Abstract
Background and Objective
Port wine stains (PWS) are heterogeneous vascular malformations that can be treated with vascular-selective pulsed dye lasers (PDL). Hypertrophic PWS, especially inadults, are consistently less responsive to PDL. Furthermore, many PWS that respond well initially to PDL treatment may reach a response plateau, becoming unresponsive to further PDL treatments, a phenomenon termed “treatment resistance.” Based on the theory of selective photothermolysis, vessels in such lesions may also bespecifically targetedwitha 755 nm laserthat has selectivity for deoxyhemoglobin as well as oxyhemoglobin and increased depth of skin penetration.
StudyDesign/PatientsandMethods
Retrospective case review of 20 patients with either hypertrophic or PDL-resistant PWS treated with a 755 nm laser alone or in combination with other lasers, including PDL.
Results
Hypertrophic PWS showed significant lightening after treatment with a 755 nm laser in combination with PDL. Most PDL-resistant PWS showed moderate improvement after treatment with either a 755 nm laser alone or in combination with another laser, including PDL. Some lesions showed only mild improvement or did not respond. Serious side effects were infrequent. Most commonly encountered complications included pain, edema, bullae, crusting, and rare scarring.
Conclusions
Alexandrite 755 nm laser can be useful for the treatment of hypertrophic and treatment-resistant PWS in adult and pediatric patients. Complications are infrequent and predictable. Careful attention tousing a fluence at or near the threshold for clinical response with this deeply penetrating laser is essential to prevent serious sequelae.
Keywords: port wine stain; resistant; PDL; alexandrite; Nd:YAG; laser; treatment; endpoint; 595 nm; 755 nm; 1,064 nm
INTRODUCTION
Port wine stains (PWS) are primarily venous micro-vascular malformations of the skin that commonly occur on the face. They are found in 0.3–0.5% of newborns, and may be associated with Sturge–Weber syndrome. PWS begin as thin macular lesions in infancy and early childhood and tend to thicken and darken with age due to decreased sympathetic innervation that produces progressive vessel ectasia. Skin lesion depth ranges from 1 to 5 mm [1–3].
PWS may be treated effectively with a variety of vascular-selective lasers. The most widely used lasers for PWS are the pulsed dye lasers (PDL), which penetrate up to 2 mm into the skin with yellow light wavelengths that are strongly absorbed by both oxyhemoglobin (HbO2) and deoxyhemoglobin (Hb). Early, thin PWS lesions, such as in newborns, respond best to this wavelength, while hypertrophic and darker lesions respond significantly less consistently [1–3]. Complete clearance of any PWS is difficult to achieve even with early intervention, and most laser-treated PWS continue to darken if left untreated [4]. Furthermore, many PWS that respond well initially to PDL treatment may reach a response plateau, becoming unresponsive to further PDL treatments, a phenomenon termed “treatment resistance” [1]. While there are likely multiple reasons for treatment resistance and incomplete clearance with PDL [5,6], one factor is the limited ability of PDL to affect ectatic vessels residing deeper than 2 mm in the skin.
For this reason, near-infrared (IR) lasers at wavelengths with greater tissue penetration and weaker absorption by HbO2 and Hb (http://omlc.ogi.edu/spectra/hemoglobin/index.html) have been used to treat hypertrophic and resistant PWS [6,7]. The long-pulsed 1,064 nm Nd:YAG and 755 nm Alexandrite lasers penetrate 50–75% deeper into the skin than the PDL [3]. Because near-IR lasers have a much lower absorption coefficient in blood than PDL, illustrated in Figure 1 (adapted from Dr. Scott Prahl: http://omlc.ogi.edu/spectra/hemoglobin), vessel damage with these devices requires higher fluences [1,3]. Li et al. [7] reported that a long-pulsed 755 nm laser is particularly effective for hypertrophic and purple PWS as compared to PDL, producing occasional hypopigmentation, but no scarring. When McGill et al. [8] compared Alexandrite, Nd:YAG, PDL, potassium titanyl phosphate (KTP), and intense pulsed light (IPL) test patches in PDL-treated vascular malformations, Alexandrite-treated test patches showed the greatest mean improvement in color, with subsequent fading in 10 of 18 treated patients. There was also significantly decreased post-treatment mean vessel diameter, which did not occur with other tested modalities. The mean pre-treatment vessel diameter was predictive of responsiveness to Alexandrite laser treatment. The KTP and Nd:YAG lasers were least effective. However, hyper-pigmentation developed in four patients and scarring in one patient after treatment with the Alexandrite laser [8].
Fig. 1.
