Abstract
The urinary tract is one of the most intractable mucosal surfaces for pathogens to colonize. In addition to the natural barriers at this site, potential pathogens have to contend with the vigorous local innate immune system. Several Toll-Like Receptors (TLRs) have been identified on epithelial cells of the bladder and the kidneys which mediate a variety of powerful immune responses. A common finding among successful uropathogens is their intrinsic ability to suppress TLR-mediated responses. As antibiotic therapy becomes increasingly ineffective, employing boosters of the innate immune system in the urinary tract may become a viable option.
Introduction
The urinary tract is one of the most intractable mucosal sites for bacteria to colonize. Considering its close proximity to the GI tract and the possibility of cross contamination by gut flora, together with the fact that urine provides a rich medium for bacterial growth, it is remarkable that urinary tract infections (UTIs) are not more frequent. The bladder makes up a large part of the urinary tract and stores urine which is surprisingly sterile. Escherichia coli, a component of the gut flora, accounts for over 85% of UTIs in healthy patients [1,2]. A consistent finding in almost all of these uropathogenic E. coli is the expression of type 1 fimbriae and its adhesin, FimH [3]. These bacteria gain a foothold in the urinary tract by binding of FimH to uroplakin 1a, a major surface moiety on superficial epithelial cells lining the bladder [4]. Much of the intractability of the bladder and the urinary tract to microbial attack is attributable to the exceptional impermeability of the epithelial cells lining the urinary tract and to the powerful flushing action of voiding urine, which readily eliminates microorganisms that successfully reach the urinary tract. There is growing evidence that another major contributing factor to the resistance of the urinary tract to microbial attack is the vigorous and multifaceted immune response mounted in the urinary tract by toll like receptors (TLRs). Although many TLRs are expressed on cells lining the urinary tract, to date only TLR4, TLR5, and TLR11 have been shown to contribute to the defense against bacterial infection in vivo. In experimental mouse models, mutants deficient or defective in TLR4, TLR5, or TLR11 are significantly impaired in their ability to clear infections at different regions of the urinary tract [5-9]. Whereas TLR4 is expressed on epithelial cells of both the bladder and the kidneys [10], TLR5 is predominantly expressed on bladder cells and TLR11 primarily on kidney cells [5,9]. Upon contact with bacteria or their products, these immune surveillance molecules evoke a variety of immune responses aimed at the early elimination of the pathogen. Here we will discuss recent insights on the remarkable ways that each of these TLRs contribute to host defense in the urinary tract. Cumulatively, these activities could begin to explain the refractivity of the urinary tract to infection.
Parallel TLR4 mediated intracellular signaling events in Bladder Epithelial Cells (BECs)
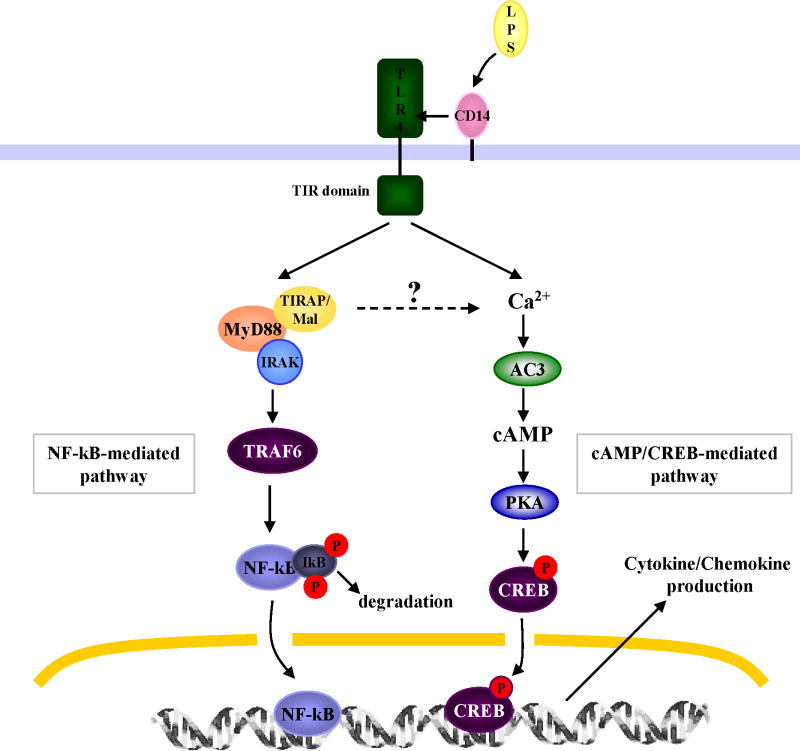
It has been known for over 20 years that mutant mice with inactive TLR4 are defective in their ability to clear UTIs. This defect was attributed to the inability of uroepithelial cells to evoke a cytokine response to gram negative bacteria, which results in limited recruitment of neutrophils to sites of infection in the urinary tract [6,11]. A similar condition of a weak immune response accompanied by inability to clear UTIs is observed in patients with asymptomatic bacteriuria [12]. A recent study demonstrating that TLR4 expression levels in these patients with asymptomatic bacteriuria is significantly lower than in age matched control subjects [12] is consistent with proposed role of TLR4 in immune responses in humans. However, the impairment in bacterial clearance seen in humans and mice with