Abstract
Multiple isolates of an alphaviruses within the western equine encephalomyelitis-serocomplex that were related closely to Ft. Morgan and its variant Buggy Creek virus were made from swallow bugs, Oeciacus vicarius Horvath (Hemiptera: Cimicidae), collected from cliff swallow (Petrochelidon pyrrhonota) nests at the Stone Lakes National Wildlife Refuge, Sacramento County, CA, during the summers of 2005 and 2006. This virus (hereafter Stone Lakes virus, family Togaviridae, genus Alphavirus, STLV) was the first record of this viral group west of the Continental Divide. STLV replicated well in Vero and other vertebrate cell cultures but failed to replicate in C6/36 cells or infect Culex tarsalis Coquillett mosquitoes. STLV failed to produce elevated viremias in adult chickens or house sparrows and was weakly immunogenic. In addition, STLV was not isolated from cliff swallow nestlings nor was antibody detected in adults collected at mist nets. We suggest that STL and related swallow bug viruses may be primarily infections of cimicids that are maintained and amplified either by vertical or nonviremic transmission and that cliff swallows may primarily be important as a bloodmeal source for the bugs rather than as an amplification host for the viruses.
Keywords: Stone Lakes virus, swallow bugs, cliff swallows, house sparrows, California
Aggregations of communally nesting or roosting birds may provide unique foci for intense arboviral amplification, especially when these avian aggregations intersect suitable vector populations during permissive climatic conditions. Cliff swallows (Petrochelidon pyrrhonota) nest during the early summer in large communal colonies throughout California and much of North America and spend the winter in South America (http://bna.birds.cornell.edu/bna/species/149/articles/introduction). Their ability to exploit bridges and culverts as nesting sites has greatly expanded their original distribution and abundance (Brown and Brown 2000). Clff swallow colonies support large populations of nest-dwelling ectoparasites (Hopla et al. 1993), including the swallow bug, Oeciacus vicarius Horvath (Hemiptera: Cimicidae). These arthropods remain relatively quiescent unless birds are present and therefore could provide an effective method for virus persistence. Large aggregations of cliff swallows also seem to be attractive to host-seeking mosquitoes, including the important arbovirus vector Culex tarsalis Coquillett (Brown and Sethi 2002).
After epidemic transmission of West Vile virus (family Flaviviridae, genus Flavivirus, WNV) was detected in Coachella Valley during 2004–2005 (Reisen et al. 2008b), Kern County during 2004–2007 (Reisen et al. 2009), and in Sacramento during 2005 (Elnaien et al. 2006), we examined cliff swallow colonies from these areas to determine their possible role in the ecology of arboviruses by testing adult birds, nestlings, and swallow bugs for evidence of infection. Previous extensive studies in California searching for alternate transmission cycles for the endemic arboviruses had largely ignored cliff swallows and their ectoparasites (Reeves et al. 1990), although other workers had studied cliff swallow blood parasites (Clark and Swinehart 1966). In 2005, we made three isolates of a rapidly growing virus on Vero cell cultures from swallow bugs collected at the Stone Lakes National Wildlife Refuge (NWR) in Sacramento County, CA. Testing the cultures using a standard multiplex reverse transcription-polymerase chain reaction (RT-PCR) for western equine encephalomyelitis virus (family Togaviridae, genus Alphavirus, WEEV), St. Louis encephalitis virus (family Flaviviridae, genus Flavivirus, SLEV) and WNV RNA produced negative results. Based on plaque morphology and the previous identification of alphaviruses from cimicids in Colorado, Nebraska, and Oklahoma (Calisher et al. 1980, Hopla et al. 1993), degenerate alphaviral primer sets were used for attempted amplification. Subsequent, genetic characterization demonstrated that these isolates from Stone Lakes NWR (hereafter Stone Lakes virus; family Togaviridae, genus Alphavirus, STLV) were related closely to Fort Morgan virus (family Togaviridae, genus Alphavirus, FMV) from Colorado (Calisher et al. 1980) and Buggy Creek Virus (family Togaviridae, genus Alphavirus, BCRV) from Oklahoma (Hopla et al. 1993). The discovery of a FMV-like virus west of the Continental Divide was novel, indicated that the distribution of these viruses was more extensive than previously documented and that these viruses may occur throughout the geographic range of their host and invertebrate vector.
The current report briefly characterizes the STLV isolates, summarizes field studies at the Stone Lakes NWR and other cliff swallow colonies in California, and describes experimental infections of adult chickens, house sparrows (Passer domesticus), and mosquitoes with STLV. Additional studies will fully sequence STLV and compare it genetically and phenotypically to multiple lineages of BCRV and FMV.
