Abstract
Objectives
Although polymorphic variations in genes of the RAS system have previously been associated with susceptibility to AAA such studies have been significantly limited by small sample sizes. This study was undertaken, using the largest case series yet reported, to determine if common genetic variants of the RAS are associated with either susceptibility or severity of AAA.
Methods and Results
The frequencies of four common genetic variants of genes related to the renin-angiotensin system were investigated in three geographically distinct, but ethnically similar, case-control cohorts, resulting in comparison of 1226 AAA cases AAA cases with 1723 controls. In all three the AGTR1 1166C allele was significantly more common in AAA patients than controls (overall adjusted OR 1.60, 95%CI 1.32-1.93, p=1.1×10-6). Overall, the ACE ID genotype was associated with AAA (OR 1.33, 95% CI 1.06-1.67, p<0.02). The AGT 268T allele appeared to have an epistatic effect on large aneurysm size.
Conclusion
This study has identified a strong and repeated association between the AGTR1 1166C allele and susceptibility to AAA, and a weaker effect associated with the ACE deletion allele, in three geographically distinct, but ethnically similar, case-control cohorts. This study highlights the key role of the RAS in AAA and emphasizes the need for replication and validation of results in suitable independent cohorts.
Keywords: abdominal aortic aneurysm (AAA), angiotensinogen (AGT), angiotensin converting enzyme (ACE), angiotensin (Ang), Bradykinin, genetic association, renin
The renin-angiotensin system (RAS) is a potent mediator of cardiovascular homeostasis. The principal effector, angiotensin II (AngII), is most widely associated with its role in the regulation of arterial blood pressure, but has been implicated in a wide range of non-pressor related actions including vascular remodeling, fibrosis, endothelial dysfunction, oxidative stress1, 2 and dyslipidemia.3 Reduction of AngII levels, by inhibition of its converting enzyme (ACE), has been shown to significantly reduce cardiovascular disease morbidity and mortality.4
Abdominal aortic aneurysm (AAA) is a common condition, being present in approximately 4% of white males between the ages of 50-79years.5 The condition is associated with a distinct familial component, with up to 20% of patients having one or more first affected relatives.6
Evidence of a link between the RAS and abdominal aortic degeneration has been shown in experimental animal models, with exogenous AngII inducing abdominal aortic aneurysms in the apolipoprotein E deficient mouse,7 and RAS blockade reducing spontaneous aortic elastic tissue degeneration in the rat, independent of changes in blood pressure.8 In humans, ACE inhibitors have been suggested to reduce AAA rupture, an effect not observed with other anti-hypertensive agents.9 Genetic polymorphisms of the RAS have been implicated as potential susceptibility candidates in a variety of vascular disorders including coronary artery disease, myocardial infarction and hypertension. The role of the RAS related polymorphisms has been previously investigated in AAA patients,10-13 Fatini and co-workers, in the largest and most recent of these studies, investigated 250 cases and reported a significant association between ACE deletion homozygotes and AAA susceptibility.10 All of the studies to date have involved small patient cohorts without adequate independent replication.
Recently, a combinational effect of ACE, AGT and AGTR1 SNPs has been reported for susceptibility to diabetic nephropathy.14 Such studies suggest that investigations of RAS genetic variations should include consideration of combinational / epistatic effects.
This study was undertaken to determine if any of four common functional variants of genes involved with the renin-angiotensin system are associated with AAA susceptibility. The largest series published to date, comprising a total of 1360 AAA cases, was examined. The results from an initial AAA cohort of 576 cases were validated in two additional geographically distinct, but ethnically similar, cohorts.
Methods
Case Control cohorts
Three geographically distinct case control series were examined in this study, resulting in comparison of a total of 1226 AAA cases and 1723 controls. The initial cohort consisted of patients from New Zealand (NZ), 80% of whom had undergone surgical AAA repair (typically AAA's >50mm in diameter). Patients were recruited from the Otago-Southland region of the country, the vast majority (>97%) being of Anglo-European ancestry as reported previously.6, 15 The control group consisted of elderly individuals without vascular disease (AAA excluded by abdominal ultrasound) from the same geographical region.15 Male United Kingdom (UK) cases were recruited from the Aneurysm Screening Programme, Chichester, West Sussex and compared with age-strata matched controls from the Second Northwick Park Heart Study (NPHS-II). Each control was at least as old as each of the cases within the strata. NPHSII is a prospective study of healthy Caucasian middle-aged men recruited from nine general practices throughout the UK. At baseline all were free of Coronary Heart Disease (CHD) and those using blood pressure or lipid lowering medication were excluded.16 The Australian cohort was recruited from the Health in Men Study (HIMS) involving over 12,200 participants in a population based trial of screening for AAAs in the state of Western Australia.17 Cases were matched to controls without AAAs from the same study.
Additional cohorts with coronary artery disease (CAD) and peripheral arterial disease (PAD)15 from the same geographical region of the New Zealand as the AAA controls groups were examined to determine the specificity of any associations with vascular disease phenotype.
The following potential demographic confounders were assessed: age, gender (New Zealand only), hypertension (a history of systolic pressure >140 mmHg or a diastolic pressure >90 mmHg or treatment with anti-hypertensive medication), dyslipidaemia (total cholesterol >6.5mmol/L or on treatment for elevated cholesterol), diabetes and smoking history, coronary heart disease (history of angina or myocardial infarction). All subjects gave written informed consent prior to being recruited and the investigations conforms with the principles outlined in the Declaration of Helsinki.
