Abstract
We generated a novel mouse model, which expresses the tetracycline-inducible transactivator under the regulation of the endogenous whey acidic protein gene. Using a tet-responsive luciferase reporter transgene, we demonstrated that the Wap-rtTA knockin allele allows a tightly controlled temporal and spatial expression of transgenes in the mammary gland in a ligand-inducible manner. The longitudinal analysis of individual females throughout their reproductive cycles using in vivo bioluminescence imaging confirmed that the expression of the Wap-rtTA knockin allele is highly upregulated during lactation. However, the extent of the transcriptional activation of the targeted Wap locus is dependent on the suckling stimulus and milk retrieval. In addition, we used WAP-rtTA/TetO-H2B-GFP double-transgenic females to monitor the presence of GFP-labeled parity-induced mammary epithelial cells (PI-MECs) during the postlactational involution period. The study shows that, unlike their progeny in mammary epithelial transplants as reported previously, PI-MECs themselves may not belong to the long-term label-retaining epithelial subtype.
Keywords: gene targeting, mammary gland development, differentiation, lactation, whey acidic protein, tetracycline-inducible transactivator, in vivo imaging
Introduction
The development of the mammary gland is a complex process in which the gland undergoes dramatic morphological changes following the onset of puberty and during each reproductive cycle. In sexually mature females, hormones and locally synthesized growth factors facilitate the elongation and branching morphogenesis of the epithelial ductal system. The altered hormonal milieu during pregnancy orchestrates the proliferation and differentiation of the milk-producing alveolar cells. After weaning of the offspring, most differentiated alveolar cells undergo apoptosis and are removed from the gland during the postlactational involution period. Using the Cre-lox technology to label differentiating epithelial cells throughout pregnancy and lactation, we found that a significant number of cells that activated the promoter of the milk protein gene Wap permanently reside at the terminal ends of ducts in the involuted gland (Wagner et al., 2002). These cells, that we named parity-induced mammary epithelial cells (PI-MECs), exhibit characteristics of stem cells and serve as alveolar progenitors in subsequent pregnancies (Matulka et al., 2007; Wagner et al., 2005). Since the mammary gland is able to repeatedly evolve through defined developmental stages in each gestation cycle, this organ serves as an excellent model system to study the biology of stem cells and molecular mechanisms that regulate cell proliferation, differentiation, and programmed cell death. A perturbation of the developmental program by endogenous or exogenous factors can lead to breast cancer, which is still one of the leading causes of cancer death in women.
Gene knockout studies in mouse models have demonstrated that prolactin (PRL) signaling though the Janus kinase 2 (Jak2) and the Signal Transducer and Activator of Transcription 5 (Stat5) plays a central role in the development of alveoli and the transcriptional activation of milk protein genes (Wagner et al., 2008). Despite the broad function of PRL in mediating the differentiation process, the expression of particular milk proteins (i.e. caseins and whey proteins) varies slightly during pregnancy. In mice, βcasein gene transcription increases rather early during pregnancy, whereas the whey acidic protein (Wap) gene is sharply upregulated during the last phase of pregnancy (Robinson et al., 1995). Consequently, the expression level of Wap is often used as a marker for an advanced differentiation profile of epithelial cells in the murine mammary gland. The transcriptional activation of Wap is principally restricted to mammary epithelial cells, and regulatory elements of this gene and other milk protein loci have been used in the past to target the expression of exogenous proteins (e.g. pharmaceutically relevant peptides, growth factors, and oncogenes) to the mammary gland (Andres et al., 1987; Gordon et al., 1987). Unlike the long terminal repeat of the mouse mammary tumor virus (MMTV-LTR), whose transcriptional activation can occur in a variety of tissues (Hennighausen et al., 1995; Wagner et al., 1997a), the promoter of Wap is being used, even across species, to confine the expression of transgenes to the mammary gland (Bayna et al., 1990; Ebert et al., 1991; Shamay et al., 1992).
Despite significant progress in the generation and analysis of tissue-specific expression models, there is only a very limited availability of transgenic strains to date that allow a temporally and spatially controlled expression of genes in the mammary gland. A reliable activation of transgenes was achieved in a ligand-inducible manner in mice that express the tetracycline-controlled transactivators (tTA and rtTA) under the MMTV-LTR (Gunther et al., 2002; Hennighausen et al., 1995). Despite differences in their spatial expression profile and a markedly improved transactivation in the MMTV-rtTA strain, the expression of both transactivators under the MMTV-LTR was not confined to the mammary gland. In comparison, the WAP-rtTA transgenic strain, which was previously generated by Utomo and colleagues (1999), exhibited little background activation in other tissues. However, its expression in the mammary gland was mosaic based on a reporter assay that detected the activation of the Cre recombinase, which is co-expressed in these mice in a doxycycline-inducible manner.
In this report we describe the generation of a novel mouse model, in which transgene expression is exclusively targeted to the mammary gland in a ligand-inducible manner. To engineer such a model, we inserted the rtTA sequence into the endogenous Wap locus by homologous recombination in embryonic stem cells. Using in vivo imaging, we performed longitudinal studies on individual WAP-rtTA knockin mice that also carry inducible reporter transgenes to a) verify the correct temporal and spatial activation of the knockin allele, and b) monitor differences in the transcriptional activation of the endogenous Wap locus under variable physiological conditions. Moreover, we used the doxycycline-inducible system to label differentiating cells during pregnancy and lactation and to monitor their presence in the involuted gland. This methodology was used to assess whether parity-induced mammary epithelial cells belong to the long-term label-retaining epithelial subtype.