low or defective expression of TLR4 may not be entirely ascribable to limited local mediator responses. Recent studies with TLR4 mutant mice have revealed that in addition to their inability to evoke limited cytokine/chemokine and neutrophil responses, these mutant mice were significantly impaired in their ability to mediate several previously unrecognized antimicrobial activities in the urinary tract [13,14]. TLR4 as well as its co-receptor, CD14, are amply expressed in the bladder of mice and humans where it appears to be exclusively found on the superficial BECs lining the lumen [14-16]. Although TLR4 is expressed in the kidneys [10,17], its role in conferring protection in this organ has not been extensively investigated. Therefore, all of the studies described here pertain to TLR4 on BECs. Upon recognition of bacterial lipopolysaccharides (LPS), TLR4/CD14 complexes on BECs are believed to follow the classical signaling pathway [18]. In the classical pathway, TLR4 initiates a signaling cascade involving TIR adaptor molecules, TIRAM/Mal and MyD88, and substrates, IRAK and TRAF6, resulting in the activation of a transcriptional factor NF-κB[18]. NF-κB first traffics to the nucleus and then stimulates transcription of various cytokines including IL-6 and IL-8, two of the major cytokines observed in the urinary tract following bacterial infection [16,19,20]. However, Song et al. have recently described a second and parallel signaling pathway triggered by TLR4 in BECs, which also results in IL-6 and IL-8 release [21]. In this second pathway, cAMP and its associated transcriptional factor, cAMP response element-binding protein (CREB), are important components. The sequence of TLR4 initiated reactions in this second pathway involves an influx of intracellular Ca2+ followed by an increase in intracellular cAMP [21]. Among the 4 isotypes of adenylyl cyclases (ACs) expressed by human BECs, AC-3 has been identified as the specific AC responsible for cAMP production following exposure to E. coli. The increase in intracellular cAMP was found to result in the phophorylation of CREB, which in turn upregulates the expression of IL-6 and IL-8 [21]. Ligation of TLR4 on BECs by bacterial LPS activates both pathways but a comparison of the kinetics of IL-6 secretion by the two pathways has revealed that the cAMP pathway is markedly faster than the classical pathway [21]. The requirement of a second and more rapidly acting pathway in BECs may be an important adaptation to promptly ward off frequent contaminants from the gut flora. A diagrammatic illustration of the two signaling pathways in BECs is depicted in Fig. 1.
Figure 1. TLR4-initiated signaling pathways in BECs.
Upon ligation by LPS, TLR4 initiates the classical signaling pathway involving NF-kB as well as a second chain of reactions where cAMP is a major substrate. The latter pathway results in a markedly faster cytokine response than the former pathway.
TLR4 mediated Inhibition of Bacterial Invasion
Another antimicrobial activity mediated by BEC TLR4 through the cAMP dependent pathway is inhibition of bacterial invasion. Because of the intense interest in the various strategies employed by bacteria for invading and surviving within host cells, the intrinsic ability of host cells to partially or completely abrogate bacterial invasion has gone largely unnoticed until now. Before describing this TLR4 mediated mechanism, it is important to first describe recent findings regarding how uropathogenic E. coli (UPEC) invade BECs.
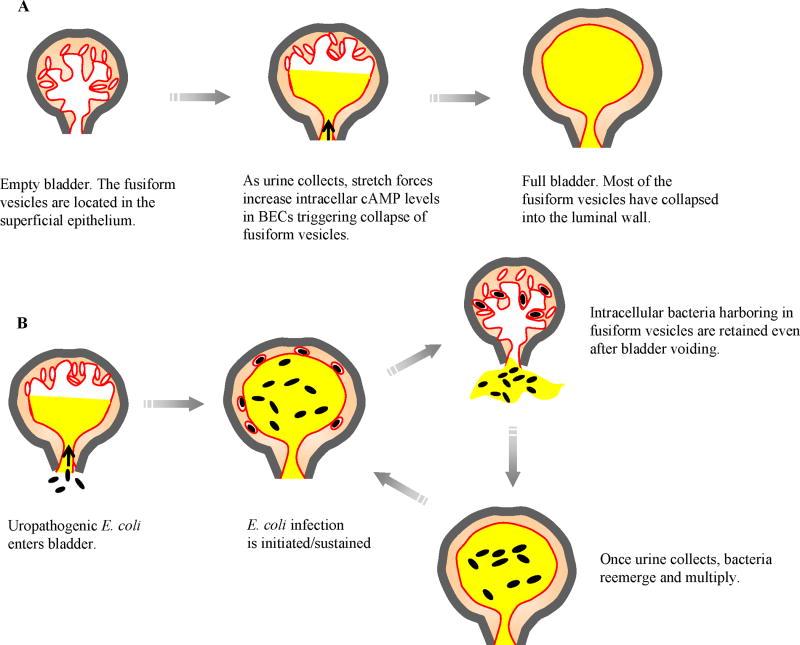
