of the levator ani muscle fibers into the portion of the perineal membrane lateral to the vagina. Panel A is a parasagittal section through the vaginal lumen, perineal body, and the ventral portion of the internal anal sphincter muscle. Ventral to the vagina, the lateral portion of the striated urethral sphincter and the ventral midline portion of the compressor urethrae and urethrovaginal sphincter muscle complex can be seen.
Figure 4.
Histologic (trichrome) sections of an adult female cadaver in sagittal plane beginning in the parasagittal plane just lateral to the urethral lumen (A) and progressing laterally to the region of the perineal membrane demonstrating the insertion of levator ani muscle (LAM) fibers directly into the cranial portion of the perineal membrane (C, D) lateral to the vagina (*). In this region, the perineal membrane can be seen to be fused with surrounding structures. Abbreviations: bulbospongiosus muscle (BBS); Bartholin's gland (BG); clitoral crus (CC); compressor urethrae/urethro-vaginal sphincter muscles (CU/UVS); levator ani muscles (LAM); perineal body (PB); striated urethral sphincter (SUS); vaginal lumen (VL); vestibular bulb (VB). (© DeLancey 2007)
Panels B and C are sections lateral to A, and show the vaginal lumen becoming the lateral vaginal wall and its connective tissue (C). Similarly, the perineal body also becomes connective tissue as the sections move laterally (C). This connective tissue lateral to the vagina and perineal body, within which the compressor urethrae and urethrovaginal sphincter muscles are contained, is the perineal membrane structure.
Panels C and D show the levator ani muscle fibers inserting into the cranial surface of the perineal membrane complex. These sections demonstrate that the structures in this region are not separate entities, but are interconnected parts of a single tissue mass.
Figure 5 shows the axial sections of the same cadaver whose coronal sections are shown in Figure 2. A comparison of the coronal and axial sections from the same cadaver demonstrates that axial section is not favorable to defining the relationships of the complex 3-dimensional structure of the perineal membrane, its attachments to the pubic bone, perineal body and pelvic viscera. Note the lower vagina, mid-urethra, levator ani and obturator internus muscles. Panel B, shows the ventral portions of the perineal membrane lateral to the urethra and vagina. The superficial transverse perineal muscle marks the dorsal edge of the perineal membrane. Although this muscle is not part of the complex, it lies on the caudal surface of the dorsal edge of the perineal membrane where fibrous connective tissue bands connect the perineal body to the ischia. The section in Panel C is caudal to the perineal membrane.
Figure 5.
Contiguous axial sections of the lower urogenital tract, including portions of the perineal membrane (PM), from the other side of the cadaver shown in Figure 1. Cross sections run from cephalic (A) to caudal (C). Abbreviations: anus (A); ischiopubic ramus (IPR); levator ani muscles (LAM); obturator internus muscle (OIM); perineal body (PB); perineal membrane (PM); rectum (R); superficial transverse perineal muscle (STPM); urethra (U); vagina (V); vestibular bulb (VB). (© DeLancey 2007)
Discussion
Although the perineal membrane has been described for well over a century, opinions have differed widely regarding its structure and relevance to pelvic organ support. In this study, rather than commenting on terminology or comparing previous descriptions, we have directly examined the anatomy of the perineal membrane and its surrounding tissues. The importance of this structure as a surgical landmark and its functional role in posterior vaginal wall support make it imperative that an accurate description is available. Examination of serial cross-sections revealed that the structure called the perineal membrane (previously referred to as the urogenital diaphragm) is a complex structure that is only one component of a larger interconnected support apparatus. In addition, this study reveals that the perineal membrane has two distinct parts; a dorsal portion and a ventral portion and that the levator ani muscle is intimately connected with this structure.
Because the perineal membrane is attached to so many surrounding structures, re-connecting the two sides as occurs during posterior colporrhaphy can re-align these structures into their normal configuration. The dorsal portion consists of bilateral connective tissue fibrous bands spanning from the lateral vagina and perineal body to the dorsal portions of the ischiopubic rami that can become separated after childbirth. In this region, the levator ani muscles are directly connected to the perineal membrane, to the perineal body and to the bulbospongiosus muscles. The levator muscle fibers insert directly into the perineal membrane connective tissue bands, and ventrally the superior fascia of the levator ani muscles is continuous with the perineal membrane tissue mass. The attachment of the levator ani muscles to the perineal membrane and perineal body means that disruption to the midline connection between the perineal membranes of each side though the perineal body 4 allows loss of perineal body support and also lateral displacement of the perineal membrane. Because the levator is fused with this area, this would result in widening of the urogenital hiatus seen in women with prolapse4. This provides an anatomical support for the idea that posterior repair, by bringing the separated perineal body and membrane attachments back to the midline, can restore the normal alignment of the levator ani muscles.