Results
A tetracycline-inducible transactivator under regulation of the endogenous Wap locus targets the expression of transgenes exclusively to the mammary gland in a ligand-inducible manner
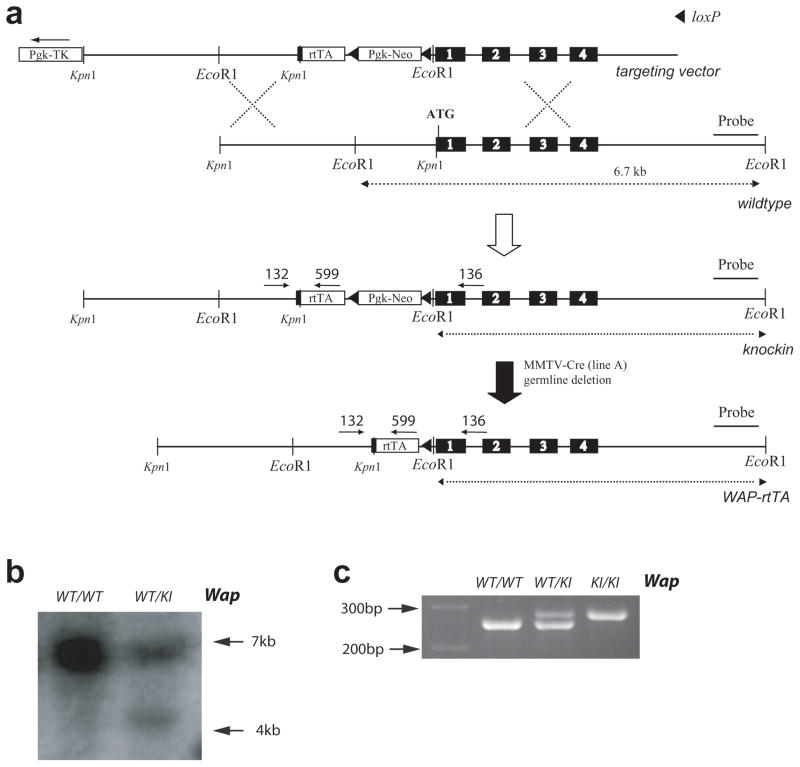
We generated genetically engineered mice with a targeted insertion of the coding sequence of the reverse tetracycline-inducible transactivator (rtTA) into the endogenous Wap gene to regulate the activation of tet-inducible transgenes in a spatially and temporarily controlled fashion in the mammary gland. The construction of the targeting vector and the insertion of the transactivator into the first coding exon of Wap by homologous recombination in embryonic stem cells are illustrated in Fig. 1 and are described in the Materials and Methods section. After transmission of the Wap-rtTA allele through the germline of chimeric animals, we needed to remove the neomycin selectable marker from the targeted Wap locus using Cre-mediated excision. For this purpose, the WAP-rtTA mice were bred with MMTV-Cre (line A) transgenic mice (Wagner et al., 1997a) to generate double transgenic females. Since the Cre recombinase under the regulation of the MMTV-LTR is active in oocytes in this line, the backcross of the WAP-rtTA/MMTV-Cre females to wildtype males allowed a simultaneous removal of the floxed neomycin selectable marker from the targeted Wap-rtTA allele and the segregation of the MMTV-Cre, which was no longer required. The resulting heterozygous WAP-rtTA knockin males and females were fertile and exhibited no phenotypic abnormalities. Female mice were able to lactate, suggesting that the expression of the transactivator protein is not toxic to mammary epithelial cells.
Fig. 1.
Targeted insertion of the rtTA into the endogenous Wap locus. (a) Strategy to knockin the rtTA coding sequence into the first exon of Wap using homologous recombination. The floxed PGK-Neo selectable marker was subsequently excised in the germline of MMTV-Cre females. (b) Southern blot analysis using an EcoR1 restriction digest in combination with a 3′ external probe to verify the correct targeted insertion of the rtTA into the Wap locus. (c) PCR analysis on genomic DNA of tail biopsies to determine the genotype of heterozygous (WT/KI) and homozygous (KI/KI) WAP-rtTA knockin mice as well as wildtype controls.
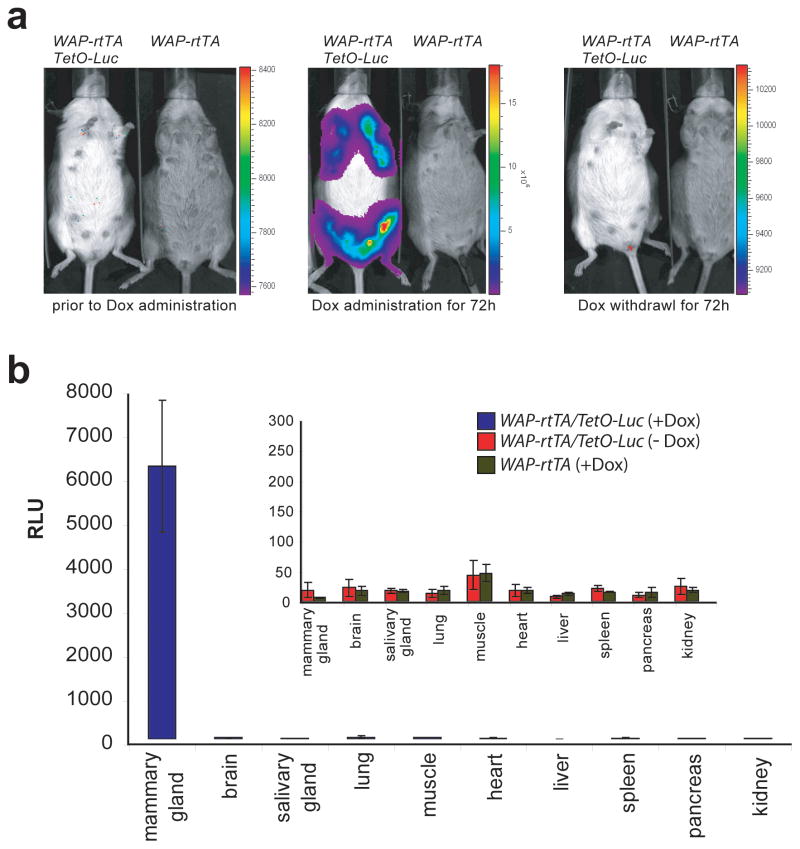
In the first experiment, we wanted to determine whether the Wap-rtTA knockin allele was able to induce the expression of tetracycline-responsive transgenes specifically within the mammary gland. For this purpose, we crossed WAP-rtTA mice with a transgenic strain that carries a luciferase reporter gene under the control of the tetracycline responsive operator (TetO-Luc) (Creamer and Wagner, unpublished). The expression of the reporter transgene was analyzed using bioluminescence imaging (IVIS200, Xenogen, Inc.) on lactating WAP-rtTA/TetO-Luc double transgenic females and their wildtype or single transgenic controls (Fig. 2A). Expression of luciferase was not detected in any of the experimental animals or their controls prior to administration of doxycycline (Dox), which is a highly active derivative of tetracycline (Fig. 2A, left panel). The same animals were subsequently fed Dox in their drinking water for 72 hours (2 mg/ml supplemented with .50 mg/ml sucrose) and examined again for luciferase expression using bioluminescence imaging. A strong activation of the reporter transgene was observed in WAP-rtTA/TetO-Luc double transgenic females but not in their single transgenic controls (Fig. 2A, middle panel). The expression of luciferase was limited to the gross area of the mammary glands, and the highest level of reporter gene activation was detectable around the nipples that contained less hair. Following the second imaging analysis, the medicated water was replaced with regular drinking water to assess the downregulation of the reporter transgene after withdrawal of doxycycline. Three days later, the animals were imaged a third time (Fig. 2A, right panel), and the expression of luciferase was not detected in the mammary glands of control mice or double transgenic females that previously exhibited a very high activation of the reporter during the treatment period with the antibiotic.