Genotyping
Four common functional polymorphisms of genes related to the RAS were examined in this study. These were the (1) Angiotensin converting enzyme intron 16 287-bp insertion deletion (ACE I/D, NCBI reference SNP ID: rs4646994), (2) Angiotensinogen exon 2 T704>C resulting in a methionine 268 to threonine substitution (AGT M268>T, rs699), (3) Angiotensin II type 1a receptor 3' UTR A1166>C (AGTR1 A1166>C, rs5186) and (4) Bradykinin receptor exon 1 5'UTR 9-bp (GGTGGGGAC) insertion deletion (BDKRB2 I/D, rs5223) polymorphisms. It should be noted that the AGT M268T SNP has previously been mis-designated M235>T. Each cohort was genotyped independently by the respective host institutions using established PCR techniques with sequence verified standards. The primer sequences and PCR conditions used are available on request.
Statistical analysis
Genotype results were compared using χ2-analysis and univariate odds ratios calculated using recessive or dominant models as appropriate. Multiple logistic regression was used to account for demographic confounders (gender, age, history of hypertension, dyslipidemia, diabetes or tobacco smoking) in the case-control association analysis. Separate logistic regression models were fitted to the data from each geographical location and then combined using meta-analysis (Comprehensive Meta Analysis, version 2.0, Biostat, NJ, USA). Both univariate genotype and multivariant adjusted odds ratio meta-analysis was performed. Results are expressed as means ± 1 standard deviation or medians with interquartile ranges (IQR). Odds ratios are expressed with 95% confidence intervals (95%CI).
Results
Population demographics
The demographics of the three case control series demonstrated consistent risk association between AAA and hypertension, dyslipidemia, smoking and other cardiovascular disease, particularly ischemic heart disease. There was no association between AAA and diabetes (Tables 1A-C). Aneurysm diameters ranged from a median size of 34mm (IQR 31-39mm) in the Australian group to 52mm (38-59mm) in the UK and 56mm (46-66mm) in the NZ groups. This result reflects the varied recruitment strategies of the three cohort studies examined, with the Australian AAA's being derived from a population screening study while the majority (80%) of NZ patients had much larger aneurysms and had undergone surgical repair. The UK group was a mixture of patients, with the majority being recruited from an aneurysm screening and surveillance programme (typically AAAs less than 50mm in diameter).
Table 1A.
Demographic data, New Zealand cohorts.
| Controls n=472 | CAD n=481 | PAD n=420 | AAA n=576 | |
|---|---|---|---|---|
| Gender (%male) | 45.8 | 73.3* | 57.4 | 78.0* |
| Age (years) | 69.8 ± 7.2 | 64.3 ± 9.7* | 70.0 ± 10.2* | 72.4 ± 8.1* |
| Infrarenal aortic diameter (mm) | 20.2 ± 3.2 | 18.7 ± 5.1* | 18.2 ± 3.4* | 60.0 ± 17.1* |
| Hypertension (%) | 30.1 | 45.3* | 67.1* | 55.2* |
| Dyslipidemia (%) | 24.1 | 52.7* | 47.2* | 35.8* |
| Diabetes (%) | 7.8 | 17.8* | 20.6* | 7.1 |
| Smoking (pack years) | 10.5 ± 19.1 | 19.1 ± 24.8* | 31.7 ± 32.0* | 29.2 ± 29.1* |
| History of coronary heart disease (%) | 0 | 100* | 25.8* | 37.7* |
| Triglycerides (mmol/L) | 2.0 ± 1.1 | 2.1 ± 1.2† | 2.3 ± 1.2* | 2.3 ± 1.2* |
| HDL-cholesterol (mmol/L) | 1.3 ± 0.4 | 1.1 ± 0.3* | 1.2 ± 0.4† | 1.1 ± 0.4* |
p-values versus controls
<0.0001,
p<0.01
Table 1C.
Demographic data, Western Australian cohorts.
| Controls Aortic diameter ≤ 25mm (n=339) | AAA Aortic diameter ≥ 30mm (n=352) | |
|---|---|---|
| Gender (%male) | 100 | 100 |
| Age (years) | 76.7 ± 2.5 | 73.5 ± 4.5* |
| Infrarenal aortic diameter (mm) | 21.3 ± 2.0 | 36.0 ± 5.8 |
| Hypertension (%) | 35.0 | 51.6* |
| Dyslipidemia (%) | 31.9 | 39.6† |
| Diabetes (%) | 7.7 | 9.9 |
| Smoking history (% ever) | 65.5 | 87.5* |
| History of Angina (%) | 18.8 | 27.7‡ |
| History of Stroke (%) | 5.3 | 12.6‡ |
p<0.0001,
p<0.04,
p<0.007
RAS genetic variations in AAA
All genotypes were in Hardy-Weinberg equilibrium, except for an under representation of BDKRB2 heterozygotes in the NZ subjects (p<0.001). In the initial analysis conducted on the NZ series, significant associations were observed between both the AGT 268 T homozygotes and AGTR1 1166 C allele carriers and AAA (Table 2A). Both of these associations remained significant when adjusted for demographic confounders (Table 3). Validation in the UK and Australian cohorts failed to confirm the AGT 268T association but did replicate the results for AGTR1 1166 C allele observation (Tables 2B and 2C). Meta-analysis resulted in an adjusted odds ratio of 1.60 (95% CI, 1.32-1.93, p=1.1×10-6) for carriers of the AGTR1 1166C allele and AAA (Table 3 and Figure 1). No additional effect was observed by addition of other putative risk markers such as the AGT 268T or ACE D alleles. The ACE genotype was not associated with significant risk in any of the individual cohorts, but overall the ID genotype was associated with an adjusted odds ratio of 1.31 (95% CI, 1.04-1.65, p<0.03).
