Synopsis
Hypertension secondary to scavenging of nitric oxide (NO) remains a limitation in the use hemoglobin based oxygen carriers (HBOCs). Recent studies suggest that nitrite reduction to NO by deoxyhemoglobin supports NO-signaling. Herein, we tested whether nitrite would attenuate HBOC-mediated hypertension using HBOC-201 (Biopure), a bovine cross-linked, low oxygen affinity hemoglobin. Similar to unmodified hemoglobin, deoxygenated HBOC-201 reduced nitrite to NO with rates directly proportional to the extent of deoxygenation. The functional importance of HBOC-201 dependent nitrite reduction was demonstrated using isolated aortic rings and a murine model of trauma, hemorrhage and resuscitation. In the former, HBOC-201 inhibited NO-donor and nitrite-dependent vasodilation when oxygenated. However, deoxygenated HBOC-201 failed to affect nitrite dependent vasodilation but still inhibited NO-donor dependent vasodilation consistent with a model in which nitrite-reduction by deoxyHBOC-201 counters NO-scavenging. Finally, resuscitation using HBOC-201 after trauma and hemorrhage, resulted in mild hypertension (~5-10mmHg). Administration of a single bolus nitrite (30-100nmol) at the onset of HBOC-201 resuscitation prevented hypertension. Nitrite had no effect on mean arterial pressure during resuscitation with lactated Ringers suggesting a role for nitrite-HBOC reactions in attenuating HBOC-mediated hypertension. Taken together these data support the concept that nitrite can be used as an adjunct therapy to prevent HBOC-dependent hypertension.
Keywords: nitric oxide, hemorrhage, resuscitation, nitrite-reductase activity, shock
INTRODUCTION
Safety concerns coupled with the lack of supply of donated RBC have led to much interest in the development of blood substitutes as alternative resuscitative agents that are capable of simultaneously improving vascular function and tissue perfusion / oxygenation. The current generation of so-called hemoglobin-based oxygen carriers (HBOC) comprise hemoglobins from different species (human and bovine) that have been chemically modified to improve structural stability (therefore extending circulatory half-life and minimizing renal toxicity) and modulate oxygen affinity (to counter the higher oxygen affinity of cell-free hemoglobin compared to erythrocytic hemoglobin) [1, 2]. Despite extensive efforts in their development, recent studies have indicated additional major concerns with the clinical use of HBOCs that stem from the ability of cell-free hemoglobins to stimulate pro-oxidative reactions and scavenge nitric oxide (NO)[3-8], an endogenously produced free radical that plays critical roles in vascular homeostasis[9, 10]. In this way, HBOC may promote hypertensive and pro-oxidative responses and thereby may exacerbate underlying tissue injury.
Nitric oxide is instrumental in vascular homeostasis mechanisms controlling blood flow, regulating mitochondrial respiration and maintaining an anti-inflammatory and anti-thrombotic environment [9, 10]. The critical role for NO is indicated by the association between loss of or aberrant NO-dependent signaling and the pathogenesis of a variety of diseases including hypertension, atherosclerosis and ischemia-reperfusion injury to name but a few[11]. Cell-free hemoglobin, in either its oxygenated or deoxygenated state, rapidly reacts with NO (k= ~107 M−1s−1)[12] which coupled with the relatively high concentrations of heme that arise from HBOC administration during resuscitative therapies (mM heme) [8], results in a significant inhibition of NO-signaling that manifests acutely with a hypertensive response. Whereas this may be of benefit in certain traumatic injuries e.g. traumatic brain injury, NO-scavenging is generally considered to be detrimental and this property remains a significant problem in the therapeutic development of HBOC[13].
Recent concepts suggest that the inorganic anion nitrite represents a reservoir for NObioactivity during hypoxia / ischemia both in tissues and the vasculature[14-18]. In this paradigm, nitrite is reduced by one electron to form NO via mechanisms that are activated during hypoxia / ischemia. Nitrite-derived NO restores NO-signaling which leads to cytoprotection in a variety of pathologies (e.g. ischemia reperfusion injury, hemorrhagic stroke, hypoxic pulmonary hypertension, hypertension) and stimulates NO-dependent physiological responses (e.g. angiogenesis) (reviewed in [13, 16-19]. How nitrite is reduced to NO during hypoxia remains an active area of investigation with the precise mechanism likely to depend on the specific tissue and degree of hypoxia. In the vascular compartment we and others have suggested that the reaction of nitrite with deoxyhemoglobin is important [20-26]. Specifically, the combination of hypoxia (more appropriately hypoxemia), deoxygenated RBC or hemoglobin and nitrite, stimulated NO-dependent vasodilatation via activation of soluble guanylate cyclase and formation of cGMP[21]. Moreover, this so-called nitrite-reductase activity of deoxyhemoglobin (see equation 1, which should be contrasted with a nitrite oxidase activity of oxyhemoglobin, see equation 2) is under allosteric control over hemoglobin oxygen affinity[27]. The latter is indicated in part by the observation that the initial rate of nitrite reduction shows a bell-shaped dependence on hemoglobin fractional saturation being maximal near the hemoglobin P50 which may allow for a coupled response by which hemoglobin-based oxygen sensing results in NO-formation at sites of tissue hypoxia[21, 23]. Interestingly, nitrite alone can stimulate vasodilation of isolated aorta[24, 28-31] which is inhibited by addition of cell-free oxyhemoglobin consistent with a role for NO in nitrite dependent vasodilation[24, 29]. However, cell-free deoxyhemoglobin has no effect despite being equally competent at inhibiting NO-dependent vasodilation [24, 32] as expected from similar rates of NO-scavenging by oxy- and deoxyhemoglobin (Eqn 3-4). Together, these data suggest that in the presence of nitrite, the nitrite-reductase activity of deoxyhemoglobin creates a balance between NO-formation and heme-based NOscavenging that dictates the degree to which NO can stimulate signaling and vasodilation.
| Equation 1 |
| Equation 2 |
| Equation 3 |
| Equation 4 |
The potential for nitrite-derived NO-formation by interaction with deoxyhemoglobin raises an intriguing possibility that co-administration of nitrite with an HBOC may result in NO-formation in tissues where HBOC are significantly deoxygenated and may therefore counter act NO-scavenging effects thereby attenuating hypertension. In this scenario therapeutic nitrite can be viewed as an NO-donor. Although many NO-donor compounds have been developed, the advantages of nitrite in this scenario is that nitrite-derived NO would be coupled to HBOC-deoxygenation and therefore produce NO at sites where increased blood flow is required, whereas other NO-donors lack site-specific release of NO. Consistent with this concept, recent studies by Yu et al show that inhaled nitric oxide (which increases circulating nitrite concentrations) and reagent nitrite itself can attenuate the hypertensive effects of either murine cell-free tetrameric hemoglobin or HBOC-201 (a glutaraldehyde cross-linked bovine hemoglobin) in a top-load murine model of resuscitation[33, 34]. Moreover, Lui et al have shown that polyethylene glycol conjugation of α-chain cross-linked hemoglobin, which results in an HBOC with suppressed vasoactivity increased the rate constant for deoxyhemoglobin mediated nitrite-reduction[35] suggesting that current strategies used to produce stable HBOC may also be used to modulate its nitrite-reductase activity and moreover that increasing such activity may be of clinical benefit in diminishing pressor effects of HBOC.