Fig. 2.
Mammary gland-specific expression of the Wap-rtTA allele. (a) In vivo bioluminescence imaging of the same lactating WAP-rtTA/TetO-Luc double transgenic female and her WAP-rtTA single transgenic control prior to and during doxycycline (Dox) administration (left and center) as well as 72h after Dox withdrawal (right). (b) Conventional luciferase assay to monitor the expression of the TetO-Luc reporter transgene in a panel of ten organs from lactating WAP-rtTA/TetO-Luc females (Dox treated and untreated) as well as WAP-rtTA single transgenic controls (N=3 of each genotype). RLU, relative light units corrected by the protein concentration, error bars represent +/− SEM.
After confirming the temporal activation of the reporter transgene in response to Dox administration, we examined the spatial expression of the luciferase reporter in order to validate the mammary-specific transcriptional activation of the Wap-rtTA knockin allele. A panel of ten organs from Wap-rtTA/TetO-Luc females treated with Dox and their untreated, double transgenic controls as well as single transgenic mice on medicated drinking water was taken on lactation day 5. Protein lysates of these tissue panels from at least three animals per experimental group were analyzed using a conventional luciferase assay. The light units obtained were normalized to the protein concentration of each individual sample. The results of this study shown in Fig. 2B demonstrate that the ligand-inducible activation of the reporter transgene occurred only in mammary tissues of Dox-treated, double transgenic females. There was no significant expression of luciferase in any other organ, and the emitted relative light units (RLU) were basically identical to those observed in Dox-treated animals that did not carry a reporter transgene. Since WAP-based transgenic strains generated by pronuclear injection can exhibit some expression of the transgene in other organs such as the brain depending on the random integration site (Wagner et al., 1997a), it is obvious that the targeted knockin approach results in a superior spatial activation of transgenes in the mammary gland with virtually no background expression in other tissues.
Collectively, the results of this initial study demonstrate that the WAP-rtTA knockin allele allows a tightly controlled temporal and spatial expression of transgenes in the mammary gland in a ligand-inducible manner. There was no leakiness in the activation of the reporter transgene in the absence of Dox, and we did not observe a transcriptional activation of the Wap-rtTA allele in any other organ besides the mammary gland.
The expression of the WAP-rtTA knockin allele is confined to mammary epithelial cells
The transcriptional activation of the endogenous Wap locus is known to occur mainly in luminal epithelial cells of the mammary gland. To confirm the correct spatial expression of the Wap-rtTA allele in this epithelial subtype, we crossed the knockin mice with a transgenic reporter strain that expresses a fusion protein of the green fluorescent protein (GFP) and the human histone H2B protein under the control of a tetracycline-responsive promoter element (TetO-H2B-GFP) (Tumbar et al., 2004). Beginning from day 14 of pregnancy, we administered Dox continuously to a subset of WAP-rtTA/TetO-H2B-GFP females, and a mammary biopsy of one inguinal gland #4 was taken on day 10 of lactation. Lactating double transgenic mice that were not fed Dox were used as negative controls. The mammary biopsies were analyzed under a fluorescent stereoscope equipped with an FITC filter set, and only the tissues from the experimental mice that were treated with Dox exhibited an intensive labeling of cell nuclei throughout the entire mammary gland (data not shown). Next, these tissues were fixed in buffered formalin and sectioned. Using immunofluorescence staining with antibodies against GFP as well as cytokeratin 8 and 14, we confirmed that the expression of the Wap-rtTA allele is confined to the mammary epithelium (Fig. 3). As anticipated, GFP-stained nuclei were almost exclusively present in the cytokeratin 8-positive (i.e. luminal) epithelial subtype (Fig. 3A). A careful examination of high-resolution pictures also revealed very few GFP-positive nuclei were also present within cells that exhibited staining of cytokeratin 14 (i.e. myoepithelial cells or basal cells that might serve as progenitors of luminal epithelial cells).
Fig. 3.
Expression of the Wap-rtTA allele is confined to mammary epithelial cells. (a) Immunofluorescent staining of the luminal epithelial cell marker cytokeratin-8 (CK8) and the H2B-GFP fusion protein in lactating mammary glands of WAP-rtTA/TetO-H2B-GFP double transgenic females either treated or not treated with doxycycline (bar represents 50 μm). (b) Immunofluorescent staining of the basal cell marker cytokeratin-14 (CK14) and the H2B/GFP fusion protein in lactating mammary glands of WAP-rtTA/TetO-H2B-GFP females either treated or not treated with doxycycline (bar represents 50 μm).
Longitudinal analysis of WAP-rtTA-mediated reporter gene expression during mammary gland differentiation
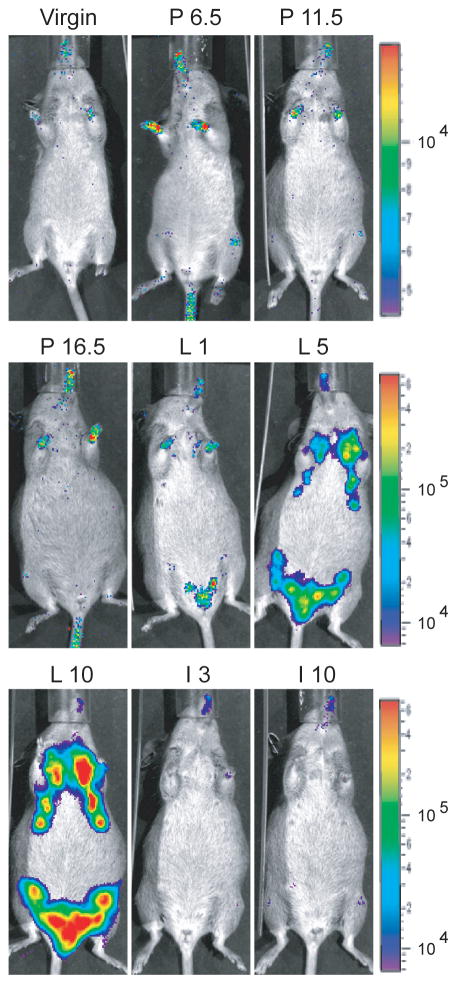
Although the expression of the whey acidic protein gene is highly upregulated during pregnancy, Robinson and co-workers (1995) have shown that the Wap locus is transcriptionally active in a subset of alveolar progenitors during estrus in sexually mature, non-pregnant females. We anticipated that the newly generated Wap-rtTA knockin allele follows the expression profile of the endogenous Wap gene, but we also learned from previous targeting experiments that minor genetic alterations in the proximity of endogenous promoters can lead to unexpected consequences in the expression profile of a gene (Rucker, III et al., 2000). To assess whether the transcriptional activation of the Wap-rtTA knockin allele occurs in the correct developmental fashion, we performed a longitudinal study on individual WAP-rtTA/TetO-Luc mice and their controls during their first gestation cycle using bioluminescence imaging (Fig. 4). Wildtype or single transgenic females as well as double transgenic animals without administration of Dox did not express luciferase at any given time point during pregnancy and lactation (data not shown). Only WAP-rtTA/TetO-Luc females that were fed doxycycline during the entire course of the study exhibited measurable luciferase activity during lactation (Fig. 4, L1–L10). The expression of the luciferase reporter gene increased steadily during the lactation period beyond day 10 post partum (data not shown). Upon weaning of the young, the ligand-inducible expression of luciferase decreased rapidly, and by the third day of involution, the expression of the reporter was undetectable (Fig. 4, I3 and I10).