Methods and Materials
Study Areas
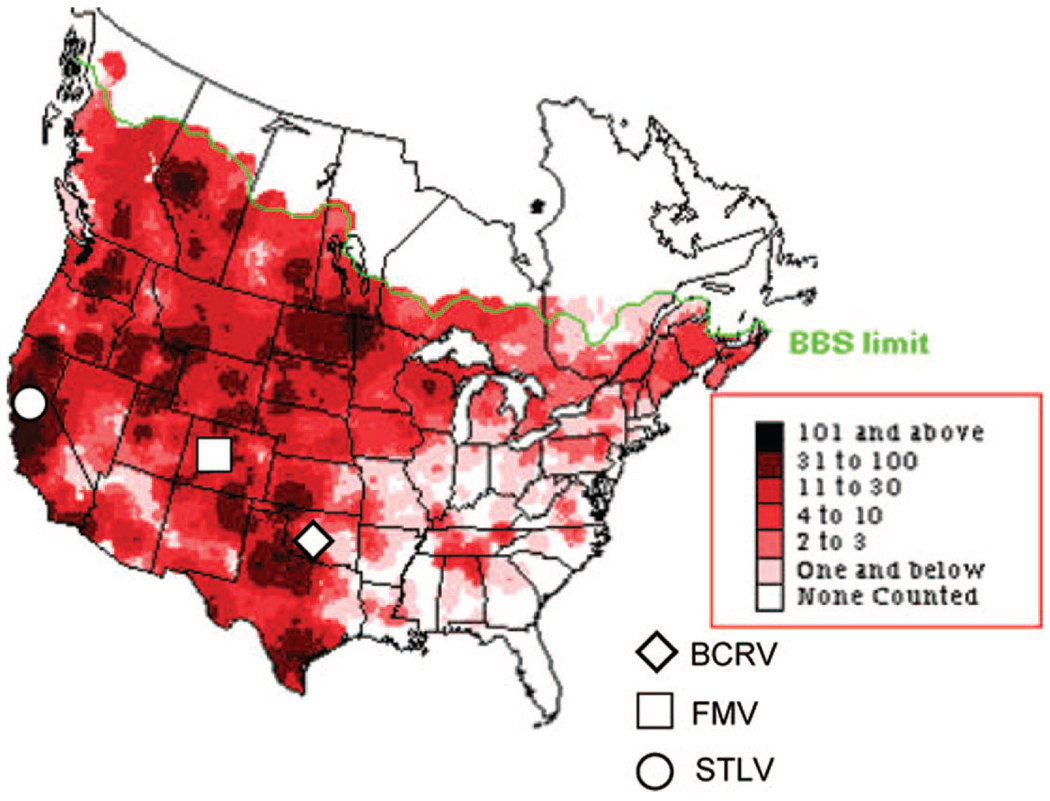
Swallow bugs were collected from active nests under a bridge along Highway 86 (N 33° 27′ 33″, W 116° 05′ 14″) in Coachella Valley, Riverside County, during May 2004 and from under a bridge along Interstate Highway 5 at Stone Lakes NWR (N 38° 21′ 55″, W 121° 28′ 50″), Sacramento County, during 2005 and 2006. In addition, cliff swallow chicks were collected from nests in the Coachella Valley, and adults were collected by mist netting in Coachella Valley, Kern County near Bakersfield (N 35° 19′ 23″, W 119° 12′ 33″) and from Stone Lakes NWR. Figure 1 shows the distribution of breeding cliff swallows in North America, including California, and the type localities of FMV, BCRV, and STLV.
Fig. 1.
Breeding Bird Survey (BBS) distribution of Cliff swallows in North America (Sauer et al. 2004). Inserted are the type localities of Ft. Morgan (FMV), Buggy Creek (BCRV), and Stone Lakes (STLV) viruses. (Online figure in color.)
Swallow Bug Collection and Processing
Swallow bugs were collected with forceps from intact or removed cliff swallow nests, grouped into pools ranging in size from 3 to 40 bugs, and then frozen at —80°C until tested for virus. During fall, after the birds had migrated, additional nests were removed and placed in the sun or under heat lamps to activate the bugs, which then were collected by hand. Pools were ground in 1–3 ml of virus diluent (phosphate-buffered saline, 15% fetal bovine serum, and antibiotics) in a tissue grinder, clarified by centrifugation, and 100 µl of homogenate inoculated onto Vero and C6/36 Aedes albopictus (Skuse) cell cultures in six-well plates. Positive cultures were inoculated into liquid Vero cell culture; held for 4 d; and then the RNA was extracted, amplified by RT-PCR, and sequenced.
RT-PCR and Sequence Analyses
Culture supernatants and aliquots from the original pools were screened by a multiplex TaqMan RT-PCR for WEEV, SLEV, and WNV RNA with negative results. Growth characteristics and plaque sizes on Vero cell culture were consistent with that of an alphavirus. Previous isolation of FMV, BCRV, and Bijou Bridge virus (family Togaviridae, genus Alphavirus, BBV) (Monath et al. 2007), alphaviruses from swallow bugs, also indicated that the unknown agent might have been an alphavirus within this complex. Therefore, alphavirus-specific primers were used for the amplification of a 1.2-kilobase fragment of the E1 through 3′ untranslated region (3′ UTR) using a degenerate forward primer in the E1 gene region as well as a reverse primer complementary to the 3′ poly-A tail present within all alphaviruses (Powers et al. 2001). Amplicons were generated successfully from Vero cultures but not from C6/36 Ae. albopictus cell cultures.
