Abstract
This study aimed to evaluate the ability of the health food supplement Cordyceps sinensis (CS) to ameliorate suppressive effects of chemotherapy on bone marrow function as a model for cancer treatment Mice were treated with Taxol (17 mg/kg body wt) one day before oral administration of a hot-water extract of CS (50 mg/kg daily) that was given daily for 3 weeks. White blood cell counts in peripheral blood of mice receiving Taxol were at 50% of normal levels on day 28 but had recovered completely in mice treated with CS. In vitro assays showed that CS enhanced the colony-forming ability of both granulocyte macrophage colony forming unit (GM-CFU) and osteogenic cells from bone marrow preparations and promoted the differentiation of bone marrow mesenchymal stromal cells into adipocytes, alkaline phosphatase–positive osteoblasts, and bone tissue. This result could be attributed to enhanced expression of Cbfa1 (core binding factor a) and BMP-2 (bone morphogenetic protein) with concurrent suppression of ODF (osteoclast differentiation factor/RANK [receptor activator of NF-κB]) ligand. In summary, CS enhances recovery of mice from leukopenia caused by Taxol treatment. It appears to do so by protecting both hematopoietic progenitor cells directly and the bone marrow stem cell niche through its effects on osteoblast differentiation.
Keywords: Cordyceps sinensis, Taxol, leucopenia, osteoblast
Introduction
Leukopenia is a common side effect of cancer therapy. The FDA has approved the use of the cytokines, granulocyte colony stimulating factor (G-CSF), granulocyte macrophage colony stimulating factor (GM-CSF), and interleukin-11 (IL-11) for the treatment of acute myelosuppression regardless of etiology. However, their clinical use is restricted to a narrow scenario, mainly because of their specificity, their intrinsic pleiotropism, and their cascade-like mechanism of action. Toxicity also limits their effectiveness (1). There is therefore a need to discover nontoxic agents that are applicable to a broader range of operational conditions and, in particular, to assist the recovery of bone marrow (BM) function compromised by cancer treatment.
Recovery of blood-forming stem cells requires not only the hematopoietic stem/progenitor cells (HSC/HPC) to be stimulated but also an appropriate microenvironment in which the cells can develop (see review in Ref. 2). Osteoblastic or vascular niches (3–5) can fulfill this function. Although the key molecular signals that trigger HSC survival and/or replication within a niche have not been well defined, a model based on HSC-osteoblast attachment has been proposed (2, 6, 7). Modulation of the HSC niche(s) through affecting mesenchymal stem cell (MSC) activities therefore offers opportunities for treating hematopoietic-related disorders, in addition to the use of hematopoietic growth factors.
In the past 20 years, as the result of the passage of the Dietary Supplement Health and Education Act of 1994 by the Congress for Food Safety and Applied Nutrition, there has been phenomenal growth in the interest in and use of complementary or alternative medicines. One of these, Cordyceps sinensis (CS), which is a fungal parasite of moth larvae (lepidoptera spp.) in the genera Hepitalus and Thitarodes (8), has been widely used in traditional Chinese medicine. It has been promoted as a popular remedy that is devoid of toxicity for the side effects of cancer treatment (see reviews in [9–11]). A broad spectrum of pharmacologic actions including the modulation of hepatic, renal, cardiovascular, immune, nervous, endocrine, and steroid systems has also been described. At the cellular level, diverse biological effects of CS such as activating macrophages (12), modulating apoptosis (13, 14), and inhibiting tumor metastasis (15, 16) have been reported. Many of these effects can be attributed to production of cytokines such as interferon (TFN)-γ, tumor necrosis factor-α (TNF-α), IL-1, IL-6, and GM-CSF (12, 17).
We have shown that CS could protect mice against radiation-induced BM failure. It accelerates recovery from radiation-induced leukopenia and enhances survival (18). The present study aimed to examine whether CS has similar effects after chemotherapy for cancer and to explore how CS affects the regeneration of HPCs. This preclinical study provides proof-of-principle that CS may be a potent remedy for leukopenia after cancer treatment and that it may act by enhancing the survival and differentiation of BM-HSCs and BM-MSCs.
Materials and Methods
Mice and Treatments
C57BL/6J mice were purchased from the National Laboratory Animal Center, Taipai, Taiwan, and housed in National Tsing-Hua University Laboratory Animal Center, Hsinchu, Taiwan. Seven- to eight-week-old male mice were used for experiments. Mice were divided into two major groups: control and Taxol treatment groups. Taxol (pacletaxel; Bristol-Myers Squibb, Princeton, NJ) was given by the intraperitoneal (ip) route in a dose of 17 mg/kg body wt. Control mice were given injections of the carrier, which was a mixture of 50% Cremophor EL (Sigma-Aldrich, St. Louis, MO) and 50% dehydrated alcohol. One day after Taxol treatment, the mice were given an extract of CS (50 mg/kg daily), prepared as described (18) or saline through an orogastric tube once a day on week days for a total of 3 weeks. At the indicated times, blood samples were collected from the tail and analyzed by MICROS ABC LC-152 animal blood counter (Horiba Co., Kyoto, Japan) according to the manufacturer’s protocol. Experiments were repeated three times with three mice from each group in every experiment. Data analyses and statistical tests were performed by using GraphPad Prism software version 3.03 (GraphPad Software, Inc., San Diego, CA). In all experiments, mice were killed via CO2 inhalation on day 30 after Taxol treatment. During the experiments, all mouse care followed the recommendations of the approved guide for the care and use of laboratory animals by the Institutional Animal Care and Use Committee (IACUC approval number: 09508) of National Tsing Hua University.