In this study we evaluated the nitrite-reductase activity of hemoglobin glutamer-250 (hemoglobin based oxygen carrier-201 (HBOC-201), Hemopure®), a purified glutaraldehyde-polymerized bovine hemoglobin and tested the potential for nitrite-therapy to attenuate HBOC-201 mediated hypertension in a murine model of trauma-hemorrhage and resuscitation. HBOC-201 is ‘T’-state stabilized hemoglobin with a higher P50 (lower oxygen affinity, ~ 38mmHg at 37°C, pH 7.4) compared to native cell-free hemoglobin (P50 ~10mmHg at 37°C, pH 7.4) that has an extended shelf, does not require cross matching or cold storage, is non toxic and has been shown to be an efficacious resuscitative agent in diverse experimental models of trauma and hemorrhagic shock but also causes significant hypertension secondary to scavenging of endogenous NO[36-38].
EXPERIMENTAL
Detailed Materials and Methods are provided in supplementary information.
Vessel Bioassay Studies
For all vessel experiments thoracic aorta from male Sprague-Dawley rats were used as previously described[24]. Aortic rings in Krebs-Henseleit buffer (KH) were pretreated with indomethacin (5μM), NG-monomethyl L-arginine (L-NMMA,100μM) and equilibrated with 95, 21, 2 or 0% O2 gas mixtures (containing 5% CO2 and balanced with N2 Following precontraction with phenylephrine (PE) the vasodilatory effects of nitrite or MahmaNONOate (MNO) were determined in the absence or presence of either human hemoglobin (hHb, 20μM heme) or HBOC-201 (20μM heme), which were added before initiating the dose-response. Vasodilatory effects of cumulative MNO or nitrite additions were determined by measuring the delta tension and expressing this as a percent relaxation with respect to the maximal PE constriction. Cumulative dose-dependent relaxation curves were fitted to a sigmoidal function -using GraphPad- from which EC50s were obtained.
During initial vasodilation studies with HBOC-201 it was observed that in the presence of nitrite, but not MNO, HBOC-201 concentrations decreased over time. Concomitant to this decrease HBOC-201 protein precipitates were noticed in the vessel bioassay chambers. Since a varying heme concentration will affect both NO-scavenging and formation processes, and hence the vessel response to vasoactive stimuli, in a sub-set of experiments we assessed the vasodilatory effect of single doses of nitrite in the absence and presence of HBOC-201. For these experiments, vessels were pre-contracted with PE and pre-equlibirated with the desired O2 containing gas. HBOC-201 was then added and 2 minutes thereafter (allowing time for HBOC-201 mixing/equilibration) a single dose of nitrite was added and changes in vessel tension recorded. Using this protocol no significant loss of HBOC-201 was observed (see results). This process was repeated using different vessel segments and different nitrite doses to assimilate a dose-dependence of nitrite-dependent vasodilation in the presence and absence of HBOC-201. No significant loss of HBOC-201 was observed in MNO-dependent vasodilation experiments, nor was any loss of human Hb observed in any experiment.
Assessment of Hb or HBOC-201 concentration and redox/ligation state
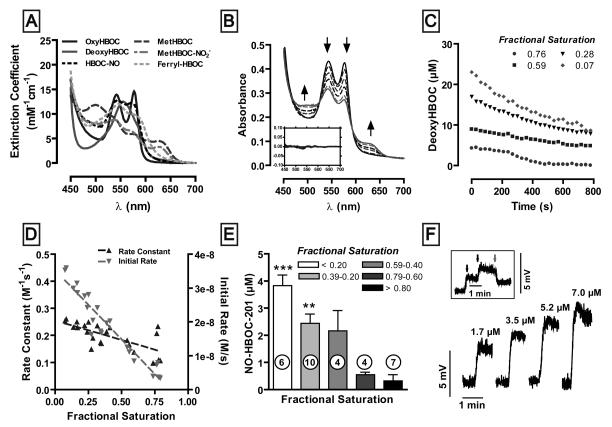
Aliquots of Hb or HBOC-201 were collected from vessel bioassay chambers after equilibration with different O2 containing gases immediately before and after addition of either nitrite or MNO and redox and ligation state determined by fitting measured visible spectra to previously acquired base spectra for oxy-, deoxy-, met-, ferryl-, nitrosyl- and metnitrite forms of both HBOC-201 and Hb (all species that may be populated during reaction with nitrite) using a least squares method as previously described[21-24]. Preparation of reference spectra is described in supplementary methods and spectra for HBOC-201 shown in Fig 1A.
Figure 1. Nitrite-reductase activity of HBOC-201 as a function of fractional saturation.
(A) Reference visible spectra of different derivatives expected to be involved in the reaction between HBOC-201 and nitrite. The individual species were synthesized at pH 7.4 as described in the supplementary methods. (B) Representative time-dependent spectral changes (indicated by arrows) after the addition of nitrite (5mM) to HBOC-201 (30μM) at 60% initial oxygen fractional saturation. Spectra were collected every 30sec for 15 min but spectra collected every 3min are shown for clarity. Inset: residuals for fits of acquired spectra to base spectra used for deconvolution. (C) Representative time dependent changes in deoxyHBOC-201 at different initial oxygen fractional saturations and after nitrite addition. (D) Rate constants and initial rates for the reaction between nitrite and deoxyHBOC-201 as a function of fractional saturation. Rate constants were determined by dividing the slope of the initial linear portion of the curve (panel C) by the initial concentration of deoxyHBOC-201 and sodium nitrite. Rate constant vs. fractional saturation: r2 = 0.47, the slope is significantly different from zero, p = 0.0003. Initial rate vs. fractional saturation: r2 = 0.91, the slope is significantly different from zero, p < 0.0001. (E) Yield of nitrosyl-HBOC (NO-HBOC-201) as a function of fractional saturation 720 s after the addition of 5 mM nitrite to 30μM HBOC-201 at pH 7.4, 37°C. Data are mean ± SEM with n-vlaues indicated on figure. P <0.0001 as determined by one-way ANOVA. ** p<0.01 and *** p<0.001 versus highest fractional saturation value as determined by Bonferroni's post test. (F) Direct formation of NO was monitored by ozone-based chemiluminescence. Shown are representative NO-formation traces produced after the addition of the indicated concentrations of HBOC-201 to 1 mM sodium nitrite in the reaction vessel. The reaction was performed in PBS at pH 7.4, 37°C in the presence of 100μM DTPA and GE antifoam in a He-purged vessel connected in line with a nitric oxide analyzer. Inset: Subsequent additions of 1.7 and 3.5 μM HBOC-201 to 1 mM sodium nitrite in the reaction vessel (blue arrows) followed by 25 μM CPTIO (red arrow).
Determination of the nitrite reductase activity of HBOC-201
Rates of deoxyheme loss at 37°C were measured to assess nitrite-reductase activity of HBOC-201. HBOC-201 (30μM) in PBS + 100μM DTPA at pH 7.4 were progressively degassed under a stream of helium or nitrogen to obtain a range of oxygen fractional saturations. The resulting solutions were transferred anaerobically to sealed spectrophotometer cuvettes and visible spectra (450-700nm) collected immediately before and every 30 seconds for 15 minutes after the addition of excess sodium nitrite (5mM, final concentration; nitrite stock solutions were prepared in degassed PBS). Concentrations of deoxyHBOC-201 as a function of time were determined by spectral deconvolution using reference spectra to different HBOC-201 redox and ligation listed above and as described [21-23]. Rate constants were calculated by dividing the slope of the initial (10%) linear portion of the curve by the initial concentration of deoxyHBOC and nitrite. Concentrations of nitrosylHBOC-201 were used as an index of NO production and were determined by spectral deconvolution after 720 seconds of nitrite addition.
