Abstract
There are no analytical methods that simultaneously quantify nicotine, cotinine, trans-3’-hydroxycotinine, nornicotine and norcotinine in human meconium. Such a method could improve identification of in utero tobacco exposure, determine if maternal dose-meconium concentration relationships exist, and whether nicotine meconium concentrations predict neonatal outcomes. The first liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry method for simultaneous quantification of these analytes in meconium was developed and validated. Specimen preparation included homogenization, enzyme hydrolysis and solid phase extraction. The linear range was 1.25 or 5 – 500 ng/g. Method applicability was evaluated with meconium collected from an in utero tobacco exposed infant.
Keywords: meconium, nicotine, cotinine, trans-3-hydroxycotinine, tobacco
INTRODUCTION
Tobacco use by pregnant women is a serious public health concern. Decreased fetal growth, impaired brain development, and increased risk of nicotine dependence in adulthood have been associated with in utero nicotine exposure.[1–3] In adults, most nicotine is converted in the liver to cotinine which is further metabolized to trans-3’-hydroxycotinine (OH-cotinine).[4] Nicotine, cotinine and OH-cotinine are conjugated to form nicotine-N-glucuronide, cotinine-N-glucuronide and OH-cotinine-O-glucuronide.[4] Demethylation of nicotine and cotinine are minor metabolic pathways.[4] Nicotine, cotinine, OH-cotinine, nornicotine, norcotinine and respective glucuronides account for 74 – 99% of a nicotine dose excreted in an adult smoker’s urine.[4] Nicotine readily crosses the placenta with less than 1% converted to cotinine.[5] No published reports describe the placental transfer of cotinine, OH-cotinine, nornicotine or norcotinine or the fetus’ metabolic capabilities, although cotinine has been detected in the placenta, amniotic and coelomic fluids.[6,7]
Several neonatal matrices are available for monitoring in utero nicotine exposure, including hair, urine, plasma and meconium. Correlations between maternal and neonatal nicotine and/or cotinine concentrations have been observed in hair, urine and plasma.[8–10] Meconium has several advantages for monitoring prenatal drug exposure including an easy, non-invasive collection and a wide window of detection. Meconium starts accumulating around twelve weeks gestational age and is generally passed within the first few days after birth. Though testing procedures exist for many illicit and therapeutic drugs, few methods exist for nicotine and metabolite analysis in meconium, perhaps because of its complex composition and analytical challenges. Most commonly, cotinine is measured by immunoassay.[11,12] Ostrea and colleagues describe a qualitative gas chromatography/mass spectrometry (GC/MS) method for cotinine and OH-cotinine in meconium; however, no specimen tested positive for OH-cotinine and limits of quantification were not provided.[13] Cotinine, but not nicotine, was detected in meconium analyzed for nicotine, cotinine and caffeine with high performance liquid chromatography-diode array (HPLC/DAD).[14]
We describe for the first time, a liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry (LC-APCI-MS/MS) method for simultaneous nicotine, cotinine, OH-cotinine, norcotinine and nornicotine analysis. This method will be useful in describing nicotine and metabolites disposition in meconium and for studies correlating analyte concentrations to neonatal outcome measures.
EXPERIMENTAL SECTION
Meconium
Meconium specimens screening less than 10 ng/g for cotinine by immunoassay were pooled and mixed thoroughly. Prior to use as calibrators or controls, pools were confirmed negative for nicotine and metabolites at the method’s lower limit of quantification (LOQ). To demonstrate method applicability, a meconium specimen was collected from a prenatally tobacco-exposed neonate whose mother was enrolled in an institutional review board-approved protocol.
Reagents and Standards
(R,S)-Norcotinine, (3’R, 5’S)-OH-cotinine, OH-cotinine-D3, (R,S)-norcotinine-D4, (R,S)-nornicotine-D4, OH-cotinine-O-D-glucuronide, cotinine-N-glucuronide, and nicotine-N-glucuronide were purchased from Toronto Research Chemicals (North York, Ontario, Canada). (−)-Nicotine, (±)-nornicotine, ammonium acetate, formic acid, and β-glucuronidase, Type IX-A from Escherichia coli were obtained from Sigma (St. Louis, MO, USA). (−)-Cotinine, (±)-cotinine-D3, (±)-nicotine-D4 were acquired from Cerilliant (Austin, TX, USA). Water, acetonitrile, potassium phosphate dibasic, potassium phosphate monobasic, sodium acetate, hydrochloric acid, dichloromethane, 2-propanol, and ammonium hydroxide were purchased from J.T. Baker (Philipsburg, NJ, USA). Methanol was obtained from Fisher Chemical (Pittsburgh, PA, USA). All solvents and reagents were HPLC or ACS grade. CleanScreen solid phase extraction columns, part ZSDAU020, were purchased from United Chemical Technologies (Bristol, PA, USA).
Instrumentation
MS/MS analysis was performed using a MDS Sciex API 3200 QTrap® triple quadrupole/linear ion trap mass spectrometer with an APCI source (Applied Biosystems, Foster City, CA, USA). The HPLC system consisted of Shimadzu LC-20AD pumps and SIL-20AC autosampler (Columbia, MD, USA). Analyst software version 1.4.1 was used for acquisition and data analysis. During specimen preparation, sonication was performed by a Branson 3510 Ultrasonicator (Danbury, CT). SPSS 13.0 for Windows (Chicago, IL, USA) was used for statistical analyses.