Cell and Colony Formation Assays
BM cells were harvested by flushing the medullary cavities of femur and tibia bones with Hanks’ balanced salt solution. Cells (1 × 106 cells/ml) were cultured in 6-well plates in 2 ml RPMI 1640 medium (GIBCO, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum (FCS), 1% penicillin/streptomycin, and 50 μM 2-mercaptoethanol (Sigma-Aldrich). For the GM-CFU colony assay, non-adherent BM cells were collected after a 24-hr incubation and their concentration was adjusted to 2 × 105 cells/ml in phosphate-buffered saline (PBS). These nonadherent BM cells were then plated (2 × 105 cells/ml, 0.3 ml) in Petri dishes (BD Falcon, San Jose, CA) with 1.1 ml per dish in the presence or absence of CS (500 μg/ml) along with premixed methylcellulose culture medium (Methocut M3234, Stem Cell Technologies, Vancouver, Canada) as described by Lin et al. (19) with final concentrations of 1% methylcellulose, 15% FCS, 1% bovine serum albumin, 10 μg/ml insulin, 200 μg/ml transferrin, 10−4 M 2-mercaptoethanol, 2 mM L-glutamine, 10 ng/ml recombinant murine IL-3, and 500 ng/ml GM-CSF (Invitrogen Biosource, Carlsbad, CA). After 7 days of incubation, GM-CFU colonies consisting of 50 or more cells were scored by using an inverted microscope.
Osteogenic cultures were established from BM cells by culture in α-MEM (Sigma-Aldrich) supplemented with 10% FCS, 1% penicillin/streptomycin, and 50 μM 2-mercaptoethanol (Sigma-Aldrich) in the presence of 50 μg/ml ascorbic acid and 10 mM β-glycerophosphate as described by Rickard et al. (20). Control, CS (500 μg/ml)-treated, and dexamethasone (10 nM)-treated cultures were established in 12-well plates (1.5 × 106 cells/ml). One day later, nonadherent cells were removed by washing three times with 5 ml α-MEM medium, and the number of attached MSCs with pseudopodia processes were counted. Complete medium, with or without the appropriate supplements, was added and changed thereafter every 2 to 3 days. After 5 days, small osteoblast colonies that formed were counted in five fields under a microscope (magnification, ×100; see pictures in the insert of Fig. 2B). After 7, 10, 14, and 28 days of culture, adipocytes were identified by the presence of oil drops and counted in 30 fields by microscopy (magnification, ×100). After 7, 10, and 14 days of culture, preosteoblast colony-forming cells were identified by positive staining for alkaline phosphatase (ALP) using the FAST RED kit (Sigma-Aldrich) (20, 21) and scored by using microscopy (magnification, ×100) to count the number in five fields. After 28 and 60 days of culture, mineralized bone nodules were identified by staining with Alizarin red S reagent (Sigma-Aldrich).
Figure 2.
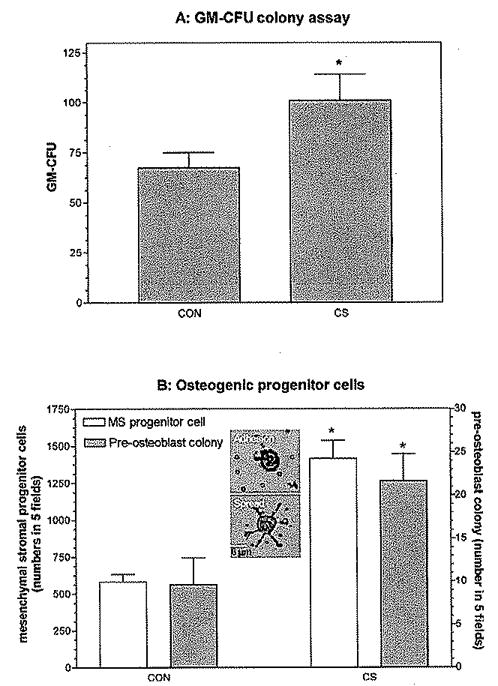
CS effects on BM cells in vitro. (A) CS enhanced the survival of GM-CFUs from BM cells. BM cells were harvested from the tibia and femur and cultured in RPMI medium for one day. Nonadherent BM cells were then taken and cultured with or without CS (500 μg/ml) in premixed methylcellulose culture medium containing IL-3 and GM-CSF. GM-CFUs were counted on day 7. (B) CS enhanced the survival and clonogenicity of MSCs. BM cells were seeded in 12-well culture plates (3 × 103/well) with or without CS (500 μg/ml). After one day, the number of adherent MSCs identified by their pseudopodia processes (see the insert) that were left on the culture plate after removal of nonadherent cells was scored in five fields by microscopy (magnification: ×100). In addition, the number of preosteoblast colonies that developed from these cells was scored on day 5 in five fields by microscopy (magnification: ×100). Values are the means ± 1 SEM for six determinations per group. Data and error bars represent the means ± 1 SEM for one representative of three experiments, as analyzed by GraphPad Prism software version 3.03. *, P < 0.01 by Student’s t test (compared with control).