Nitric oxide detection by chemiluminescence
Direct formation of NO was monitored by ozone-based chemiluminescence using a nitric oxide analyzer (NOA 280i Sievers). Sodium nitrite (1 mM) in PBS + 100μM DTPA and 50μL GE Antifoam (Sievers) pH 7.4 were equilibrated at 37°C under anoxic conditions in a sealed chamber directly connected to the analyzer before the addition of HBOC-201.
Cyclic GMP measurement
Aortic rings in KH buffer were equilibrated with gas mixtures containing 0% and 95% O2 (supplemented with 5% CO2) at 37°C and pH 7.45 in the presence of 5μM indomethacin, 100μM L-NMMA, 100μM isobutylmethylxanthine (IBMX), 100nM PE and 0.00067% (v/v) SE-15 antifoam. HBOC-201 (20μM heme) was added and allowed to equilibrate (1 min) before addition of nitrite (25μM). Rings were collected after 10 minutes, blotted dry, weighed and frozen in liquid nitrogen. cGMP was measured in ring homogenates by ELISA according to manufacturer's indications (Cayman Chemical, Ann Arbor, MI. USA.).
Trauma-Hemorrhage and resuscitation model
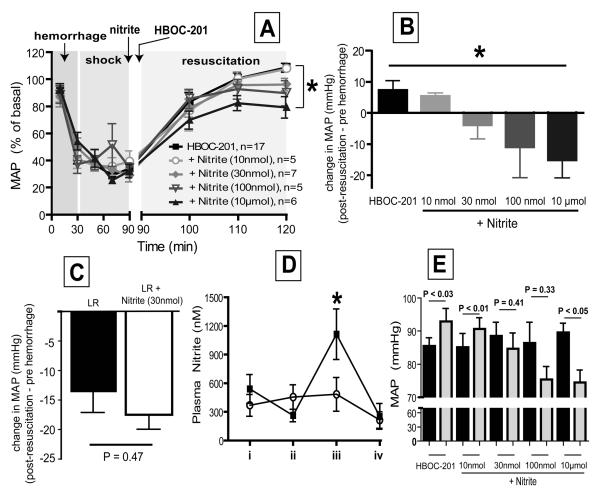
C57Bl/6 male mice were anesthetized initially by inhalation of 5% isoflurane in air, and then reduced to minimal concentration for maintenance (<1%). All procedures were performed under standard aseptic conditions and according to UAB IACUC approved protocols. The abdomen and groins were shaved and washed with povidone-iodine (10%). A 2-cm midline laparotomy was performed to induce soft-tissue trauma. The incision was closed in two layers (fascia/muscle and skin) and bathed in 1% lidocaine for analgesia. Both femoral arteries were cannulated with catheters (Braintree Scientific, Braintree, MA). Systemic arterial pressure was continuously monitored through one arterial line while hemorrhage and resuscitation was performed via the other. Mice were bled over 30 minutes to a mean arterial pressure (MAP) of 25 ± 5 mm Hg. This blood pressure was maintained for a further 60 minutes by additional bleeding as required. At the end of the 90 minute hemorrhagic shock period, animals were resuscitated over 30 minutes with either lactated Ringers (LR) or HBOC-201 formulation equal to total bleedout volume (~60% of total blood volume). Nitrite was administered in a bolus form (100μL i.v; nitrite stock solutions were 0.1mM, 0.3mM, 1mM, 100mM) immediately prior to initiation of HBOC-201 infusion. Nitrite doses indicated in Figure 6 correspond to amount of nitrite administered. One higher dose of nitrite (100μl of 1M stock solution) was also evaluated in preliminary experiments (n=2) but this induced death within the resuscitation phase and therefore excluded from analysis (not shown). All experiments were carried out for at least 120 minutes post-resuscitation. Arterial blood samples were obtained at designated intervals for nitrite measurement and assessment of PaO2, PaCO2 , fractional saturation and methemoglobin.
Figure 6. Effects of nitrite on HBOC-201 induced hypertension during hemorrhagic shock and resuscitation.
A) Changes in MAP expressed as a percent change from pre-hemorrhage levels are shown. Time course is separated into three phases of hemorrhage, shock and resuscitation as described in methods. Nitrite was administered 15-30 sec before HBOC-201, which was administered continuously over 30min during the resuscitation phase. Nitrite doses indicate amount added. For the sakes of clarity, only data at select times are shown and represent mean ± SEM (n's are indicated on the figure and refer to individual mice). *P < 0.0001 by 2wayANOVA for resuscitation phase. B) Shown is the change in MAP post-resuscitation (averaged from 120-124min) with respect to its pre-hemorrhage (0 min) value. Data are mean ± SEM (n = 5-17). *P < 0.03 by 1way ANOVA. C) Shown is the change in MAP post-resuscitation (averaged from 120-124min) with respect to its pre-hemorrhage (0 min) value after resuscitation with lactate ringers (LR) or LR + nitrite administered immediately before the resuscitation phase. Data are mean ± SEM (n = 4-7). Indicated P-value calculated by unpaired t-test D) Plasma nitrite levels during hemorrhage shock and resuscitation with HBOC-201 alone (○) or HBOC-201 + nitrite (30nmol) (■). i = pre-hemorrhage, ii = end of shock phase and immediately before addition of nitrite, iii = 30 mins after HBOC-201 or HBOC-201 and nitrite resuscitation, iv = 120 mins post completion of resuscitation. Data are mean ± SEM (n=4-6). *P < 0.05 relative to HBOC-201 alone at indicated time point by 2way ANOVA with Bonferrroni post test. E) MAP pre-hemorrhage (black bars) and 30 mins post resuscitation (gray bars). Data show mean ± SEM (n= 5-17). P-values calculated by paired t-test for pre-hemorrhage vs post-resuscitation.
Measurement of nitric oxide metabolites
Plasma nitrite, S-nitrosothiols and N-nitroso compounds were measured as previously described[39]. Upon collection, RBC and plasma were immediately separated by centrifugation (2000g, 1min). Plasma was then mixed 1:1 with a solution containing NEM (1mM final concentration), DTPA (100μM, final concentration) and incubated at room temperature 2mins before snap freezing in liquid nitrogen. Samples were then thawed on ice and in the dark within 4hr of collection and nitrite, S-nitrosothiol and N-nitroso species measured.
Statistical Analysis
Dose-dependent vessel relaxation responses were fitted to sigmoidal curves and data analyzed by 2-way repeated measures ANOVA with Bonferroni post-test. Changes in circulating nitrite levels during hemorrhagic shock and resuscitation with HBOC-201 with or without exogenous nitrite addition were analyzed by 2-way ANOVA with Bonferroni post test. To evaluate if nitrite-therapy attenuated HBOC-201 mediated hypertension during hemorrhagic shock and resuscitation, repeated-measures ANOVA was determined for each of the three experimental periods (hemorrhage, shock and resuscitation). Statistical significance in vessel bioassays and biochemical experiments was assessed by either repeated-measures ANOVA or one-way ANOVA followed by Bonferroni post-tests. P-values less than 0.05 were considered significant in all cases and analyses performed using GraphPad Prism Software (San Diego, CA, USA).