Preparation of Standard Solutions
Powdered standards were reconstituted to a known concentration using the manufacturer’s recommended solvent. A 125 µg/mL stock solution in methanol was stored at −20°C. Dilutions of stock solution in methanol created 25 – 10,000 ng/mL working solutions. Final standard concentrations in meconium were 1.25, 2.5, 5, 7.5, 50, 100, 350 and 500 ng/g when fortifying 0.5 g meconium with 25 µL working standards.
Quality control solutions were prepared in a similar manner, but from different manufacturer lots than for calibrators. A 100 µg/mL stock solution in methanol was stored at −20°C and diluted to produce 160, 1,600, and 8,000 ng/mL working solutions corresponding to 8 (low), 80 (medium), and 400 ng/g (high) quality control concentrations when fortifying 0.5 g meconium with 25 µL working quality control solutions.
Deuterated analogs of each analyte were combined and diluted with methanol to 12,500 ng/mL, creating the internal standard stock solution. A 1,000 ng/mL working internal standard solution was prepared by diluting stock solution with methanol. The final deuterated internal standard concentration after fortifying 0.5 g meconium with 25 µL working solution was 50 ng/g.
Procedures
Specimen Preparation
Blank or authentic meconium (0.5 ± 0.01 g) was weighed into a 15 mL polypropylene tube. Twenty-five µL of standard or quality control solution (if appropriate) and 25 µL internal standard solution were added. For homogenization, 2 mL methanol with 0.01% formic acid (w/v) were added; specimens were vortexed vigorously and sonicated 1 h, vortexing every 10 min. Specimens were centrifuged at 8,000g for 10 min. The supernatant was transferred to a round bottom screw-top tube and evaporated to dryness under nitrogen at 40°C. The residue was reconstituted in 1 mL 0.1 M potassium phosphate buffer, pH 6.8. β-Glucuronidase prepared in 0.1 M potassium phosphate buffer was added to a final concentration of 5,000 Units/mL phosphate buffer. Specimens were incubated for 18 h at 37°C in a reciprocating water bath, and centrifuged quickly for 1 min at 1,000g. Two mL of 2 M sodium acetate buffer, pH 5.5 were added prior to solid phase extraction.
Solid Phase Extraction. CleanScreen DAU solid phase extraction columns were conditioned with 3 mL methanol, 3 mL water and 2 mL 2 M sodium acetate buffer, pH 5.5. Specimens were loaded and allowed to flow by gravity alone. Columns were washed with 2 mL water, dried 1 min, washed with 1.5 mL 0.2 M aqueous hydrochloric acid, dried 5 min, washed with 2 × 1 mL methanol, and dried 5 min. Analytes were eluted with freshly prepared 6 × 1 mL dichloromethane: 2-propanol: ammonium hydroxide (78:20:2, v/v/v). Eluates were dried under nitrogen at 40°C after the addition of 100 µL 1% hydrochloric acid in methanol (v/v). Samples were reconstituted in 200 µL methanol with 0.01% formic acid (w/v) and transferred to glass autosampler vials.
Mass Spectrometry
Atmospheric pressure chemical ionization operating in positive mode was used for all analytes. Compound-specific optimization of MS/MS parameters was performed via direct infusion of 100 ng/mL reference solution in methanol using a syringe pump. Optimization results for the two most abundant ion transitions per analyte in multiple reaction monitoring scan mode (MRM) are given in Table 1. Source parameters were set to 30 psi curtain gas, 35 psi auxiliary gas, 50 psi nebulizer gas, medium collision gas, 3.0 µA nebulizer current, and 550°C source temperature after flow injection analysis source optimization. Quadrupoles one and three were set to unit resolution for period one and low for period two to increase sensitivity.
Table 1.