Fig. 4.
Longitudinal analysis of the Wap-rtTA-mediated activation of the TetO-Luc reporter transgene in the mammary gland at various stages of the first gestation cycle using in vivo bioluminescence imaging. The animal was fed doxycycline throughout this experiment. V, virgin; P6.5 through P16.5, pregnancy day 6.5 through day 16.5 post coitus; L1 through L10, lactation day 1 through day 10; I3 and I10, involution day 3 and day 10.
Using bioluminescence imaging, we determined that the regulation of the Wap-rtTA knockin allele occurs correctly in the anticipated manner during the gestation cycle. Nonetheless, the overall expression was less than expected, in particular, during the late pregnancy and at parturition when the endogenous Wap locus is upregulated. It is reasonable to suggest that a) a ligand-inducible expression might exhibit some delay in a reporter assay, and b) a portion of the photons emitted from the luciferase are absorbed in the skin and hair that could lead to a reduced sensitivity of the bioluminescence imaging. In addition, we considered the possibility that the transcriptional activation of the knockin allele is lower compared to the endogenous Wap locus. To address this issue, we performed a semi-quantitative RT-PCR assay on mammary tissues from lactating heterozygous (WT/KI) and homozygous (KI/KI) WAP-rtTA mice (Fig. 5A and 5B). The PCR primers used in this assay amplify exons 3 and 4 of the spliced Wap mRNA that are transcribed in both the wildtype locus and the knockin allele (Fig. 5A). The results shown in Fig. 5B demonstrate that in a homozygous configuration (KI/KI), the expression of the targeted alleles is reduced compared to heterozygous females that carry one wildtype allele of Wap (WT/KI). This observation suggests that the presence of a cDNA and a remaining loxP site within the 5′ UTR of the first exon led to a reduction in the expression of the targeted Wap allele.
Fig. 5.
Transcriptional activation of the targeted Wap-rtTA locus and the wildtype Wap gene. (a) RT-PCR strategy. (b) RT-PCR assay to determine the transcriptional activation of the wildtype and targeted Wap-rtTA knockin loci in both heterozygous (WT/KI) and homozygous (KI/KI) knockin mice. A WAP-rtTA knockin mouse that still carries the neomycin cassette between the WAP promoter and the rtTA (neo/neo) was used as an additional control. (c) Immunofluorescence staining of the Wap protein (green) in lactating mammary glands of heterozygous and homozygous WAP-rtTA knockin mice. Conventional WAP knockout mice (KO/KO) served as negative controls. The histological sections were counterstained with DAPI (blue). Bar, 50 μm.
In addition to the transcriptional analysis, we used the tissues from the heterozygous and homozygous WAP-rtTA knockin mice to examine the expression of the Wap protein in these glands. The immunostaining with an antibody against the whey acidic protein shows that the knockin of the rtTA sequence immediately upstream of the ATG translation initiation codon of Wap leads to a knockout of the protein (Fig. 5C). This is not problematic since a) the Wap protein is not required for mammary gland development and differentiation (Triplett et al., 2005), and b) one Wap-rtTA allele is sufficient to drive a ligand-inducible expression of transgenes as demonstrated in figures 2 through 4.
Gene transcription on demand: The transcriptional activation of the Wap locus is greatly modified by the extend of suckling and milk retrieval
During the bioluminescence analyses of Dox-treated WAP-rtTA/TetO-Luc double transgenic females, we noticed a dramatic variation in the intensity of emitted photons between lactating females. We observed that dams with smaller litter sizes exhibited a reduced expression of luciferase. To examine this phenomenon in a controlled setting, we measured the luciferase activity of two WAP-rtTA/TetO-Luc females on lactation day 5 during their first two gestation cycles. The litter sizes of both females were adjusted for 8 and 3 pups, respectively. The same females received the inverse number of pups during the second lactation period. The results of this experiment shown in Fig. 6A clearly demonstrate that the litter size has a dramatic impact on the transcriptional activation of the Wap locus. A more intense suckling stimulus associated with milk retrieval leads to a significantly higher expression of this milk protein gene. Interestingly, the focal expression of luciferase in individual mammary glands of dams with fewer offspring might indicate that pups have a selective preference for particular glands.
Fig. 6.
The transcriptional activation of the Wap locus is greatly modified by the extent of suckling and milk retrieval. (a) In vivo bioluminescence imaging on two WAP-rtTA/TetO-Luc females on lactation day 5 during their first two gestation cycles. The litter sizes of both females were adjusted for 8 and 3 pups, respectively. (b) Decline in milk protein gene expression in individual mammary glands in response to milk stasis. The nipples of the left #3, #4 and #5 mammary glands were sealed at day 10 of lactation. Bioluminescence images were taken immediately before closing the glands and 72 hours later.
The Wap locus is a prime target of prolactin signaling through the Jak2/Stat5 pathway (Liu et al., 1997; Wagner et al., 2004). A decrease in circulating levels of this hormone is associated with the initiation of programmed cell death during the postlactational involution period. Upon weaning of the young, the expression of milk protein genes declines, and this process precedes the collapse of the alveolar architecture shortly thereafter (Li et al., 1996; Lund et al., 1996). Several studies have shown that intrinsic factors in each of the individual mammary glands are activated in response to milk stasis. These factors are able to initiate pro-apoptotic signals and inhibit the activation of Stat5 even in the presence of high levels of circulating lactogenic hormones (Li et al., 1997; Wagner et al., 1997b). To assess whether we can monitor the decline in milk protein gene expression in individual mammary glands in response to milk stasis, we sealed the nipples of the left #3, #4 and #5 mammary glands at day 10 of lactation. Bioluminescence images were taken immediately before closing the glands and 72 hours later (Fig. 6B). Within this short time period, the expression of luciferase declined significantly in the sealed glands but not in the other seven glands that were suckled. This observation indicates that we are able to indirectly measure over a time course the steady-state expression level of the Wap locus in individual mammary glands using bioimaging.