Cliff Swallow Sampling and Testing
Adult cliff swallows were collected by mist netting near colonies, banded using U.S. Geological Survey (USGS) bands, bled (100 µl of blood sample taken by jugular puncture) using 28-gauge needles, and released at the site of capture. To collect nestlings, a hole was cut into the side of each mud nest; the chicks were removed with tongs; and a blood sample taken as described above, except that 50 µl was taken when the nestlings were very small. Nestlings were returned to the nests immediately after bleeding, the hole filled with a Styrofoam plug, and the nest was numbered. For antibody testing, blood samples were expelled into 0.9 ml of saline, held at ambient temperature, and clarified by centrifugation. Sera initially were screened using an enzyme immunoassay (EIA) with crude antigen prepared from Vero cell culture of the BFS1703 strain of WEEV and the Kern217 strain of SLEV (Chiles and Reisen 1998). After STLV was isolated, a crude antigen was prepared from a Vero cell culture of STLV and some sera retested by EIA using this antigen. Samples positive by EIA were confirmed by plaque reduction neutralization test (PRNT) using STLV. Positive sera were considered to be confirmed if they neutralized >80% of >75 plaque-forming units (PFU) of STLV at a dilution of ≥1:20. For virus isolation from nestlings and some adults, blood samples were expelled into 0.4 ml of virus diluent, frozen immediately on dry ice, and then held at —80°C until tested by Vero cell plaque assay as described above; these samples also were screened for antibody by EIA after heat inactivation at 56°C for 30 min. Sera considered positive for SLEV or WNV had PRNT90 end point titers >4× the competing virus.
Avian Experimental Infections
To evaluate and optimize our EIA as well as provide positive control sera, alphavirus seronegative adult chickens >22 wk old were inoculated with 3–4 log10 PFU of STLV (isolate 33B; see Results) and BFS1703 strain of WEEV. Birds were bled daily for 5 d to detect viremia and then at 4–6 wk to detect EIA and PRNT antibody levels. Previous studies demonstrated viremias of 4–5 d duration for FMV in cliff swallows nestlings and PRNT antibody in after hatching year (AHY) and hatching year (HY) house sparrows and cliff swallows (Hayes et al. 1978, Scott et al. 1984). To interpret the results of our field seroprevalence surveys, we inoculated adult house sparrows from Bakersfield, Kern County, with 4.5 log10 PFU of STLV. Data from birds previously inoculated with the COA592 strain of WEEV (Reisen et al. 2003) were included for comparison. Birds in each group first were prebled to rule out previous natural infection with WEEV or STLV, then daily for 7 d postinoculation to monitor viremia response, and then weekly for 6 wk to monitor the antibody response. In addition, we conducted cross PRNTs with WEEV, STLV and BCRV (strain H628 isolated from a swallow bug collected in Oklahoma, provided by C. R. Brown) with both chicken and house sparrow sera.
Mosquito Experimental Infections
Because BCRV has been detected in a pool of field-collected Cx. tarsalis mosquitoes (C. R. Brown, unpublished data), we attempted to experimentally determine their vector competence for STLV. Female Cx. tarsalis from the Yolo County colony were starved for 24 h and then offered solutions containing a 1:5 dilution of stock virus in heparinized chicken blood supplemented with 2.5% sucrose and warmed to 37°C in a Hemotek membrane feeding system (Discovery Workshops, Accrington, Lancashire, United Kingdom). We used the STLV33 isolate made from a swallow bug from Sacramento County, CA, at Vero cell passage 2. Titer of virus taken from the Hemotek block after blood feeding was 5.5 log10 PFU/ml. Blood fed females were transferred to clean cages and held at 26°C for 14 d after which transmission was attempted using the capillary tube method (Aitken 1977). Expectorates were expelled into 0.3 ml of virus diluent, and both mosquito bodies and diluent were frozen at −80°C until tested for infection and transmission, respectively, using a plaque assay on Vero cells.
Ethics
The collection and infection of wild birds with encephalitis viruses was done under Protocol 11184 approved by the Institutional Animal Care and Use Committee of the University of California, Davis, CA Resident Scientific Collection Permit 801049-02 by the State of California Department of Fish and Game, and Federal Fish and Wildlife Permit MB082812-0. Collection, banding and release of wild birds was done under USGS Master Station Bird Banding Permit 22763 and Protocol 11188 approved by the Institutional Animal Care and Use Committee of the University of California, Davis. Use of arboviruses was approved under Biological Use Authorization 0554 by Environmental Health and Safety of the University of California, Davis, and USDA Permit 47901.
Results
Isolation and Characterization
The first isolations of STLV made in California were from swallow bugs collected during July 2005 from the Stone Lakes NWR (Table 1). Three of 16 pools produced low tittered cytopathic effect (CPE) on Vero cell cultures at 3–4 d postinoculation (titer range, 1.3–2.5 log10 PFU/ml). Previous attempts to isolate virus from the Coachella Valley cliff swallow colony by using Vero cell culture were unsuccessful as were attempts at Stone Lakes NWR after the cliff swallows departed during fall 2005 (0 of 35 pools; P < 0.01). However, the following summer 10 additional isolations were made from 30 pools of swallow bugs (minimum infection rate = 37 per 1,000 bugs tested) collected from 12 nests at the Stone Lakes NWR. The temporal pattern of isolation success and the titers of the virus within these pools (2.3–6.3 log10 PFU/ml) suggested that virus amplification was greatest and isolation most effective during summer when adult and nestling birds were present and least effective after the birds had fledged and migrated south for the winter (Table 1A). Similar findings were reported for other strains of BCRV (Moore et al. 2007, Brown et al. 2009).
Table 1.