The ability of BM cells to give rise to osteoclasts was tested by culturing them in 6-well plates in 2 ml (1 × 106/ml) RPMI1640 medium (GIBCO) supplemented with 10% heat-inactivated FCS, 1% penicillin/streptomycin, and 50 μM 2-mercaptoethanol (Sigma-Aldrich) (22) with or without CS (500 μg/ml). After 7 days, nonadherent cells were removed by washing three times with PBS. The attached cells were then fixed with 10% formaldehyde in PBS, treated with ethanol-acetone (50:50), and stained for tartrate-resistant acid phosphatase (TRAP, a marker enzyme of osteoclasts) (22) by using a TRAP staining kit (acid phosphatase staining kit, Sigma-Aldrich). TRAP-positive multinucleated giant cells containing more than three nuclei were counted as osteoclasts.
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
Cbfa (core binding factor a), BMP (bone morphogenetic protein), and ODF (osteoclast differentiation factor/RANK [receptor activator of NFκB]) ligand mRNA expression was assessed by RT-PCR. Total cellular RNA was extracted from mesenchymal stromal cells after in vitro CS treatment for 3 or 5 days. Total cellular RNA was extracted with the RNA easy Mini Kit (QIAGEN, Valencia, CA) according to the manufacturer’s instructions. Reverse transcription and PCR were conducted by using AMV reverse transcriptase (QIAGEN) and Taq DNA polymerase (QIAGEN), respectively. The primer pairs were picked by the Primer3 Web-based primer prediction program (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3.cgi) and synthesized by Invitrogen Co. (Carlsbad, CA). They were ACCAAGTAGCCAGGTTCAA and TCTCAGTGAGGGATGAAATG for the murine Cbfa1 gene (GenBank access number NM009820), TCGTGGAACATTAGCATGGA and CCTCTCCCAATCTGGTTCAA for the murine ODFIRANKL gene (GenBank access number NM011613), TGGAAGTGGCCCATTTAGAG and TGACGCTTTTCTCGTTTGTG for the murine BMP2 gene (GenBank access number NM007553), and AACTTTGGCATTGTGGAAGG and ACACATTGGGGGTAGGAACA for the murine GAPDH gene (GenBank access number BC083149).
Real-Time PCR
The expression level of BMP2 and RANKL mRNA expression was quantized by real-ime PCR. The primer pairs for BMP2 (GAGGTGTTCGATGGACGTGAT and AGAACATGCGGTTGCCTGTAG), RANKL (ACTGGTCGGGCAATTCTGAA and GGGTTGGACACCTGAATGCT), and β-actin (CCACCAGACAACACTGTGTTG and AGAGGTATCCTGACCCTGAAG) were designed by Primer express software v3.0 (Applied Biosystems). SYBR Green 1 dye (Applied Biosystems) was used as a reporter, and the reactions were performed by the ABI prism 7300 PCR machine. The quantitation analysis was performed by ABI 7300 systems sequence detection software v1.4 (Applied Biosystems).
Results
CS Prevents Taxol-Induced Leukopenia
Injection of a single dose of Taxol (17 mg/kg body wt given by the ip route; about 91 mg/m2) caused a severe loss of WBCs from the peripheral blood of mice (Fig. 1). The WBC number reached its nadir (mean, 6.2 × 103/mm3; range, 0.6–12.4) 7 days after the start of Taxol treatment. This result is not dissimilar to the mean nadir of 1.3 × 103/mm3 and range of 0 to 13.4 found in patients receiving an infusion course of 135 mg/m2 over 3 hrs. Oral administration of CS water extracts (50 mg/kg daily, 5 days per week for 3 weeks) alone did not affect WBC counts, but it did significantly minimize the extent of Taxol-induced leukopenia (P < 0.01). Whereas Taxol-treated mice had only half the normal number of WBCs in the peripheral blood after 28 days, WBCs in those receiving CS had recovered completely (P < 0.01).
Figure 1.
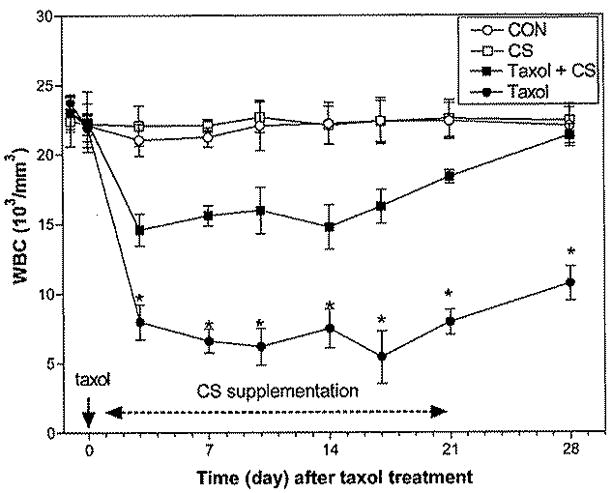
Taxol (17mg/kg) treatment given by the ip route to mice caused a dramatic decrease in peripheral WBCs with 50% recovery by day 28. Oral administration of CS (50 mg/kg daily, 5 days per week for 3 weeks) reduced the WBC nadir and enhanced recovery to control levels within a month. Data are represented by the mean ± 1 SD for 9 mice from three repeated experiments, as analyzed by GraphPad Prism software version 3.03, *, P < 0.01 by ANOVA test (compared with control mice that received saline).