Collectively, the experiments with alternating litter sizes and the locally induced milk stasis demonstrate that the steady-state transcriptional activity of the Wap locus is a complex process that involves the action of hormones and intrinsic factors, mechanical stimuli as well as the extent of milk withdrawal from individual mammary glands.
A small subset of alveolar cells retains labeled chromosomes during the involution period
Our previous studies have suggested that parity-induced mammary epithelial cells (PI-MECs) contain a subpopulation of cells with characteristics of multipotent progenitors (Matulka et al., 2007; Wagner et al., 2002). Upon transplantation, some of the progeny of the self-renewing PI-MECs become label-retaining epithelial cells in mammary ducts (Smith, 2005). Consequently, we asked whether a few PI-MECs themselves, and not just their progeny in transplants, belong to the small population of label-retaining epithelial cells. To address this question, we used the Dox-regulated expression of the H2B-GFP fusion protein described by Tumbar et al. (2004) in combination our WAP-rtTA mice to visualize label-retaining cells in the mammary gland.
In an initial experiment, we examined the dynamics of nuclear staining using the H2B-GFP fusion protein. Dox was administered to WAP-rtTA/TetO-H2B-GFP double transgenic females on day 10 of lactation for five days. Mammary gland biopsies taken prior to and during the Dox treatment were analyzed under a fluorescent stereoscope. Subsequently, paraffin embedded sections of these tissues were stained with an antibody against GFP (Fig. 7A). While GFP was undetectable in the mammary gland prior to Dox treatment at day 10 of lactation, the ligand-inducible expression of the H2B-GFP fusion protein resulted in a strong nuclear staining of many secretory epithelial cells on lactation day 15. Since alveolar cells rarely proliferate at such an advanced stage of lactation, this observation suggests that the H2B-GFP fusion protein is able to translocate to the nucleus independently of cell division.
Fig. 7.
A small subset of Wap-rtTA-expressing cells is retained in the involuting mammary gland. (a,b) Immunofluorescent staining of the H2B-GFP fusion protein (green) in lactating mammary tissue (L10 and L15) of WAP-rtTA/TetO-H2B-GFP females. DAPI (blue) was used as a counter stain. Bar, 50 μm. The timing of Dox treatment is illustrated in the upper panel (c) Analysis of nuclear H2B-GFP staining on freshly isolated mammary tissue using a fluorescence stereoscope. The female was treated with Dox during pregnancy until day 3 of lactation, followed by forced involution and Dox removal for 14 days. Bar, 50 μm.
In a second experiment, we examined the nuclear incorporation of the H2B-GFP fusion protein during late pregnancy and early lactation when a significant subset of alveolar cells still proliferates. WAP-rtTA/TetO-H2B-GFP females were fed Dox during pregnancy and until day 10 of lactation. After performing a mammary gland biopsy, Dox was withdrawn for five days, and a second mammary gland was taken from the euthanized animal. After staining paraffin-embedded sections of these glands with GFP, we noticed that on day 15 of lactation a significant number of GFP-labeled nuclei were still present in cells that no longer expressed the TetO-H2B-GFP transgene (Fig. 7B). This observation suggests that after the initial pulse, the nuclear labeling is retained in secretory alveolar cells of the lactating gland prior to remodeling during the postlactational involution period.
In the last experiment, we wanted to determine whether labeled alveolar cells were still present after completion of the postlactational involution period. We induced the expression of the TetO-H2B-GFP reporter transgene throughout pregnancy and until day 3 of lactation in several double transgenic females. After performing mammary gland biopsies, Dox was withdrawn and the pups were removed from their mothers. The remaining mammary glands were analyzed 14 and 21 days later. The analysis of freshly isolated tissues under the fluorescent stereoscope (Fig. 7C) and the subsequent immunofluorescent staining of GFP on formalin-fixed sections (not shown) revealed that there was still a small number of epithelial cells with GFP-labeled nuclei present in the mammary glands 14 days and three weeks after weaning of the pups. We were however unable to unambiguously identify GFP-labeled cells four weeks after Dox removal and forced involution (data not shown). This observation might suggest that PI-MECs themselves do not belong to the long-term label-retaining epithelial subtype, which comprises less than 2% of all mammary epithelial cells (Booth et al., 2006).
Discussion
A WAP-rtTA-mediated and ligand-inducible activation of transgenes is superior to the generation of conventional transgenic mice carrying transgenes under regulation of milk protein gene promoters
For over two decades, the promoter of the Wap gene has been used by a number of investigators to target the expression of growth regulators and oncogenes as well as pharmaceutically relevant proteins specifically to the mammary gland of transgenic rodents and farm animals. A successful generation of Wap-based transgenic lines that are activated in the correct spatial and temporal manner, however, can be cumbersome. While approximately one half of the founder lines express the transgene to some extent in gland, there is generally a strong variation in the expression level and the timing of transgene activation between the lines. Depending on the random integration site, the expression of Wap promoter-driven transgenes can often be detected in other organs. For example, only one out of eight WAP-Cre transgenic lines that we generated previously (Wagner et al., 1997a) expressed the recombinase specifically in the developing mammary gland with negligible expression in the brain. The other seven lines exhibited significant Cre activity in other tissues including testes. In this report, we describe the generation of knockin mice that express the reverse tetracycline-inducible transactivator under the regulation of the endogenous Wap gene. The Wap-rtTA allele mediates a stringently controlled temporal and spatial expression of reporter transgenes (luciferase and GFP) in the mammary gland in a ligand-inducible manner. The preliminary analysis of more than eight newly generated founder lines from three different tet-regulated constructs shows that, unlike conventional Wap-based transgenes, the expression of TetO-driven genes are less dependent on the random integration site (Creamer, Lin, Zhang, and Wagner; unpublished). In fact, all these lines express their transgene in a ligand-dependent manner in tissues where the transactivator is present. This includes primary cell lines derived from embryonic tissues that were subsequently infected with a retroviral vector expressing the tTA. This strategy for screening expressing lines is significantly faster than the generation and analysis of lactating females that carry regulatory elements of WAP or other milk protein gene promoters. Hence, the availability of WAP-rtTA mice makes the generation of conventional WAP transgenics obsolete. In addition, tet-inducible transgenes have the advantage that they can be utilized with other rtTA/tTA-expressing strains to elucidate biological functions of genes at distinct stages of organogenesis or in different cell types of the same organ or in other tissues. WAP-rtTA mice will be particularly valuable to examine the function of genes in differentiating alveolar cells of the mammary gland during pregnancy and lactation. The widespread expression of the Wap-rtTA allele throughout the lactating mammary gland will also allow the examination of genes that play a role in the remodeling of the mammary gland during the involution period. Although we were unable to detect the expression of reporter genes in virgin WAP-rtTA mice at various stages of the estrus cycle using a conventional luciferase assay (data not shown), crosses of this strain with transgenic lines that express oncogenes in a tet-regulatable manner will show whether or not the WAP-rtTA is expressed in a small subset of hormone-dependent alveolar precursors in nulliparous females as suggested previously (Robinson et al., 1995).