Summary of field sampling: virus isolation attempts from swallow bugs (A) and testing blood samples from cliff swallows for virus and antibody (B)
| Date | Locality | Sample | Nests | Pools | Bugs | Isolates | Titer rangea |
|---|---|---|---|---|---|---|---|
| A. Cliff swallow bug isolation attempts | |||||||
| May 2004 | Hwy 86 Coachella | Hand-picked | 8 | 9 | 90 | 0 | |
| June 2005 | Stone Lakes NWR | Hand-picked | nd | 40 | 1–6 | 0 | |
| July 2005 | Stone Lakes NWR | Hand-picked | 9 | 16 | nd | 3 | 1.3–2.5 |
| Oct. 2005 | Stone Lakes NWR | Hand-picked | 7 | 12 | 202 | 0 | |
| Nov. 2005 | Stone Lakes NWR | Hand-picked | 17 | 23 | 376 | 0 | |
| July 2006 | Stone Lakes NWR | Hand-picked | 12 | 30 | 270 | 10 | 2.3–6.3 |
| EIA (P/N >2) |
|||||||
| n | Isolation | STLV | WEEV | Other viruses | |||
| B. Cliff swallows tested for virus and antibody | |||||||
| 1996–2003 | Coachella Valley | Mist net | 43 | nd | nd | 0 | 0 |
| Kern County | Mist net | 261 | nd | nd | 0 | 0 | |
| May 2004 | Hwy 86 Coachella | Nestling-blood | 30 | 0 | nd | 0 | 0 |
| Nestling-oral swab | 28 | 0 | nd | 0 | 0 | ||
| June 2004 | Stone Lakes NWR | Mist net | 123 | nd | 0 | 0 | 1 SLEV, 1 WNV |
| June 2005 | Stone Lakes NWR | Mist net | 104 | 0 | 6 | 0 | 1 SLEV |
| June 2006 | Stone Lakes NWR | Mist net | 76 | nd | 1 | 4 | 1 WNV |
| July 2006 | Stone Lakes NWR | Mist net | 52 | nd | 0 | 0 | 0 |
nd, not done or no data.
Log10 PFU/ml.
During initial isolation and characterization, we found that STLV grew well on Vero cell culture but failed to amplify in C6/36 cells. This was unexpected, because C6/36 Ae. albopictus cells are generally permissive for the growth of most arboviruses (Kuno 2007). Direct sequence analyses of amplicon products (E1–3′UTR)from three Vero cell isolates made during summer 2005, demonstrated a 100% genetic identity, with a relatively minor 4.4 and 5.6% nucleotide divergence from single strains of FMV and BCRV sequences, respectively. Bootstrapping replicates indicated that the three STLV isolates reproducibly clustered within their own lineage among WEEV-serocomplex viruses isolated from cliff swallow bugs. Additional genetic studies are planned to compare the STLV variant with multiple strains of BCRV using the same viral envelope glycoprotein-coding region, covering the entire PE2 gene used previously (Pfeffer et al. 2006).
Avian Infection
Attempts to isolate STLV or other viruses from either oral swabs or blood samples collected from nestling cliff swallows in Coachella Valley were unsuccessful; virus also was not found in 90 swallow bugs collected concurrently and tested by Vero cell culture (Table 1B). In addition, virus was not isolated from 104 blood samples from adult cliff swallows collected in June 2005 at Stones Lakes NWR. We attempted to detect evidence of past infection by testing sera from adult swallows for antibodies (Table 1B). Overall, sera from 659 birds were tested for evidence of previous WEEV-serocomplex virus infection, of which four gave a weak positive reaction by EIA (positive/negative [P/N] well ratio range 2.1–2.4) that could not be confirmed by PRNT using WEEV or STLV. In addition, four birds had positive EIAs with a flavivirus antigen, and these were confirmed as either SLEV or WNV, with PRNT titers ≥1:40. Of these same 659 sera, 355 were retested by EIA using a crude STLV antigen made in Vero cells, of which six birds had a weak positive reaction (P/N range = 2.1–2.6). We again failed to confirm these EIA positives by PRNT using STLV; birds EIA positive for STLV were different from those birds that were weakly positive by EIA for WEEV antigen (see above). House sparrows were not observed nesting or collected by mist net at our Coachella, Kern, or Stone Lakes cliff swallow study sites, although they were abundant at other habitats in these general areas (Reisen et al. 2008b, Reisen et al. 2009).
Avian Experimental Infections
Adult chickens inoculated with 3.3 log10 PFU of STLV failed to produce a viremia on days 1–5 postinfection (pi) above our plaque assay detection threshold titer of 1.7 log10 PFU/ml. This negative finding was expected based on previous infections with WEEV (Reisen et al. 1994). Chickens were held 6 wk, then bled, and sera were tested for antibodies to STLV and WEEV by both EIA and PRNT (Table 2). Responses measured by EIAs to WEEV were distinct from STLV. It is interesting that the EIA response seemed markedly greater than the PRNT response. Because PRNT titers remained ≤1:20 on two tests, it was not possible to do cross-PRNTs among viral strains to determine antigenic relatedness.
Table 2.
Antibody response of adult chickens inoculated with WEEV or STLV measured by EIA (A) or PRNT (B) against WEEV, BCRV, and STLV viruses
| Infecting virus |
Antigen or virus |
|||
|---|---|---|---|---|
| WEEV | BCRV | STLV | ||
| A. EIA results (P/N ratio) | ||||
| Band | ||||
| 1889 | WEEV | 5.2 | 0.9 | nd |
| 1890 | WEEV | 7.7 | 1.7 | nd |
| 1298 | STLV | 1.0 | 7.1 | 2.9 |
| 1401 | STLV | 1.9 | 10.1 | 2.9 |
| 1403 | STLV | 1.0 | 4.9 | 3.5 |
| B. PRNT results (reciprocal of end point titer) | ||||
| Band | ||||
| 1889 | WEEV | 20 | 20 | <20 |
| 1890 | WEEV | <20 | <20 | <20 |
| 1298 | STLV | <20 | 20 | 20 |
| 1401 | STLV | <20 | <20 | 20 |
| 1403 | STLV | <20 | <20 | <20 |
nd, not done.