There is a growing consensus that invasion of the superficial epithelial cells of the bladder is an essential aspect of infection by UPEC [22]. Seeking intracellular refuge within BECs is perhaps the only way that UPEC can avoid elimination by either urine or neutrophils in the bladder. That UPEC can invade BECs is remarkable considering that they do not have any special invasive traits and would have to penetrate the highly impregnable scalloped shaped plaques, also known as asymmetric unit membranes, on the apical surface of the superficial BECs [23]. The first clue to the underlying mechanism for how E. coli invade BECs came from the identification of uroplakin 1a, a major component of the plaques on the superficial BECs as the receptor for type 1 fimbrial FimH of UPEC [4]. Uroplakin 1a is found within cellular entities called lipid raft microdomains which are dynamic assemblies of proteins and lipids that float freely within the liquid-disordered bilayer of cellular membranes. Upon ligation, these lipid raft microdomains can cluster to form larger, ordered platforms with intrinsic endocytic ability [24-26]. Bacterial FimH-mediated aggregation of lipid raft associated Uroplakin 1a on the apical surface of BECs is presumably the trigger for lipid raft mediated bacterial invasion. Several other components of lipid rafts on BECs such as caveolin-1 and Rac-1 have also been implicated in E. coli entry [24]. Since the BECs employed for these in vitro studies are human BEC lines that poorly express uroplakin1a the nature of the FimH receptor on these cells has been of considerable interest. Recently, the putative FimH receptor on these cells was identified to be the integrin heterodimer α3β1 [27] which, like uroplakin 1a, is lipid raft associated [28]. A much better understanding of E. coli invasion of BECs has emerged from recent in vivo studies of bladder infections by UPEC. E. coli internalized by superficial BECs were revealed to be encased within fusiform vesicles which are a dynamic pool of cAMP regulatable discoid shaped vesicles [13]. These vesicles, which are highly enriched in lipid raft components including uroplakin 1a, have a critical function in regulating bladder volume [23]. By collapsing into the luminal plasma membrane, they provide the extra membrane required for bladder distension [23]. There appears to be an association between lipid raft-mediated entry of E. coli and exocytosis of fusiform vesicles. Based on the observation that E. coli invasion is significantly reduced in BECs whose expression of Rab27b, a mediator of vesicle exocytosis, is reduced, it is theorized that fusiform vesicle exocytosis is a prerequisite for E. coli invasion [13]. Taken together, the lipid raft mediated invasion of BECs involves deposition of bacteria within fusiform vesicles through an endocytic process that is closely associated with exocytosis of fusiform vesicles. A model showing how fusiform vesicles function in regulating bladder volume and how UPEC coopt these functions to cause and sustain UTIs is depicted in Fig. 2. Several studies from the Hultgren laboratory have demonstrated that certain intracellular UPEC can multiply within their intracellular compartment to form “intracellular bacterial communities” (IBCs) some of which can then switch into a quiescent phase that persists intracellularly for indefinite periods of time [29-33]. A recent survey of UPEC isolates revealed that over 80% of them exhibited the potential to form IBCs in the mouse bladder [32]. It has been proposed that the resurrection of these quiescent forms of UPEC is coincident with recurrence of UTIs [34,35].
Figure 2. Cooption of fusiform vesicles of superficial BECs by UPEC.
(A) Fusiform vesicles are intracellular vesicles that regulate bladder volume. When bladder volume increases as urine collects, fusiform vesicles collapse into luminal surface membrane providing the necessary membrane. (B) Proposed model of how harboring of UPEC within fusiform vesicles sustains bladder infections. Once UPEC enter the lumen of bladder, they rapidly multiply in the urine. A fraction of these bacteria gain access into fusiform vesicles. When urine is voided all bacteria are eliminated except those within fusiform vesicles. Once urine collects in the bladder, bacteria remerge from collapsing fusiform vesicles and rapidly multiply to previous levels.