Changes in the transcriptional activation of the Wap locus in response to external stimuli and their implications for studying mammary gland development
Our experiments with alternating litter sizes and the locally induced milk stasis in WAP-rtTA mice revealed a number of interesting findings that have significant implications in the design of experimental studies that are aimed to address the functional differentiation of the mammary gland. First, the transcriptional activation of Wap still increases with the nutritional demand of the growing young beyond day 10 of lactation. This finding is in agreement with our previous observation on Wap-deficient mice that showed that this milk protein is particularly important for the nourishment of the offspring during the second half of lactation (Triplett et al., 2005). Thus far, in most published reports, the expression levels of Wap were determined almost exclusively at parturition or within the first ten days of lactation to assess the degree of functional differentiation of the gland. Hence, the true potential for the transcriptional activation of Wap remains greatly underestimated in these studies. Second, it is generally known that fewer pups thrive better on a female as assessed by their average weight gain due to increased milk consumption. Nonetheless, the litter size had a profound impact on the transcriptional activation of the Wap locus. This finding emphasizes the need for adjusting the number of pups suckling on a dam before comparing the expression of Wap and possibly other genes as markers for functional differentiation. Most published studies using wildtype or genetically engineered mice, including our own, were often not stringently controlled for the litter size. Third, the ability to indirectly measure over a time course the steady-state expression level of the Wap locus in individual mammary glands using bioimaging opens new avenues for analyzing functional differentiation. This methodology could be used to examine in real-time mammary gland development in mutant strains that exhibit precocious or delayed alveolar proliferation and differentiation. As demonstrated in the sealed nipple experiment, this method could also be employed to monitor the effects of growth factors and their downstream mediators on the remodeling of the gland during the involution period (i.e. delayed or precocious involution).
The vast majority of parity-induced mammary epithelial cells (PI-MECs) do not belong to the long-term label-retaining epithelial subtype
Using a H2B-GFP fusion protein, Kanda and colleagues (1998) labeled nucleosomes to monitor the dynamics of chromosomes in cultured cells. Subsequently, Tumbar et al. (2004) expressed this fusion protein in mice in a doxycycline-regulated manner (TetO-H2B-GFP) to visualize label-retaining cells in the stem cell niche of the skin. Similar to using BrdU or tritium-labeled thymidine in a pulse chase experiment, the H2B-GFP fusion protein is chased out of the chromosomes of dividing cells that no longer express the TetO-H2B-GFP transgene. Consequently, cells that divide infrequently will retain the label over an extended period. Although the precise mechanism for the label retention is still under debate (i.e. slow cycling cells versus the immortal strand theory), this type of pulse chase experiment using the ligand-inducible TetO-H2B-GFP transgene could be adapted to virtually any type of tissue including the mammary epithelium to mark progenitor cells. In this study, we used the newly generated WAP-rtTA mice to label PI-MECs to assess whether a subset of these cells are able to retain the H2B-GFP protein in their nucleosomes for an extended period. The fusion protein remained present in nuclei of mammary epithelial cells at day 15 of lactation following a timed tet-inducible activation of the TetO-H2B-GFP transgene during pregnancy and the first ten days of lactation. This prolonged retention of the GFP label might suggests that alveolar cells that express endogenous Wap are still capable of DNA replication and incorporation of the H2B-GFP protein into nucleosomes. The premise that proliferation and the initiation of functional differentiation are not entirely separate events is supported by the fact that a subset of proliferating cells can still be found in the lactating gland several days after parturition (Wagner et al., 1997b). Nonetheless, we also consider the possibility that unincorporated H2B-GFP could have a prolonged turnover rate compared to luciferase, which was virtually absent after 72 hours of Dox withdrawal (Fig. 2A). Unlike the simplified portrayal of the H2B-GFP labeling method of progenitors during DNA replication (Blanpain et al., 2007), a fraction of the nuclear H2B-GFP fusion protein might also be incorporated into the nucleus without cell division in vitro (Brennand et al., 2007). The initial experiment, in which we induced the expression of the TetO-H2B-GFP transgene during mid-lactation (day 10–15), revealed that the fusion protein was able to translocate to the nucleus independently of cell proliferation. Previously, Kimura and Cook (2001) used GFP-labeled variants of the core histones to study their kinetics in HeLa cells. They found that the inner core of the nucleosome is relatively stable, whereas H2B on the surface of active nucleosomes exchanges continually, in particular in transcriptionally active chromatin. If this is correct, it might be feasible to hypothesize that a fraction of the nuclear H2B-GFP protein is also incorporated into chromosomes without cell division in mammary epithelial cells of WAP-rtTA/TetO-H2B-GFP mice at lactation day 15. The extent of a constitutive exchange of histone proteins in differentiated mammary epithelial cells in vivo remains to be elucidated. While this process could impose some technical limitations in discriminating proliferating from non-proliferating cells, it might be less important for the staining of long-term label-retaining cells.
Following the Dox-inducible expression of the H2B-GFP protein during pregnancy and early lactation, we were able to detect a small number of epithelial cells with GFP-labeled nuclei in the involuted mammary gland 14 days and three weeks after Dox withdrawal and weaning of the pups. This experiment provides additional experimental evidence for the existence of PI-MECs in the involuted gland using an entirely different approach. However, the tet-system appears to be less sensitive, and unlike the Cre/lox technology, it does not allow a permanent labeling of PI-MECs and their descendants. Then again, the tet-system provided the opportunity to address the question whether PI-MECs belong to the small population of label-retaining epithelial cells that comprise approximately 2% of the entire mammary epithelium. According to Booth and Smith (2006), these cells are asymmetrically cycling mammary stem/progenitor cells, and a significant subset of them expresses nuclear steroid receptors. Since we were unable to unambiguously identify GFP-labeled cells four weeks after Dox removal and forced involution, we conclude that the vast majority of PI-MECs may not belong to the label-retaining epithelial subtype. In the involuted gland, PI-MECs normally reside at the terminal end of ducts and alveolar units, which is a highly proliferative compartment that undergoes constant changes with each estrus cycle (i.e. approximately every four days in a mouse). While PI-MECs may not be slow cycling cells, they still possess properties of multipotent progenitors as demonstrated in transplantation studies (Matulka et al., 2007; Wagner et al., 2002).