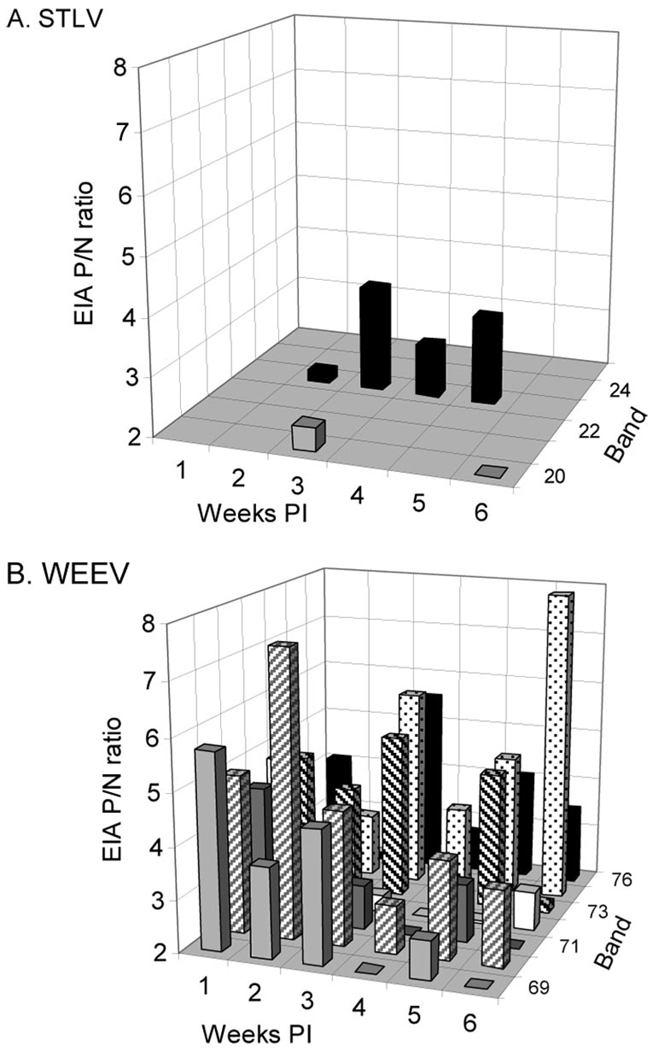
Few house sparrows inoculated with 4.5 log10 PFU of STLV produced detectable viremias on day 1 pi (Table 3); all samples were negative on 2–7 dpi, suggesting that the low titered day-1 viremias may have been residual inocula and not replicating infections. In contrast, house sparrows inoculated with 1.9 log10 PFU of the COA592 strain of WEEV produced a mean ± SE viremia of 6.5 ± 0.96 log10 PFU/ml on day 1 pi (Reisen et al. 2003). STLV also was not markedly immunogenic in adult house sparrows. Similar to chickens, PRNT titers on week 6 pi were marginally detectable, with most titers ≤1:20; a single bird had a titer of 1:40 (Table 3). In house sparrows, our EIA produced intermittent results using homologous antigen, with few sera having EIA P/N ratios >2.0 (Fig. 2A). In marked contrast, house sparrows infected with WEEV produced EIA P/N ratios consistently positive on all bleed dates (Fig. 2B). Data for WEEV indicated that our assays were sensitive and that our EIA detector antibody readily recognized antibody from this bird species (Table 2).
Table 3.
Viremia and antibody responses of six adult house sparrows inoculated with 4.5 log10 PFU/ml of STLV (PRNT was measured 6 wk postinfection)
| Band | Viremiaa | STLV PRNTb | BCRV PRNTb |
|---|---|---|---|
| 20 | <1.7 | <20 | 20 |
| 21 | 2.0 | 40 | 20 |
| 22 | <1.7 | 20 | 20 |
| 23 | 2.3 | 20 | <20 |
| 24 | 2.4 | 20 | <20 |
| 25 | <1.7 | 20 | <20 |
Viremia in log10 PFU/ml on day 1 postinfection. Minimal detection threshold for plaque assay was 1.7 log10 PFU/ml.
Reciprocal of endpoint neutralization of 80% of >75 plaques of STLV or BCRV.
Fig. 2.
Antibody response of individual house sparrows to STLV (A) and WEEV (B). EIA optical density ratio for antigen P and N wells are presented for each bird by band number on weeks 1–6 postinoculation.
Mosquito Vector Competence
Twenty females that blood fed on the STLV33 viral strain survived the 2-wk incubation period at 26°C and were tested for viral infection with negative results. Because females were not infected, expectorate samples were not tested. In contrast, 22 and 21 of 25 Cx. tarsalis females per group that fed on comparable titers of the IMP181 or BFS1703 strains of WEEV, respectively, became infected (Reisen et al. 2008a).