Tandem Mass Spectrometry Parameters for Nicotine and Metabolites in Meconium
| Period 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Q1 Mass (amu) |
Q3 Mass (amu) |
Dwell Time (msec) |
DPa (volts) |
Entrance Potential (volts) |
CEPb (volts) |
Collision Energy (volts) |
CXPc (volts) |
|
| OH-cotinined | 193.2 | 80.2* | 150 | 46 | 8.5 | 14.00 | 35 | 8 |
| 193.2 | 134.1 | 150 | 46 | 8.5 | 14.00 | 25 | 8 | |
| OH-cotinine-D3 | 196.2 | 80.2* | 150 | 46 | 12.0 | 14.00 | 39 | 6 |
| 196.2 | 134.0 | 150 | 46 | 12.0 | 14.00 | 27 | 6 | |
| Cotinine | 177.2 | 80.0* | 150 | 41 | 3.0 | 12.00 | 37 | 4 |
| 177.2 | 98.2 | 150 | 41 | 3.0 | 12.00 | 25 | 4 | |
| Cotinine-D3 | 180.2 | 80.0* | 150 | 36 | 3.0 | 14.00 | 31 | 4 |
| 180.2 | 101.2 | 150 | 36 | 3.0 | 4.00 | 27 | 4 | |
| Norcotinine | 163.2 | 80.2* | 150 | 51 | 10.0 | 10.00 | 33 | 6 |
| 163.2 | 118.1 | 150 | 41 | 10.0 | 10.00 | 29 | 6 | |
| Norcotinine-D4 | 167.2 | 84.2* | 150 | 46 | 10.5 | 12.00 | 33 | 6 |
| 167.2 | 139.2 | 150 | 46 | 10.5 | 12.00 | 27 | 6 | |
| Nornicotine | 149.2 | 80.2* | 150 | 36 | 4.5 | 12.00 | 27 | 4 |
| 149.2 | 130.1 | 150 | 36 | 4.5 | 12.00 | 25 | 4 | |
| Nornicotine-D4 | 153.25 | 134.1 | 150 | 36 | 4.5 | 14.43 | 27 | 4 |
| 153.25 | 84.1* | 150 | 36 | 4.5 | 14.43 | 29 | 4 | |
| Period 2 | ||||||||
| Nicotine | 163.2 | 84.2* | 250 | 36 | 4.5 | 12.00 | 29 | 8 |
| 163.2 | 132.0 | 250 | 36 | 5.5 | 12.00 | 21 | 6 | |
| Nicotine-D4 | 167.2 | 136.0* | 250 | 36 | 6.0 | 10.00 | 21 | 4 |
| 167.2 | 121.2 | 250 | 36 | 6.0 | 10.00 | 35 | 6 | |
Denotes quantifier transition
Declustering Potential
Collision Entrance Potential
Cell Exit Potential
trans-3’-Hydroxycotinine
Liquid Chromatography
Separation was achieved using a Synergi Polar RP column (150 × 2.0 mm, 4 µm) attached to a guard column of the same packing material. Column temperature was maintained at 30°C and flow rate was set to 0.4 mL/min. Mobile phases were (A) 0.01 M ammonium acetate, pH 6.8 and (B) acetonitrile with 0.01% formic acid (w/v). Gradient conditions were as follows: 15% B for 1 min, increased to 40% over 2 min, increased to 95% over 1 min, decreased to 80% over 0.5 min, held at 80% for 1.5 min, decreased to 15% over 4 min and re-equilibrated at 15% for 2 min. Autosampler temperature was set to 15°C and 20 µL were injected.
Data analysis
Calibration by deuterated internal standardization was performed using simple least squares regression with 1/x weighting. Peak area ratios of target analytes and respective internal standards were calculated at each concentration. The most abundant transition for each analyte was used for quantification; the second transition served as a qualifier (Table 1).
Validation
Specificity, sensitivity, linearity, intra- and inter-day precision and accuracy, recovery, matrix effect, hydrolysis efficiency, carryover, interference and analyte stability were evaluated. Specificity was assessed by relative retention time and quantifier and qualifier transition peak area ratios. Transition peak area ratios for quality control and participant specimens were required to be within ± 20% of the average calibrator transition peak area ratio. Sensitivity was determined by the limits of detection (LOD) and LOQ. The LOD and LOQ were empirically determined as the lowest concentration with a signal to noise ratio of at least 3:1 and 10:1 for the quantifier transition, respectively. Linearity was evaluated using a least square regression line calculated from all standard concentrations and expressed by the squared correlation coefficient (R2). Individual calibrator concentrations were recalculated against the full calibration curve and were required to be within 20% of target. In addition, the R2 value for the full curve was required to be greater than 0.98 for each analyte on every analysis.
Accuracy and precision were assessed using three quality control concentrations spanning the dynamic linear range (8, 80 and 400 ng/g). Intra-day precision and accuracy were determined on five replicates of each concentration. Inter-day precision and accuracy were evaluated for five replicates analyzed on four separate days (n=20). Imprecision was expressed as % relative standard deviation (RSD) of the calculated concentrations; ANOVA analysis was performed to assess the effect of day of analysis on precision. Accuracy was defined as % relative error from target concentration. To accommodate the complex composition of meconium, acceptable inaccuracy was extended from 20% to 25% of target concentration.
Analytical recovery and matrix effect were evaluated using the three set system described by Matuszewski et al. [15]. The first set consisted of meconium samples fortified with quality control and internal standard solutions prior to solid phase extraction. Set 2 was meconium specimens fortified with quality control and internal standard solutions after solid phase extraction. The third set was quality control and internal standard solutions prepared “neat” in mobile phase. Analytical recovery, expressed as a percentage, was calculated by dividing average peak areas from Set 1 and Set 2. Matrix effect was calculated by dividing the average peak area from Set 2 by Set 3; the value was converted to a percentage and subtracted from 100 to represent the amount of signal suppressed by the presence of matrix. To evaluate the effect of different sources of meconium on matrix effects and analyte quantification, negative meconium from six neonates were fortified with low quality control and internal standard solutions after solid phase extraction. As in the previous determination of matrix effect, peak area ratios from the six specimens were compared to five low quality control and internal standard solutions prepared neat. The % RSD of the calculated concentrations was also determined. To evaluate whether hydrolysis affects free analytes, internal standards or matrix effect, quality control concentrations were analyzed in triplicate with and without the addition of β-glucuronidase. Average peak areas of hydrolyzed and nonhydrolyzed specimens were compared. Hydrolysis efficiency was assessed by preparing glucuronidated quality control solutions containing molar equivalents of 8, 80 and 400 ng/g free nicotine, cotinine and OH-cotinine. One low, medium and high glucuronidated quality control specimen was analyzed per run and calculated concentrations of free drug were compared to target concentration.