The different parts of the perineal membrane complex suggest different functions. It would appear that the dorsal portion is related to the support of the perineal body and lateral vaginal wall through its attachment to the ischiopubic rami.4 It seems likely that during the second stage of labor the dorsal portion, by attaching the vagina and perineal body, participates in holding these structures in place while the head dilates the introitus. It is often stated that the perineal membrane supports structures during normal activities, but this is not logical since considerable descent of the perineal body during straining occurs in normal women when the levator ani muscles are relaxed.11 Therefore it may be implied that the perineal body, via its attachment to the dorsal portion of the perineal membrane complex, limits downward motion when the levator ani muscles are relaxed, but that it is normal muscle tone that maintains perineal position.
The ventral portion is contiguous with the urethral supportive apparatus as previously discussed by Milley and Nichols.12 Whether or not the urethral supports and perineal membrane are considered different supports or the same structure is an arbitrary distinction about which experts will probably always have honest disagreements. The real need is for an accurate understanding of the structural mechanics of this region. This need is not addressed by arguments about nomenclature. In this region, it is not possible to understand structure and function under the historical paradigm that the perineal membrane is a sheet of tissue.
Although there has been some recent research and reassessment of the perineal membrane,7,10 the traditional, century-old paradigm persists in most anatomy and surgical textbooks.7 Recent research clearly states that there is no deep transverse perineal muscle in the female, and that the urogenital diaphragm concept of urogenital sphincter muscles sandwiched between two layers of fascia is also incorrect.5,7,10 Our study agrees with these two points. However, these recent reassessments of the perineal membrane continue to describe a single sheet of fascia spanning the ischiopubic rami denoting a boundary between superficial and deep perineal spaces. Oelrich describes a thin layer of perineal membrane separating the superficial compartment, containing the erectile tissues and their muscles, from the “urethrovaginal compartment” which is continuous with the pelvic cavity.5 A more recent paper describes the perineal membrane as extending between the pubic arch and the ischiopubic rami creating a boundary between the superficial and deep perineal spaces.7 Our study describes a ventral portion of the perineal membrane and emphasizes the fact that the perineal membrane is not a free-standing structure in this region. It is part of a multifaceted complex of interconnected to tissues and structures. It does not create a deep perineal space in the ventral portion. It is difficult to visualize a structure that is a solid tissue mass intermingling with other tissues and structures when the structure is called a “membrane”. The fact that misleading terms can lead to misleading concepts has long been known having been captured by the father of the scientific method; Francis Bacon over 300 years ago: “Whereas the meaning ought to govern the term, the term in effect governeth the meaning”.13 This has certainly been true of the term urogenital diaphragm.
We should consider why this area, smaller than the palm of a hand, has been so confusing. Dissection in this area is difficult because of the dense connective tissue present and because there are not natural cleavage planes that allow structures to be easily separated from one another. Perhaps as important, however, has been the longstanding and practice of considering the female a variant of male anatomy. In the male, the idea that the perineal membrane is a continuous sheet perforated by the urethra is not only plausible, but mechanically sound. In the female the situation is completely different because of the presence of the vagina. It is interesting that in the area of the human body that actually defines the anatomical differences between the sexes, the presumption that one sex is a variant of the other has been applied. The anatomy in the male and female must be separately described.
There are limitations to any anatomical study that must be considered in interpreting their results. Anatomical material, either embalmed or fresh, has topographic distortion because of fixation and loss of muscle tone.14 MRI scans show anatomy in normal women that is helpful in understanding topographic relations, but is limited in resolution of fine detail and only shows part of the structures involved. We have chosen to focus primarily on cross-sectional anatomy because it avoids the distortion that dissection creates and also avoids the “creative” portion of dissection where dissection is often carried out to make a structure look like what textbooks suggest. We recognize that examining cross sections can be challenging for those who do not have prior experience in evaluating serial cross-sectional images, but cross-sectional images remain the gold standard of evaluating pelvic floor structure as Lawson has previously pointed out.15 Careful study of these images allows the undisturbed anatomy to be studied. We have especially depended on histological cross-section because it allows tissue type (striated muscle, smooth muscle, dense connective tissue, loose areolar connective tissue) to be characterized. This study has addressed the constant features seen in all specimens. There is considerable individual variation seen in this region. A larger study will be needed to address these variations and their relation to such issues as vaginal parity. For dissection we have chosen adult cadavers in the age ranges relevant to the occurrence of pelvic organ prolapse.