Discussion
The discovery of STLV, a virus in the WEEV-serocomplex closely related to FMV and BCRV, was serendipitous, but indicated that this group of viruses may be widely distributed throughout the range of O. vicarius. Based on partial sequencing data, three isolates from STLV were identical and showed <6% divergence from FMV and BCRV, indicating close relatedness. At this point, we feel that STLV should be considered a strain of BCRV until further genetic and serological evaluations are completed. Our current serological studies were inhibited by the low PRNT responses of chickens and house sparrows following experimental inoculation.
The ecology of STLV differs markedly from WEEV. Based on our review of the available literature and attempts to infect adult house sparrows experimentally, we concluded that STLV is primarily an infection of swallow bugs. These data and conclusions are in agreement with similar recent experimental infections of house sparrows with BCRV (Huyvaert et al. 2008) that suggest use of nestling birds may be critical for cimicid infection and transmission. Both viral strains were weakly immunogenic, and EIA and PRNT responses in adult chickens and house sparrows were marginal in comparison with WEEV. In agreement, comparatively few adult swallows collected by mist netting were EIA positive, and none of these EIA positives could be confirmed by PRNT. In marked contrast, FMV was isolated repeatedly from sequential bleeds from naturally infected house sparrow nestlings, was isolated significantly more frequently from house sparrow than cliff swallow adults, and neutralizing antibodies were present in both avian species (Hayes et al. 1978, Scott et al. 1984). Our results suggest that transmission of STLV in California from cimicid to bird to cimicid may rely on nestling house sparrow/cliff swallow that may be considerably more susceptible than adults, producing elevated viremias and frequently succumbing to infection (Scott et al. 1984).
Like BCRV (Moore et al. 2007), STLV replication within swallow bugs seems limited to periods of the year when birds are present and the bugs are metabolically and reproductively active. Attempts to isolate virus from bugs during winter were unsuccessful, and titers of virus within pools of our initial isolations made in 2004 just after the birds had fledged were low (1.3–2.5 log10 PFU/ml) compared with isolates made from bugs collected from active nests during 2005 (2.3–6.5 log10 PFU/ml). We did not isolate virus from cliff swallow nestlings or from mist netted HY and AHY birds; however, FMV (Scott et al. 1984) and BCRV have been isolated repeatedly from cliff swallow nestlings east of the Continental Divide. It is interesting that the viremia titers and isolation rates at their study areas were always higher in nestling house sparrows, an aggressive invading European bird species that frequently exploits cliff swallow nests. Based on field data, house sparrows seemed more susceptible to and more frequently infected with FMV infection than cliff swallows (Scott et al. 1984). However, adult house sparrows were relatively refractory to experimental infection with BCRV (Huyvaert et al. 2008) and STLV reported here. Virus did not seem to replicate and few plaques were found in viremia samples taken from birds during 1 and 2 dpi. More surprisingly, these viruses did not seem to be consistently immunogenic, producing infrequent and low PRNT titers. These experimental infection results may help explain our inability to find adult birds with PRNT antibody, although a few had low P/N EIA ratios. In addition, there were no house sparrows at our California nesting colonies, and it may be that highly susceptible nestling house sparrows are necessary to amplify virus but succumb to infection negating the collection of antibody positive adults (Huyvaert et al. 2008).
Research on the FMV-complex, cliff swallows, and swallow bugs has left important questions unanswered. For example, are the viruses within the FMV-complex distinct, having evolved separation because of cliff swallow colony isolation (Pfeffer et al. 2006) or are they variants of FMV, similar to different lineages within WEEV (Kramer and Fallah 1999). Although considerable infection and field studies have been done with house sparrows, experimental infections with adult and nestling cliff swallows need to be performed to describe their viremia and antibody responses to fully assess the potential of this avian species to serve as an amplification host. Current data indicate that this virus may not replicate within birds and that repeated isolations from cliff swallow nestlings could have been from nonpropagative viremias created by repeated feeding by large numbers of highly infectious swallow bugs on this small sized host, similar to WNV viremias created by blood feeding mosquitoes on house finches (Reisen et al. 2007). This could allow for continued and high frequency nonviremic transmission when the bugs were active, similar to that seen by cofeeding ticks infected with tick-borne encephalitis virus (Labuda et al. 1993) or with Thogoto virus (Jones et al. 1990). In addition, FMV/STLVs seem to be passaged transtadially and transgenerationally within swallow bug populations (Brown et al. 2009); however, this has not been shown for WEEV in either Culex (Hardy et al. 1979) or Aedes (Kramer et al. 1998) mosquitoes and has been documented infrequently for alphaviruses (Kay 1982).
Failed vector competence studies with Cx. tarsalis mosquitoes and culture attempts with Aedes albopictus C6/36 cells indicated that STLV and related viruses may be very specific for cimicid bugs for replication. It is interesting that these viruses grew well in mammalian baby hamster kidney and Vero cells but did not replicate well in intact birds following needle inoculation. In marked contrast, WEEV grows well in all these cell types and hosts.
Our attempts to isolate WNV or other arboviruses from cliff swallows adults or nestlings were negative, indicating that the colonies we sampled were not a site of marked WNV amplification. This was especially interesting, because WNV has been repeatedly active throughout Coachella Valley (Reisen et al. 2008b), Kern County (Reisen et al. 2009), and Sacramento County, including Stone Lakes NWR (Wright et al. 2006, 2007), and previously infected birds were detected during this study. Also interesting was the recovery of cliff swallows previously infected with SLEV during summers 2004 and 2005. Few birds in California have been found with evidence of SLEV infection, because there has been no SLEV transmission documented in California since the invasion by WNV in 2003.