Acceptable carryover was defined as no quantifiable transition peaks in a solvent injection immediately following a specimen containing two times the upper limit of quantification (ULOQ). Potential interferences with illicit and common therapeutic drugs and minor tobacco alkaloids, anabasine and anatabine, were evaluated by adding compounds into low quality control specimens. A compound did not interfere if the low quality control quantified within ±20% of target, had stable retention time and transition ratios within ±20% of the average calibrator transition ratio. Potential interfering compound concentrations were chosen to be approximately two times the highest meconium concentration reported in the literature. If no data were available, 1,000 ng/g was chosen. Minor tobacco alkaloids anabasine and anatabine concentrations in urine are 1 – 2 orders of magnitude below nicotine concentrations; therefore, 100 ng/g of each compound was chosen for the interference experiment.[16,17] Table 2 includes the compounds and concentrations tested in the interference study. Analyte stability in meconium was evaluated at each quality control concentration under three conditions: 24 h at room temperature, 72 h at 4°C, and three freeze-thaw cycles at −20°C. To ensure proper incorporation of analytes, quality control solution was pipetted into the 15 mL polypropylene tube, meconium added, and tubes centrifuged. On the day of analysis, internal standard was added to each tube before homogenization, and specimens were analyzed as usual. Calculated concentrations of stability specimens were compared to quality control specimens prepared on the day of analysis. Autosampler stability was assessed by reinjecting quality control specimens after 24 h and comparing calculated concentrations to original values.
Table 2.
Compounds Evaluated for Potential Interference with Nicotine and Metabolites in Meconium by LC-APCI-MS/MS
| Concentration (ng/g) | Compounds |
|---|---|
| 100 | Anabasine, anatabine |
| 1,000 | Δ9Tetrahydrocannabinol, 11-hydroxy-Δ9−tetrahydrocannabinol, 11-nor-9-carboxy-Δ9-tetrahydrocannabinol, diazepam, lorazepam, oxazepam, alprazolam, nitrazepam, flunitrazepam, temazepam, nordiazepam, clonidine, ibuprofen, pentazocine, caffeine, diphenhydramine, chlorpheniramine, brompheniramine, acetylsalicylic acid, acetaminophen, phencyclidine |
| 2,000 | Amphetamine, methamphetamine |
| 3,000 | Morphine, normorphine, morphine-3-glucuronide, morphine-6-glucuronide, codeine, norcodeine, 6-acetylmorphine, acetylcodeine, hydrocodone, hydromorphone, oxycodone, noroxycodone, oxymorphone, noroxymorphone |
| 10,000 | Cocaine, benzoylecgonine, norcocaine, norbenzoylecgonine, ecgonine ethyl ester, ecgonine methyl ester, anhydroecgonine methyl ester, ecgonine, cocaethylene, norcocaethylene, meta-hydroxycocaine, para-hydroxycocaine, meta-hydroxybenzoylecgonine, para-hydroxybenzoylecgonine |
Beside standard laboratory practices, no additional safety precautions were required for this procedure.
RESULTS
Calibration and Validation
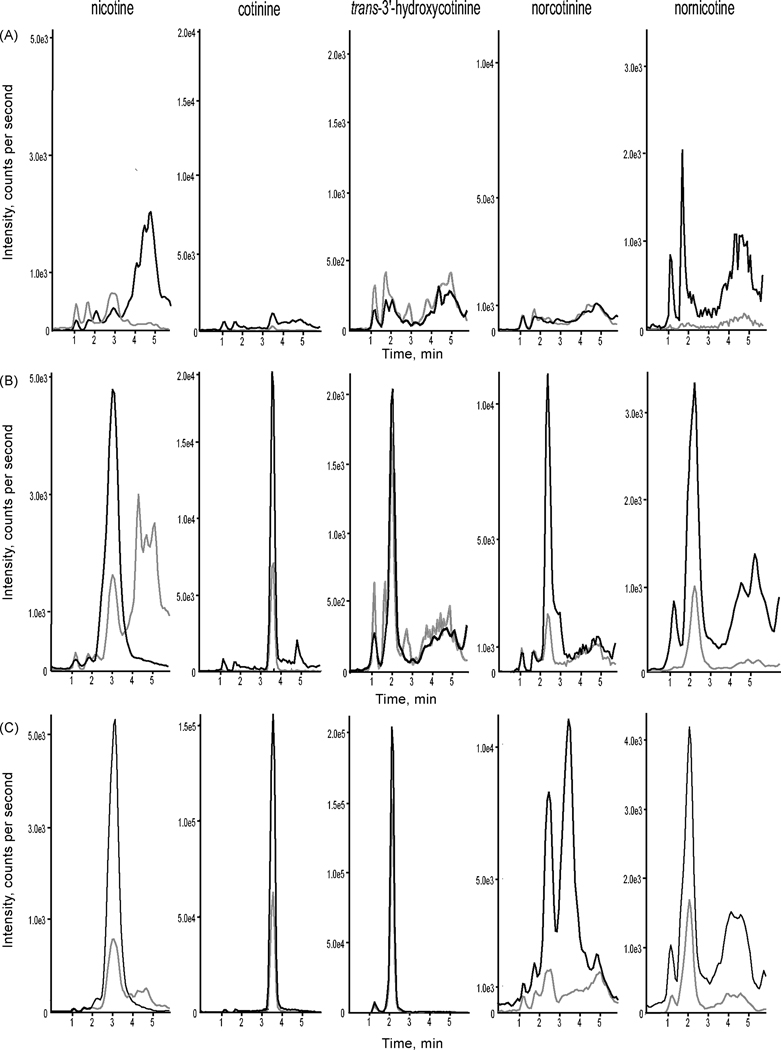
Calibration using deuterated internal standardization was possible for each analyte. Representative chromatograms of extracted blank meconium, low quality control and a positive participant specimen are shown in Figure 1. All compounds eluted within 6 min and each chromatographic run was completed in 12 min. Norcotinine and nicotine have the same molecular weight (162 amu) and produce similar transitions, making chromatographic resolution necessary.