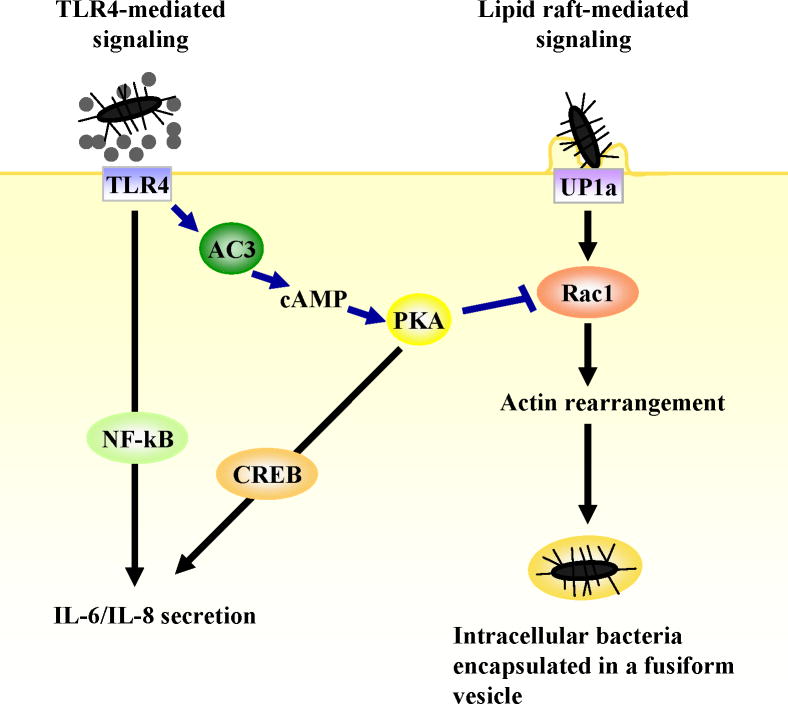
Invasion by type 1 fimbriated UPEC is actively resisted by the BECs through a mechanism triggered by TLR4. In vivo demonstration of the magnitude of this TLR4 mediated resistance in BECs comes from the finding that invasion of the bladders of TLR4 mutant (C3H/HeJ) mice by type 1 fimbriated E. coli or Klebsiella pneumoniae was 10-12 fold greater than that seen in wild type mice (C3H/HeN) (wt) [14]. The TLR4 initiated mechanism for resisting bacterial invasion also involves the signaling pathway described earlier in which cAMP is a major product. Intracellular cAMP, through a downstream effector Protein kinase A (PKA), was found to inhibit the activation of Rac-1, a component of lipid raft domains of BECs and a necessary element for actin remodeling and bacterial invasion (Fig. 3). Presumably, the invasion-abrogating defenses in BECs lining the lumen are triggered when TLR4 molecules make contact with LPS shed from bacteria, an event likely to be initiated as soon as bacteria enter the bladder. Presumably, if the contaminating bacteria are not able to overcome cellular actions that impede BEC penetration, they will be rapidly eliminated. That BECs are invaded by type 1 fimbriated E. coli in spite of its TLR4 mediated resistance argue that expression of type 1 fimbriae is a trait evolved by UPEC to invade BECs and bypass their TLR4 defenses. The ability of TLR4 to suppress invasion of mammary epithelial cells by mastitis causing E. coli was recently reported [36] indicating that this TLR4 associated response may not be restricted to the urinary tract.
Figure 3. TLR4 mediated inhibition of lipid raft mediated bacterial invasion of UPEC.
Lipid raft mediated bacterial uptake is initiated when FimH on UPEC ligates uroplakin 1a molecules found within lipid raft domains of the BEC plasma membranes. Once UPEC are internalized, they are encased in nondegradative compartments exhibiting properties of fusiform vesicles. The cAMP signaling pathway activated when TLR4 senses LPS abrogates this activity by acting on Rac-1, which is essential for actin remodeling.
TLR4 mediated Expulsion of Bacteria from Infected BECs
BECs continue to fight UPEC even after the bacterial invasion. If the infected BECs are allowed to incubate over a period of 18 hrs, the majority of intracellular E. coli are eventually expelled in a piece-meal fashion without any appreciable loss of host cell or bacterial viability [13]. This activity also appears to be TLR4 dependent, because bacterial expulsion is significantly reduced in infected BECs silenced in TLR4 expression by RNA interference (unpublished). The mechanism of bacterial expulsion involves the TLR4 signaling pathway where intracellular cAMP is a major byproduct. Since intracellular cAMP is a powerful trigger for exocytosis of fusiform vesicles including those harboring intracellular E. coli[13], it is not surprising that TLR4-induced cAMP can cause bacterial expulsion. This capacity of BECs to sense and then expel intracellular bacteria represents another novel function for TLR4.
If increased cAMP levels within infected BECs can trigger bacterial expulsion, can artificially increasing intracellular cAMP levels with cAMP agonists be of therapeutic value against UTIs? This question was recently examined by Bishop et al [13]. They reported that treating mice with forskolin, a potent enhancer of intracellular cAMP, caused bladder fusiform vesicles to collapse into the apical plasma membrane with subsequent reduction of bacteria in UPEC infected bladders [13]. Forskolin was effective in experimental models of both acute and chronic UTIs [13]. Recurrent UTIs have been linked to persistence of quiescent forms of bacteria within bladder and other urinary tract cells. In their intracellular location, the bacteria are protected from antibiotics and the host's immune cells. It is conceivable that co-administration of antibiotics and forskolin would be an effective way to eradicate persisting intracellular bacteria in patients with recurrent UTIs.