There is much to be learned and re-learned from continued examination of anatomical material. This process must be re-discovered by each generation. The domination of books over bodies that existed for over a millennium where in Galenic anatomy was taught by “anatomists” who read Galen's text and had unlearned demonstrators point out the anatomy to the audience can lead to perpetuation of error. During this era, the book was considered right and the body was considered wrong. Vesalius, who revolutionized anatomical teaching by teaching directly from the body, recognized the problems of book-based learning. The advent of modern imaging and wide availability of inexpensive pictures of true anatomy should inspire us to constantly consult the body for the truth.
Acknowledgments
We gratefully acknowledge support from the Office for Research on Women's Health's SCOR on Sex and Gender Factors Affecting Women's Health and the National Institute of Child Health and Human Development through grants P50 HD044406 and R01 HD 38665 that have made this research possible.
References
- 1.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–6. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 2.DeLancey JOL. Correlative study of paraurethral anatomy. Obstet Gynecol. 1986;68:91–7. [PubMed] [Google Scholar]
- 3.DeLancey JOL. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166:1717–28. doi: 10.1016/0002-9378(92)91562-o. [DOI] [PubMed] [Google Scholar]
- 4.DeLancey JOL. Structural anatomy of the posterior compartment as it relates to rectocele. Am J Obstet Gynecol. 1999;80:815–23. doi: 10.1016/s0002-9378(99)70652-6. [DOI] [PubMed] [Google Scholar]
- 5.Oelrich TM. The striated urogenital sphincter muscle in the female. Anat Rec. 1983;205:223–32. doi: 10.1002/ar.1092050213. [DOI] [PubMed] [Google Scholar]
- 6.Federative Committee on Anatomical Terminology . Thieme. Stuttgart, Germany: 1998. Terminologia Anatomica. [Google Scholar]
- 7.Mirilas P, Skandalakis JE. Urogenital diaphragm: an erroneous concept casting its shadow over the sphincter urethrae and deep perineal space. J Am Coll Surg. 2004;198:279–90. doi: 10.1016/j.jamcollsurg.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Henle J. 1873. Handbuch der Systematischen Anatomie des Menschen. Bd. II. Druck und Verlag von Friedrich Vieweg und Sohn, Braunschweig.
- 9.McVay CB. Anson & McVay Surgical Anatomy. 6th ed vol 2. WB Saunders Co; Philadelphia: 1984. p. 895.p. 899. [Google Scholar]
- 10.Dorschner W, Biesold M, Schmidt F, Stolzenburg JU. The dispute about the external sphincter and the urogenital diaphragm. J Urol. 1999;162:1942–45. doi: 10.1016/S0022-5347(05)68074-3. [DOI] [PubMed] [Google Scholar]
- 11.Henry MM, Parks AG, Swash M. The pelvic floor musculature in the descending perineum syndrome. Br J Surg. 1982;69:470–2. doi: 10.1002/bjs.1800690813. [DOI] [PubMed] [Google Scholar]
- 12.Milley PS, Nichols DH. The relationship between the pubo-urethral ligaments and the urogenital diaphragm in the human female. Anat Rec. 1971;170:281–3. doi: 10.1002/ar.1091700304. [DOI] [PubMed] [Google Scholar]
- 13.Bacon F. Of unity and religion, Essays, Civil and Moral. P.F. Collier and Sons; New York: 1937. p. 162. [Google Scholar]
- 14.Richter K. Lebendige Anatomie der Vagina. Geburtshilfe Frauenheilkd. 1966;26:1213–23. [PubMed] [Google Scholar]
- 15.Lawson JO. Pelvic anatomy. I. Pelvic floor muscles. Ann R Coll Surg Engl. 1974;54:244–52. [PMC free article] [PubMed] [Google Scholar]