Figure 1.
Representative ion chromatograms. Multiple reaction monitoring (MRM) scans of (A) blank meconium, (B) a low quality control specimen fortified with all analytes at 8 ng/g, (C) meconium from a tobacco in utero exposed neonate containing 196.8 ng/g trans-3’-hydroxycotinine, 94.7 ng/g cotinine, 4.4 ng/g norcotinine, 10.2 ng/g nornicotine, and 101.4 ng/g nicotine. Quantitative and qualitative transitions are designated by black and gray lines, respectively.
LODs for nicotine, cotinine, OH-cotinine, and norcotinine were 1.25 ng/g and 5 ng/g for nornicotine. LOQs for cotinine, OH-cotinine, and norcotinine were 1.25 ng/g and 5 ng/g for nicotine and nornicotine; the upper limit of linearity was 500 ng/g for all analytes. A least squares regression line with a 1/x weighting factor was constructed for each analyte with R2 >0.99. All calibrators quantified within 20% of target concentration.
Precision and accuracy were assessed at 8, 80 and 400 ng/g; Table 3 contains intra- and inter-day imprecision and accuracy data for each concentration. Intra-day imprecision (RSD) was less than 10% for all analytes (N=5). An ANOVA study comparing analyte concentrations grouped by day of analysis showed statistically significant differences (P<0.05) for analytes at low, medium and high concentrations, except OH-cotinine and cotinine at 80 ng/g; maximum F3,16 values at low, medium, and high concentrations were 71.4, 37.9, and 15.1. However, these differences were less than 25% from target and considered clinically insignificant. Inter-day imprecision (N=20) was less than 19.9% for each compound. Accuracy was within 20% of target, except for OH-cotinine, norcotinine, and nornicotine at medium and high concentrations that were within 25% of target.
Table 3.
Nicotine and Metabolites in Meconium by LC-APCI-MS/MS: Imprecision and Accuracy
| Intra-day imprecision (% RSDa, N=5) |
Inter-day imprecision (% RSD, N=20) |
Accuracy (% of target, N=20) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Low | Medium | High | Low | Medium | High | Low | Medium | High | |
| OH-cotinineb | 7.8 | 7.4 | 5.5 | 16.6 | 5.5 | 5.2 | 84.7 | 77.6 | 77.0 |
| Cotinine | 5.8 | 7.4 | 5.1 | 19.9 | 5.8 | 5.2 | 88.0 | 97.2 | 94.2 |
| Norcotinine | 7.7 | 3.9 | 3.9 | 9.8 | 5.3 | 4.5 | 82.3 | 77.8 | 76.6 |
| Nornicotine | 16.9 | 5.5 | 3.9 | 19.8 | 7.8 | 5.9 | 90.5 | 79.0 | 77.8 |
| Nicotine | 10.7 | 6.7 | 6.8 | 10.8 | 7.4 | 6.8 | 105.6 | 93.4 | 93.2 |
Relative standard deviation
trans-3’-Hydroxycotinine
Mean recovery of all analytes ranged from 56.2 – 95.7% (Table 4). Table 4 displays ion suppression caused by matrix effect; values ranged from 40.9 – 63.9% for low and 16.7 – 40.4% for medium and high concentrations evaluated in one meconium source. Mean matrix effect in six different meconium pools ranged from 41.0 – 72.7% at 8 ng/g analyte and 50 ng/g internal standard concentrations. The % RSD for low quality control analyte concentrations fortified in six meconium sources was 11%. These data demonstrate that the method is effective at precisely quantifying nicotine, cotinine, OH-cotinine, nornicotine and norcotinine in this highly complex matrix. Average hydrolysis efficiencies for OH-cotinine-O-glucuronide, cotinine-N-glucuronide and nicotine-N-glucuronide were 15, 80 and 90%, respectively. Peak area differences of less than 20% were detected between hydrolyzed and nonhydrolyzed specimens. No analyte peaks were detected in methanol injected after assaying a specimen containing two times the upper limit of quantification. Common therapeutic and illicit drugs and minor tobacco alkaloids did not interfere with nicotine and metabolites; low quality control concentrations were within 20% of target and transition ratios were within 20% of the average calibrator transition ratio. Analyte stability under four conditions was assessed (Table 5). Losses of less than 15% were observed for storage for 72 h at 4°C and for 24 h at 15°C autosampler storage. Room temperature storage and repeated freeze-thaw cycles should be avoided, as stability ranged from 73.9 – 106.0% of freshly extracted specimens.
