Abstract
Energy-restricted low glycemic load diets are being used increasingly for weight loss. However, the long-term effects of such regimens on mood and cognitive performance are not known. We assessed the effects of low glycemic load (LG) and high glycemic load (HG) energy-restricted diets on mood and cognitive performance during 6 months of a randomized controlled trial when all food was provided. Subjects were 42 healthy overweight adults (age 35 ± 5 y; BMI 27.8 ± 1.6 kg/m2) with a mean weight loss of 8.7 ± 5.0% that did not differ significantly by diet randomization. Mood was assessed by using the Profile of Mood States (POMS) questionnaire. Cognitive performance was assessed by using computerized tests of simple reaction time, vigilance, learning, short-term memory and attention, and language-based logical reasoning. Worsening mood outcome over time was observed in the HG diet group compared to the LG for the depression subscale of POMS (P=0.009 after including hunger as a covariate). There was no significant change over time in any cognitive performance values. These findings suggest a negative effect of an HG weight loss diet on sub-clinical depression but, in contrast to a previous suggestion, provide no support for differential effects of LG versus HD diets on cognitive performance.
Keywords: High glycemic load, Low glycemic load, Mood, Cognition, Energy metabolism
1. Introduction
There remains controversy over whether weight loss impacts mood and cognition [1] and [2], and intentional weight loss has been associated with both positive and negative mood and cognition outcomes [3], [4], [5], [6], [7], [8], [9], [10], [11] and [12]. This divergence of findings suggests that unrecognized factors may be contributing to between-study variability, and in particular the amount and quality of dietary carbohydrate may be important.
A wide range of dietary carbohydrate, fat and protein contents have been employed in weight loss diets to date. Consistent with the suggestion that dietary carbohydrate may impact mood outcomes, some [13], [14], [15] and [16] though not all [17], [18] and [19] single-meal studies have reported negative effects of lower carbohydrate intake on mood parameters such as energy level, alertness, and response to stress in individuals susceptible to depression [15]. Observational studies have also reported significant inverse associations between symptoms of depression and carbohydrate intake among free-living individuals [20] and [21]. However, there is lack of information on the effects of dietary macronutrient composition during weight loss on mood in long-term studies controlling for energy intake, and those studies also provide conflicting results [22], [23] and [24]. In addition, there is almost no information from studies providing food rather than recommending dietary changes, although self-reported information on dietary intake is recognized to be highly inaccurate [25] and [26]. Finally, there is no information on the effects of weight loss diets differing in glycemic load (GL), a newer and widely used dietary index that quantifies the amount of carbohydrate in relation to its ability to raise blood glucose [27].
Inconclusive results have also been obtained for the effects of weight loss on cognitive function, with improved, neutral and negative outcomes reported [12], [8] and [5]. As with studies on mood, variability in dietary carbohydrate may have influenced the outcomes obtained. Halyburton et al. [22] reported negative effects of very low carbohydrate intake on cognitive variables, especially in more complex tests. This finding is consistent with studies in rats showing cognitive decline in animals consuming very high fat (and therefore lower carbohydrate) diets [28]. Concerning GL, to our knowledge there are no long-term studies of the effects of this variables on cognition during weight loss. However, two single-meal studies in children and adults with type 2 diabetes [29] and [30] reported that low GL breakfasts prevent normal morning decline in cognitive performance, suggesting the potential for long-term benefits. As with studies of mood, these investigations are too limited to allow for consistent conclusions, and further studies are needed.
We describe here an investigation examining the effects of HG and LG diets consumed during weight loss on mood and cognition. This study formed part of a randomized controlled trial comparing diets with different GL for their ability to facilitate sustained adherence to a caloric restriction (CR) regimen. Because all food was provided for the first 6 months, this study provided a unique opportunity to assess the effects of GL during weight loss on mood and cognition under much more controlled dietary conditions than are usually possible.
2. Methods
2.1.Study participants
Forty-six healthy overweight (body mass index [BMI] 25–29.9 kg/m2) adults ages 20–42 years were recruited for this study as described elsewhere [31]. Potential subjects underwent a medical evaluation and laboratory tests at screening, and to be eligible were required to be free from serious disease or other conditions that could influence outcomes including diabetes, cancer, coronary heart disease, endocrine disorders, depression (defined as score of >20 on the Beck Depression Inventory [32]), other serious psychiatric diagnoses, and eating disorders. Additional exclusion criteria included a high level of physical activity (>12 hrs/wk), recent weight fluctuations (>15 lbs in the last year), high dietary restraint scores (>17) measured by the Eating Inventory, and inability to complete an acceptable dietary record. All subjects gave written, informed consent prior to participating, and were provided with a stipend. The study was conducted at the Metabolic Research Unit (MRU) of the Jean Mayer USDA Human Nutrition Research Center on Aging (HNRCA) at Tufts University with approval by the Tufts-New England Medical Center Institutional Review Board. It is a registered clinical trial (# NCT00099099) and was monitored by an external monitoring board.
2.2. Protocol
The study consisted of a 7-week baseline period followed by a 12 month intervention [31]. Subjects were instructed to maintain weight during the baseline period and consume their typical diet, while baseline outcome assessments were made including determination of energy requirements by the doubly-labeled water (DLW) method over 28 days [33]. Each subject was then randomized to receive an HG or LG diet and one of two levels of CR (10% or 30% relative to baseline energy requirements). By design, 34 subjects were randomized to the 30% CR groups and 12 subjects to the 10% CR groups; the unequal group sizes were intentional because the primary purpose of 10% groups was to provide experience with recruiting, retaining and obtaining outcomes in a control group with a smaller deficit in dietary energy for a future larger trial. Both the 10% and 30% CR groups consumed significantly less energy during CR than at baseline (p<0.01) and lost similar amounts of weight [31] and thus were combined in analyses. All food and caloric beverages were provided during the first 6 months of the intervention, and during the second 6 months, subjects were expected to maintain their assigned diet regimen at home. Only data from the baseline and first 6 months of intervention (42 subjects completed the study to 6 months) are presented here, as this was the time period when information on dietary intake was most accurate. Subjects visited the research center regularly throughout the study for a variety of activities including attending regular behavioral support groups, individual meetings with the study dietitian, safety monitoring and outcomes testing.