Effects of CS on BM Cells in Culture
The ability of CS to prevent Taxol-induced leukopenia suggested that it affected the survival and differentiation of HPCs and/or protected the stromal niche in which they grow. A series of in vitro colony-forming and differentiation assays were performed by using BM cells to examine these possibilities.
CS (500 μg/ml) increased granulocyte-macrophage colony formation from nonadherent BM cells cultured for 1 week in premixed methylcellulose culture medium containing IL-3 and GM-CSF (Fig. 2A), It also increased the number of attached MSCs with pseudopodia (insert of Fig. 2B) and the small preosteoblast colonies that formed from them after 5 days of culture (Fig. 2B). When the MSCs were further cultured in osteogenic differentiation medium, CS increased the numbers of ALP-positive osteoblast cells at day 10, oil-forming adipocytes at day 14, and bony tissue accumulation at day 28 (Fig. 3), CS (500 μg/ml) was only slightly less effective than the addition of 10 nM dexamethasone (20, 23) in causing osteoblastic differentiation (Fig. 3A).
Figure 3.

CS accelerates the differentiation of osteoblasts. (A) Osteoblast differentiation in CS-supplemented cultures showed enhanced ALP-positive osteoblasts at day 10 of culture, more oil-forming adipocytes (arrow) at day 14 of culture, and and stronger Alizarin red S–stained bone tissue at day 28 of culture. The CS influence on osteoblast differentiation was as effective as that of dexamethasone (1 μg/ml). (B) The time course of the appearance of ALP-positive osteoblasts in these osteoblast differentiation cultures with or without CS (500 μg/ml) was assessed. Cells were stained for ALP after 7, 10, and 14 days, and the numbers of ALP-positive cells per 100 cells were counted. CS enhanced the number of ALP-positive cells at all time points. (C) Oil-forming adipocytes were counted (30 fields) at 7, 10, 14, 21, and 28 days. CS promoted differentiation of adipocytes earlier than did control. Data and error bars represent the means ± 1 SEM for one representative of three experiments, as analyzed by GraphPad Prism software version 3.03. *, P < 0.01 by Student’s t test (compared with control). A color figure is available in the online version of the journal.
These data indicate that CS supplement enhances the survival of both HSCs and MSCs. Furthermore, the scores for ALP-positive osteoblasts (Fig. 3B) and adipocytes (Fig. 3C) at different time points indicated that CS not only enhanced the survival of precursor cells but also accelerated osteogenic differentiation. For example, adipocyte numbers peaked at 14 days in the CS supplemented cultures instead of at 21 days in control cultures (Fig. 3B).
CS Affects the mRNA Levels of Cbfa1, BMP-2, and ODF/RANK Ligand Expressed by Osteoblasts
To confirm that CS influences osteoblast differentiation, we examined the expression of the marker gene, Cbfa1, in MSC cultures by using RT-PCR (Fig. 4A), Cbfa1 is a transcription factor that drives osteoblast lineage determination at an early differentiation stage (24, 25). CS enhanced the expression of Cbfa1 mRNA after 3 days of osteoblastic culture, an event that occurred only at 5 days under normal conditions (Fig. 4A). In addition to Cbfa1, BMP-2 mRNA expression was enhanced by CS (Fig. 4A, B) BMP-2 plays a significant role in MSC differentiation (26, 27) and is required for Cbfa1-dependent induction of the osteoblast phenotype (28).
Figure 4.
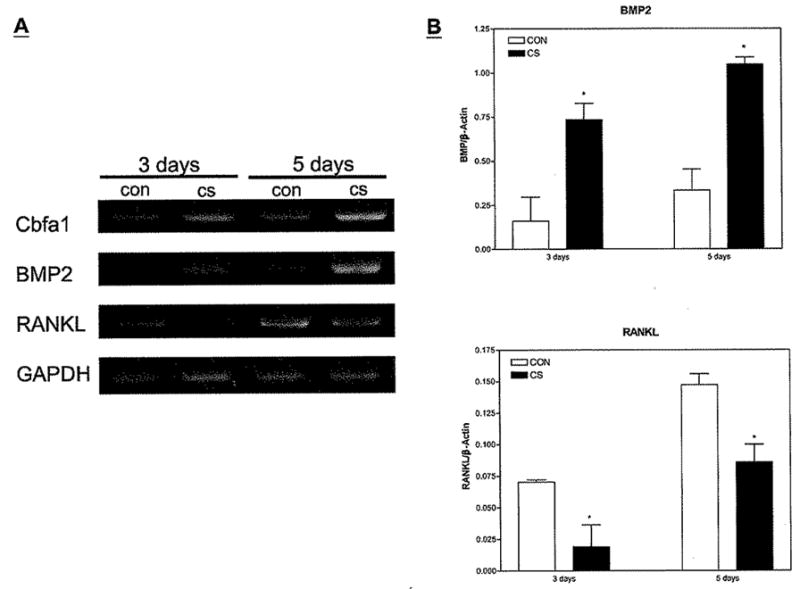
Expression of Cbfa1, BMP-2, ODF, and GAPDH mRNA by BM-MSCs. BM cells were seeded in 12-well culture plates (3 × 103/well). The hematopoietic progenitor cells were removed after one day of culture. The attached cells were collected at 3 and 5 days after the culture. (A) Expression of Cbfa1, BMP-2, ODF, and GADPH was evaluated by RT-PCR. CS enhanced the expression of Cbfa1 and BMP-2 but reduced the expression of ODF, GADPH was used as an internal control, (B) Real-time PCR was used to quantify the mRNA levels of BMP-2 and RANKL genes. Data and error bars represent the means ± 1 SD of three experiments, as analyzed by GraphPad Prism software version 3.03. *, P < 0.01 by Student’s t test (compared with control).