Materials and Methods
Generation of genetically engineered mice with a Wap-rtTA knock-in allele (Waptm2(rtTA)Kuw; MGI:3799057)
The source for the genomic DNA of the Wap gene was described previously (Triplett et al., 2005). The coding sequence of the rtTA was inserted into the KpnI site located in the 5′ UTR of Wap. A 2.4 kb fragment of the promoter and the rtTA were subcloned into the pLoxpNeo targeting vector that contained a PGK-TK cassette and a floxed PGK-Neo selectable marker. A 3.7 kb KpnI/DraI fragment, which included all four exons of Wap, served as the 3′ homology region. The targeting vector was linearized using NotI and electroporated into RW-4 cells. The selection and expansion of ES cell clones was performed by the UNMC Mouse Genome Engineering Core Facility. A detailed Southern blot procedure using a 3′ external probe to detect targeted ES cell clones was described previously (Triplett et al., 2005). The EcoRI Southern blot analysis yielded two distinct bands of 6.7 kb (wildtype allele) and 4.7 kb in size (knock-in allele). After screening 98 ES cell clones, we identified 24 correctly targeted clones (approximately 25%). Two correctly targeted ES cell clones (7A3 and 7C1) were injected into C57/Bl6 blastocycts for the production of chimeric mice. After verification of the transmission of the Wap-rtTA construct to the next generation, the targeted allele of the founder line 7A3 was transmitted through the germline of MMTV-Cre (line A) transgenic females to remove the PGK-Neo selectable marker.
Genotyping Protocols
A PCR assay using three primers (132 5′-TAG AGC TGT GCC AGC CTC TTC -3′; 136 5′-GTT CTC CAA GCC ACA CCC GG -3′; and 599 5′-GCC AAT ACA GTG TAG GCT GC -3′) was developed to genotype mice that carry one or two Wap-rtTA knock-in alleles. The amplicon for the knockin allele (primers 132 and 599) is approximately 290 bp, whereas the presence of a wildtype Wap locus (primers 132 and 136) produces a band of approximately 250 bp (Fig. 1C). The TetO-Luc transgenic mice were genotyped using primer sets that amplify a 313 bp fragment of the luciferase gene (1965 5′-TGG AAC CGC TGG AGA GCA ACT G-3′ and 1966 5′-GTA GGC TGC GAA ATG TTC ATA CTG-3′). TetO-H2B-GFP transgenic mice (Tumbar et al., 2004) were obtained from the Jackson Laboratory (stock # 005104). All animals used in the studies were treated humanely and in accordance with federal guidelines and institutional policies.
Luciferase reporter assays
The expression and activity of the luciferase reporter gene was determined using in vivo bioluminescence imaging (IVIS200, Caliper Life Sciences, Alameda, CA). According to the manufacture’s recommendations, luciferin (1 mg D-luciferin potassium salt in 0.2 ml 1× PBS) was injected intraperitoneally ten minutes prior to the imaging procedure. The mice were kept under anesthesia (isoflurane) during the acquisition of the images that were collected at intervals ranging from ten seconds to four minutes. To assess the expression of the luciferase reporter gene in various organs, we collected a panel of ten tissues from three lactating females of each experimental group (see Fig. 2B). The tissues were snap-frozen in liquid nitrogen and homogenized using a pre-chilled porcelain mortar and pestle. We used the Dual Luciferase Assay Kit (Promega, Madison, WI) and a Perkin Elmer 1420 Victor3V luminometer (Perkin Elmer, Waltham, MA) to measure the light units in each individual sample. Concurrently, a Bradford assay was used to determine the protein concentrations and to normalize the relative light units (RLU) accordingly.
Immunohistochemistry
The immunofluorescent staining of cytokeratins 8 and 14 as well as the whey acidic protein on mammary gland tissues was performed as described previously (Matulka et al., 2007; Triplett et al., 2005). We used a primary antibody from Aves Labs, Inc (Tigard, OR) to label the GFP in histological sections (chicken anti-GFP, GFP-1020, 1:500 dilution). An Alexa Fluor 488-conjugated goat anti-chicken secondary antibody from Invitrogen (A11039, 1:1000 dilution) was used to visualize the GFP. Images of histological slides were taken on a Zeiss AxioImager microscope (Carl Zeiss, Inc., Germany) equipped with a SPOT FLEX camera (Diagnostic Instruments, Inc., Sterling Heights, MI).
RT-PCR for rtTA and Wap mRNA
The isolation of RNA and the synthesis of cDNA was described previously (Wagner et al., 2004). To specifically amplify the transcript from the WAP-rtTA knockin allele by PCR, we used primers that span the 3′ end of the rtTA (412 5′-CGC TAG ACG ATT TCG ATC TGG -3′) and exon 3 of the targeted Wap gene (1869 5′-TGA CAC GGT CGA CGT TGC AGC -3′). The PCR primers located in exons 3 and 4 of Wap were able to detect transcripts that originated from the wildtype and the targeted Wap locus (1836 5′-TGG AAT CTA CTC CAA ACG ATC AG -3′ and 1834 5′-CTT GCT GTA TAG ACT TGG GTG GT -3′).
Acknowledgments
We thank the members of the UNMC Mouse Genome Engineering Core Facility for maintaining embryonic stem cell clones and for performing the blastocysts injections. This work was supported, in part, by the Public Health Service grant CA117930 from the National Cancer Institute. Additional financial support provided to K.U.W. by the Nebraska Research Initiative was imperative to finance the generation and maintenance the genetically engineered mice. B.A.C. received a graduate fellowship through the UNMC Cancer Research Training Program that is funded by a grant from the National Cancer Institute (CA009476). Currently, B.A.C. receives a Program of Excellence Graduate Assistantship through the UNMC Graduate Studies Office.
Abbreviations
- Cre
Cre-recombinase
- Jak2
Janus kinase 2
- MEC
mammary epithelial cell
- MMTV-LTR
long terminal repeat of the mouse mammary tumor virus
- Neo
neomycin
- PGK
phosphoglycerate kinase
- PI-MEC
parity-induced mammary epithelial cell
- PRL
prolactin
- rtTA
reverse tetracycline-controlled transactivator
- Stat5
signal transducer and activator of transcription 5
- TetO
tetracycline-responsive operator
- TK
thymidine kinase
- WAP
whey acidic protein
Footnotes
Author contributions: B.A.C. and K.-U.W. designed research; B.A.C., A.A.T. and K.-U.W. performed research and analyzed data; B.A.C. and K.-U.W. wrote the paper.
The authors declare no conflict of interest.