2.2.1. Study diets
The LG and HG study diets differed in GL and macronutrient composition (Table 1), but were matched for fiber content and energy density, and were also determined to be equivalently palatable in pilot testing of the regimens. Both diets consisted of generally lower energy dense foods and limited liquid calories, and 3 meals and 1 snack were provided daily. Subjects were expected to fully consume the provided food, and document any deviations from the plan (additions or subtractions). All food was picked up at the research center twice weekly. In addition to the primary randomization, subjects were also randomized briefly within groups to extra dietary fiber (Fiber One cereal by General Mills, providing 20 g/d fiber) from either 5–24 weeks or 11–24 weeks of CR [34] and given the option to substitute 1000 kcal/week of foods of their choice for provided foods from 15–24 or 21–24 weeks. There was no effect of either of these short-term secondary randomizations on weight change or hunger during either the period of randomization or over the entire study (data not shown). Subjects also received a one-a-day multivitamin throughout the study to ensure no micronutrient deficiencies.
Table 1.
Composition of the provided diets*
| High Glycemic Load Diet (HG) |
Low Glycemic Load Diet (LG) |
|
|---|---|---|
| % Energy from Carbohydrate/Protein/Fat |
60/20/20 | 40/30/30 |
| Dietary Fiber (g/1000 kcal) | 15 | 15 |
| Glycemic Load * (g/1000 kcal) | 116 | 45 |
| Energy Density (kcal/g) | 1.0 | 1.0 |
[34].
2.2.2. Behavioral & nutritional support
Biweekly behavioral support groups were conducted, with subjects attending groups based on a pre-planned schedule regardless of diet randomization. Standard topics were discussed including self-monitoring, adherence, menu design, meal planning, grocery shopping, differentiating between hunger and non-hunger cues, relapse control, social and family support, practical strategies for social situations, and eating outside the home. During alternate weeks, subjects attended biweekly individual consulting sessions with the study dietitian.
2.3. Mood and cognitive performance assessments
A battery of tests assessing mood and cognitive function was administered at baseline and month 6 of CR to capture 6 dimensions of mood and a broad spectrum of cognitive functions. To standardize testing conditions for factors such as hunger and testing environment, tests were made approximately 10.30 am after subjects had slept overnight at the research center, and were conducted in the subjects’ private bedroom. No caffeine was allowed on testing days prior to testing, and those subjects who did normally consume coffee in the morning were restricted to 2 cups daily throughout the investigation. Technical difficulties with encrypted data reduced available cognitive function data to 28 individuals.
2.3.1. Profile of mood states
The Profile of Mood States (POMS) questionnaire is a widely used, standardized instrument which can be computer-based or self-administered on paper [35]. The POMS questionnaire has been used in various fields including experimental psychology, exercise physiology and psychiatry, and to assess the effects of dietary constituents on behavioral state [36]. For this investigation, subjects used the computer-based format [8] and [37] and rated a series of mood-related adjectives on a 5-point scale in response to the question, “How are you feeling right now?” The possible response adjectives factor into 6 mood subscales: tension-anxiety, depression-dejection, anger-hostility, vigor-activity, fatigue-inertia and confusion-bewilderment. The subscales are considered individually, and are also used to create a composite total mood disturbance score by summing the individual scores (with vigor-activity reverse scored).
2.3.2. Cognitive function
The specific computer-based tests used to assess cognition were chosen to test a spectrum of functions from simple to more complex tasks without creating excess study burden. Practice sessions for the tests were conducted to familiarize subjects with the specifics of testing one week prior to each actual test session, and were identical to the regular test sessions except one longer-duration test (visual vigilance) was shortened from 25 to 5 minutes. All tests were administered in a comfortable private room in the research center. They were presented in standardized order as listed.
2.3.3. Grammatical reasoning test
This test assessed language-based logical reasoning [38]. On each trial, a logical statement, such as “A is preceded by B”, was followed by the letters “AB” or “BA” on the computer screen. Statements can be positive/negative or active/passive, and a given letter may precede/follow the other letter. Subjects attempted to determine whether or not each statement correctly described the order of the two letters. The “T” key on the keyboard was pressed for correct (true) response, and the “F” key was pressed for incorrect (false) responses. The subjects had 20 seconds to press a key, or a score of no response was recorded. Parameters recorded included correct and incorrect responses, no response and response time (seconds). Each session included 32 trials.
2.3.4. Four-choice visual reaction time test
This test assessed the ability to respond rapidly and accurately to simple visual stimuli. Subjects were presented with a series of randomly repeating visual stimuli at 1 of 4 different spatial locations on the computer screen [39]. They indicated the correct spatial location of each stimulus by pressing one of the four corresponding keys on the keyboard. Parameters recorded included reaction time (milliseconds), correct responses and time-out errors.
2.3.5. Repeated acquisition test
This test assessed motor learning, attention and short-term memory [40]. Subjects learned a sequence of 12 keystrokes, using the four arrow keys on the computer. The outline of an empty rectangle was presented on the screen at the beginning of a trial. Each correct response filled in a portion (1/12) of the rectangle from left to right with a solid block. Each incorrect response blanked the screen for 0.05 seconds. When the screen returned, the subject was at the same point as before the incorrect response. The subject learned the correct sequence by trial and error. When a sequence was correctly completed, the rectangle became full, the screen blanked, and another empty rectangle appeared for the next trial. A session ended when the subject completed 15 correct sequences (15 trials). Incorrect responses and time to complete each trial were recorded.