In contrast to increasing expression of osteogenic differentiation factors, CS decreased expression of the osteoclast differentiation factor ODF (RANKL) by osteoblasts (Fig. 4A, B). RANKL is a member of the TNF family, and osteoblasts express it in response to several osteotropic factors. It is critical for TRAP (tartrate-resistant acid phosphatase) production and differentiation of osteoclast precursor cells into multinucleated osteoclasts (29, 30) from their monocytic/macrophage precursors (see a review in [30]).
CS Affects Hematopoietic Differentiation
The finding that CS suppressed ODF (RANKL) mRNA expression in osteoblastic cells indicates that it may inhibit the differentiation of HSCs into osteoclasts (30–32). To determine whether this was the case, an osteoclast differentiation assay was performed. Osteoclasts develop through the interaction of HSCs with MSCs over 7 days of culture, can be identified by TRAP staining, and are characterized as multinucleated cells (22). The fact that this differentiation was inhibited by the addition of CS in a dose-dependent manner (Fig. 5) along with the suppressed ODF production by MSCs (Fig. 4) suggests that CS may increase the size of the MSC niche to increase HSC production (3, 4). This is consistent with the finding of more hematopoietic cells in HSC and MSC coculture systems in the presence of CS supplementation (Fig. 6). CS may therefore act both directly and indirectly on HSCs to promote their survival and proliferation and counter chemotherapy-induced BM suppression.
Figure 5.
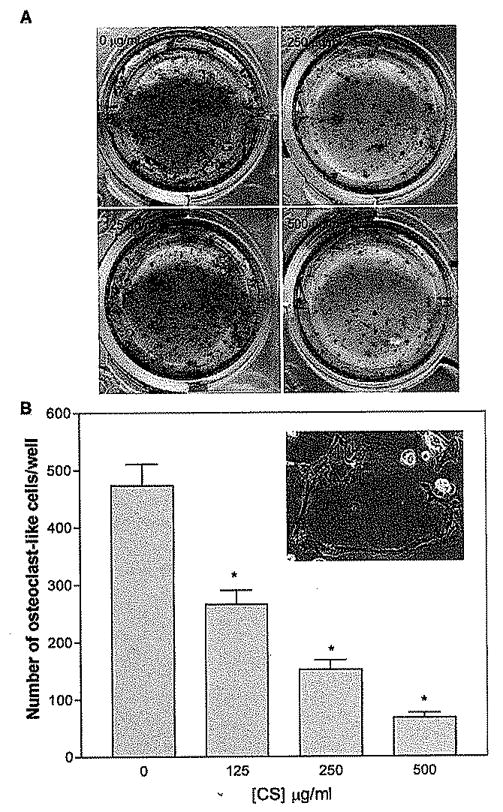
Influence of CS on osteoclast differentiation, (A) A CS dose-dependent reduction of tartrate-resistant acid phosphatase (TRAP)-stained cells was found in 7-day BM cultures. (B) The number of osteoclasts, evidenced by multinucleated giant cells containing more than three nuclei (insert), were counted and plotted against CS concentration. A reverse dose-dependent fashion was found. Values are means ± 1 SEM of six determinations. One represented data from three repeated experiments were shown. *, P < 0.01 by ANOVA (compared with control without CS addition).
Figure 6.