Table 2A.
Renin-Angiotensin System genotypes New Zealand cohorts.
| Controls | AAA | Univariate OR (95% CI) | |
|---|---|---|---|
| AGT M268>T (rs699) | |||
| MM | 176 (37.3) | 193 (34.0) | 1.00 |
| MT | 241 (51.1) | 257 (45.2) | 0.97 (0.74-1.27, p=0.84) |
| TT | 55 (11.7) | 118 (21.8) | 1.96 (1.34-2.86, p<0.0006) |
| T allele frequency | 0.372 (0.34-0.40) | 0.434 (0.40-0.46) | p<0.005 |
| ACE I/D (rs4646994) | |||
| II | 85 (22.5) | 112 (21.3) | 1.00 |
| ID | 177 (46.8) | 257 (49.0) | 1.10 (0.78-1.55, p=0.58) |
| DD | 116 (30.7) | 156 (29.7) | 1.02 (0.71-1.48, p=0.91) |
| D allele frequency | 0.541 (0.50-0.58) | 0.542 (0.51-0.57) | p=0.97 |
| AGTR1 A1166>C (rs5186) | |||
| AA | 252 (54.1) | 266 (47.3) | 1.00 |
| AC | 177 (38.0) | 252 (44.8) | 1.35 (1.04-1.75, p<0.03) |
| CC | 37 (7.9) | 44 (7.8) | 1.13 (0.70-1.80, p=0.62) |
| C allele carrier | 214 (45.9) | 296 (52.7) | 1.31 (1.02-1.68, p<0.04) |
| C allele frequency | 0.269 (0.24-0.30) | 0.303 (0.28-0.33) | p=0.10 |
| BDKRB2 I/D (rs5223) | |||
| II | 151 (33.1) | 184(32.4) | 1.00 |
| ID | 172 (37.6) | 217 (38.2) | 1.04 (0.77-1.39, p=0.82) |
| DD | 134 (29.3) | 167 (29.4) | 1.02 (0.75-1.40, p=0.89) |
| D allele frequency | 0.481 (0.45-0.51) | 0.485 (0.46-0.51) | p=0.87 |
Table 3.
Adjusted odds ratio for AAA association in New Zealand, United Kingdom and Australian AAA cohorts
| NZ-AAA*† | UK-AAA* | AUSTRALIA-AAA* | Meta-analysis | |
|---|---|---|---|---|
| AGT M268T | ||||
| TT (recessive) | 2.3 (1.42-3.73, p<0.0008) | 0.45 (0.22-0.91, p=0.03) | 1.02 (0.61-1.69, p=0.94) | 1.23 (0.89-1.68, p=0.20) |
| ACE I/D | ||||
| ID | 1.45 (0.96-2.18, p=0.08) | 1.33 (0.92-1.94, p=0.13) | 1.16 (0.76-1.76, p=0.50) | 1.31 (1.04-1.65, p<0.03) |
| DD | 1.28 (0.82-1.98, p=0.28) | 1.26 (0.83-1.92, p=0.27) | 1.40 (0.87-2.27, p=0.17) | 1.31 (1.01-1.69, p<0.05) |
| AGTR1 A1166>C | ||||
| AC | 1.53 (1.09-2.13, p<0.02) | 1.62 (1.18-2.21, p<0.003) | 1.53 (1.02-2.30, p<0.05) | 1.56 (1.28-1.92, p=1.2×10-5) |
| CC | 1.52 (0.84-2.75, p=0.16) | 1.79 (1.07-2.99, p<0.03) | 1.89 (1.02-3.50, p<0.05) | 1.72 (1.25-2.40, p<0.002) |
| C carrier | 1.53 (1.11-2.10, p<0.01) | 1.65 (1.23-2.22, p<0.001) | 1.61 (1.10-2.36, p<0.02) | 1.60 (1.32-1.93, p=1.1×10-6) |
| BDKRB2 I/D | ||||
| ID | 0.95 (0.67-1.34, p=0.75) | 0.85 (0.59-1.22, p=0.38) | 1.04 (0.69-1.58, p=0.84) | 0.94 (0.76-1.16, p=0.55) |
| DD | 0.92 (0.64-1.34, p=0.67) | 1.17 (0.78-1.76, p=0.44) | 0.81 (0.50-1.32, p=0.40) | 0.97 (0.76-1.23, p=0.80) |
adjusted for age, history of hypertension, hypercholesterolemia, diabetes and smoking (pack years).
plus gender.
Table 2B.