TLR5 and TLR11 mediated cytokine/chemokine responses
To date several potent immune responses to UPEC in the urinary tract have been described that are clearly TLR4 independent [5,9,17] but it is not known how these responses are initiated. Recent in vivo studies have implicated two more TLRs in immune responses of the urinary tract. TLR5, which recognizes bacterial flagella, is expressed at high levels by epithelial cells in the bladder and although expressed in the kidneys, its levels are relatively low[5]. Compared to wt mice, TLR5 -/- mice are significantly more susceptible to UPEC UTIs [5]. Whereas bacterial numbers in the bladders of wt mice were consistently less than in TLR5-/- mice, interestingly there were no differences in bacterial numbers in the kidneys in the early days of the infection [5]. The different rates of bacterial clearance in bladders and kidneys could be related to differential expression levels of TLR5. The bladder has been shown to respond vigorously to bacterial flagellin by generating a wide range of proinflammatory molecules including KC (CXCL1), MIP2 (CXCL2), MIP-1 (CCL2), IL-6 and TNF [5]. These powerful chemical mediators can promote the early recruitment of neutrophils and other phagocytic cells leading to reduced bacterial numbers in the bladder. Because of low levels of TLR5 expression in the kidneys, such a vigorous inflammatory response may not be present and consequently clearance rates at this site may not be as effective as in the bladder. UPEC may be able to sense local TLR5 expression levels because it was recently reported that flagellar expression by UPEC is switched “off” in the bladders and is switched “on” in the ureters and kidneys coincident with bacterial ascension to these sites [37,38]. The intrinsic ability of UPEC to differentially regulate flagellar expression based on local TLR expression levels could be an important adaptation to evade local innate immune defenses.
TLR11 is expressed mainly in the kidneys and the liver of mice [9]. Although TLR11 sequences are present in the genome of mice and humans, the human genome sequence in the NCBI data base indicates the presence of stop codons in the putative TLR11 ORF, indicating that TLR11 might not be expressed in humans [9]. Nevertheless, studies of TLR11-/- mice have revealed that compared to wt mice, they are markedly more susceptible to kidney infections by UPEC [9]. Although the definitive bacterial ligand of TLR11 is not known, it is present on UPEC strains but not on nonpathogenic E. coli or gram positive bacteria [9]. The inability of the kidneys of TLR11-/- mice to clear UPEC was ascribed to the impaired ability of epithelial and immune cells at this site to respond to UPEC strains [9]. Although only 3 TLRs have been discussed here, many other TLRs potentially contribute significantly and in various different ways to the net defense of the urinary tract.
Immune Evasion by Uropathogenic Bacteria
In spite of the vigorous immune responses mounted by TLRs, UPEC appear to have evolved corresponding mechanisms to overcome or evade them. An emerging theme from the examination of a broad phylogenetic range of UPEC isolates is that these bacteria are capable of suppressing cytokine and chemokine responses of uroepithelial cells including TLR initiated signaling events [39]. These “suppressive” factors include rfa, rfb, and surA. The rfa and rfb operons encode LPS biosynthetic genes, while surA encodes a periplasmic cis-trans prolyl isomerase important in the biogenesis of outer membrane proteins and fimbriae [40,41]. How these bacterial components suppress cellular responses of uroepithelial cells is largely unknown but the presence of multiple TLR4 initiated pathways in BECs for the production of proinflammatory mediators may be a specific adaptation of these cells to avoid bacteria-induced shut down of immunomodulatory mediator responses. In addition to suppressing cytokine/chemokine production by BECs, UPEC can selectively activate various components of the TLR4 signaling pathway in order to modulate these responses. For example, the P fimbriae expressed by UPEC can activate the TRIF-related adaptor molecule (TRAM) without activating MyD88 adaptor in kidney epithelial cells [42]. This ability of bacterial fimbriae to modify chemokine responses is illustrated in a study that revealed that whereas E. coli expressing P fimbriae evoked CCL2 and CCL5 chemokines, type 1 fimbriated E. coli evoked CXCL1 and CXCL8 chemokines [43]. Although a complex and integrated immune system exists in the urinary tract it is also clear that uropathogens have evolved equally intricate mechanisms to overcome or evade these systems.
Conclusions
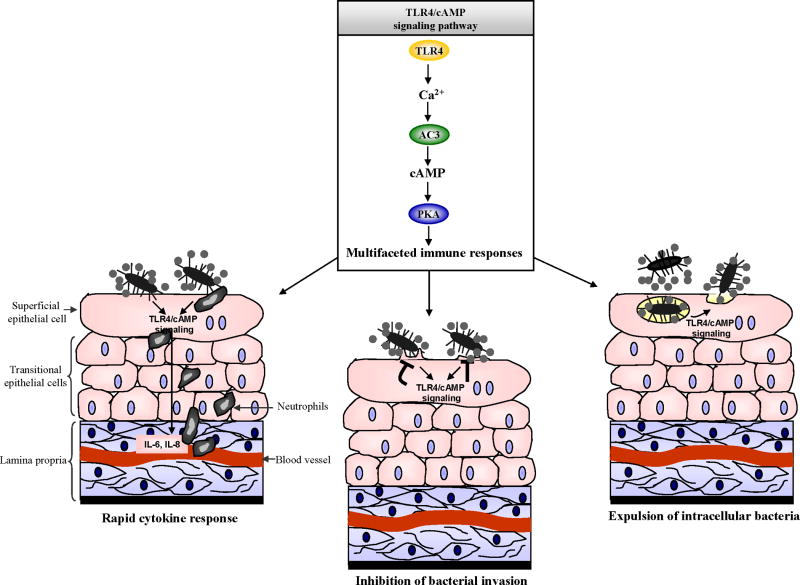
The upper and lower urinary tract is lined by various TLRs and at least three of these TLRs have been implicated in triggering powerful innate immune responses during experimental UPEC induced UTIs. Although recruitment of neutrophils and other immune cells to the infection site appears to be the primary function of these TLRs, TLR4 on BECs can also actively inhibit bacterial invasion and expel UPEC from infected BECs. These novel TLR4 initiated activities are mediated by a unique signaling pathway where the well known secondary messenger, cAMP, is a major substrate (Fig. 4 depicts multiple TLR4/cAMP mediated immune functions). As new TLR mediated immune functions are being discovered, it is also becoming clear that uropathogens have evolved factors directed at evading, suppressing, or modulating several of these functions. For example, UPEC appear to restrict expression of virulence factors such as flagella to sites such as the kidney where expression of their complementary TLR is low. There is a growing need for the development of novel strategies for the treatment of UTIs. Antibiotics are becoming increasingly ineffective against uropathogens because of multiple drug resistance and their intrinsic ability to harbor within BECs as quiescent forms. A major focus of future study should be the development of small molecule compounds to boost local TLR initiated innate immune responses during infection. These could include TLR ligands or agents such as forskolin that boost intracellular levels of cAMP, a substrate of the TLR4 signaling pathway. In the future, strategies to boost the innate immune system of the urinary tract could complement or even replace antibiotic therapy.