Table 4.
Nicotine and Metabolites in Meconium by LC-APCI-MS/MS: Recovery and Matrix Effect
| Recovery (%, N=5) |
Matrix effect (% of signal suppressed, N=5) |
|||||
|---|---|---|---|---|---|---|
| Low | Medium | High | Low | Medium | High | |
| OH-cotininea | 60.8 | 73.8 | 76.6 | 53.8 | 23.8 | 32.5 |
| OH-cotinine-D3b | 56.2 | 69.5 | 67.9 | 57.8 | 28.2 | 32.4 |
| Cotinine | 84.1 | 89.0 | 95.7 | 56.3 | 33.4 | 32.7 |
| Cotinine-D3 | 75.2 | 87.1 | 81.3 | 59.2 | 31.3 | 28.9 |
| Norcotinine | 78.2 | 83.3 | 89.4 | 53.0 | 19.9 | 28.1 |
| Norcotinine-D4 | 76.1 | 80.3 | 81.0 | 55.0 | 30.4 | 32.5 |
| Nornicotine | 86.0 | 74.2 | 72.9 | 40.9 | 16.8 | 20.2 |
| Nornicotine-D4 | 61.2 | 65.8 | 64.1 | 61.2 | 36.6 | 33.6 |
| Nicotine | 64.8 | 82.8 | 79.4 | 63.9 | 30.1 | 40.4 |
| Nicotine-D4 | 57.7 | 75.8 | 71.7 | 59.5 | 21.0 | 24.9 |
trans-3’-Hydroxycotinine
Deuterated internal standard concentrations were 50 ng/g.
Table 5.
Nicotine and Metabolites in Meconium by LC-APCI-MS/MS: Stability Data
| % of freshly extracted quality control concentrations (N=5) | ||||||
|---|---|---|---|---|---|---|
| 24 h room temperature | 72 h 4°C | |||||
| Low | Medium | High | Low | Medium | High | |
| OH-cotininea | 103.2 | 76.2 | 98.4 | 87.2 | 96.6 | 103.6 |
| Cotinine | 97.4 | 86.8 | 95.8 | 94.8 | 93.2 | 104.4 |
| Norcotinine | 106.8 | 79.5 | 94.0 | 102.2 | 95.0 | 102.4 |
| Nornicotine | 90.1 | 74.5 | 93.6 | 89.6 | 88.0 | 96.5 |
| Nicotine | 97.6 | 74.5 | 97.3 | 94.1 | 99.6 | 119.0 |
| 3 freeze thaw cycles | 24 h 15°C autosampler | |||||
| Low | Medium | High | Low | Medium | High | |
| OH-cotinine | 106.0 | 82.3 | 97.2 | 91.2 | 100.6 | 101.1 |
| Cotinine | 100.4 | 85.3 | 94.0 | 100.2 | 103.2 | 100.1 |
| Norcotinine | 97.9 | 83.4 | 94.1 | 96.2 | 100.3 | 102.5 |
| Nornicotine | 97.8 | 73.9 | 88.1 | 87.9 | 103.0 | 108.3 |
| Nicotine | 75.1 | 97.1 | 120.4 | 102.9 | 98.4 | 101.6 |
trans-3’-Hydroxycotinine
APPLICATION OF METHOD
To demonstrate method applicability, a prenatally tobacco-exposed neonate’s meconium specimen was analyzed. The specimen contained 196.8 ng/g OH-cotinine, 94.7 ng/g cotinine, 4.4 ng/g norcotinine, 10.2 ng/g nornicotine, and 101.4 ng/g nicotine; corresponding chromatograms are displayed in Figure 1. The neonate’s mother reported smoking 1 to 5 cigarettes per day throughout pregnancy.