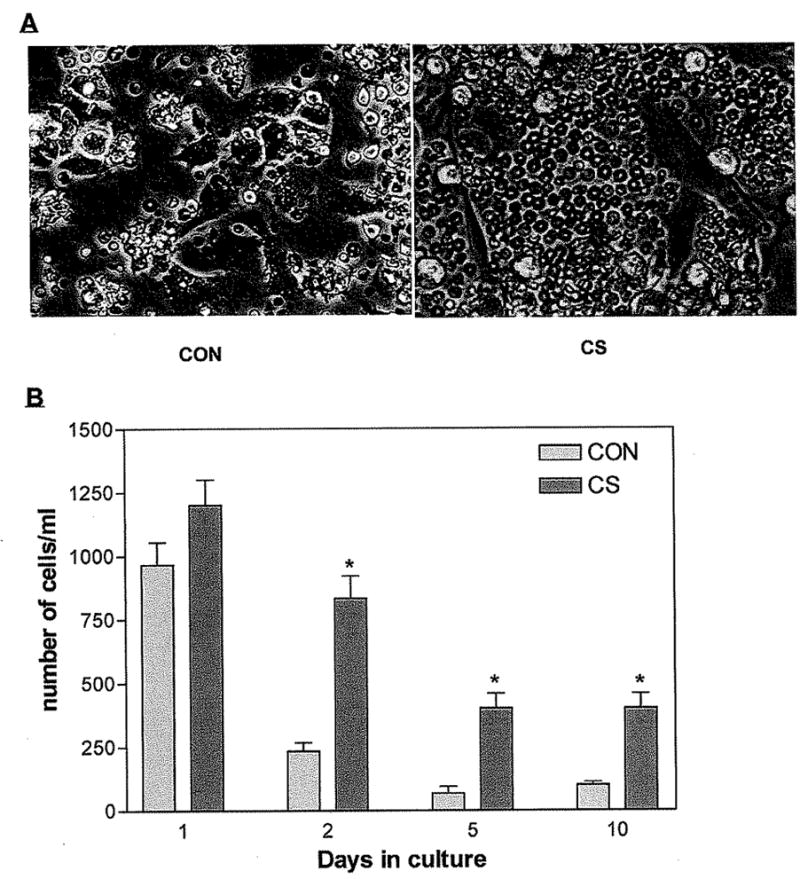
Mesenchymal stromal cells support the growth and differentiation of hematopoietic cells. (A) Hematopoietic cells are still detectable after 10 days in cultures containing FCS only, when they were cocultured with MSCs. (B) The number of hematopoietic cells was further increased in the culture medium containing CS (500 μg/ml). Values are means ± 1 SEM of six determinations. One represented data from three repeated experiments were shown. *, P < 0.01 by Student’s t test (compared with control).
Discussion
Leukopenia is a frequent side effect of many forms of cancer therapy, including chemotherapy and radiotherapy. In this study, we extended our previous findings on in vivo administration of a Chinese herbal medicine, Cordyceps sinensis, to show that it not only promotes the recovery from radiation-induced leukopenia (18) but also rescues mice from Taxol-induced leukopenia. This study also provides evidence that CS influences the survival and differentiation of HSCs both directly and indirectly by affecting the MSC niche.
We believe that this is the first report to show that CS affects the differentiation of BM-HSCs and BM-MSCs. GM-CFUs and preosteoblast colony numbers were increased by the addition of CS to nonadherent and adherent BM cells, respectively. Furthermore, an osteoblast differentiation assay demonstrated that CS could promote the differentiation of MSCs into bone tissue. Although the precise mechanism by which CS affects osteoblast differentiation is unclear, the enhanced expression of Cbfa1 and BMP-2 mRNA indicates that CS acts at early stage on osteoblastic progenitors. In contrast, we also found that CS retards the development of osteoclasts. This is probably due to its inhibitory effect on the expression of ODF (RANKL) in osteoblasts. RANKL produced by marrow osteoblasts causes osteoclast precursors within the monocytic/macrophage precursor pool to express TRAT and form multi-nucleated osteoclasts (29, 30). The delicate balance between osteoblast and osteoclast differentiation is crucial during bone development, homeostasis, and repair (see a review in [30]) but may also determine the size of the niche in which HSCs develop. By suppressing HSC differentiation to osteoclast lineages, CS may also switch them to make other blood cell lineages by enhancing Cbfa1 and BMP-2 expression by MSCs.
The hematopoietic system and bone have an intimate relationship that is manifested in multiple ways. Osteoblasts produce hematopoietic growth factors such as GM-CSF and hepatocyte growth factor (33–35) that cooperatively permit the survival of hematopoietic progenitors. Taichman and Emerson (33, 36) first proposed that the osteoblast may have a role in hematopoietic recovery. This has been further supported by Zhang et al. (3) and Calvi et al. (4), who used genetic strategies to increase the size of the osteoblast population in specific regions of bone. They found that this caused parallel increases in the HSC population and that bone not only provides cavities in which HSC can differentiate into specialized blood cells but also that osteoblasts lining the trabecular bones provide “niches” for HSC development (3, 4). Calvi et al. (4) also demonstrated that osteoblastic cells were a regulatory component of the niche in vivo that influences HSC function through Notch activation. The ability of CS water extracts to promote the recovery of leukopenia after cancer therapy may therefore partially be through its effects on osteoblast clonogenicity, which includes inhibiting their differentiation into osteoclasts, as well as a direct effect in increasing the colony-forming ability of HSCs. However, this study does not exclude the possibility that CS directly protects blood leukocytes or BM stem cells from Taxol cytotoxicity.