Literature Cited
- Andres AC, Schonenberger CA, Groner B, Hennighausen L, LeMeur M, Gerlinger P. Ha-ras oncogene expression directed by a milk protein gene promoter: tissue specificity, hormonal regulation, and tumor induction in transgenic mice. Proc Natl Acad Sci U S A. 1987;84:1299–1303. doi: 10.1073/pnas.84.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayna EM, Rosen JM. Tissue-specific, high level expression of the rat whey acidic protein gene in transgenic mice. Nucleic Acids Res. 1990;18:2977–2985. doi: 10.1093/nar/18.10.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007;128:445–458. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth BW, Smith GH. Estrogen receptor-alpha and progesterone receptor are expressed in label-retaining mammary epithelial cells that divide asymmetrically and retain their template DNA strands. Breast Cancer Res. 2006;8:R49. doi: 10.1186/bcr1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand K, Huangfu D, Melton D. All beta Cells Contribute Equally to Islet Growth and Maintenance. PLoS Biol. 2007;5:1520–1529. doi: 10.1371/journal.pbio.0050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert KM, Selgrath JP, DiTullio P, Denman J, Smith TE, Memon MA, Schindler JE, Monastersky GM, Vitale JA, Gordon K. Transgenic production of a variant of human tissue-type plasminogen activator in goat milk: generation of transgenic goats and analysis of expression. Biotechnology. 1991;9:835–838. doi: 10.1038/nbt0991-835. [DOI] [PubMed] [Google Scholar]
- Gordon K, Lee E, Vitale JA, Smith AE, Westphal H, Hennighausen L. Production of human tissue plasminogen activator in transgenic mouse milk. Biotechnology. 1987;24:425–428. [PubMed] [Google Scholar]
- Gunther EJ, Belka GK, Wertheim GB, Wang J, Hartman JL, Boxer RB, Chodosh LA. A novel doxycycline-inducible system for the transgenic analysis of mammary gland biology. FASEB J. 2002;16:283–292. doi: 10.1096/fj.01-0551com. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Wall RJ, Tillmann U, Li M, Furth PA. Conditional gene expression in secretory tissues and skin of transgenic mice using the MMTV-LTR and the tetracycline responsive system. J Cell Biochem. 1995;59:463–472. doi: 10.1002/jcb.240590407. [DOI] [PubMed] [Google Scholar]
- Kanda T, Sullivan KF, Wahl GM. Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr Biol. 1998;8:377–385. doi: 10.1016/s0960-9822(98)70156-3. [DOI] [PubMed] [Google Scholar]
- Kimura H, Cook PR. Kinetics of core histones in living human cells: little exchange of H3 and H4 and some rapid exchange of H2B. J Cell Biol. 2001;153:1341–1353. doi: 10.1083/jcb.153.7.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Hu J, Heermeier K, Hennighausen L, Furth PA. Apoptosis and remodeling of mammary gland tissue during involution proceeds through p53-independent pathways. Cell Growth Differ. 1996;7:13–20. [PubMed] [Google Scholar]
- Li M, Liu X, Robinson G, Bar-Peled U, Wagner KU, Young WS, Hennighausen L, Furth PA. Mammary-derived signals activate programmed cell death during the first stage of mammary gland involution. Proc Natl Acad Sci U S A. 1997;94:3425–3430. doi: 10.1073/pnas.94.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- Lund LR, Romer J, Thomasset N, Solberg H, Pyke C, Bissell MJ, Dano K, Werb Z. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development. 1996;122:181–193. doi: 10.1242/dev.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matulka LA, Triplett AA, Wagner KU. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol. 2007;303:29–44. doi: 10.1016/j.ydbio.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Robinson GW, McKnight RA, Smith GH, Hennighausen L. Mammary epithelial cells undergo secretory differentiation in cycling virgins but require pregnancy for the establishment of terminal differentiation. Development. 1995;121:2079–2090. doi: 10.1242/dev.121.7.2079. [DOI] [PubMed] [Google Scholar]
- Rucker EB, III, Dierisseau P, Wagner KU, Garrett L, Wynshaw-Boris A, Flaws JA, Hennighausen L. Bcl-x and Bax regulate mouse primordial germ cell survival and apoptosis during embryogenesis. Mol Endocrinol. 2000;14:1038–1052. doi: 10.1210/mend.14.7.0465. [DOI] [PubMed] [Google Scholar]
- Shamay A, Pursel VG, Wilkinson E, Wall RJ, Hennighausen L. Expression of the whey acidic protein in transgenic pigs impairs mammary development. Transgenic Res. 1992;1:124–132. doi: 10.1007/BF02528777. [DOI] [PubMed] [Google Scholar]
- Smith GH. Label-retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development. 2005;132:681–687. doi: 10.1242/dev.01609. [DOI] [PubMed] [Google Scholar]
- Triplett AA, Sakamoto K, Matulka LA, Shen L, Smith GH, Wagner KU. Expression of the whey acidic protein (Wap) is necessary for adequate nourishment of the offspring but not functional differentiation of mammary epithelial cells. Genesis. 2005;43:1–11. doi: 10.1002/gene.20149. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utomo AR, Nikitin AY, Lee WH. Temporal, spatial, and cell type-specific control of Cre-mediated DNA recombination in transgenic mice. Nat Biotechnol. 1999;17:1091–1096. doi: 10.1038/15073. [DOI] [PubMed] [Google Scholar]
- Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development. 2002;129:1377–1386. doi: 10.1242/dev.129.6.1377. [DOI] [PubMed] [Google Scholar]
- Wagner KU, Krempler A, Triplett AA, Qi Y, George NM, Zhu J, Rui H. Impaired alveologenesis and maintenance of secretory mammary epithelial cells in Jak2 conditional knockout mice. Mol Cell Biol. 2004;24:5510–5520. doi: 10.1128/MCB.24.12.5510-5520.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KU, Rui H. Jak2/Stat5 signaling in mammogenesis, breast cancer initiation and progression. J Mammary Gland Biol Neoplasia. 2008;13:93–103. doi: 10.1007/s10911-008-9062-z. [DOI] [PubMed] [Google Scholar]
- Wagner KU, Smith GH. Pregnancy and stem cell behavior. J Mammary Gland Biol Neoplasia. 2005;10:25–36. doi: 10.1007/s10911-005-2538-1. [DOI] [PubMed] [Google Scholar]
- Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, Li M, Furth PA, Hennighausen L. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 1997a;25:4323–4330. doi: 10.1093/nar/25.21.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KU, Young WS, Liu X, Ginns EI, Li M, Furth PA, Hennighausen L. Oxytocin and milk removal are required for post-partum mammary-gland development. Genes and Function. 1997b;1:233–244. doi: 10.1046/j.1365-4624.1997.00024.x. [DOI] [PubMed] [Google Scholar]