Renin-Angiotensin System genotypes United Kingdom cohorts.
| Controls | AAA | Univariate OR (95% CI) | |
|---|---|---|---|
| AGT M268>T (rs699) | |||
| MM | 58 (33.9) | 105 (35.5) | 1.00 |
| MT | 78 (45.6) | 158 (53.4) | 1.00 (0.55-1.82, p=0.99) |
| TT | 35 (20.5) | 33 (11.2) | 0.45 (0.21-0.98, p=0.05) |
| T allele frequency | 0.433 (0.380-0.487) | 0.378 (0.339-0.419) | p=0.07 |
| ACE I/D (rs4646994) | |||
| II | 238 (26.5) | 64 (21.6) | 1.00 |
| ID | 423 (47.2) | 157 (52.9) | 1.33 (0.92-1.94, p=0.13) |
| DD | 236 (26.3) | 76 (25.6) | 1.26 (0.83-1.92, p=0.27) |
| D allele frequency | 0.499 (0.475-0.522) | 0.520 (0.479-0.561) | p=0.29 |
| AGTR1 A1166>C (rs5186) | |||
| AA | 488 (53.5) | 126 (42.3) | 1.00 |
| AC | 348 (38.2) | 139 (46.6) | 1.62 (1.18-2.21,p=0.003) |
| CC | 76 (8.3) | 33 (11.1) | 1.79 (1.07-2.99, p=0.03) |
| C allele carrier | 424 (46.5) | 172 (57.7) | 1.65 (1.23-2.22, p=0.001) |
| C allele frequency | 0.274 (0.254-0.295) | 0.344 (0.306-0.384) | p=0.004 |
| BDKRB2 I/D (rs5223) | |||
| II | 215 (24.8) | 72 (24.4) | 1.00 |
| ID | 442 (51.0) | 140 (47.5) | 0.85 (0.59-1.22, p=0.38) |
| DD | 209 (24.1) | 83 (28.1) | 1.17 (0.78-1.76, p=0.44) |
| D allele frequency | 0.497 (0.473-0.520) | 0.519 (0.477-0.560) | p=0.19 |
Table 2C.
Renin-Angiotensin System genotypes Western Australian cohorts.
| Controls Aortic diameter ≤25mm | AAA Aortic diameter ≥30mm | Univariate OR (95% CI) | |
|---|---|---|---|
| AGT M268>T (rs699) | |||
| MM | 110 (32.4) | 100 (28.6) | 1.00 |
| MT | 160 (47.2) | 188 (53.7) | 1.29 (0.92-1.82, p=0.14) |
| TT | 69 (20.4) | 62 (17.7) | 0.99 (0.64-1.53, p=0.99) |
| T allele frequency | 0.440 (0.40-0.48) | 0.446 (0.41-0.48) | p=0.82 |
| ACE I/D (rs4646994) | |||
| II | 92 (27.1) | 81 (23.1) | 1.00 |
| ID | 164 (48.2) | 171 (48.7) | 1.18 (0.82-1.71, p=0.37) |
| DD | 84 (24.7) | 99 (28.2) | 1.34 (0.88-1.03, p=0.17) |
| D allele frequency | 0.488 (0.45-0.53) | 0.526 (0.49-0.56) | p=0.16 |
| AGTR1 A1166>C (rs5186) | |||
| AA | 159 (52.0) | 111 (40.8) | 1.00 |
| AC | 117 (38.2) | 120 (44.1) | 1.47 (1.03-2.09), p<0.04 |
| CC | 30 (9.8) | 41 (15.1) | 1.96 (1.15-3.33), p<0.02 |
| C allele carrier | 147 (48.0) | 161 (59.2) | 1.57 (1.13-2.18), p<0.008 |
| C allele frequency | 0.289 (0.25-0.33) | 0.371 (0.33-0.41) | p=0.003 |
| BDKRB2 I/D (rs5223) | |||
| II | 95 (27.9) | 93 (26.7) | 1.00 |
| ID | 159 (46.6) | 171 (49.1) | 1.10 (0.77-1.57, p=0.95) |
| DD | 87 (25.5) | 84 (24.1) | 0.99 (0.65-1.49, p=0.61) |
| D allele frequency | 0.488 (0.45-0.53) | 0.487 (0.45-0.52) | p=0.96 |
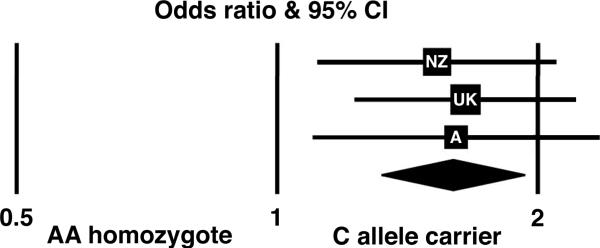
AGTR1 A1166>C and AAA susceptibility.