A critical issue is which component of CS is responsible for its multiple effects and is the same component active in all cases. Polysaccharides seem to be responsible for many CS effects (37–39). The β-glucan polysaccharide extract from the fruit body of the Maitake mushroom (Grifola frondosa) also enhanced hematopoietic BM cell growth and differentiation into colony-forming cells (19), similar to what we found for CS in this study. We have been working to identify the CS ingredient(s) responsible for stimulating BM, but our preliminary data indicate that multiple components express activity (data not shown) and that these individual components are less effective than the whole extract and have a higher risk of side effects in vivo. This has been a major issue for many other traditional Chinese medicines. Multiple ingredients may be necessary, perhaps because they balance the side effects associated with a single compound (40) and stimulate multiple pathways and multiple cell types, as was seen in this study.
Nevertheless, this study demonstrates the potential of CS, a traditional Chinese medicine, as a remedy for leukopenia after cancer therapy and shows that it can promote the differentiation of HSCs both directly and indirectly through its action on osteoblast differentiation.
Acknowledgments
This work is supported by the NSC95-2320-B-007-005 grant to C.S.C. J.-H.H. is supported by NSC93-2314-B-182-030, CMRPG1001, and SMRPG340021. W.H.M. acknowledges support from the National Institutes of Health (NCI ROI CA-101752) and the Department of Defense (W81XWH-04-1-0126).
References
- 1.Schooltink H, Rose-John S. Cytokines as therapeutic drugs. J Interferon Cytokine Res. 2002;22:505–516. doi: 10.1089/10799900252981981. 2. Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood 105:2631–2639. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishme Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 3.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 4.Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grunewald E, Cheng T, Dombkowski D, Calvi LM, Rittling SR, Scadden DT. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005;201:1781–1791. doi: 10.1084/jem.20041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu J, Emerson SG. A new bone to pick: osteoblasts and the haematopoietic stem-cell niche. Bioessays. 2004;26:595–599. doi: 10.1002/bies.20052. [DOI] [PubMed] [Google Scholar]
- 7.Kinjo N, Zang M. Morphological and phylogenetic studies on Crodyceps sinensis distributed in southwestern China. Myoscience. 2001;41:567–574. [Google Scholar]
- 8.Zhu JS, Halpern GM, Jones K. The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis: part I. J Altern Complement Med. 1998;4:289–303. doi: 10.1089/acm.1998.4.3-289. [DOI] [PubMed] [Google Scholar]
- 9.Zhu JS, Halpern GM, Jones K. The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis: part II. J Altern Complement Med. 1998;4:429–457. doi: 10.1089/acm.1998.4.3-289. [DOI] [PubMed] [Google Scholar]
- 10.Ng TB, Wang HX. Pharmacological actions of Cordyceps, a prized folk medicine. J Pharm Pharmacol. 2005;57:1509–1519. doi: 10.1211/jpp.57.12.0001. [DOI] [PubMed] [Google Scholar]
- 11.Koh JH, Yu KW, Suh HJ, Choi YM, Ahn TS. Activation of macrophages and the intestinal immune system by an orally administered decoction from cultured mycelia of Cordyceps sinensis. Biosci Biotechnol Biochem. 2002;66:407–411. doi: 10.1271/bbb.66.407. [DOI] [PubMed] [Google Scholar]
- 12.Yang LY, Huang WJ, Hsieh HG, Lin CY. H1-A extracted from Cordyceps sinensis suppresses the proliferation of human mesangial cells and promotes apoptosis, probably by inhibiting the tyrosine phosphorylation of Bcl-2 and Bcl-XL. J Lab Clin Med. 2003;141:74–83. doi: 10.1067/mlc.2003.6. [DOI] [PubMed] [Google Scholar]
- 13.Buenz Ej, Bauer BA, Osmundson TW, Motley TJ. The traditional Chinese medicine Cordyceps sinensis and its effects on apoptotic homeostasis. J Ethnopharmacol. 2005;96:19–29. doi: 10.1016/j.jep.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura K, Konoha K, Yamaguchi Y, Kagota S, Shinozuka K, Kunimoto M. Combined effects of Cordyceps sinensis and Methotrexate on hematogenic lung metastasis in mice. Receptors Channels. 2003;9:329–334. doi: 10.3109/713745176. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K, Yamaguchi Y, Kagota S, Kwon YM, Shinozuka K, Kunimoto M. Inhibitory effect of Cordyceps sinensis on spontaneous liver metastasis of Lewis lung carcinoma and B16 melanoma cells in syngeneic mice. Jpn J Pharmacol. 1999;79:335–341. doi: 10.1254/jjp.79.335. [DOI] [PubMed] [Google Scholar]
- 16.Chen YJ, Shiao MS, Lee SS, Wang SY. Effect of Cordyceps sinensis on the proliferation and differentiation of human leukemic U937 cells. Life Sci. 1997;60:2349–2359. doi: 10.1016/s0024-3205(97)00291-9. [DOI] [PubMed] [Google Scholar]