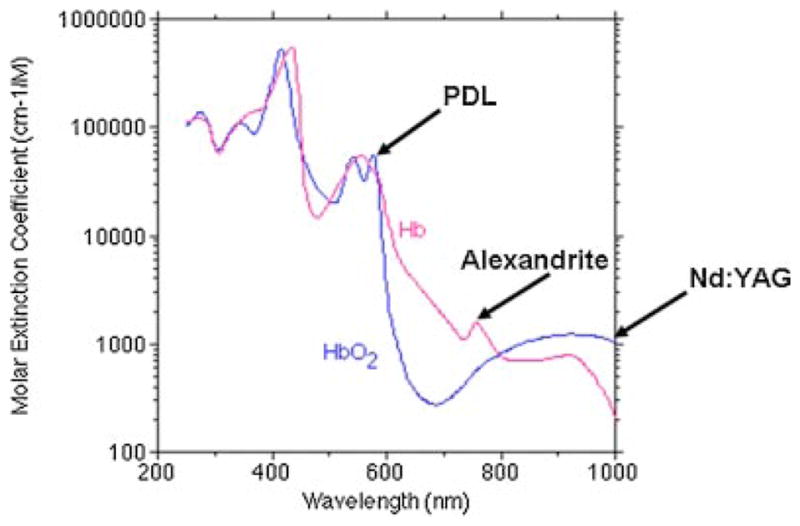
Optical absorption spectrum of hemoglobin and deoxyhemoglobin, adapted from Dr. Scott Prahl: http://omlc.ogi.edu/spectra/hemoglobin.
Many deeply situated PWS may be treated effectively with the 1,064 nm Nd:YAG laser [1]. However, a 755 nm wavelength may provide two advantages over a 1,064 nm wavelength. First, the 755 nm absorption coefficient of Hb is greater than that of HbO2; in theory, the Alexandrite laser therefore preferentially damages veins more than arteries. The opposite is true for Nd:YAG lasers at 1,064 nm, a wavelength with stronger absorption by HbO2 than Hb. Second, there is a very narrow therapeutic window for the 1,064 nm wavelength. In a comparative trial of Nd:YAG and PDL lasers for adult PWS, Yang et al. [1] reported that Nd:YAG laser fluences greater than about 1.2× minimal purpura dose (MPD) can produce extensive deep dermal damage and result in scarring. The authors also reported that the Nd:YAG laser MPD varied greatly among patients, and within each PWS. PWS are heterogeneous lesions, such that in practice, the narrow therapeutic fluence range of Nd:YAG reduces both efficacy and safety. Formation of methemoglobin (metHb), an oxidized species that appears during laser-induced heating of blood vessels, may largely account for the narrow therapeutic fluence range of 1,064 nm Nd:YAG lasers [9]. At 1,064 nm, metHb has much stronger absorption than either HbO2 or Hb. The Nd:YAG laser can therefore induce “runaway” absorption in blood, which tends to produce an all-or-nothing fluence–response relationship [9]. The effect of metHb formation is, in theory, less pronounced at 755 nm.
The purpose of this study was to evaluate in adult and pediatric patients with either hypertrophic or PDL-resistant PWS the treatment response to a 755 nm laser alone or in combination with other lasers, including PDL. This was done by a retrospective case review. Safety was also evaluated by searching for adverse events, such as scarring or dyspigmentation.
MATERIALS AND METHODS
We identified patients referred to the Massachusetts General Hospital Laser and Cosmetic Dermatology Center, and Beckman Laser Institute and Medical Clinic, for treatment of challenging PWS. All patients with PWS who underwent treatment with a 755 nm laser alone or in combination with another laser modality were selected. Case charts were reviewed, specifically for history of each PWS lesion, prior treatments and responses, and treatment parameters with a 755 nm laser, PWS responses, and complications. Responses to laser therapy as documented in the patient charts by the treating physician on the day of each visit were reviewed.
Patient characteristics are summarized in Table 1. Patients with hypertrophic PWS (n = 3) were male and female adults between the ages of 44 and 59 years, with Fitzpatrick skin types 1 and 2. Lesions were located on the face in the right or left V1/V2 distribution. One patient had features of Sturge–Weber syndrome. None were previously treated with PDL. These patients were treated with Alexandrite laser alone, or in combination with concurrent or alternating PDL. Patients with resistant PWS (n = 17) were male and female, ranging in age from 5 months to 46 years, with Fitzpatrick skin types 1–3. Most patients were children between the ages of 2 and 16 years (12 patients), with one baby of 5 months of age. Most lesions were located on the face in the right or left V1/V2 distribution. Two patients had features of Sturge–Weber syndrome. All were treated previously with PDL, with 2–45 sessions, with most patients reaching a treatment plateau. They were subsequently treated with a 755 nm laser alone, or with concurrent or alternating sessions of PDL or another laser modality. Most pediatric patients were treated in an operating room (OR) setting under general anesthesia. Older patients (16–46 years) were treated in an office setting with local anesthesia, sedatives, and perioperative narcotic analgesics as needed. Intra-ocular eye shields were used for lesions in immediate proximity to ocular structures.