2.3.6. Scanning visual vigilance test
This test assessed visual vigilance, which is the ability to sustain attention during relatively boring, continuous tasks that generate minimal cognitive load [41]. The subject continuously scanned the computer screen to detect the occurrence of an infrequent, difficult-to-detect stimulus that appeared for 2 seconds at random intervals and locations on the screen. On average, a stimulus was presented once per minute. Upon detection, the subject pressed the keyboard space bar as rapidly as possible. Parameters recorded include correct responses, reaction time (milliseconds) and false alarms.
2.4. Other testing
2.4.1. Body height and weight
Height was determined at the beginning of the study to ±0.1 cm using a wall-mounted stadiometer. Fasting weight was assessed at weekly intervals to ±0.01 kg using a calibrated electronic scale (DETECTO-Cardinal Scale Manufacturing Co. Model CN-20, Webb City, MO) with subjects wearing a preweighed hospital gown.
2.4.2. Dietary restraint
The Eating Inventory [42] assesses restraint, disinhibition, and hunger and was administered at baseline and at 3 month intervals during the intervention.
2.5. Statistical analyses
The data were analyzed by using Analysis of Covariance models with 6 month value as the response, diet as the study factor, and baseline value as a covariate. Other covariates were CR level (10% or 30%), percent weight loss and gender. Because randomization to 10% and 30% CR level did not significantly influence energy intake or weight loss (10% subjects tended to leave some of their provided food, while 30% subjects tended to eat all their provided food), data summaries combine both CR levels. Overall body weight change was expressed as a percent change ([final – initial/initial] * 100). Pearson’s correlations were used to test for associations between changes in weight and changes in mood and cognition. Paired and independent t-tests were used to test for within and between diet group differences on behavioral outcomes. All analyses were performed using SPSS version 14.0 or SAS 9.1.3 (SAS Institute, Cary, NC, USA). A two-tailed, 0.05 level of probability was used to determine statistical significance. Unless otherwise noted, values are expressed as means ± standard deviations.
3. Results
3.1. Sample characteristics
Table 2 summarizes the characteristics of the subjects at baseline. There was no statistically significant difference between the groups for any variables. The mean percent weight loss for all completing subjects (n=42) was 8.7 ± 5.0%. Percent weight change did not differ significantly by diet type, CR level, or gender.
Table 2.
Characteristics of the subjects
| HG Diet* (4M, 16F) |
LG Diet (7M, 15F) |
||
|---|---|---|---|
| Baseline | |||
| Age (y) | 34.9±4.3 | 34.6±5.5 | |
| Height (cm) | 168.6±10.2 | 170.4±10.7 | |
| Body weight (kg) | 79.2±11.5 | 81.3±10.1 | |
| BMI (kg/m2) | 27.7±1.7 | 27.9±1.4 | |
| Intervention | |||
| % weight change 0–6 mo | −8.5 ± 4.6 | −8.9 ± 5.4 | |
Data are means±SD (s). HG, high glycemic; LG, low glycemic; BMI, body mass index.
No significant differences between diet groups.
3.2. Mood
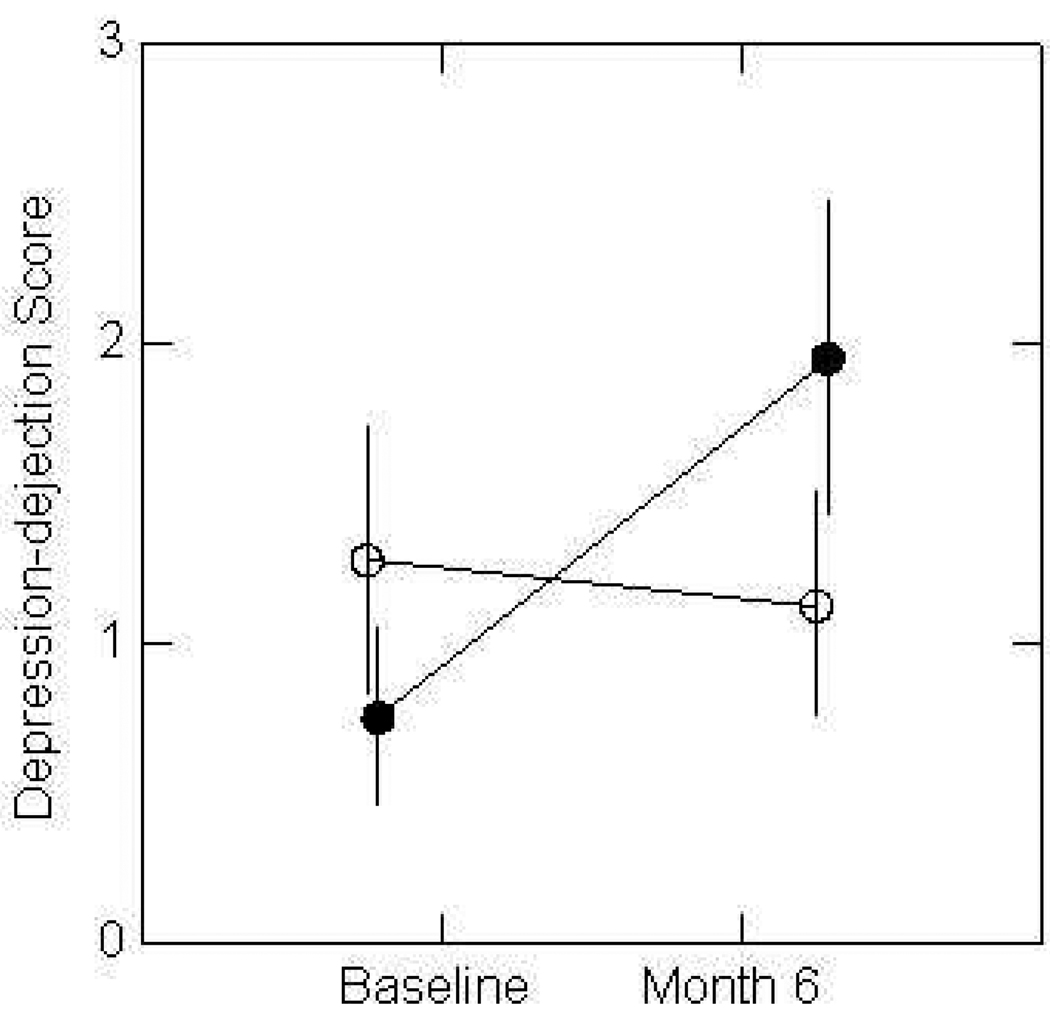
Mood data are summarized in Table 3. In analyses controlling for CR level, percent weight change and gender, there was no significant diet by time interaction for total mood disturbance (Table 3). However, there was a diet by time interaction for the depression-dejection subscale (p=0.04), and after also adding hunger at baseline and 6 months as covariates (because of suspected differences in hunger between the two diets) the significance of the interaction increased (to p=0.009). As shown in Figure 1, randomization to the HG diet was associated with a poorer mood outcome for depression at the sub-clinical level compared to the LG diet. There was also trend to significance for the diet by time interaction in the confusion subscale (p=0.07) and an increase in significance when hunger was added as a covariate (to p=0.055). Since the number of subjects available for analysis of mood data was greater than for cognitive function, the depression subscale analyses were also repeated using only the subset of individuals in whom cognitive function measurements were made, on the grounds that consistent findings in the smaller population would support conclusions drawn in the cognitive function studies. Despite the smaller population size in this analysis, the diet by time interaction approached significance (p=0.08) and differences between groups in changes in mean values over time increased (HG at baseline and 6 mo were 2.0 and 1.9, respectively, and values for LG were 0.6 and 2.6).