DISCUSSION
This validated method is the first to simultaneously quantify nicotine, cotinine, OH-cotinine, nornicotine and norcotinine in meconium. Previous investigators identified only cotinine in meconium by immunoassay, GC/MS and HPLC/DAD.[11–14] Some radioimmunoassays (RIA) and enzyme-linked immunosorbent assays (ELISA) designed to quantify cotinine in other matrices are approximately 30% cross-reactive to OH-cotinine [18,19]; cross-reactivity of OH-cotinine in meconium, specifically, is unknown. Consequently, immunoassay testing may overestimate cotinine concentrations present in meconium. Cotinine, but not OH-cotinine, was detected at an unspecified LOD in meconium by a qualitative GC/MS method described by Ostrea et al.[13] Highly polar OH-cotinine may not have been sufficiently recovered from meconium with these authors’ solid phase extraction procedure, or endogenous meconium compounds could have inhibited necessary derivatization. In an HPLC/DAD assay[14], cotinine, nicotine and caffeine were detected at 10 ng/g in fortified meconium, with analyte recoveries greater than 85%. Our LC-APCI-MS/MS method allows cotinine quantification as low as 1.25 ng/g. Interestingly, with HPLC/DAD, no nicotine was observed in thirty authentic meconium specimens, though eleven were cotinine-positive.[14] The authors attribute nicotine’s absence to its rapid conversion to cotinine. In contrast to these reports, an authentic meconium specimen analyzed by our method contained 101.4 ng/g nicotine, 94.7 ng/g cotinine, 196.8 ng/g OH-cotinine, 4.4 ng/g norcotinine, and 10.2 ng/g nornicotine.
Though ion suppression was observed, the presence of deuterated internal standards mitigated the overall effect. Meconium is a highly complex matrix and, although specimen preparation was more extensive and solid phase extraction performed, matrix effect could not be eliminated, but compensated for with deuterated internal standards for each analyte. In spite of matrix suppression, sensitivity was very good. Limits of detection ranged from 1.25 to 5 ng/g; these concentrations are lower than previously reported. In addition, accuracy and precision do not seem to be negatively affected by matrix effect. Low, medium and high quality control concentrations quantified within 25% of target and imprecision was less than 20% in this complex biological matrix. Moreover, variations in matrix effects of different meconium sources do not seem to affect quantification. The % RSD of calculated concentrations was less than 11% in six neonates’ meconium fortified with low quality control solution.
Approximately three days were needed for meconium weighing, specimen preparation, and LC-APCI-MS/MS and data analyses. Analysis with and without enzymatic hydrolysis is necessary to estimate free and total analyte concentrations, extending the time and amount of specimen required for each patient.
As with other nicotine matrices, finding adequately blank matrix proved challenging due to environmental exposure and dietary nicotine sources. Commercially available meconium is not screened for cotinine; therefore, testing meconium pools prior to use as calibrators and/or quality control specimens is imperative.
Direct analysis of glucuronide conjugates was investigated as an alternative to time-consuming and costly hydrolysis of meconium specimens. When methanolic glucuronide standards were injected on column, peaks corresponding to OH-cotinine-, nicotine- and cotinine-glucuronide were observed to elute quickly, with retention times less than 1 min. However, glucuronidated analytes were not recovered from buffer fortified with glucuronide conjugates and subjected to solid phase extraction. Most likely, the more polar glucuronides were eluted during the wash steps. Additionally, glucuronide conjugates were not visible in meconium extracts fortified with glucuronide standards after solid phase extraction. This suggests that analyte signals were suppressed by endogenous meconium compounds present early in the analytical run. For these reasons, enzymatic hydrolysis and subsequent analysis of unbound analytes was chosen. Possibly, alternative chromatographic and solid-phase extraction parameters could separate glucuronide compounds from meconium, allowing direct analysis.
Alternative hydrolysis procedures were evaluated including strong hydrolysis with 1.1 M sodium hydroxide for 35 min at 75°C, but OH-cotinine-glucuronide hydrolysis did not improve and artifactual nornicotine production was observed. Overnight incubation with β-glucuronidase Type IX-A yielded 80–90% hydrolysis of cotinine- and nicotine-N-glucuronide. Hydrolyzed specimens’ OH-cotinine concentrations should be interpreted with caution, as OH-cotinine-O-glucuronide hydrolysis efficiency was only 15%, and total OH-cotinine may not be fully recovered. Studies in urine and plasma have not reported OH-cotinine-O-glucuronide hydrolysis efficiencies, likely because authenticated OH-cotinine-O-glucuronide only recently became commercially available.[20–22] In order to estimate the extent of glucuronidation of nicotine, cotinine and OH-cotinine, meconium specimens were analyzed twice, with and without hydrolysis.
Quantification of nicotine and metabolites in meconium will aid in identifying prenatally tobacco-exposed infants, determining the disposition of tobacco analytes in this neonatal matrix, and identifying developmental toxicity of in utero tobacco exposure. In addition, this new method may help determine if the magnitude and frequency of prenatal tobacco exposure correlates with analyte meconium concentrations, and more importantly, if these concentrations are predictive of neonatal outcome measures. Cotinine concentrations in meconium determined by immunoassay are correlated to the degree of active maternal smoking and birth weight and are useful in predicting lower respiratory tract infections.[12,23] Nicotine and/or other previously untested metabolites may prove to be invaluable biomarkers of in utero tobacco exposure.
CONCLUSION
The first LC-APCI-MS/MS method for simultaneous quantification of nicotine, cotinine, OH-cotinine, norcotinine and nornicotine in meconium was developed and validated. Methanolic homogenization, enzymatic hydrolysis and solid phase extraction provided adequate recovery of nicotine and metabolites. LC-APCI-MS/MS analysis was sensitive and specific. Method validation results for accuracy, precision, linearity, matrix effect and carryover were acceptable. This method will be used to quantify nicotine and metabolites in in utero tobacco-exposed neonates.