Forest plot of adjusted odds ratios for the New Zealand (NZ), United Kingdom (UK) and Australian (A) series. Carriers of the AGTR1 1166C allele had a strong, reproducible AAA association with an overall odds ratio of 1.60 (1.32-1.93, p=1.1×10-6)
AAA size and RAS genotype
Aneurysm size was weakly associated with AGT M268>T genotype in the NZ (p<0.04) and UK (p=0.07) AAA cohorts. In the New Zealand AAA cohort this was shown to be an epistatic interaction between the AGT M268>T and AGTR1 A1166>C genotypes. In AGTR1 A homozygotes, carriers of the AGT 268T allele had significantly larger aneurysms than AGT 268M homozygotes (64.3± 18.5mm (n=158) versus 56.3 ±, 16.8mm (n=79), p<0.004). Amongst AGTR1 1166A homozygotes, the AGT 268T allele had an adjusted (demographic confounders) odds ratio for aneurysm size of 1.29 (95%CI 1.07-1.56, p<0.009) compare to AGT 268M homozygotes. This interaction was not present in AGTR1 1166C allele carriers, nor was it significant in the UK or Australian cohorts, though it should be noted that both of these cohorts had significantly smaller aneurysms with a narrower range of sizes compare with the New Zealand aneurysm cohort. No associations were observed between either the BDKRB2 I/D or ACE I/D genotypes and AAA size.
Specificity of genetic associations with AAA
The genotype and allele frequency of the four polymorphisms investigated were also compared with NZ CAD and PAD patients to determine the specificity of AAA associations (supplementary table 1). The ACE D allele was significantly more common in CAD subjects than all other groups with an adjusted odds ratio of 2.6 (95%CI 1.7-4.0, p<0.0001) versus controls. Carriers of the AGTR1 1166C allele were not significantly more common in CAD and PAD compared with controls, however PAD patients did have significantly fewer C alleles (p<0.006) and C carrier genotypes (p<0.02) than the AAA group. CAD patients were significantly less frequent carriers of the BDKRB2 deletion allele than either controls or other vascular disease groups (p<0.0001).
Discussion
Abdominal aortic aneurysm is a common condition being responsible for 1.3% of all deaths in 65-85 year old white males.18 The reported prevalence rates varies depending on factors such as the threshold aortic diameter used to define an AAA and age. If the most widely accepted value of 30mm is applied, 1% of women and 4.2% of men between the ages of 50 to 79 years are affected in a (north American) predominantly white population.5 In British males over the age of 65 this rate increases to 7.6%.19 Moreover, AAA has a distinct familial component with up to 20% of patients having one or more first affected relatives.6 Verloes et al concluded that AAAs have an autosomal dominant pattern of inheritance with up to 40% penetrance, and the identification of the genes underlying the familial risk is of clinical importance.20
The principal observation of this study was that the identification of the common 1166C variant of the angiotensin II type 1a receptor gene showed a significant association with AAA in three independent, geographically distinct but ethnically similar, case-control cohorts. This strong consistent association was independent of known AAA demographic confounders, including age, gender, hypertension, dyslipidemia and smoking. The AGTR1 1166C risk association was not seen in all forms of cardiovascular disease, with the allele not being significantly associated with either peripheral or coronary artery disease. The notion that AAA and atherosclerotic occlusive PAD are clinically distinct pathophysiological entities is well established. As has previously been reported for the MMP9 C-1562T SNP21 the greatest variation in AGTR1 1166C genotype was observed between AAA and peripheral arterial disease subjects.
The AGT 268T allele is associated with a 10-20% increase in plasma AGT.22 Such elevated AGT levels have been shown to alter renin kinetics and result in higher AngII production.23 Such observations may provide some mechanistic insight into the apparent AGT 268 T allele epistatic aneurysm size association observed in New Zealand AAA patients. While this effect was not replicated in the other two AAA cohorts, it should be noted that the New Zealand cohort had not only the largest number of patients but that they also had significantly larger aneurysms, with a greater range of values, than either the UK or Australian cohorts. Based on these results future AAA surveillance studies should consider including AGT 268 and AGTR1 1166 analysis to determine a possible epistatic influence of these genotypes on aneurysm growth rates.
The ACE intron 16 287-bp deletion allele has been previously associated with AAA susceptibility in a 250 patient cohort.10 While meta-analysis of all 1360 cases in this present study did indicate greater risk for heterozygotes for this polymorphism and AAA, this was at best a weak association, and risk in deletion allele homozygotes was not higher. One explanation for this would be if ACE DD subjects developed other types of cardiovascular disease, before AAA could develop, and there is support for this in the literature24 and from our study, where the DD genotype was associated with CAD. The OR for AAA seen here is similar to that reported by us in a meta-analysis of previously published data.25
This study aimed to replicate the observations of the initial (New Zealand) case-control study in two independent cohorts. While there were differences in the mode of case identification, ranging from a population-based screening project to a clinical case selection, and consequent variability in the disease phenotype (most notably aneurysm size), all the cases meet the accepted definition of an AAA (aortic diameter > 30mm). A limitation of this study was that the UK control population was not screened for concurrent AAA. However, both the New Zealand and Australian control groups were confirmed AAA free and the potential under-ascertainment in the UK control group (conservatively estimated at 7.6%19) could only result in a weakening of the true association. While the New Zealand controls were slightly younger than their case counterparts it is well established that individuals with aortic diameters less than 25mm at such ages are unlikely to subsequently develop an aneurysm.26
Since this study involved comparing genetic variation in independent population groups it is reassuring to note a high degree of concordance between allele frequencies within the three cases-control cohorts. The most significant polymorphism in the context of this present study, AGTR1 A1166>C, has a reported C allele frequency of 0.301 and 0.299 in 858 French and 395 Spanish subjects respectively,27 further demonstrating the stability, and therefore potential utility, of these SNP's within populations of European ethnicity.