Table 3.
Total mood score and subscales of mood assessed by Profile of Mood States at baseline and 6 months in subjects randomized to HG and LG diets
| Time | HG (n=20) |
LG (n=22) |
|
|---|---|---|---|
| Tension | BL | 4.1±0.5 | 5.2±0.7 |
| 6 mo | 5.4±0.7 | 4.7±0.5 | |
| Depression* | BL | 1.0±0.4 | 1.7±0.6 |
| 6 mo | 2.6±0.7 | 1.5±0.5 | |
| Anger | BL | 1.7±0.6 | 2.5±0.7 |
| 6 mo | 1.3±0.4 | 1.5±0.6 | |
| Vigor | BL | 14.9±1.6 | 12.3±1.1 |
| 6mo | 15.3±1.1 | 12.6±1.4 | |
| Fatigue | BL | 5.0±1.2 | 5.2±0.9 |
| 6 mo | 3.9±0.8 | 4.7±0.8 | |
| Confusion† | BL | 4.6±0.5 | 4.7±0.5 |
| 6 mo | 4.2±0.4 | 4.0±0.5 | |
| Total Mood | BL | 1.5±3.3 | 7.0±3.6 |
| Disturbance | 6 mo | 1.9±2.7 | 3.9±3.4 |
Data are means±SEM (s). POMS, The Profile of Mood States (subscales have 0–4 scale and to calculate total mood disturbance, the subscales are summed with the vigor-activity subscale reversed); BL, baseline; HG, high glycemic; LG, low glycemic; mo, month.
Change over time significantly different between groups when controlling for gender, CR level and % weight change: p = 0.04; when also controlling for baseline and change in hunger P=0.009.
Change over time approaches significance when controlling for gender, CR level and % weight change: p ≤ 0.07; when also controlling for baseline and change in hunger P=0.055.
Figure 1.
Changes in depression subscale of POMS in volunteers randomized to HG or LG provided diets for 6 months. The score increased on HG diet compared to LG diet after controlling for %CR, % weight loss and gender (p=0.04). Possible range of depression scores 0–60. Error bars represent ±1.0 SEM, n=20 HG and n=22 LG). HG = dotted line; LG = solid line.
3.3. Cognitive function
Cognitive function data are summarized in Table 4. When comparing baseline with month 6 no test differed significantly between baseline and month 6 and there was no diet×time interaction. In addition, percent weight change did not correlate with any change score for cognitive function.
Table 4.
Cognitive performance variables at baseline and 6 months in subjects randomized to HG and LG diets
| Time | HG (n=12) |
LG (n=16) |
|
|---|---|---|---|
| Grammatical Reasoning | |||
| # Correct Responses | BL | 26.8±1.6 | 25.8±1.4 |
| 6 mo | 27.2±1.3 | 27.4±1.2 | |
| # Incorrect Responses | BL | 5.2±1.6 | 6.1±1.4 |
| 6 mo | 4.7±1.3 | 4.5±1.2 | |
| # No response | BL | 0.1±0.1 | 0.1±0.1 |
| 6 mo | 0.2±0.1 | 0.1±0.1 | |
| Response time (sec) | BL | 4.4±0.3 | 4.3±0.4 |
| 6 mo | 4.0±0.3 | 3.8±0.4 | |
| Four Choice Reaction Time | |||
| Reaction time (msec) | BL | 509.7±21.1 | 547.0±17.1 |
| 6 mo | 510.8±29.5 | 540.4±18.4 | |
| # Correct responses | BL | 392.0±2.7 | 392.2±1.5 |
| 6 mo | 391.5±1.8 | 393.3±1.6 | |
| # Time-out errors | BL | 0.0±0.0 | 0.2±0.1 |
| 6 mo | 0.2±0.2 | 0.1±0.1 | |
| Repeated Acquisition | |||
| # Incorrect Responses | BL | 5.6±1.1 | 5.4±0.6 |
| 6 mo | 4.9±0.9 | 4.7±0.6 | |
| Time to complete (sec) | BL | 18.3±2.2 | 18.4±2.1 |
| 6 mo | 14.6±1.4 | 16.7±1.7 | |
| Visual vigilance | |||
| # Correct Responses | BL | 14.2±2.1 | 13.8±1.9 |
| 6 mo | 14.5±1.4 | 13.0±1.7 | |
| Reaction Time (sec) | BL | 1.2±0.1 | 1.2±0.1 |
| 6 mo | 1.3±0.1 | 1.2±0.1 | |
| # False alarms | BL | 5.0±1.4 | 3.3±0.6 |
| 6 mo | 4.1±0.5 | 4.1±1.1 |
Data are means±SEM (s); BL, baseline; HG, high glycemic; LG, low glycemic; mo, month. There were no significant differences between groups in changes from baseline.