Figure 4. A model depicting multiple immune outcomes of TLR4/cAMP signaling pathway in BECs.
TLR4/cAMP signaling triggers rapid cytokine/chemokine secretion, inhibition of UPEC invasion, and UPEC expulsion from infected BECs.
Acknowledgments
We thank Alison Hofmann (Department of Pediatrics, Duke University Medical Center) and Yuvon Mobley (Post-Baccalaureate Research Education Program, Duke University Medical Center) for critical reading of this manuscript. Our studies were supported by National Institutes of Health grants AI 056101, AI 150021, and DK 050814.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am. 1997;11:551–581. doi: 10.1016/s0891-5520(05)70373-1. [DOI] [PubMed] [Google Scholar]
- 2.Svanborg C, Godaly G. Bacterial virulence in urinary tract infection. Infect Dis Clin North Am. 1997;11:513–529. doi: 10.1016/s0891-5520(05)70371-8. [DOI] [PubMed] [Google Scholar]
- 3.Abraham SN, Sun D, Dale JB, Beachey EH. Conservation of the D-mannose-adhesion protein among type 1 fimbriated members of the family Enterobacteriaceae. Nature. 1988;336:682–684. doi: 10.1038/336682a0. [DOI] [PubMed] [Google Scholar]
- 4.Zhou G, Mo WJ, Sebbel P, Min G, Neubert TA, Glockshuber R, Wu XR, Sun TT, Kong XP. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. J Cell Sci. 2001;114:4095–4103. doi: 10.1242/jcs.114.22.4095. [DOI] [PubMed] [Google Scholar]
- 5.Andersen-Nissen E, Hawn TR, Smith KD, Nachman A, Lampano AE, Uematsu S, Akira S, Aderem A. Cutting edge: Tlr5-/- mice are more susceptible to Escherichia coli urinary tract infection. J Immunol. 2007;178:4717–4720. doi: 10.4049/jimmunol.178.8.4717. [DOI] [PubMed] [Google Scholar]; Using a murine model of UTI, the authors demonstrate for the first time the importance of TLR5 in regulating innate immune response in the urinary tract. Bladders of wild type mice showed high levels of cytokine and chemokine expression in response to flagellin, which was linked to increased inflammation and subsequent bacterial clearance.
- 6.Hagberg L, Hull R, Hull S, McGhee JR, Michalek SM, Svanborg EC. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect Immun. 1984;46:839–844. doi: 10.1128/iai.46.3.839-844.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schilling JD, Mulvey MA, Vincent CD, Lorenz RG, Hultgren SJ. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J Immunol. 2001;166:1148–1155. doi: 10.4049/jimmunol.166.2.1148. [DOI] [PubMed] [Google Scholar]
- 8.Svanborg C, Bergsten G, Fischer H, Godaly G, Gustafsson M, Karpman D, Lundstedt AC, Ragnarsdottir B, Svensson M, Wullt B. Uropathogenic Escherichia coli as a model of host-parasite interaction. Curr Opin Microbiol. 2006;9:33–39. doi: 10.1016/j.mib.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 10.Samuelsson P, Hang L, Wullt B, Irjala H, Svanborg C. Toll-like receptor 4 expression and cytokine responses in the human urinary tract mucosa. Infect Immun. 2004;72:3179–3186. doi: 10.1128/IAI.72.6.3179-3186.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shahin RD, Engberg I, Hagberg L, Svanborg EC. Neutrophil recruitment and bacterial clearance correlated with LPS responsiveness in local gram-negative infection. J Immunol. 1987;138:3475–3480. [PubMed] [Google Scholar]
- 12.Ragnarsdottir B, Samuelsson M, Gustafsson MC, Leijonhufvud I, Karpman D, Svanborg C. Reduced toll-like receptor 4 expression in children with asymptomatic bacteriuria. J Infect Dis. 2007;196:475–484. doi: 10.1086/518893. [DOI] [PubMed] [Google Scholar]; Human patients afflicted with recurrent bacteriuria are shown to have reduced TLR4 expression in their neutrophils. The inability of these patients to clear UTIs mimics the inability of TLR4 mutant mice to clear UPEC infections. This represents one of the first indications of the contribution of TLR4 in protecting humans against UTIs.