Although the AGTR1 1166C allele has been previously associated with the development of essential hypertension28 and increased aortic stiffness in hypertensive patients,29 these associations appear inconsistent.30 In this study there was no association between this SNP and hypertension, nevertheless hypertension was included in the logistic regression model and the association between AGTR1 1166C and AAA susceptibility remained independent. Previously the AGTR1 A1166>C SNP has been reported as non-functional and in putative linkage disequilibrium with a variant that alters receptor sensitivity, resulting in an increased response to angiotensin II.31 Recently the 3' UTR 1166 site has in fact been shown to be functional as part of a cis-regulatory site involved with microRNA gene silencing.32 The 1166C allele has impaired microRNA binding and a consequent impairment of translation attenuation resulting in increased receptor density. Such AGTR1 receptor alterations have a number of possible effects which may influence AAA susceptibility including; enhanced MMP production, increased ECM formation, SMC migration, oxidation of LDL and the formation of ROS leading to activation of intracellular signaling cascades (NFkB, MAP and JAK/STAT).33 Angiotensin II is key player in vascular remodeling within the intima, as reported in atheromatous disease.34 It is possible to speculate that an exaggerated response to vascular injury, as might be seen in AGTR1 1166C, could more easily spill over from the confines of the atherosclerotic intima to the media and adventitia, which is characteristic of AAA disease.
Recent studies in coronary heart disease (CHD) susceptibility have highlighted the need for replication of genetic associations in suitably sized independent populations.35, 36 While large CHD cohorts are relatively common in the literature, AAA genetic associations studies have been severely hampered by inadequate sample sizes. AAA is far less common than CHD, is often asymptomatic prior to rupture and occurs in older patient populations, all of which combine to make the establishment of large cohorts for analysis more difficult. Nevertheless, this study demonstrates the benefits of combining case-control cohort data to obtain appropriately powered genetic association analysis.
This study strongly confirms the key role of the RAS in the risk of AAA found in smaller samples by others.10, 12 The potential for slowing AAA disease progression with medication targeting the RAS has already been suggested by a large therapeutic study.9 Further functional work is now needed to better understand the pathophysiology behind these findings. In addition, this study highlights the potential utility of candidate based genetic association studies and emphasizes the need for replication and validation of results in suitable independent cohorts.
Supplementary Material
Table 1B.
Demographic data, United Kingdom cohorts.
| NPHS-II Controls n=912 | AAA n=298 | |
|---|---|---|
| Gender (%male) | 100 | 100 |
| Age (years) | 72.2 ± 3.1 | 70.6 ± 4.9* |
| Hypertension (%) | 54.7 | 60.7 |
| Dyslipidemia (%) | 33.8 | 48.3* |
| Diabetes (%) | 11.7 | 9.7 |
| Smoking (% ex-smoker) | 45.8 | 55.7 |
| Smoking (% current-smoker) | 28.3 | 25.8 |
| History of coronary heart disease (%) | 12.8 | 33.5* |
p<0.001
Acknowledgements
Special thanks to all participants and staff who contributed to the cohort studies examined in this work (the Otago Vascular Genetics Study, the WA AAA Program and the Health In Men Study and the Second Northwick Park Heart Study). We would like to thank Mrs Vicky Phillips for her highly skilled technical assistance and patient recruitment. Drs Michael Williams and Ian Thomson for aiding in the New Zealand coronary and peripheral artery disease cohorts. Ms Jade Hampel for genotyping assistance of the Australian samples and staff at the cardiovascular genetics department, UCL for their technical support in genotyping the UK cohort. Hilary Ashton and Stephanie Druce, Scott Research Unit, for their support in patient recruitment and data collection.
Sources of Funding This work was supported by grants from the Health Research Council (New Zealand), The National Heart Foundation of New Zealand, The British Heart Foundation and the WA study was supported by NHMRC Project Grant 303232 and National Institutes of Health Grant R01 HL080010-01. PN and JG are supported by NHMRC Practitioner Fellowships 458505 and 431503.
Footnotes
Disclosures None.