4. Discussion
Previous studies have suggested beneficial effects of low glycemic index and low GL weight loss diets on mood and cognition [13], [14], [15], [16], [20], [21], [29] and [30]. However, studies to date have all been short-term, and there is no information on longer-term effects in individuals actually losing weight. The results of this unique investigation with provided food (and therefore highly controlled dietary intake) indicate no differential effects of HG and LG diets on cognitive performance during weight loss, and no adverse effects of weight loss generally for the parameters assessed. However, whereas some previous long-term studies have suggested negative effects of very low carbohydrate (and therefore low GL) ketogenic diets on mood, or positive effects of HG diets, the opposite trend was observed in this study. Specifically, we observed that volunteers randomized to the LG diet had no change in the depression subscale of POMS during weight loss, whereas volunteers randomized to the HG diet experienced a negative change. This study used a relatively small population and therefore further studies are needed, but the results are consistent with the suggestion that moderately lower carbohydrate and low GL eating patterns may be protective against negative mood change during weight loss whereas negative changes occur if a conventional HG diet is consumed. In addition, the results lend support to accumulating evidence of broadly beneficial health effects of low GL diets compared to HG diets in weight management [43], [44] and [45].
Because this investigation of mood and cognition was part of a larger randomized controlled trial investigating the metabolic effects of CR, subjects were provided with all food, caloric beverages and a multivitamin supplement for 6 months. Thus, the effects of the different diets were studied under exceptionally controlled dietary conditions and are likely to be due to randomizations and consequent macronutrient intakes, rather than to potential confounding factors such as inadequate micronutrient intakes [46]. It should be noted that, although data from two levels of CR (10% and 30%) were combined in the analyses with CR level used as a covariate, there was no significant effect of CR level on weight loss because 10%CR subjects did not typically consume all provided food.
Concerning our observations of effects of LG versus HG diets on mood, we observed these effects when measuring the acute rather than long-term effects of food on mood, out of concern that the long-term instruments may provide less sensitive indicators of mood change within the normal range. A significant diet by time interaction (p=0.04) for the depression subscale of the POMS, with depression increasing on the higher carbohydrate HG diet over time compared to no change on the lower carbohydrate LG diet. The magnitude of the changes did not result in any clinical levels of depression, and the functional effects of changes of the magnitude observed is not known and requires further study. However, to our knowledge, these results are the first to highlight a potentially beneficial effect of an LG diet on sub-clinical depression during weight loss.
Concerning underlying mechanisms influencing depression symptomology when consuming HG or LG diets, we speculated that hunger might be a factor in the results obtained. Previous studies by our group have shown that consumption of HG meals provokes adverse hormonal changes and alterations in the availability of metabolic fuels such as glucose and free fatty acids that exacerbate hunger [47] and [45]. In addition, increased fluctuations in blood glucose (which occur with consumption of HG diets) are associated with negative effects on mood in some [19], [48] and [49] though not all [50] studies. These observations, combined with reports that feelings of vigor are negatively related to hunger sensations [51], and that consumption of extreme hunger-promoting diets is associated with negative mood [52], made increased hunger when consuming an HG diet a potential candidate for the results obtained in this study. However, there was no effect of the HG versus LG diets on hunger (assessed with the Eating Inventory). Furthermore, controlling for hunger in models assessing the effect of diet in changes in mood parameters over time actually increased the significance of the diet by time interaction for depression (from p=0.04 to p=0.009). The negative mood effect of the HG diet in this study was thus apparently not due to increased hunger, but given the small size of our study further investigations are needed.
Our results are also relevant to the carbohydrate-depression hypothesis [53] [54] and [55], which postulates that high carbohydrate diets improve mood through increased delivery of tryptophan (a precursor of the mood-modulating neurotransmitter serotonin) to the brain. Support for the hypothesis has been provided by self-reports of higher carbohydrate intake associated with reduced levels of depressive symptoms [20] and [21], but conversely meals containing as little as 4% protein appear to counteract any carbohydrate-induced rise in the plasma ratio of tryptophan to other large neutral amino acids that controls uptake into the brain [56], and high-carbohydrate and high-protein breakfasts can cause substantial differences in plasma tryptophan ratios [57]. However, if valid, the substantially higher carbohydrate HG diet tested in this study would be expected to yield more positive mood outcomes than the LG diet. In contrast, we saw the opposite effect, with our higher-carbohydrate HG diet resulting in a worsening of mood over time compared to the LG diet.
In contrast to the significant association between diet randomization and changes in the assessment of depression by POMS we observed no differences between dietary groups in changes in cognitive variables. In order to effectively investigate cognition, we selected a battery of cognitive tests that assessed a broad range of cognitive functions including reaction time, vigilance, learning, working memory and reasoning. Several previous studies have reported that weight loss is associated with poorer sustained attention, diminished immediate recall and longer simple reaction times [58] and [59], and suggested that the cognitive requirements of calorie counting and increased preoccupation with body weight may create measurable cognitive deficits in other areas [60]. Despite such potentially negative suggested effects of weight loss on cognitive function, our results support other previous work suggesting no adverse effects of moderate CR on cognition [8] and [12]. These results were obtained in a subgroup of the whole study population, and require confirmation in a larger study. Nevertheless, the apparent negative effects of weight loss on cognitive function in other studies may be less reliable, due to confounding factors such as non compliance with dietary recommendations and possibly suboptimal micronutrient intakes [61], [62] and [63].