- 13.Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med. 2007;13:625–630. doi: 10.1038/nm1572. [DOI] [PubMed] [Google Scholar]; This paper reveals the underlying mechanism of how UPEC circumvents the bladder barrier by invading and harboring in cAMP-regulatable fusiform vesicles within bladder epithelial cells. The use of forskolin, an inducer of cAMP as a therapeutic agent against UTI is demonstrated.
- 14.Song J, Bishop BL, Li G, Duncan MJ, Abraham SN. TLR4 initiated and cAMP mediated abrogation of bacterial invasion of the bladder. Cell Host Microbe. 2007;1:287–298. doi: 10.1016/j.chom.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrate for the first time, the in vivo and in vitro ability of TLR4 to block invasion of BECs by UPEC. They demonstrate that the pathway involves cAMP mediated inhibition of Rac-1 a critical component of actin rearrangement.
- 15.Miyazaki J, Kawai K, Oikawa T, Johraku A, Hattori K, Shimazui T, Akaza H. Uroepithelial cells can directly respond to Mycobacterium bovis bacillus Calmette-Guerin through Toll-like receptor signalling. BJU Int. 2006;97:860–864. doi: 10.1111/j.1464-410X.2006.06026.x. [DOI] [PubMed] [Google Scholar]
- 16.Schilling JD, Martin SM, Hunstad DA, Patel KP, Mulvey MA, Justice SS, Lorenz RG, Hultgren SJ. CD14- and Toll-like receptor-dependent activation of bladder epithelial cells by lipopolysaccharide and type 1 piliated Escherichia coli. Infect Immun. 2003;71:1470–1480. doi: 10.1128/IAI.71.3.1470-1480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chassin C, Goujon JM, Darche S, du Merle L, Bens M, Cluzeaud F, Werts C, Ogier-Denis E, Le Bouguenec C, Buzoni-Gatel D, et al. Renal collecting duct epithelial cells react to pyelonephritis-associated Escherichia coli by activating distinct TLR4-dependent and -independent inflammatory pathways. J Immunol. 2006;177:4773–4784. doi: 10.4049/jimmunol.177.7.4773. [DOI] [PubMed] [Google Scholar]
- 18.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Agace W, Hedges S, Andersson U, Andersson J, Ceska M, Svanborg C. Selective cytokine production by epithelial cells following exposure to Escherichia coli. Infect Immun. 1993;61:602–609. doi: 10.1128/iai.61.2.602-609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hang L, Wullt B, Shen Z, Karpman D, Svanborg C. Cytokine repertoire of epithelial cells lining the human urinary tract. J Urol. 1998;159:2185–2192. doi: 10.1016/S0022-5347(01)63303-2. [DOI] [PubMed] [Google Scholar]
- 21.Song J, Duncan MJ, Li G, Chan C, Grady R, Stapleton A, Abraham SN. A Novel TLR4-Mediated Signaling Pathway Leading to IL-6 Responses in Human Bladder Epithelial Cells. PLoS Pathogens. 2007;3:e60. doi: 10.1371/journal.ppat.0030060. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrates the existence of a novel TLR4 initiated and NF-kB independent signaling pathway in BECs resulting in a very rapid cytokine response. Other components of this signaling pathway include two prominent secondary messengers cAMP and Ca2+.
- 22.Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc Natl Acad Sci U S A. 2000;97:8829–8835. doi: 10.1073/pnas.97.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apodaca G. The uroepithelium: not just a passive barrier. Traffic. 2004;5:117–128. doi: 10.1046/j.1600-0854.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 24.Duncan MJ, Li G, Shin JS, Carson JL, Abraham SN. Bacterial penetration of bladder epithelium through lipid rafts. J Biol Chem. 2004;279:18944–18951. doi: 10.1074/jbc.M400769200. [DOI] [PubMed] [Google Scholar]
- 25.Duncan MJ, Shin JS, Abraham SN. Microbial entry through caveolae: variations on a theme. Cell Microbiol. 2002;4:783–791. doi: 10.1046/j.1462-5822.2002.00230.x. [DOI] [PubMed] [Google Scholar]
- 26.van der Goot FG, Harder T. Raft membrane domains: from a liquid-ordered membrane phase to a site of pathogen attack. Semin Immunol. 2001;13:89–97. doi: 10.1006/smim.2000.0300. [DOI] [PubMed] [Google Scholar]
- 27.Eto DS, Jones TA, Sundsbak JL, Mulvey MA. Integrin-Mediated Host Cell Invasion by Type 1-Piliated Uropathogenic Escherichia coli. PLoS Pathog. 2007;3:e100. doi: 10.1371/journal.ppat.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]; A new receptor for UPEC FimH on human bladder epithelial cell lines is described. Since uroplakin 1a is going to be overwhelmingly expressed in the plasma membrane of superficial BECs, the functional relevance of this receptor is at present unclear. Nevertheless, this finding is important for studies where human epithelial cell lines, which poorly express uroplakin 1a, are used.