RESULTS
Nitrite reduction by HBOC-201
We first tested whether HBOC-201 could reduce nitrite and whether this process is regulated by oxygen fractional saturation. Nitrite was added to HBOC-201 at different oxygen saturations and changes in visible absorbance spectra followed as a function of time. Fig 1B shows a representative experiment performed at 60% oxygen saturation. Time-dependent changes in deoxyHBOC-201 were calculated (to assess nitrite-reductase activity, Fig 1C) from experimentally measured spectra by least squares deconvolution (shown in Fig 1A). Fig 1D shows the initial rate and calculated rate constant for nitrite reduction (using initial nitrite and deoxyhemoglobin concentrations) as a function of oxygen fractional saturation. Unlike human Hb which shows a bell-shaped dependence of initial rate vs. fractional saturation [21, 23], the initial rate for nitrite-reduction by HBOC-201 shows a significant inverse and linear relationship with fractional saturation. This is reflected in a similar profile for rate constant which also significantly increases as fractional saturation decreases. Consistent with nitrite-reduction, NO was a product of nitrite deoxy-HBOC-201 reactions as determined by formation of nitrosyl-HBOC-201 (Fig 1E) which increased at lower fractional saturations and by the formation of free NO under anoxic conditions (Fig 1F). The latter was confirmed by loss of signal in response to the NO-scavenger CPTIO (Fig 1F, Inset).
Effects of human Hb and HBOC-201 oxygen fractional saturation on NO- and nitrite-mediated vasodilation of aortic rings
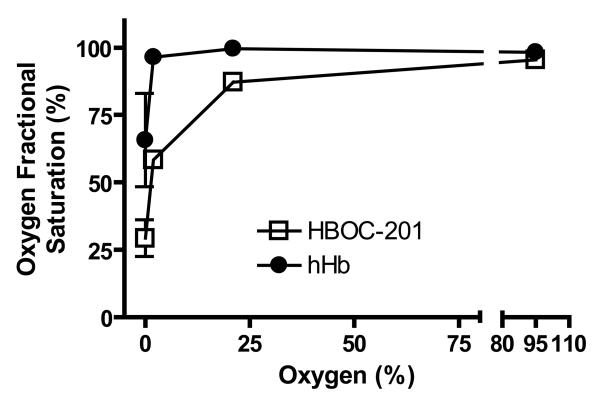
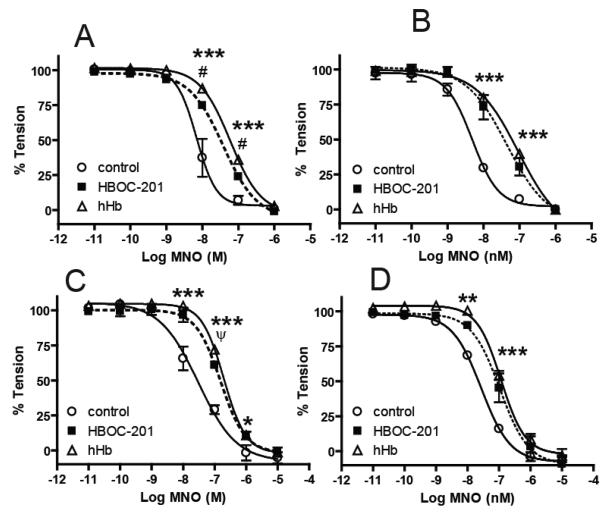
To assess if nitrite-reduction by HBOC-201 regulates NO-signaling we employed the experimental model of vasodilation of isolated aortic rings as previously described [24]. This approach, which provides a sensitive read-out of NO-dependent signaling (i.e. vasodilation) is amenable to performing experiments at different oxygen tensions and hence HBOC-201 fractional saturations. Fig 2 shows that the fractional saturation of either HBOC-201 or cell-free human hemoglobin attained in vessel bioassay chambers equilibrated with 95%, 21%, 2% or 0% oxygen ranged from ~95-30% and ~100-65% respectively. Consistent with HBOC-201 having a higher P50 value, HBOC-201 was deoxygenated compared to hHb at oxygen tensions ≤21%. To allow comparison of HBOC-201 and hHb we chose to study the effects of HBOC-201 and hHb at 95%, 21%, 2% and 0% oxygen to provide a range of overlapping fractional saturations for each hemoglobin preparation. To evaluate if the oxygen fractional saturation of HBOC-201 affects NO-scavenging, we tested the effects of HBOC-201 on vasodilation elicited by the NO-donor MahmaNONOate (MNO). Fig 3A-D shows that NO-dependent vasodilation was inhibited at all fractional saturations by both HBOC-201 and hHb as indicated by significant right shifts in MNO-dose dependent vasodilation. These data indicate that, similar to hHb, both oxygenated and deoxygenated HBOC-201 are competent in inhibiting NO-dependent signaling in the vascular compartment.
Figure 2. Fractional Saturations of HBOC-201 and hHb at different oxygen tensions.
Aliquots of hHb or HBOC-201 were collected from vessel bioassay baths perfused with gas mixtures comprising either 95%, 21%, 2% or 0% O2 + 5% CO2 and balanced with N2. Fractional saturation was determined by UV-Vis spectroscopy coupled with spectral deconvolution. Data shown are mean ± SEM (n=5-9).
Figure 3. Effects of hHb and HBOC-201 on cumulative NO-dependent vasodilation at different oxygen tensions.
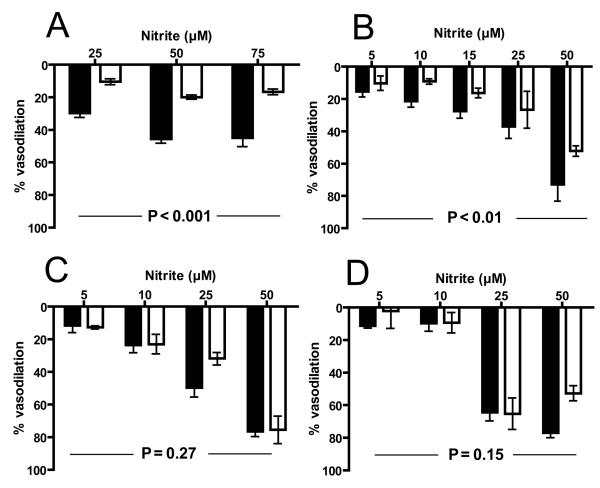
MNO-dependent vasodilation was assessed in the absence or presence of either HBOC-201 (20μM) or hHb (20μM) at either 95% (Panel A), 21% (Panel B), 2% (Panel C) or 0% O2 (Panel D). Lines show best fits using sigmoidal fitting alogirithms and data represent mean ± SEM (n=2-3). In some cases errors are smaller than symbol size. ***P =< 0.001, **P =< 0.01, *P = < 0.05 for HBOC-201 and hHb relative to control. #P = < 0.01, ψP = < 0.05 for HBOC-201 relative to hHb. P-values calculated by 2-way RM-ANOVA with Bonferroni post-test.
We next tested the effects of HBOC-201 and hHb on nitrite-dependent vasodilation. Consistent with previous studies showing a role for NO in nitrite-mediated vasodilation, hHb inhibited vasodilation at high (at 2%, 21% and 95% O2) oxygen fractional saturations (Supplementary Fig 1). Under deoxygenated conditions however, hHb failed to inhibit nitrite-dependent vasodilation. We have shown previously that this failure to inhibit NO-dependent effects at lower fractional saturations can be explained by the nitrite-reductase activity of deoxyhemoglobin with the resultant NO-formation counteracting heme based NO-scavenging[24]. Supplementary Fig 1 shows results from experiments testing the effects of HBOC-201 on nitrite-dependent vasodilation. However, in the course of these experiments it was noted that in the presence of nitrite, the concentration of HBOC-201 was decreasing and moreso at higher oxygen tensions. A systematic evaluation of HBOC-201 concentrations as a function of time demonstrated that the HBOC-201 concentration decreased ~40% during the course of nitrite-dependent vasodilation experiments at both 95% and 21% oxygen (Supplementary Figure 2A). Interestingly, nitrite had no effect on the concentration of hHb (supplementary figure 2A) and the loss of HBOC-201 did not occur in the presence of MNO (supplementary Fig 2B) suggesting a specific interaction between nitrite, higher oxygen tensions and HBOC-201 that leads to HBOC-201 decomposition. The changing HBOC-201 concentration during these experiments precludes interpretation of effects on nitrite-dependent vasodilation. We therefore employed a modified protocol in which the vasodilatory effects of single doses of nitrite were assessed in the presence or absence of HBOC-201. Using this protocol which resulted in nitrite – HBOC-201 incubation times of 3.9 ± 0.4 min (mean ± SEM, n= 7) versus 38.1 ± 1.4 min (mean ± SEM, n= 17) for nitrite-dose cumulative studies (shown in supplementary Fig 1) no significant loss of HBOC-201 occurred at any oxygen tension (not shown). Figure 4 shows that using this approach HBOC-201 significantly inhibited nitrite-dependent vasodilation at 95% and 21% oxygen, but did not affect nitrite-dependent vasodilation at either 2% or 0% oxygen. Consistent with this data, nitrite increased cGMP levels to similar extents both in the absence and presence of HBOC-201 at 0% (Figure 5).