In conclusion, we observed no effect of consuming a provided HG or LG weight-loss diet for 6 months on changes in cognitive function. However, randomization to the HG diet was associated with a relatively negative change in subclinical depression symptomology over time compared to randomization to the LG diet. Given that adverse changes in mood may negatively impact a long-term commitment to healthy eating habits and weight control, our findings suggest a potentially important psychological benefit of consuming LG diets for weight loss, and further studies in this area are warrented.
Acknowledgements
We thank our volunteers for their dedicated participation in the study, and the outstanding staff of the metabolic research unit for their expert help. Supported by National Institutes of Health grant U01-AG20480, U.S. Department of Agriculture under agreement No. 58-1950-4-401, K23 DK61506 from the National Institutes of Diabetes and Digestive and Kidney Diseases, and Boston Obesity Nutrition Research Center (BONRC) H150001. R Cheatham was supported by a NIH T32 grant (#DK62032-11). Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture, the Food and Drug Administration or the U.S. Army.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.French SA, Jeffery RW. Consequences of dieting to lose weight: effects on physical and mental health. Health Psychol. 1994;13:195–212. doi: 10.1037//0278-6133.13.3.195. [DOI] [PubMed] [Google Scholar]
- 2.Ness-Abramof R, Apovian CM. Diet modification for treatment and prevention of obesity. Endocrine. 2006;29:5–9. doi: 10.1385/endo:29:1:5. [DOI] [PubMed] [Google Scholar]
- 3.Foster GD, Wadden TA, Peterson FJ, Letizia KA, Bartlett SJ, Conill AM. A controlled comparison of three very-low-calorie diets: effects on weight, body composition, and symptoms. Am J Clin Nutr. 1992;55:811–817. doi: 10.1093/ajcn/55.4.811. [DOI] [PubMed] [Google Scholar]
- 4.Shukitt-Hale B, Askew EW, Lieberman HR. Effects of 30 days of undernutrition on reaction time, moods, and symptoms. Physiol Behav. 1997;62:783–789. doi: 10.1016/s0031-9384(97)00236-9. [DOI] [PubMed] [Google Scholar]
- 5.Buffenstein R, Karklin A, Driver HS. Beneficial physiological and performance responses to a month of restricted energy intake in healthy overweight women. Physiol Behav. 2000;68:439–444. doi: 10.1016/s0031-9384(99)00222-x. [DOI] [PubMed] [Google Scholar]
- 6.Keys A, Brozek J, Henschel A, Mickelson O, Taylor H. The biology of human starvation. Minneapolis: University of Minnesota Press; 1950. [Google Scholar]
- 7.Brehm BJ, Seeley RJ, Daniels SR, D'Alessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88:1617–1623. doi: 10.1210/jc.2002-021480. [DOI] [PubMed] [Google Scholar]
- 8.Bryan J, Tiggemann M. The effect of weight-loss dieting on cognitive performance and psychological well-being in overweight women. Appetite. 2001;36:147–156. doi: 10.1006/appe.2000.0389. [DOI] [PubMed] [Google Scholar]
- 9.Burley VJ, Kreitzman SN, Hill AJ, Blundell JE. Across-the-day monitoring of mood and energy intake before, during, and after a very-low-calorie diet. Am J Clin Nutr. 1992;56:277S–278S. doi: 10.1093/ajcn/56.1.277S. [DOI] [PubMed] [Google Scholar]
- 10.Wadden TA, Vogt RA, Andersen RE, Bartlett SJ, Foster GD, Kuehnel RH, Wilk J, Weinstock R, Buckenmeyer P, Berkowitz RI, Steen SN. Exercise in the treatment of obesity: effects of four interventions on body composition, resting energy expenditure, appetite, and mood. J Consult Clin Psychol. 1997;65:269–277. doi: 10.1037//0022-006x.65.2.269. [DOI] [PubMed] [Google Scholar]
- 11.Wing RR, Epstein LH, Marcus MD, Kupfer DJ. Mood changes in behavioral weight loss programs. Psychosomatic Res. 1984;28:189–196. doi: 10.1016/0022-3999(84)90019-9. [DOI] [PubMed] [Google Scholar]
- 12.Kretsch MJ, Green MW, Fong AKH, Elliman NA, Johnson HL. Cognitive effects of a long-term weight reducing diet. Int J Obesity. 1997;21:14–21. doi: 10.1038/sj.ijo.0800353. [DOI] [PubMed] [Google Scholar]
- 13.Holt SH, Delargy HJ, Lawton CL, Blundell JE. The effects of high-carbohydrate vs high-fat breakfasts on feelings of fullness and alertness, and subsequent food intake. Int J Food Sci Nutr. 1999;50:13–28. doi: 10.1080/096374899101382. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd HM, Rogers PJ, Hedderley DI, Walker AF. Acute effects on mood and cognitive performance of breakfasts differing in fat and carbohydrate content. Appetite. 1996;27:151–164. doi: 10.1006/appe.1996.0042. [DOI] [PubMed] [Google Scholar]
- 15.Markus CR, Panhuysen G, Tuiten A, Koppeschaar H, Fekkes D, Peters ML. Does carbohydrate-rich, protein-poor food prevent a deterioration of mood and cognitive performance of stress-prone subjects when subjected to a stressful task? Appetite. 1998;31:49–65. doi: 10.1006/appe.1997.0155. [DOI] [PubMed] [Google Scholar]
- 16.Pasman WJ, Blokdijk VM, Bertina FM, Hopman WP, Hendriks HF. Effect of two breakfasts, different in carbohydrate composition, on hunger and satiety and mood in healthy men. Int J Obes Relat Metab Disord. 2003;27:663–668. doi: 10.1038/sj.ijo.0802284. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd HM, Green MW, Rogers PJ. Mood and cognitive performance effects of isocaloric lunches differing in fat and carbohydrate content. Physiol Behav. 1994;56:51–57. doi: 10.1016/0031-9384(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 18.Wells AS, Read NW, Uvnas-Moberg K, Alster P. Influences of fat and carbohydrate on postprandial sleepiness, mood, and hormones. Physiol Behav. 1997;61:679–686. doi: 10.1016/s0031-9384(96)00519-7. [DOI] [PubMed] [Google Scholar]