- 28.Echarri A, Muriel O, Del Pozo MA. Intracellular trafficking of raft/caveolae domains: Insights from integrin signaling. Semin Cell Dev Biol. 2007 doi: 10.1016/j.semcdb.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 30.Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, Footer MJ, Hultgren SJ. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci U S A. 2004;101:1333–1338. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Justice SS, Lauer SR, Hultgren SJ, Hunstad DA. Maturation of intracellular Escherichia coli communities requires SurA. Infect Immun. 2006;74:4793–4800. doi: 10.1128/IAI.00355-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garofalo CK, Hooton TM, Martin SM, Stamm WE, Palermo JJ, Gordon JI, Hultgren SJ. Escherichia coli from urine of female patients with urinary tract infections is competent for intracellular bacterial community formation. Infect Immun. 2007;75:52–60. doi: 10.1128/IAI.01123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]; A survey of UPEC isolates reveals that over 80% of them can potentially form intracellular bacterial communities.
- 33.Wright KJ, Seed PC, Hultgren SJ. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell Microbiol. 2007;9:2230–2241. doi: 10.1111/j.1462-5822.2007.00952.x. [DOI] [PubMed] [Google Scholar]
- 34.Eto DS, Sundsbak JL, Mulvey MA. Actin-gated intracellular growth and resurgence of uropathogenic Escherichia coli. Cell Microbiol. 2006;8:704–717. doi: 10.1111/j.1462-5822.2006.00691.x. [DOI] [PubMed] [Google Scholar]
- 35.Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci U S A. 2006;103:14170–14175. doi: 10.1073/pnas.0602136103. [DOI] [PMC free article] [PubMed] [Google Scholar]; Here the authors demonstrate that intracellular bacteria can switch into a quiescent phase of growth and that activating these quiescent forms within transitional BECs with cationic compounds can result in resurrection of infection in mice.
- 36.Gonen E, Vallon-Eberhard A, Elazar S, Harmelin A, Brenner O, Rosenshine I, Jung S, Shpigel NY. Toll-like receptor 4 is needed to restrict the invasion of Escherichia coli P4 into mammary gland epithelial cells in a murine model of acute mastitis. Cell Microbiol. 2007 doi: 10.1111/j.1462-5822.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- 37.Lane MC, Alteri CJ, Smith SN, Mobley HL. Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc Natl Acad Sci U S A. 2007;104:16669–16674. doi: 10.1073/pnas.0607898104. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a biophotonic imaging technique and comparative quantitative PCR, the authors demonstrate the ability of UPEC to tightly regulate expression of flagella in the bladder and kidneys during ascending UTI.
- 38.Lane MC, Simms AN, Mobley HL. complex interplay between type 1 fimbrial expression and flagellum-mediated motility of uropathogenic Escherichia coli. J Bacteriol. 2007;189:5523–5533. doi: 10.1128/JB.00434-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Billips BK, Forrestal SG, Rycyk MT, Johnson JR, Klumpp DJ, Schaeffer AJ. Modulation of Host Innate Immune Response in the Bladder by Uropathogenic Escherichia coli. Infect Immun. 2007;75:5353–5360. doi: 10.1128/IAI.00922-07. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comparing clinical isolates with laboratory E. coli isolates, the authors show that UPEC isolates specifically suppress the innate immune response of uropepithelial cells including cytokine/chemokine secretion and neutrophil recruitment.
- 40.Hunstad DA, Justice SS, Hung CS, Lauer SR, Hultgren SJ. Suppression of bladder epithelial cytokine responses by uropathogenic Escherichia coli. Infect Immun. 2005;73:3999–4006. doi: 10.1128/IAI.73.7.3999-4006.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Justice SS, Hunstad DA, Harper JR, Duguay AR, Pinkner JS, Bann J, Frieden C, Silhavy TJ, Hultgren SJ. Periplasmic peptidyl prolyl cis-trans isomerases are not essential for viability, but SurA is required for pilus biogenesis in Escherichia coli. J Bacteriol. 2005;187:7680–7686. doi: 10.1128/JB.187.22.7680-7686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischer H, Yamamoto M, Akira S, Beutler B, Svanborg C. Mechanism of pathogen-specific TLR4 activation in the mucosa: fimbriae, recognition receptors and adaptor protein selection. Eur J Immunol. 2006;36:267–277. doi: 10.1002/eji.200535149. [DOI] [PubMed] [Google Scholar]
- 43.Godaly G, Otto G, Burdick MD, Strieter RM, Svanborg C. Fimbrial lectins influence the chemokine repertoire in the urinary tract mucosa. Kidney Int. 2007;71:778–786. doi: 10.1038/sj.ki.5002076. [DOI] [PubMed] [Google Scholar]; The ability of fimbriae expressed on UPEC to differentially modulate the chemokine and cytokine responses mediated by TLR4 on uroepithelial cells is described.