References
- 1.Dzau VJ. Theodore Cooper Lecture: Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37:1047–1052. doi: 10.1161/01.hyp.37.4.1047. [DOI] [PubMed] [Google Scholar]
- 2.Schieffer B, Schieffer E, Hilfiker-Kleiner D, Hilfiker A, Kovanen PT, Kaartinen M, Nussberger J, Harringer W, Drexler H. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: potential implications for inflammation and plaque instability. Circulation. 2000;101:1372–1378. doi: 10.1161/01.cir.101.12.1372. [DOI] [PubMed] [Google Scholar]
- 3.Singh BM, Mehta JL. Interactions between the renin-angiotensin system and dyslipidemia: relevance in the therapy of hypertension and coronary heart disease. Arch Intern Med. 2003;163:1296–1304. doi: 10.1001/archinte.163.11.1296. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 5.Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, Makaroun MS, Barone GW, Bandyk D, Moneta GL, Makhoul RG. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med. 2000;160:1425–1430. doi: 10.1001/archinte.160.10.1425. [DOI] [PubMed] [Google Scholar]
- 6.Rossaak JI, Hill TM, Jones GT, Phillips LV, Harris EL, van Rij AM. Familial abdominal aortic aneurysms in the Otago region of New Zealand. Cardiovasc Surg. 2001;9:241–248. doi: 10.1016/s0967-2109(00)00140-x. [DOI] [PubMed] [Google Scholar]
- 7.Daugherty A, Rateri DL, Cassis LA. Role of the renin-angiotensin system in the development of abdominal aortic aneurysms in animals and humans. Ann NY Acad Sci. 2006;1085:82–91. doi: 10.1196/annals.1383.035. [DOI] [PubMed] [Google Scholar]
- 8.Huang W, Alhenc Gelas F, Osborne-Pellegrin MJ. Protection of the arterial internal elastic lamina by inhibition of the renin-angiotensin system in the rat. Circ Res. 1998;82:879–890. doi: 10.1161/01.res.82.8.879. [DOI] [PubMed] [Google Scholar]
- 9.Hackam DG, Thiruchelvam D, Redelmeier DA. Angiotensin-converting enzyme inhibitors and aortic rupture: a population-based case-control study. Lancet. 2006;368:659–665. doi: 10.1016/S0140-6736(06)69250-7. [DOI] [PubMed] [Google Scholar]
- 10.Fatini C, Pratesi G, Sofi F, Gensini F, Sticchi E, Lari B, Pulli R, Dorigo W, Azas L, Pratesi C, Gensini GF, Abbate R. ACE DD genotype: a predisposing factor for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2005;29:227–232. doi: 10.1016/j.ejvs.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 11.Hamano K, Ohishi M, Ueda M, Fujioka K, Katoh T, Zempo N, Fujimura Y, Okamura A, Rakugi H, Higaki J, Ogihara T, Esato K. Deletion polymorphism in the gene for angiotensin-converting enzyme is not a risk factor predisposing to abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;18:158–161. doi: 10.1053/ejvs.1999.0873. [DOI] [PubMed] [Google Scholar]
- 12.Pola R, Gaetani E, Santoliquido A, Gerardino L, Cattani P, Serricchio M, Tondi P, Flore R, Grande M, Carbonin P, Fadda G, Pola P. Abdominal aortic aneurysm in normotensive patients: association with angiotensin-converting enzyme gene polymorphism. Eur J Vasc Endovasc Surg. 2001;21:445–449. doi: 10.1053/ejvs.2001.1339. [DOI] [PubMed] [Google Scholar]
- 13.Yeung JM, Heeley M, Gray S, Lingam MK, Manning G, Nash JR, Donnelly R. Does the angiotensin-converting enzyme (ACE) gene polymorphism affect rate of abdominal aortic aneurysm expansion? Eur J Vasc Endovasc Surg. 2002;24:69–71. doi: 10.1053/ejvs.2002.1693. [DOI] [PubMed] [Google Scholar]
- 14.Osawa N, Koya D, Araki S, Uzu T, Tsunoda T, Kashiwagi A, Nakamura Y, Maeda S. Combinational effect of genes for the renin-angiotensin system in conferring susceptibility to diabetic nephropathy. J Hum Genet. 2007;52:143–151. doi: 10.1007/s10038-006-0090-5. [DOI] [PubMed] [Google Scholar]
- 15.Jones GT, van Rij AM, Cole J, Williams MJ, Bateman EH, Marcovina SM, Deng M, McCormick SP. Plasma lipoprotein(a) indicates risk for 4 distinct forms of vascular disease. Clin Chem. 2007;53:679–685. doi: 10.1373/clinchem.2006.079947. [DOI] [PubMed] [Google Scholar]
- 16.Miller GJ, Bauer KA, Barzegar S, Foley AJ, Mitchell JP, Cooper JA, Rosenberg RD. The effects of quality and timing of venepuncture on markers of blood coagulation in healthy middle-aged men. Thromb Haemostasis. 1995;73:82–86. [PubMed] [Google Scholar]
- 17.Norman PE, Jamrozik K, Lawrence-Brown MM, Le MT, Spencer CA, Tuohy RJ, Parsons RW, Dickinson JA. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. Brit Med J. 2004;329:1259. doi: 10.1136/bmj.38272.478438.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365:1577–1589. doi: 10.1016/S0140-6736(05)66459-8. [DOI] [PubMed] [Google Scholar]
- 19.Scott RA, Wilson NM, Ashton HA, Kay DN. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Brit J Surg. 1995;82:1066–1070. doi: 10.1002/bjs.1800820821. [DOI] [PubMed] [Google Scholar]
- 20.Verloes A, Sakalihasan N, Koulischer L, Limet R. Aneurysms of the abdominal aorta: familial and genetic aspects in three hundred thirteen pedigrees. J Vasc Surg. 1995;21:646–655. doi: 10.1016/s0741-5214(95)70196-6. [DOI] [PubMed] [Google Scholar]