- 19.Nabb SL, Benton D. The effect of the interaction between glucose tolerance and breakfasts varying in carbohydrate and fibre on mood and cognition. Nutritional Neuroscience. 2006;9:161–168. doi: 10.1080/10284150600955099. [DOI] [PubMed] [Google Scholar]
- 20.De Castro JM. Macronutrient relationships with meal patterns and mood in the spontaneous feeding behavior of humans. Phys & Beh. 1987;39:561–569. doi: 10.1016/0031-9384(87)90154-5. [DOI] [PubMed] [Google Scholar]
- 21.Pellegrin KL, O'Neil PM, Stellefson EJ, Fossey MD, Ballenger JC, Cochrane CE, Currey HS. Average daily nutrient intake and mood among obese women. Nutr Res. 1998;18:1103–1112. [Google Scholar]
- 22.Halyburton AK, Brinkworth GD, Wilson CJ, Noakes M, Buckley JD, Keogh JB, Clifton PM. Low- and high-carbohydrate weight-loss diets have similar effects on mood but not cognitive performance. Am J Clin Nutr. 2007;86:580–587. doi: 10.1093/ajcn/86.3.580. [DOI] [PubMed] [Google Scholar]
- 23.Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity. 2007;15:421–429. doi: 10.1038/oby.2007.531. [DOI] [PubMed] [Google Scholar]
- 24.McClernon FJ, Yancy WJ, Eberstein JA, Atkins RC, Westman EC. The effects of a low-carbohydrate ketogenic diet and a low-fat diet on mood, hunger, and other self-reported symptoms. Obesity. 2007;15:182–187. doi: 10.1038/oby.2007.516. [DOI] [PubMed] [Google Scholar]
- 25.Schoeller DA. How accurate is self-reported dietary energy intake? Nutr Rev. 1990;48:373–379. doi: 10.1111/j.1753-4887.1990.tb02882.x. [DOI] [PubMed] [Google Scholar]
- 26.Caballero B, Clay T, Davis S, Pathways Study Research Group Pathways: a school-based, randomized controlled trial for the prevention of obesity in American Indian schoolchildren. Am J Clin Nutr. 2003;78:1030–1038. doi: 10.1093/ajcn/78.5.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997;277:472–477. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- 28.Winocur G, Greenwood CE. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiology of Aging. 2005;26:46–49. doi: 10.1016/j.neurobiolaging.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Papanikolaou Y, Palmer H, Binns MA, Jenkins DJ, Greenwood CE. Better cognitive performance following a low-glycaemic-index compared with a high-glycaemic-index carbohydrate meal in adults with type 2 diabetes. Diabetologia. 2006;49:855–862. doi: 10.1007/s00125-006-0183-x. [DOI] [PubMed] [Google Scholar]
- 30.Ingwersen J, Defeyter MA, Kennedy DP, Wesnesc KA, Scholey AB. A low glycaemic index breakfast cereal preferentially prevents children's cognitive performance from declining throughout the morning. Appetite. 2007;49:240–244. doi: 10.1016/j.appet.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, Lichtenstein AH, Dallal GE, Dutta C, Bhapkar MV, DeLany JP, Saltzman E, Roberts SB. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr. 2007;84:1023–1030. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- 32.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatr. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 33.Roberts SB. Use of the doubly labeled water method for measurement of energy expenditure, total body water, water intake, and metabolizable energy intake in humans and small animals. Can J Physiol Pharm. 1989;67:1190–1198. doi: 10.1139/y89-189. [DOI] [PubMed] [Google Scholar]
- 34.*Gilhooly CH* DS, Golden JK, McCrory MA, Rochon J, DeLany JP, Freed AM, Fuss PJ, Dallal GE, Saltzman E, Roberts SB. Use of cereal fiber to facilitate adherence to a human caloric restriction program. Aging Clin Exp Res. 2008;20:513–520. doi: 10.1007/bf03324878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNair DM, Lorr M, Droppleman LF. Profile of Mood States Manual. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 36.Lieberman HKR, Prasad C. In: Fundamental issues and methods in nutritional neuroscience. Taylor and Francis Group, editor. Boca Raton: 2005. [Google Scholar]
- 37.O'Connor PJ. Evaluation of four highly cited energy and fatigue mood measures. J Psychosom Res. 2004;57:435–441. doi: 10.1016/j.jpsychores.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Baddeley AD. A three-minute reasoning test based on grammatical transformation. Psychosomatic Science. 1968;10:341–342. [Google Scholar]
- 39.Dollins AB, Lynch HJ, Wurtman RJ, Deng MH, Kischka KU, Gleason RE, Lieberman HR. Effect of pharmacological daytime doses of melatonin on human mood and performance. Psychopharmacology (Berl) 1993;112:490–496. doi: 10.1007/BF02244899. [DOI] [PubMed] [Google Scholar]
- 40.Lieberman HR, Falco CM, Slade SS. Carbohydrate administration during a day of sustained aerobic activity improves vigilance, as assessed by a novel ambulatory monitoring device, and mood. Am J Clin Nutr. 2002;76:120–127. doi: 10.1093/ajcn/76.1.120. [DOI] [PubMed] [Google Scholar]
- 41.Lieberman HR, Coffee B, Kobrick J. A vigilance task sensitive to the effects of stimulants, hypnotics and environmental stress--the scanning visual vigilance tes. Behavior Research Methods, Instruments and Computers. 1998;30:416–422. [Google Scholar]