- 17.Liu WC, Wang SC, Tsai ML, Chen MC, Wang YC, Hong JH, McBride WH, Chiang CS. Protection against radiation-induced bone marrow and intestinal injuries by Cordyceps sinensis - a Chinese herbal medicine. Radiation Res. 2006;166:900–907. doi: 10.1667/RR0670.1. [DOI] [PubMed] [Google Scholar]
- 18.Lin H, She YH, Cassileth BR, Sirotnak F, Cunningham Rundles S. Maitake beta-glucan MD-fraction enhances bone marrow colony formation and reduces doxorubicin toxicity in vitro. Int Immunopharmacol. 2004;4:91–99. doi: 10.1016/j.intimp.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Rickard DJ, Sullivan TA, Shenker BJ, Leboy PV, Kazhdan I. Induction of rapid osteoblast differentiation in rat bone marrow stromal cell cultures by dexamethasone and BMP-2. Dev Biol. 1994;161:218–228. doi: 10.1006/dbio.1994.1022. [DOI] [PubMed] [Google Scholar]
- 20.Rosa AL, Beloti MM. Effect of cpTi surface roughness on human bone marrow cell attachment, proliferation, and differentiation. Braz Dent J. 2003;14:16–21. doi: 10.1590/s0103-64402003000100003. [DOI] [PubMed] [Google Scholar]
- 21.Rubin J, Murphy T, Nanes MS, Fan X. Mechanical strain inhibits expression of osteoclast differentiation factor by murine stromal cells. Am J Physiol Cell Physiol. 2000;278:C1126–1132. doi: 10.1152/ajpcell.2000.278.6.C1126. [DOI] [PubMed] [Google Scholar]
- 22.Jorgensen NR, Henriksen Z, Sorenson OH, Civitelli R. Dexamethasone, BMP-2, and 1, 25-dihydroxy vitamin D enhance a more differentiated osteoblast phenotype: validation of an in vitro model for human bone marrow-derived primary osteoblasts. Steroids. 2004;69:219–226. doi: 10.1016/j.steroids.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Otto F, Thornell AP, Crompton T, Denzei A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 24.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 25.Guicheux J, Lemonnier J, Ghayor C, Suzuki A, Palmer G, Caverzasio J. Activation of p38 mitogen-activated protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their implication in the stimulation of osteoblastic cell differentiation. J Bone Miner Res. 2003;18:2060–2068. doi: 10.1359/jbmr.2003.18.11.2060. [DOI] [PubMed] [Google Scholar]
- 26.Noth U, Tuli R, Seghatoleslami R, Howard M, Shah A, Hall DJ, Hickok NJ, Tuan RS. Activation of p38 and Smads mediates BMP-2 effects on human trabecular bone-derived osteoblasts. Exp Cell Res. 2003;291:201–211. doi: 10.1016/s0014-4827(03)00386-0. [DOI] [PubMed] [Google Scholar]
- 27.Phimphilai M, Zhao Z, Boules H, Roca H, Franceschi RT. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J Bone Miner Res. 2006;21:637–646. doi: 10.1359/JBMR.060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy SV. Regulatory mechanisms operative in osteoclasts. Crit Rev Eukaryot Gene Expr. 2004;14:255–270. doi: 10.1615/critreveukaryotgeneexpr.v14.i4.20. [DOI] [PubMed] [Google Scholar]
- 29.Bruzzaniti A, Baron R. Molecular regulation of osteoclast activity. Rev Endocr Metab Disord. 2006;7:123–139. doi: 10.1007/s11154-006-9009-x. [DOI] [PubMed] [Google Scholar]
- 30.Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, Miyata T, Anderson DM, Suda T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med. 1999;190:1741–1754. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, Koga T, Martin TJ, Suda T. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci U S A. 1990;87:7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med. 1994;179:1677–1682. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taichman RS, Reilly MJ, Emerson SG. Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. Blood. 1996;87:518–524. [PubMed] [Google Scholar]
- 34.Taichman R, Reilly M, Verma R, Ehrenman K, Emerson S. Hepatocyte growth factor is secreted by osteoblasts and cooperatively permits the survival of haematopoietic progenitors. Br J Haematol. 2001;112:438–448. doi: 10.1046/j.1365-2141.2001.02568.x. [DOI] [PubMed] [Google Scholar]
- 35.Taichman RS, Reilly MJ, Emerson SG. The hematopoietic microenvironment: osteoblasts and the hematopoietic microenvironment. Hematology. 2000;4:421–426. [PubMed] [Google Scholar]
- 36.Li SP, Yang FQ, Tsim KW. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J Pharm Biomed Anal. 2006;41:1571–1584. doi: 10.1016/j.jpba.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 37.Li SP, Zhao KJ, Ki ZN, Song ZH, Dong TT, Lo CK, Cheung JK, Zhu SQ, Tsim KW. A polysaccharide isolated from Cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sci. 2003;73:2503–2513. doi: 10.1016/s0024-3205(03)00652-0. [DOI] [PubMed] [Google Scholar]
- 38.Koh JH, Suh HJ, Ahn TS. Hot-water extract from mycelia of Cordyceps sinensis as a substitute for antibiotic growth promoters. Biotechnol Lett. 2003;25:585–590. doi: 10.1023/a:1022893000418. [DOI] [PubMed] [Google Scholar]
- 39.Wang CY, Chiao MT, Yen PJ, Huang WC, Hou CC, Chien SC, Yeh KC, Yang WC, Shyur LF, Yang NS. Modulatory effects of Echinacea purpurea extracts on human dendritic cells: a cell- and gene-based study. Genomics. 2006;88:801–808. doi: 10.1016/j.ygeno.2006.08.011. [DOI] [PubMed] [Google Scholar]