Acknowledgments
We thank Sandra Garcia and Keira Simmons, CVEC, for assistance with virus isolation and serological testing. Funding for these studies was provided by the Pacific Southwest Regional Center for Excellence AI65359 and National Institutes of Health National Institute of Allergy and Infectious Diseases grant AI55607.
References Cited
- Aitken THG. An in vitro feeding technique for artificially demonstrating virus transmission by mosquitoes. Mosq. News. 1977;37:130–133. [Google Scholar]
- Brown CR, Brown MB. Nest spacing in relation to settlement time in colonial cliff swallows. Anim. Behav. 2000;59:47–55. doi: 10.1006/anbe.1999.1277. [DOI] [PubMed] [Google Scholar]
- Brown CR, Sethi RA. Mosquito abundance is correlated with cliff swallow (Petrochelidon pyrrhonota) colony size. J. Med. Entomol. 2002;39:115–120. doi: 10.1603/0022-2585-39.1.115. [DOI] [PubMed] [Google Scholar]
- Brown CR, Moore AT, Knutie SA, Komar N. Overwintering of infectious Buggy Creek virus (Togaviridae: Alphavirus) in Oeciacus vicarius (Hemiptera: Cimicidae) in North Dakota. J. Med. Entomol. 2009;46:391–394. doi: 10.1603/033.046.0227. [DOI] [PubMed] [Google Scholar]
- Brown CR, Moore AT, Young GR, Padhi A, Komar N. Isolation of Buggy Creek virus (Togaviridae: Alphavirus) from field-collected eggs of Oeciacus vicarius (Hemiptera: Cimicidae) J. Med. Entomol. 2009;46:375–379. doi: 10.1603/033.046.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher CH, Monath TP, Muth DJ, Lazuick JS, Trent DW, Francy DB, Kemp GE, Chandler FW. Characterization of Fort Morgan virus, an alphavirus of the western equine encephalitis virus complex in an unusual ecosystem. Am. J. Trop. Med. Hyg. 1980;29:1428–1440. doi: 10.4269/ajtmh.1980.29.1428. [DOI] [PubMed] [Google Scholar]
- Chiles RE, Reisen WK. A new enzyme immunoassay to detect antibodies to arboviruses in the blood of wild birds. J. Vector Ecol. 1998;23:123–135. [PubMed] [Google Scholar]
- Clark GW, Swinehart B. Blood parasitism in cliff swallows from the Sacramento Valley. J. Protozool. 1966;13:395–397. doi: 10.1111/j.1550-7408.1966.tb01928.x. [DOI] [PubMed] [Google Scholar]
- Elnaien D-EA, Kelley K, Wright SA, Laffey R, Yoshimura G, Armijos V, Reed M, Goodman G, Reisen WK, Brown DA. Epidemic amplification of West Nile virus in Sacramento and Yolo counties, June–September 2005. Proc. Calif. Mosq. Vector Control Assoc. 2006;74:18–20. [Google Scholar]
- Hardy JL, Reeves WC, Bruen JP, Presser SB. Vector competence of Culex tarsalis and other mosquito species for western equine encephalomyelitis virus. Arctic Trop. Arboviruses. 1979;10:157–171. [Google Scholar]
- Hayes RO, Francy DB, Lazuick JS, smith GC, Gibbs EPJ. Role of the swallow bug (Oeciacus vicarius) in the natural cycle of a western equine encephalitis-related alphavirus. J. Med. Entomol. 1978;14:257–262. [Google Scholar]
- Hopla CE, Francy DB, Calisher CH, Lazuick JS. Relationship of cliff swallows, ectoparasites and an alphavirus in west-central Oklahoma. J. Med. Entomol. 1993;30:267–272. doi: 10.1093/jmedent/30.1.267. [DOI] [PubMed] [Google Scholar]
- Huyvaert KP, Moore AT, Panella NA, Edwards EA, Brown MB, Komar N, Brown CR. Experimental inoculation of House sparrows (Passer domesticus) with Buggy Creek virus. J. Wildl. Dis. 2008;44:331–340. doi: 10.7589/0090-3558-44.2.331. [DOI] [PubMed] [Google Scholar]
- Jones LD, Davies CR, Williams T, Cory J, Nuttall PA. Non-viraemic transmission of Thogoto virus: vector efficiency of Rhipicephalus appendiculatus and Amblyomma variegatum. Trans. R. Soc. Trop. Med. Hyg. 1990;84:846–848. doi: 10.1016/0035-9203(90)90104-m. [DOI] [PubMed] [Google Scholar]
- Kay BH. Three modes of transmission of Ross River virus by Aedes vigilax (Skuse) Aust. J. Exp. Biol. Med. Sci. 1982;60:339–344. doi: 10.1038/icb.1982.37. [DOI] [PubMed] [Google Scholar]
- Kramer LD, Fallah HM. Genetic variation among isolates of western equine encephalomyelitis virus from California. Am. J. Trop. Med. Hyg. 1999;60:708–713. doi: 10.4269/ajtmh.1999.60.708. [DOI] [PubMed] [Google Scholar]
- Kramer LD, Reisen WK, Chiles RE. Vector competence of Aedes dorsalis (Diptera: Culicidae) from Morro Bay, California, for western equine encephalomyelitis virus. J. Med. Entomol. 1998;35:1020–1024. doi: 10.1093/jmedent/35.6.1020. [DOI] [PubMed] [Google Scholar]