- 42.Stunkard A, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of Psychiatric Research. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 43.Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297:2092–2102. doi: 10.1001/jama.297.19.2092. [DOI] [PubMed] [Google Scholar]
- 44.McMillan-Price J, Petocz P, Atkinson F, O'Neill K, Samman S, Steinbeck K, Caterson I, Brand-Miller J. Comparison of 4 diets of varying glycemic load on weight loss and cardiovascular risk reduction in overweight and obese young adults: a randomized controlled trial. Arch Intern Med. 2006;166:1466–1475. doi: 10.1001/archinte.166.14.1466. [DOI] [PubMed] [Google Scholar]
- 45.Pittas AG, Hariharan R, Stark PC, Hajduk CL, Greenberg AS, Roberts SB. Interstitial glucose level is a significant predictor of energy intake in free-living individuals with healthy body weight. J Nutr. 2005;135:1070–1074. doi: 10.1093/jn/135.5.1070. [DOI] [PubMed] [Google Scholar]
- 46.Kretsch MJ, Fong AK, Green MW, Johnson HL. Cognitive function, iron status, and hemoglobin concentration in obese dieting women. Eur J Clin Nutr. 1998;52:512–518. doi: 10.1038/sj.ejcn.1600598. [DOI] [PubMed] [Google Scholar]
- 47.Ludwig DS, Majzoub JA, Al-Zahrani A, Dallal GE, Blanco I, Roberts SB. High glycemic index foods, overeating, and obesity. Pediatrics. 1999;103:26. doi: 10.1542/peds.103.3.e26. [DOI] [PubMed] [Google Scholar]
- 48.Owens D, MacDonald I, Benton D, Sytnik N, Tucker P, Folkard S. A preliminary investigation into individual differences in the circadian variation of meal tolerance: effects on mood and hunger. Chronobiology International. 1996;13:435–447. doi: 10.3109/07420529609020914. [DOI] [PubMed] [Google Scholar]
- 49.Gold AE, MacLeod KM, Frier BM, Deary IJ. Changes in mood during acute hypoglycemia in healthy participants. J Pers Soc Psychol. 1995;68:498–504. doi: 10.1037//0022-3514.68.3.498. [DOI] [PubMed] [Google Scholar]
- 50.Rodin J, Wack J, Ferrannini E, DeFronzo RA. Effect of insulin and glucose on feeding behavior. Metabolism. 1985;34:826–831. doi: 10.1016/0026-0495(85)90106-4. [DOI] [PubMed] [Google Scholar]
- 51.Fischer K, Colombani PC, Wenk C. Metabolic and cognitive coefficients in the development of hunger sensations after pure macronutrient ingestion in the morning. Appetite. 2004;42:49–61. doi: 10.1016/S0195-6663(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 52.Keys A, Brozek J, Henschel A, Mickelsen O, Taylor HL. A Report from the Laboratory of Physiological Hygiene. Minneapolis, MN: University of Minnesota 1945 University of Minnesota Minneapolis, MN; 1945. Experimental Starvation in Man. [Google Scholar]
- 53.Lieberman HR, Wurtman J, Chew B. Changes in mood after carbohydrate consumption among obese individuals. Am J Clin Nutr. 1986;44:772–778. doi: 10.1093/ajcn/44.6.772. [DOI] [PubMed] [Google Scholar]
- 54.Wurtman RJ, Wurtman JJ. Carbohydrates and depression. Sci Am. 1989;260:68–75. doi: 10.1038/scientificamerican0189-68. [DOI] [PubMed] [Google Scholar]
- 55.Fernstrom JD, Wurtman RJ. Control of brain serotonin levels by the diet. Adv Biochem Psychopharmacol. 1974;11:133–142. [PubMed] [Google Scholar]
- 56.Teff KL, Young SN, Blundell JE. The effect of protein or carbohydrate breakfasts on subsequent plasma amino acid levels, satiety and nutrient selection in normal males. Pharmacol Biochem Behav. 1989;34:829–837. doi: 10.1016/0091-3057(89)90282-7. [DOI] [PubMed] [Google Scholar]
- 57.Wurtman RJ, Wurtman JJ, Regan MM, McDermott JM, Tsay RH, Breu JJ. Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios. Am J Clin Nutr. 2003;77:128–132. doi: 10.1093/ajcn/77.1.128. [DOI] [PubMed] [Google Scholar]
- 58.Green M, Rogers P, Elliman N, Gatenby S. Impairment of cognitive performance associated with dieting and high levels of dietary restraint. Physiology & Behavior. 1994;55:447–452. doi: 10.1016/0031-9384(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 59.Rogers PJ, Green MW. Dieting, dietary restraint and cognitive performance. Br J Clin Psychol. 1993;32(Pt 1):113–116. doi: 10.1111/j.2044-8260.1993.tb01034.x. [DOI] [PubMed] [Google Scholar]
- 60.Baddeley R, Abbott LF, Booth MC, Sengpiel F, Freeman T, Wakeman EA, Rolls ET. Responses of neurons in primary and inferior temporal visual cortices to natural scenes. Proc Biol Sci. 1997;264:1775–1783. doi: 10.1098/rspb.1997.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ashley JM, Herzog H, Clodfelter S, Bovee V, Schrage J, Pritsos C. Nutrient adequacy during weight loss interventions: a randomized study in women comparing the dietary intake in a meal replacement group with a traditional food group. Nutr J. 2007;6:12. doi: 10.1186/1475-2891-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Green MW, Rogers PJ. Selective attention to food and body shape words in dieters and restrained nondieters. Int J Eat Disord. 1993;14:515–517. doi: 10.1002/1098-108x(199312)14:4<515::aid-eat2260140417>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 63.Green MW, Rogers PJ. Impairments in working memory associated with spontaneous dieting behaviour. Psychol Med. 1998;28:1063–1070. doi: 10.1017/s0033291798007016. [DOI] [PubMed] [Google Scholar]