- 21.Jones GT, Phillips VL, Harris EL, Rossaak JI, van Rij AM. Functional matrix metalloproteinase-9 polymorphism (C-1562T) associated with abdominal aortic aneurysm. J Vasc Surg. 2003;38:1363–1367. doi: 10.1016/s0741-5214(03)01027-9. [DOI] [PubMed] [Google Scholar]
- 22.Jeunemaitre X, Soubrier F, Kotelevtsev YV, Lifton RP, Williams CS, Charru A, Hunt SC, Hopkins PN, Williams RR, Lalouel JM, Corvol P. Molecular basis of human hypertension: role of angiotensinogen. Cell. 1992;71:169–180. doi: 10.1016/0092-8674(92)90275-h. [DOI] [PubMed] [Google Scholar]
- 23.Bohlender J, Menard J, Ganten D, Luft FC. Angiotensinogen concentrations and renin clearance: implications for blood pressure regulation. Hypertension. 2000;35:780–786. doi: 10.1161/01.hyp.35.3.780. [DOI] [PubMed] [Google Scholar]
- 24.Keavney B, McKenzie C, Parish S, Palmer A, Clark S, Youngman L, Delepine M, Lathrop M, Peto R, Collins R. Large-scale test of hypothesised associations between the angiotensin-converting-enzyme insertion/deletion polymorphism and myocardial infarction in about 5000 cases and 6000 controls. International Studies of Infarct Survival (ISIS) Collaborators. Lancet. 2000;355:434–442. doi: 10.1016/s0140-6736(00)82009-7. [DOI] [PubMed] [Google Scholar]
- 25.Thompson AR, Drenos F, Hafez H, Humphries SE. Candidate gene association studies in abdominal aortic aneurysm disease: A review and meta-analysis. Eur J Vasc Endovasc Surg. 2008;35:19–30. doi: 10.1016/j.ejvs.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 26.Wilmink AB, Hubbard CS, Day NE, Quick CR. The incidence of small abdominal aortic aneurysms and the change in normal infrarenal aortic diameter: implications for screening. Eur J Vasc Endovasc Surg. 2001;21:165–170. doi: 10.1053/ejvs.2000.1285. [DOI] [PubMed] [Google Scholar]
- 27.Ng MC, Wang Y, So WY, Cheng S, Visvikis S, Zee RY, Fernandez-Cruz A, Lindpaintner K, Chan JC. Ethnic differences in the linkage disequilibrium and distribution of single-nucleotide polymorphisms in 35 candidate genes for cardiovascular diseases. Genomics. 2004;83:559–565. doi: 10.1016/j.ygeno.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Wang WY, Zee RY, Morris BJ. Association of angiotensin II type 1 receptor gene polymorphism with essential hypertension. Clin Genet. 1997;51:31–34. doi: 10.1111/j.1399-0004.1997.tb02410.x. [DOI] [PubMed] [Google Scholar]
- 29.Benetos A, Topouchian J, Ricard S, Gautier S, Bonnardeaux A, Asmar R, Poirier O, Soubrier F, Safar M, Cambien F. Influence of angiotensin II type 1 receptor polymorphism on aortic stiffness in never-treated hypertensive patients. Hypertension. 1995;26:44–47. doi: 10.1161/01.hyp.26.1.44. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Erdmann J, Regitz-Zagrosek V, Kurzinger S, Hense HW, Schunkert H. Evaluation of three polymorphisms in the promoter region of the angiotensin II type I receptor gene. J Hypertens. 2000;18:267–272. doi: 10.1097/00004872-200018030-00005. [DOI] [PubMed] [Google Scholar]
- 31.van Geel PP, Pinto YM, Voors AA, Buikema H, Oosterga M, Crijns HJ, van Gilst WH. Angiotensin II type 1 receptor A1166C gene polymorphism is associated with an increased response to angiotensin II in human arteries. Hypertension. 2000;35:717–721. doi: 10.1161/01.hyp.35.3.717. [DOI] [PubMed] [Google Scholar]
- 32.Martin MM, Buckenberger JA, Jiang J, Malana GE, Nuovo GJ, Chotani M, Feldman DS, Schmittgen TD, Elton TS. The Human Angiotensin II Type 1 Receptor +1166 A/C Polymorphism Attenuates MicroRNA-155 Binding. J Biol Chem. 2007;282:24262–24269. doi: 10.1074/jbc.M701050200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Grote K, Drexler H, Schieffer B. Renin-angiotensin system and atherosclerosis. Nephrology, Dialysis, Transplantation. 2004;19:770–773. doi: 10.1093/ndt/gfh030. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt-Ott KM, Kagiyama S, Phillips MI. The multiple actions of angiotensin II in atherosclerosis. Regul Peptides. 2000;93:65–77. doi: 10.1016/s0167-0115(00)00178-6. [DOI] [PubMed] [Google Scholar]
- 35.Helgadottir A, Thorleifsson G, Manolescu A, Gretarsdottir S, Blondal T, Jonasdottir A, Jonasdottir A, Sigurdsson A, Baker A, Palsson A, Masson G, Gudbjartsson DF, Magnusson KP, Andersen K, Levey AI, Backman VM, Matthiasdottir S, Jonsdottir T, Palsson S, Einarsdottir H, Gunnarsdottir S, Gylfason A, Vaccarino V, Hooper WC, Reilly MP, Granger CB, Austin H, Rader DJ, Shah SH, Quyyumi AA, Gulcher JR, Thorgeirsson G, Thorsteinsdottir U, Kong A, Stefansson K. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 36.McPherson R, Pertsemlidis A, Kavaslar N, Stewart A, Roberts R, Cox DR, Hinds DA, Pennacchio LA, Tybjaerg-Hansen A, Folsom AR, Boerwinkle E, Hobbs HH, Cohen JC. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–1491. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.