Figure 4. Effects of HBOC-201 on bolus nitrite-dependent vasodilation at different oxygen tensions.
To avoid time-dependent loss of HBOC-201 in nitrite-dependent vasodilation experiments, aortic segments were exposed to single additions of nitrite at indicated concentrations in the presence (□) or absence (■) of HBOC-201 (20μM) and at either 95% (Panel A), 21% (Panel B), 2% (Panel C) or 0% O2 (Panel D) as described in methods. Data represent mean ± SEM (n=3-8). Indicated P-values were calculated by 2-way RM-ANOVA
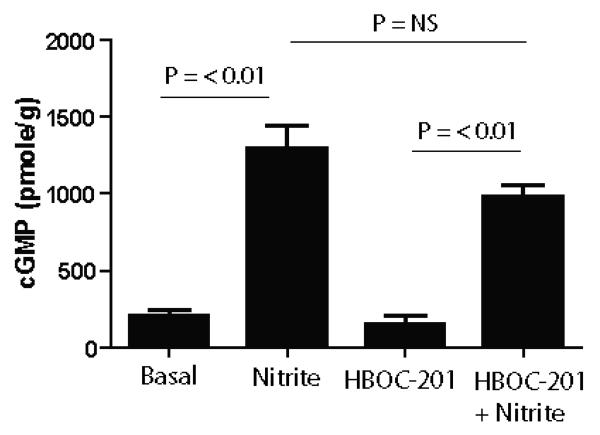
Figure 5. Effects of HBOC-201 on bolus nitrite-dependent increases in cGMP.
Rat aortic segments were equilibrated in KH buffer at pH 7.4 and 37°C with a gas mixture containing 0% O2 and 5% CO2. Vessels were pretreated with indomethacin (5μM), L-NMMA (100μM), IBMX (100μM) and 0.00067% SE-15 antifoam in the presence or absence of 20μM HBOC-201 before the addition of either 25μM sodium nitrite or vehicle controls (PBS). Vessel rings were collected after 10 minutes and tissue cGMP levels determined by enzyme linked immunoanalysis. Data are mean ± SEM (n=3-4), p<0.0001 as determined by one-way ANOVA and shown p values determined by Bonferroni's post test.
Nitrite administration attenuates HBOC-201 resuscitation mediated hypertension during trauma-hemorrhage
We next tested if nitrite-therapy could prevent HBOC-201 mediated hypertension in a murine model of trauma-hemorrhagic shock and resuscitation. Consistent with previous studies, resuscitation with HBOC-201 increased mean arterial pressure above pre hemorrhage levels underscoring its hypertensive effects (Fig 6A-B). However, in a dose-dependent manner, one bolus administration of nitrite immediately before resuscitation prevented HBOC-201 mediated hypertension (Fig 6A), with MAP returning to pre-hemorrhage levels with 30nmol nitrite (Fig 6B and Fig 6E). Fig 6C shows that at the end of resuscitation with LR alone, MAP remains ~15mmHg below pre-hemorrhage levels and that bolus addition of nitrite (30nmol) immediately before resuscitation did not have any further effect on MAP relative to LR alone. Fig 6D shows that at the end of resuscitation with 30nmol nitrite and HBOC-201, circulating nitrite was significantly increased ~2-3 fold with nitrite therapy compared to HBOC-201 alone and returned to basal levels after 2hr post-resuscitation. No significant changes in circulating S-nitrosothiols nor N-nitroso compounds were observed under this condition (not shown). Importantly, compared to the effects of HBOC-201 alone, 30nmol nitrite did not change fractional saturation, PaO2 nor PCO2 post resuscitation (table 1) indicating that nitrite can improve hemodynamics without affecting O2 and CO2 carrying functions of hemoglobin (Table 1). To assess safety of nitrite administration we measured changes in MAP, metHb and serum levels of HBOC-201. Fig 6E shows a paired analysis of the effects of different doses of nitrite on MAP at the end of resuscitation relative to pre-hemorrhage. MAP increased significantly with HBOC-201 alone and HBOC-201 + nitrite (10nmol), whereas with 10μmol nitrite a significant decrease in MAP was observed. No significant changes in MAP were observed with 30nmol or 100nmol nitrite. Supplementary Figure 3 shows that with HBOC-201 alone metHb increased to ~2-3% 2 hr post-resuscitation. Nitrite at concentrations of ≤100 nmol did not affect this increase in metHb. Only at the highest dose tested (10μmol) did metHb increase significantly beyond that observed with HBOC-201 alone. Finally, since vessel bioassay studies indicated an interaction of relatively high concentrations of nitrite and oxyHBOC-201 resulting in the decomposition of the latter, we measured serum hemoglobin (indicating HBOC-201) ~3-4hr post resuscitation in HBOC-201 alone and HBOC-201 + nitrite (30 nmol) groups. Table 1 shows that nitrite administered at this dose which was able to normalize hypertension, did not cause any loss of HBOC-201.
Table 1. Effects of nitrite on PaO2, PaCO2 and blood oxygen fractional saturation during hemorrhagic shock and resuscitation.
Data show mean ± SEM with indicated P-values calculated by unpaired t-test for comparison between HBOC-201 and HBOC-201 + nitrite (30nmol). Serum HBOC-201 measurements were made 251 ± 7.8 and 236.9 ± 11.2 min post HBOC-201 administration in HBOC-201 alone and HBOC-201 + nitrite groups respectively.
| HBOC-201 | HBOC-201 + Nitrite (30nmol) |
P-value (n-value) | |
|---|---|---|---|
| PaO2 (mmHg) | 80.2 ± 16.8 | 80.1 ± 15.3 | 0.99 (7) |
| PaCO2 (mmHg) | 43 ± 4.5 | 47.9 ± 2.4 | 0.35 (7) |
| Oxygen fractional saturation (%) |
73.5 ± 5.3 | 69.3 ± 5 | 0.58 (7) |
| Serum HBOC-201 (g/dL) |
4.95 ± 0.24 | 4.71 ± 0.13 | 0.43 (8) |
DISCUSSION
Recent developments have highlighted the potential for NO-formation from hemoglobin and nitrite in mediating vascular function and offering a novel therapeutic strategy to attenuate hypertensive effects of HBOC[13, 20, 21, 25, 33-35, 40-43]. Using HBOC-201 as a model HBOC that has been extensively studied, the goal of the present work was to evaluate the nitrite-reductase activity of HBOC-201 and test if NO-derived from this process is capable of maintaining NO-dependent vasodilation both ex-vivo (aortic ring studies) and in-vivo during trauma hemorrhage and resuscitation.