ACKNOWLEDGMENT
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jaddoe VW, Verburg BO, de Ridder MA, Hofman A, Mackenbach JP, Moll HA, Steegers EA, Witteman JC. Am. J. Epidemiol. 2007;165:1207. doi: 10.1093/aje/kwm014. [DOI] [PubMed] [Google Scholar]
- 2.Buka SL, Shenassa ED, Niaura R. Am. J. Psychiatry. 2003;160:1978. doi: 10.1176/appi.ajp.160.11.1978. [DOI] [PubMed] [Google Scholar]
- 3.Roza SJ, Verburg BO, Jaddoe VW, Hofman A, Mackenbach JP, Steegers EA, Witteman JC, Verhulst FC, Tiemeier H. Eur. J. Neurosci. 2007;25:611. doi: 10.1111/j.1460-9568.2007.05393.x. [DOI] [PubMed] [Google Scholar]
- 4.Hukkanen J, Jacob P, 3rd, Benowitz NL. Pharmacol. Rev. 2005;57:79. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 5.Sastry BV, Chance MB, Hemontolor ME, Goddijn-Wessel TA. Pharmacology. 1998;57:104. doi: 10.1159/000028231. [DOI] [PubMed] [Google Scholar]
- 6.Luck W, Nau H, Hansen R, Steldinger R. Dev. Pharmacol. Ther. 1985;8:384. doi: 10.1159/000457063. [DOI] [PubMed] [Google Scholar]
- 7.Jauniaux E, Gulbis B, Acharya G, Thiry P, Rodeck C. Obstet. Gynecol. 1999;93:25. doi: 10.1016/s0029-7844(98)00318-4. [DOI] [PubMed] [Google Scholar]
- 8.Kohler E, Bretschneider D, Rabsilber A, Weise W, Jorch G. Hum. Exp. Toxicol. 2001;20:1. doi: 10.1191/096032701669841404. [DOI] [PubMed] [Google Scholar]
- 9.Mercelina-Roumans PE, Schouten H, Ubachs JM, van Wersch JW. Eur. J. Clin. Chem. Clin. Biochem. 1996;34:525. doi: 10.1515/cclm.1996.34.7.525. [DOI] [PubMed] [Google Scholar]
- 10.Eliopoulos C, Klein J, Phan MK, Knie B, Greenwald M, Chitayat D, Koren G. JAMA. 1994;271:621. [PubMed] [Google Scholar]
- 11.Dempsey D, Moore C, Deitermann D, Lewis D, Feeley B, Niedbala RS. Forensic Sci. Int. 1999;102:167. doi: 10.1016/s0379-0738(99)00052-3. [DOI] [PubMed] [Google Scholar]
- 12.Sherif NA, Kamel SM, Al-Ashkar OS, Sharaki OA, Seif EA, Hegazy EA. East. Mediterr. Health J. 2004;10:96. [PubMed] [Google Scholar]
- 13.Ostrea EM, Knapp DK, Romero AI, Montes M, Ostrea AR. J. Pediatr. 1994;124:471. doi: 10.1016/s0022-3476(94)70378-7. [DOI] [PubMed] [Google Scholar]
- 14.Baranowski J, Pochopien G, Baranowska I. Journal of Chromatography B: Biomedical Sciences and Applications. 1998;707:317. doi: 10.1016/s0378-4347(97)00619-1. [DOI] [PubMed] [Google Scholar]
- 15.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Anal. Chem. 2003;75:3019. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Iba MM, Weisel CP. Clin. Chem. 2004;50:2323. doi: 10.1373/clinchem.2004.038489. [DOI] [PubMed] [Google Scholar]
- 17.Hoofnagle AN, Laha TJ, Rainey PM, Sadrzadeh SM. Am. J. Clin. Pathol. 2006;126:880. doi: 10.1309/LQ8U3UL956ET324X. [DOI] [PubMed] [Google Scholar]
- 18.Zuccaro P, Pichini S, Altieri I, Rosa M, Pellegrini M, Pacifici R. Clin. Chem. 1997;43:180. [PubMed] [Google Scholar]
- 19.Schepers G, Walk RA. Arch. Toxicol. 1988;62:395. doi: 10.1007/BF00293630. [DOI] [PubMed] [Google Scholar]
- 20.Byrd GD, Chang KM, Greene JM, deBethizy JD. Drug Metab. Dispos. 1992;20:192. [PubMed] [Google Scholar]
- 21.Benowitz NL, Jacob P, 3rd, Fong I, Gupta S, Pharmacol J. Exp. Ther. 1994;268:296. [PubMed] [Google Scholar]
- 22.Ghosheh OA, Browne D, Rogers T, de Leon J, Dwoskin LP, Crooks PA. J. Pharm. Biomed. Anal. 2000;23:543. doi: 10.1016/s0731-7085(00)00339-3. [DOI] [PubMed] [Google Scholar]
- 23.Nuesslein TG, Beckers D, Rieger CH. Hum. Exp. Toxicol. 1999;18:283. doi: 10.1191/096032799678840057. [DOI] [PubMed] [Google Scholar]