- Kuno G. Host range specificity of flaviviruses: correlation with in vitro replication. J. Med. Entomol. 2007;44:93–101. doi: 10.1603/0022-2585(2007)44[93:hrsofc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Labuda M, Jones LD, Williams T, Danielova V, Nuttall PA. Efficient transmission of tick-borne encephalitis virus between cofeeding ticks. J Med. Entomol. 1993;30:295–299. doi: 10.1093/jmedent/30.1.295. [DOI] [PubMed] [Google Scholar]
- Monath TP, Lazuick JS, Cropp CB, Rush WA, Calisher CH, Kinney RM, Trent DW, Kemp GE, Bowen GS, Francy DB. Recovery of Tonate virus (“Bijou Bridge” strain), a member of the Venezuelan equine encephalomyelitis virus complex, from cliff swallow nest bugs (Oeciacus vicarius) and nestling birds in North America. Am. J. Trop. Med. Hyg. 2007;29:969–983. doi: 10.4269/ajtmh.1980.29.969. [DOI] [PubMed] [Google Scholar]
- Moore AT, Edwards EA, Brown MB, Komar N, Brown CR. Ecological correlates of buggy creek virus infection in Oeciacus vicarius, southwestern Nebraska, 2004. J Med. Entomol. 2007;44:42–49. doi: 10.1603/0022-2585(2007)44[42:ecobcv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Pfeffer M, Foster JE, Edwards EA, Brown MB, Komar N, Brown CR. Phylogenetic analysis of Buggy Creek virus: evidence for multiple clades in the Western Great Plains, United States of America. Appl. Environ. Microbiol. 2006;72:6886–6893. doi: 10.1128/AEM.00868-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers AM, Brault AC, Shirako Y, Strauss EG, Kang W, Strauss JH, Weaver SC. Evolutionary relationships and systematics of the alphaviruses. J. Virol. 2001;75:10118–10131. doi: 10.1128/JVI.75.21.10118-10131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves WC, Asman SM, Hardy JL, Milby MM, Reisen WK. Epidemiology and control of mosquito-borne arboviruses in California, 1943–1987. Sacramento, CA: California Mosquito Vector Control Association; 1990. [Google Scholar]
- Reisen WK, Presser SB, Lin J, Enge B, Hardy JL, Emmons RW. Viremia and serological responses in adult chickens infected with western equine encephalomyelitis and St. Louis encephalitis viruses. J. Am. Mosq. Control Assoc. 1994;10:549–555. [PubMed] [Google Scholar]
- Reisen WK, Chiles RE, Martinez VM, Fang Y, Green EN. Experimental infection of California birds with western equine encephalomyelitis and St. Louis encephalitis viruses. J. Med. Entomol. 2003;40:968–982. doi: 10.1603/0022-2585-40.6.968. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Fang Y, Martinez V. Is nonviremic transmission of West Nile virus by Culex mosquitoes (Diptera: Culicidae) nonviremic? J. Med. Entomol. 2007;44:299–302. doi: 10.1603/0022-2585(2007)44[299:intown]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Fang Y, Brault AC. Limited interdecadal variation in mosquito (Diptera: Culicidae) and avian host competence for Western equine encephalomyelitis virus (Togaviridae: Alphavirus) Am. J. Trop. Med. Hyg. 2008a;78:681–686. [PubMed] [Google Scholar]
- Reisen WK, Lothrop HD, Wheeler SS, Kensington M, Gutierrez A, Fang Y, Garcia S, Lothrop B. Persistent West Nile virus transmission and the displacement St. Louis encephalitis virus in southeastern California, 2003–2006. J. Med. Entomol. 2008b;45:494–508. doi: 10.1603/0022-2585(2008)45[494:pwnvta]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen WK, Carroll BD, Takahashi R, Fang Y, Garcia S, Martinez VM, Quiring R. Repeated West Nile virus epidemic transmission in Kern County, California, 2004–2007. J. Med. Entomol. 2009;46:139–157. doi: 10.1603/033.046.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer JR, Hines JE, Fallon J. The North American Breeding Bird Survey, Results and Analysis 1966–2003. Version 2004. 1. Laurel, MD: USGS Patuxent Wildlife Research Center; 2004. [Google Scholar]
- Scott TW, Bowen GS, Monath TP. A field study of the effects of Fort Morgan virus, an arbovirus transmitted by swallow bugs, on the reproductive success of cliff swallow and symbiotic house sparrows in Morgan County, Colorado, 1976. Am. J. Trop. Med. Hyg. 1984;33:981–991. doi: 10.4269/ajtmh.1984.33.981. [DOI] [PubMed] [Google Scholar]
- Wright SA, Armijos V, Wheeler SS, Kelley K, Harvey T, Reisen WK, Elnaimen D-EA, Brown DA. Local amplification of WNV in wild bird populations in Sacramento and Yolo Counties, California. Proc. Mosq. Vector Control Assoc. Calif. 2006;74:16–17. [Google Scholar]
- Wright SA, Wheeler SS, Perez B, Armijos V, Kelley K, Reisen W, Macedo PA. Avian herd immunity and WNV in Sacramento County. Proc. Mosq. Vector Control Assoc. Calif. 2007;75:23–24. [Google Scholar]