The differential reactivities of oxyhemoglobin and deoxyhemoglobin with nitrite have been acknowledged for many years [27] with oxyhemoglobin oxidizing nitrite to nitrate and deoxyhemoglobin reducing nitrite to NO. Recent insights into the mechanisms of this oxygen-linked reactivity of hemoglobin have shown that nitrite reduction is modulated via allosteric regulation of Hb conformation states (‘R’ or ‘T’)[21-23]. Specifically, the rate of nitrite reduction by Hb shows a bell-shaped dependence on fractional saturation with the maximum around the Hb P50. This property has been demonstrated with cell free and erythrocytic human hemoglobin[21, 23], rabbit[44], murine and most recently adult and fetal sheep hemoglobin[45] The importance of P50 in nitrite reduction can be explained by considering that the rate is controlled by i) the availability of deoxyhemes for nitrite binding and ii) the average intrinsic heme redox potential; with a more negative redox potential (associated with R-state Hb) facilitating electron transfer and resulting in increased rate constants for nitrite reduction. Additional factors have been suggested to control nitrite reductase activity including steric factors that regulate heme accessibility for nitrite[46] and mechanisms that regulate how nitrite may enter the RBC[32]. Consistent with the hypothesized faster rates of nitrite-reduction by R-state deoxyheme, fetal hemoglobin (which is more ‘R’ state compared to adult hemoglobin) reduces nitrite ~2-fold faster than adult hemoglobin[45] and increasing additions of PEG chains to cross-linked hemoglobin also increases the rate constant for nitrite reduction in a manner proportional to the ‘R’ state character [35].
HBOC-201 represents an interesting hemoglobin with respect to nitrite-reduction since it is ‘T’-state stabilized resulting in a higher P50, a therapeutic goal that improves oxygen delivery[47]. Extrapolating from the data shown in Fig 1D, the rate constant for nitrite-reduction by fully deoxygenated HBOC-201 is 0.25M−1s−1 at 37°C. Reported rate constants for either deoxygenated cell free human Hb or cell-free sheep hemoglobin at 25°C are 0.25 M−1s−1 and 0.28 M−1s−1 respectively. Considering that these numbers were obtained at 25°C, it is reasonable to expect that these reaction rates will be higher at 37°C. As a result we propose that the rate constant for nitrite reduction by HBOC-201 is slower compared to either deoxygenated cell free human Hb or cell-free sheep hemoglobin consistent with ‘T’-state character of HBOC-201. This is further reflected in the dependence of the rate constant and initial rate of nitrite reduction on HBOC-201 oxygen fractional saturation. For native hemoglobin the rate constant for nitrite reduction increases with fractional saturation paralleling ‘R’-state configuration[21, 23]. With HBOC-201 however the rate constant was linear and inversely proportional to the oxygen fractional saturation, a relationship that was also observed with the initial rate (Fig 1D). Whereas the increased initial rate is expected based on deoxyheme concentrations, the mechanistic basis of the observed increase in the rate constant for nitrite-reduction with HBOC-201 deoxygenation remains unclear. We have not measured nitrite-reductase kinetics of native bovine hemoglobin precluding comparison of HBOC-201 with the unmodified (and non-‘T’-state stabilized) control and also note that we cannot exclude an effect of steric effects introduced by glutaraldehyde modification but present these data to underscore the potential to modulate nitrite-reductase activity of any given HBOC by both controlling deoxyheme concentrations (P50)and intrinsic rate constant for nitrite reduction (R vs. T-state character).
Importantly, we also observed NO-formation from the nitrite-deoxyHBOC-201 reaction (Fig 1E and 1F). Recent insights suggest that nitrite-reduction to NO occurs via the intermediate formation of N2O3 secondary to metheme-nitrite reactions with NO[48]. We have not explored this mechanism in this study focusing specifically on the ability of HBOC-201 to produce NO from nitrite and evaluate the potential of this process in mediating vascular NO-dependent signaling. The latter was assessed first using isolated aortic ring bioassays. We employed an experimental design that allows the testing of how the interplay between nitrite, NO and HBOC-201 at different fractional saturations affects nitrite-dependent vasodilation. Consistent with our previous study[24], hHb inhibited MNO-dependent vasodilation at all tested fractional saturations due to rapid heme-based NO-scavenging. These observations were reprised with HBOC-201 supporting NO-scavenging as the primary mechanism by which this modified Hb causes hypertension. With nitrite, hHb inhibited relaxation only at high oxygen fractional saturations with deoxygenated hHb having no effect. We have shown previously that nitrite-dependent vasodilation at all oxygen tensions tested is mediated through NO-formation and interpreted the lack of an inhibitory affect of deoxygenated hHb as evidence for a counterbalancing formation of NO at low oxygen tensions via nitrite-reduction. A similar response is observed with HBOC-201 (Fig 3). Interestingly, we had to employ a modified protocol (bolus-addition) to assess effects of HBOC-201 fractional saturation on nitrite-meditated vasodilation that could be performed without any loss of HBOC-201. When performing cumulative nitrite dose-dependent experiments significant loss of HBOC-201 was noted. Moreover, this effect predominated at high oxygen tensions and was specific to HBOC-201 and nitrite. It is likely that HBOC-201 decomposition occurs secondary to protein denaturation. The latter has been documented especially when Hb undergoes redox cycling reactions that lead to the intermediate population of higher heme oxidation states and free radicals (e.g. ferryl, ferryl associated free radical [3]) that can form inter- and intramolecular cross links and ultimately heme and protein degradation. Likewise, nitrite reaction with oxygenated hemoglobin involves heme redox cycling and formation of protein based radicals[49] and higher nitrogen oxides all of which may contribute to HBOC-201 denaturation. We have not tested this hypothesis here and highlight this denaturation data since clearly any potential interaction that leads to HBOC-201 (or other HBOC) denaturation will not only likely limit therapeutic efficacy of HBOC-201 but may contribute to tissue injury through release of free iron and generation of reactive species. It is important therefore to bring attention to the possibility of a toxic effect of high dose nitrite therapy and HBOC-201, and the potential use of supplemental oxygen in situations when nitrite and HBOC-201 are co-administered. We note however that in our in vivo studies (discussed below) no evidence of HBOC-201 decomposition due to an interaction with nitrite therapy was observed (see Table 1). Moreover, the doses of nitrite used in vivo that attenuated HBOC-201 dependent hypertension are lower than the doses achieved in ex-vivo cumulative dose-dependent vasodilation studies (~100μM final).
Biochemical and ex-vivo vasodilation studies suggested that HBOC-201 mediated nitrite-reduction and NO formation may be harnessed to prevent vasoconstriction in vivo. To test this we used a murine model of trauma, controlled hemorrhage and resuscitation. Consistent with previous reports normovolemic resuscitation with HBOC-201 led to a mild but significant hypertensive response of ~8mmHg. This response remains at the center of the proposed toxicity associated with administration of HBOC's and has thus far largely limited translation of these compounds as front-line resuscitative agents. Normalizing HBOC-201 mediated hypertension remains a key therapeutic goal therefore. We show data that nitrite at low doses (<30nmol) is able to prevent HBOC-201-mediated hypertension. Nitrite may stimulate NO-signaling by hemoglobin independent, tissue dependent mechanisms [28, 50] a role for which cannot be excluded by our data. However, the observation that nitrite (30 nmol) failed to affect MAP with LR resuscitation but did promote a hypotensive effect with HBOC-201 resuscitation suggests a role for nitrite reactions with HBOC-201 in redressing the balance for NO-signaling in vivo.
Nitrite dose response data also demonstrates the presence of a therapeutic window in which nitrite can be administered during HBOC-201 resuscitation which prevents hypertension without causing hypotension, the latter being observed at >100nmol (Fig 6E). Other concerns with the use of nitrite as a therapy include formation of metHb which would compromise oxygen delivery and potentially contribute to heme-based oxidative stress. However, within the nitrite dose range in which hypertension was attenuated without causing hypotension, metHb only increased to 2-3% which importantly was no different to that observed in the absence of nitrite. Also, no differences in metHb formation with nitrite doses of 0-100nmol were observed suggesting that increased metHb formation at the expense of ferrous heme (which rapidly scavenges NO) is unlikely to account for the nitrite-dose dependent attenuation of hypertension. Using 30 nmol, nitrite levels at end of resuscitation were higher than basal but still relatively low (~1μM) and within 2 hr of resuscitation nitrite levels decrease to baseline consistent with a relatively short half-life of nitrite in the mammalian circulation (~10-30min) a property that would further limit potential toxicity of nitrite administration.
In summary we present data that support the use of nitrite as an adjunct therapy to attenuate HBOC-dependent hypertension. We propose that HBOC-mediated nitrite reduction counters NO-scavenging and hypertensive effects. These data further support the notion that the effectiveness of any HBOC as a nitrite reductase in vivo can be affected by either altering the oxygen affinity and/or intrinsic nitrite reductase activity. Relative to other α-chain cross-linked hemoglobins [35], HBOC-201 is a slower nitritereductase (i.e. lower rate constant for nitrite reductase activity) however, we speculate that due to its high P50, sufficient deoxygenation occurs in vivo to sustain significant nitrite reductase activity in vivo. These data underscore the potential flexibility afforded by the ability to influence nitrite reductase activity by altering intrinsic redox potential and / or oxygen affinity maybe critical in the therapeutic development of the next generation of HBOCs.
Supplementary Material
ACKNOWLEDGEMENTS
RPP and JDK are co-inventors on an NIH - UAB provisional patent application for use of nitrite salts with HBOCS.
FUNDING
We acknowledge funds from the Naval Medical Research Center (JK), American Heart Association Grant in Aid (Southeast Affiliate) (RPP) and American Heart Association Predoctoral Fellowship (Southeast Affiliate) (DAV). MV is funded by an institutional NRSA T32 training grant
REFERENCES
- 1.Buehler PW, Alayash AI. All hemoglobin-based oxygen carriers are not created equally. Biochim Biophys Acta. 2008;1784:1378–1381. doi: 10.1016/j.bbapap.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Winslow RM. Cell-free oxygen carriers: scientific foundations, clinical development, and new directions. Biochim Biophys Acta. 2008;1784:1382–1386. doi: 10.1016/j.bbapap.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 3.Alayash AI. Oxygen therapeutics: can we tame haemoglobin? Nat Rev Drug Discov. 2004;3:152–159. doi: 10.1038/nrd1307. [DOI] [PubMed] [Google Scholar]
- 4.Doherty DH, Doyle MP, Curry SR, Vali RJ, Fattor TJ, Olson JS, Lemon DD. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat Biotechnol. 1998;16:672–676. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- 5.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. Jama. 2008;299:2304–2312. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel RP. Biochemical aspects of the reaction of hemoglobin and NO: implications for Hb-based blood substitutes. Free Radic Biol Med. 2000;28:1518–1525. doi: 10.1016/s0891-5849(00)00259-8. [DOI] [PubMed] [Google Scholar]
- 7.Rohlfs RJ, Bruner E, Chiu A, Gonzales A, Gonzales ML, Magde D, Magde MD, Jr., Vandegriff KD, Winslow RM. Arterial blood pressure responses to cell-free hemoglobin solutions and the reaction with nitric oxide. J Biol Chem. 1998;273:12128–12134. doi: 10.1074/jbc.273.20.12128. [DOI] [PubMed] [Google Scholar]
- 8.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. Jama. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 9.Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol. 2002;53:503–514. [PubMed] [Google Scholar]
- 10.Moncada S. Nitric oxide: discovery and impact on clinical medicine. J R Soc Med. 1999;92:164–169. doi: 10.1177/014107689909200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torok J. Participation of nitric oxide in different models of experimental hypertension. Physiol Res. 2008;57:813–825. doi: 10.33549/physiolres.931581. [DOI] [PubMed] [Google Scholar]
- 12.Lancaster JR., Jr. Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc Natl Acad Sci U S A. 1994;91:8137–8141. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lancaster JJ, H A, Kerby JD, Patel RP. The hemoglobin-nitric oxide axis: Implications for transfusion therapeutics. Transfusion alternatives in transfusion medicine. 2007;9:273–280. [Google Scholar]
- 14.Butler AR, Feelisch M. Therapeutic uses of inorganic nitrite and nitrate: from the past to the future. Circulation. 2008;117:2151–2159. doi: 10.1161/CIRCULATIONAHA.107.753814. [DOI] [PubMed] [Google Scholar]
- 15.Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, Cannon RO, 3rd, Kelm M, Wink DA, Espey MG, Oldfield EH, Pluta RM, Freeman BA, Lancaster JR, Jr., Feelisch M, Lundberg JO. The emerging biology of the nitrite anion. Nat Chem Biol. 2005;1:308–314. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- 16.Kevil CG, Patel RP. Preserving vessel function during ischemic disease: new possibilities of inorganic nitrite therapy. Expert Rev Cardiovasc Ther. 2008;6:1175–1179. doi: 10.1586/14779072.6.9.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 18.van Faassen EE, Bahrami S, Feelisch M, Hogg N, Kelm M, Kim-Shapiro DB, Kozlov AV, Li H, Lundberg JO, Mason R, Nohl H, Rassaf T, Samouilov A, Slama-Schwok A, Shiva S, Vanin AF, Weitzberg E, Zweier J, Gladwin MT. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev. 2009 doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calvert JW, Lefer DJ. Myocardial protection by nitrite. Cardiovasc Res. 2009 doi: 10.1093/cvr/cvp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 21.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Jr., Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang KT, Keszler A, Patel N, Patel RP, Gladwin MT, Kim-Shapiro DB, Hogg N. The reaction between nitrite and deoxyhemoglobin. Reassessment of reaction kinetics and stoichiometry. J Biol Chem. 2005;280:31126–31131. doi: 10.1074/jbc.M501496200. [DOI] [PubMed] [Google Scholar]
- 23.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isbell TS, Gladwin MT, Patel RP. Hemoglobin oxygen fractional saturation regulates nitrite-dependent vasodilation of aortic ring bioassays. Am J Physiol Heart Circ Physiol. 2007;293:H2565–2572. doi: 10.1152/ajpheart.00759.2007. [DOI] [PubMed] [Google Scholar]
- 25.McNulty PH, Scott S, Kehoe V, Kozak M, Sinoway LI, Li J. Nitrite consumption in ischemic rat heart catalyzed by distinct blood-borne and tissue factors. Am J Physiol Heart Circ Physiol. 2008;295:H2143–2148. doi: 10.1152/ajpheart.00050.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagababu E, Ramasamy S, Abernethy DR, Rifkind JM. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J Biol Chem. 2003;278:46349–46356. doi: 10.1074/jbc.M307572200. [DOI] [PubMed] [Google Scholar]
- 27.Gladwin MT, Grubina R, Doyle MP. The new chemical biology of nitrite reactions with hemoglobin: R-state catalysis, oxidative denitrosylation, and nitrite reductase/anhydrase. Acc Chem Res. 2009;42:157–167. doi: 10.1021/ar800089j. [DOI] [PubMed] [Google Scholar]
- 28.Alzawahra WF, Talukder MA, Liu X, Samouilov A, Zweier JL. Heme proteins mediate the conversion of nitrite to nitric oxide in the vascular wall. Am J Physiol Heart Circ Physiol. 2008;295:H499–508. doi: 10.1152/ajpheart.00374.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalsgaard T, Simonsen U, Fago A. Nitrite-dependent vasodilation is facilitated by hypoxia and is independent of known NO-generating nitrite reductase activities. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.01298.2006. [DOI] [PubMed] [Google Scholar]
- 30.Luchsinger BP, Rich EN, Yan Y, Williams EM, Stamler JS, Singel DJ. Assessments of the chemistry and vasodilatory activity of nitrite with hemoglobin under physiologically relevant conditions. J Inorg Biochem. 2005;99:912–921. doi: 10.1016/j.jinorgbio.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Modin A, Bjorne H, Herulf M, Alving K, Weitzberg E, Lundberg JO. Nitrite-derived nitric oxide: a possible mediator of ‘acidic-metabolic’ vasodilation. Acta Physiol Scand. 2001;171:9–16. doi: 10.1046/j.1365-201X.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- 32.Vitturi DA, Teng X, Toledo JC, Matalon S, Lancaster J, Jr, Patel RP. Regulation of nitrite transport in red blood cells by hemoglobin oxygen fractional saturation. Am J Physiol Heart Circ Physiol. 2009 doi: 10.1152/ajpheart.01303.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation. 2008;117:1982–1990. doi: 10.1161/CIRCULATIONAHA.107.729137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu B, Volpato GP, Chang K, Bloch KD, Zapol WM. Prevention of the pulmonary vasoconstrcitor effects of HBOC-201 in awake lambs by continuously breathing nitric oxide. Anesthesiology. 2009;110:113–122. doi: 10.1097/ALN.0b013e318190bc4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lui FE, Dong P, Kluger R. Polyethylene glycol conjugation enhances the nitrite reductase activity of native and cross-linked hemoglobin. Biochemistry. 2008;47:10773–10780. doi: 10.1021/bi801116k. [DOI] [PubMed] [Google Scholar]
- 36.Fitzpatrick CM, Biggs KL, Atkins BZ, Quance-Fitch FJ, Dixon PS, Savage SA, Jenkins DH, Kerby JD. Prolonged low-volume resuscitation with HBOC-201 in a large-animal survival model of controlled hemorrhage. J Trauma. 2005;59:273–281. doi: 10.1097/01.ta.0000174730.62338.88. discussion 281-273. [DOI] [PubMed] [Google Scholar]
- 37.Lee R, Neya K, Svizzero TA, Vlahakes GJ. Limitations of the efficacy of hemoglobin-based oxygen-carrying solutions. J Appl Physiol. 1995;79:236–242. doi: 10.1152/jappl.1995.79.1.236. [DOI] [PubMed] [Google Scholar]
- 38.York GB, Eggers JS, Smith DL, Jenkins DH, McNeil JD, Mueller D, Josephs JD, Kerby JD. Low-volume resuscitation with a polymerized bovine hemoglobin-based oxygen-carrying solution (HBOC-201) provides adequate tissue oxygenation for survival in a porcine model of controlled hemorrhage. J Trauma. 2003;55:873–885. doi: 10.1097/01.TA.0000092681.17874.6F. [DOI] [PubMed] [Google Scholar]
- 39.Lang JD, Jr., Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, Liu Y, Jhala N, Crowe DR, Smith AB, Cross RC, Frenette L, Kelley EE, Wilhite DW, Hall CR, Page GP, Fallon MB, Bynon JS, Eckhoff DE, Patel RP. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007;117:2583–2591. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blood AB, Power GG. In vitro and in vivo kinetic handling of nitrite in blood: effects of varying hemoglobin oxygen saturation. Am J Physiol Heart Circ Physiol. 2007;293:H1508–1517. doi: 10.1152/ajpheart.01259.2006. [DOI] [PubMed] [Google Scholar]
- 41.Maher AR, Milsom AB, Gunaruwan P, Abozguia K, Ahmed I, Weaver RA, Thomas P, Ashrafian H, Born GV, James PE, Frenneaux MP. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation. 2008;117:670–677. doi: 10.1161/CIRCULATIONAHA.107.719591. [DOI] [PubMed] [Google Scholar]
- 42.Piknova B, Keszler A, Hogg N, Schechter AN. The reaction of cell-free oxyhemoglobin with nitrite under physiologically relevant conditions: Implications for nitrite-based therapies. Nitric Oxide. 2009;20:88–94. doi: 10.1016/j.niox.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minneci PC, Deans KJ, Shiva S, Zhi H, Banks SM, Kern S, Natanson C, Solomon SB, Gladwin MT. Nitrite reductase activity of hemoglobin as a systemic nitric oxide generator mechanism to detoxify plasma hemoglobin produced during hemolysis. Am J Physiol Heart Circ Physiol. 2008;295:H743–754. doi: 10.1152/ajpheart.00151.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensen FB. Nitric oxide formation from the reaction of nitrite with carp and rabbit hemoglobin at intermediate oxygen saturations. FEBS J. 2008;275:3375–3387. doi: 10.1111/j.1742-4658.2008.06486.x. [DOI] [PubMed] [Google Scholar]
- 45.Blood AB, Tiso M, Verma ST, Lo J, Joshi MS, Azarov I, Longo LD, Gladwin MT, Kim-Shapiro DB, Power GG. Increased nitrite reductase activity of fetal versus adult ovine hemoglobin. Am J Physiol Heart Circ Physiol. 2009;296:H237–246. doi: 10.1152/ajpheart.00601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roche CJ, Dantsker D, Samuni U, Friedman JM. Nitrite reductase activity of sol-gel-encapsulated deoxyhemoglobin. Influence of quaternary and tertiary structure. J Biol Chem. 2006;281:36874–36882. doi: 10.1074/jbc.M603914200. [DOI] [PubMed] [Google Scholar]
- 47.Pearce LB, Gawryl MS. The pharmacology of tissue oxygenation by biopure's hemoglobin-based oxygen carrier, Hemopure (HBOC-201) Adv Exp Med Biol. 2003;530:261–270. doi: 10.1007/978-1-4615-0075-9_25. [DOI] [PubMed] [Google Scholar]
- 48.Basu S, Wang X, Gladwin MT, Kim-Shapiro DB. Chemiluminescent detection of S-nitrosated proteins: comparison of tri-iodide, copper/CO/cysteine, and modified copper/cysteine methods. Methods Enzymol. 2008;440:137–156. doi: 10.1016/S0076-6879(07)00808-7. [DOI] [PubMed] [Google Scholar]
- 49.Keszler A, Piknova B, Schechter AN, Hogg N. The reaction between nitrite and oxyhemoglobin: a mechanistic study. J Biol Chem. 2008;283:9615–9622. doi: 10.1074/jbc.M705630200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Webb AJ, Milsom AB, Rathod KS, Chu WL, Qureshi S, Lovell MJ, Lecomte FM, Perrett D, Raimondo C, Khoshbin E, Ahmed Z, Uppal R, Benjamin N, Hobbs AJ, Ahluwalia A. Mechanisms underlying erythrocyte and endothelial nitrite reduction to nitric oxide in hypoxia: role for xanthine oxidoreductase and endothelial nitric oxide synthase. Circ Res. 2008;103:957–964. doi: 10.1161/CIRCRESAHA.108.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.