TABLE 1.
Patient Characteristics and Treatment Responses
| No. | Age (years) |
Sex | Location | SWS | PDL | Response/ description |
Plateau with PDL |
755 nm Tx # |
Treated with PDL |
Response (lightening) |
Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypertrophic PWS | |||||||||||
| 1 | 44 | F | R V2 | N | 0 | Lesion hypertrophic and dark | N | 2 | Y | Significant | None |
| 2 | 59 | M | L V2 | N | 0 | Lesion hypertrophic and dark | N | 2 | Y | Significant | None |
| 3 | 46 | M | L V1 | Y | 0 | Lesion hypertrophic and dark | N | 4 | Y | Significant | None |
| Resistant PWS | |||||||||||
| 1 | 2 | F | R V1/V2 | N | 14 | Mild on face, significant on temple | Y | 9 | Y | Moderate | None |
| 2 | 16 | M | R V1/V2 | N | 13 PDL, 1 Nd:YAG | Significant | Y | 3 | N | Moderate | None |
| 3 | 37 | F | R V1/V2 | N | Multiple | Mild | Y | 4 | N | Moderate | None |
| 4 | 10 | F | R V1/V2 | N | 45 | Moderate | Y | 8 | Y | Moderate | None |
| 5 | 16 | M | L V2 | N | 2 | Mild | Y | 8 | Y | Moderate | Isolated scar, improved; temporary hypopigmentation |
| 6 | 24 | F | Trunk | N | 3 | Moderate | Y | 3 | N | Moderate | None |
| 7 | 9 | F | L V2 | N | 18 | Significant | Y | 3 | N | Moderate | None |
| 8 | 7 | M | L V2 | N | 6 | Moderate | N* | 10 | Y | Moderate | None |
| 9 | 5months | M | R V3 | N | 8 | Moderate | Y | 3 | Y | Moderate | None |
| 10 | 28 | M | L V1/V2 | N | 14 | Moderate | Y | 5 | Y | Moderate | Blister, crusting, isolated scar |
| 11 | 4 | F | L V1/V2 | N | 12 | Moderate | Y | 5 | Y | Moderate | None |
| 12 | 2 | M | L V2 | N | 6 | Moderate | Y | 4 | N | Moderate | None |
| 13 | 46 | F | Forehead | N | 19 | Significant | N | 6 | N | Significant | None |
| 14 | 5 | F | L V1/V2 | N | 8 | Significant | N** | 2 | Y | Mild | None |
| 15 | 5 | F | L V1/V2 | N | 12 | Significant | Y | 2 | N | Mild | Blister |
| 16 | 2 | F | R V1/V2 | Y | 13 | Significant | Y | 2 | N | Mild | None |
| 17 | 12 | F | R V2 | N | 20 | Significant | Y | 2 | N | None | None |
Darkened/thickened over time.
Darkened over 1.5 years.
The target treatment endpoint with the Alexandrite 755 nm laser—transient gray discoloration of the skin followed by lasting purpura—was attempted as much as possible for all PWS, and achieved in nearly all cases. Indeed, different fluences and spot sizes were necessary to achieve the same tissue endpoint in each PWS or portion of an individual PWS. No areas were double-pulsed.
Pulse duration with the 755 nm laser was 3 milliseconds, with spot sizes of 8–12 mm, concurrent cryogen spray of 40–60 milliseconds spurt duration with 40 milliseconds delay, and sometimes combined with forced cold air-cooling. Fluences ranged from 35 to 100 J/cm2, with most between 60 and 85 J/cm2, adjusted as needed to achieve the correct treatment endpoint at each session for each lesion.
RESULTS
All three adult patients with hypertrophic PWS had significant lightening after treatment with Alexandrite laser, either in combination with PDL after two sessions (Fig. 2); or Alexandrite laser alternating with PDL (four sessions with Alexandrite laser, two sessions with PDL). There were no complications. Side effects were the predictable sequelae of selective photothermolysis for PWS: purpura, edema, erythema, and mild-to-moderate pain, followed by healing with lightening of the PWS. The combination of Alexandrite and PDL in the same session produced more rapid and significant lightening of the hypertrophic PWS without increased adverse events.
Fig. 2.
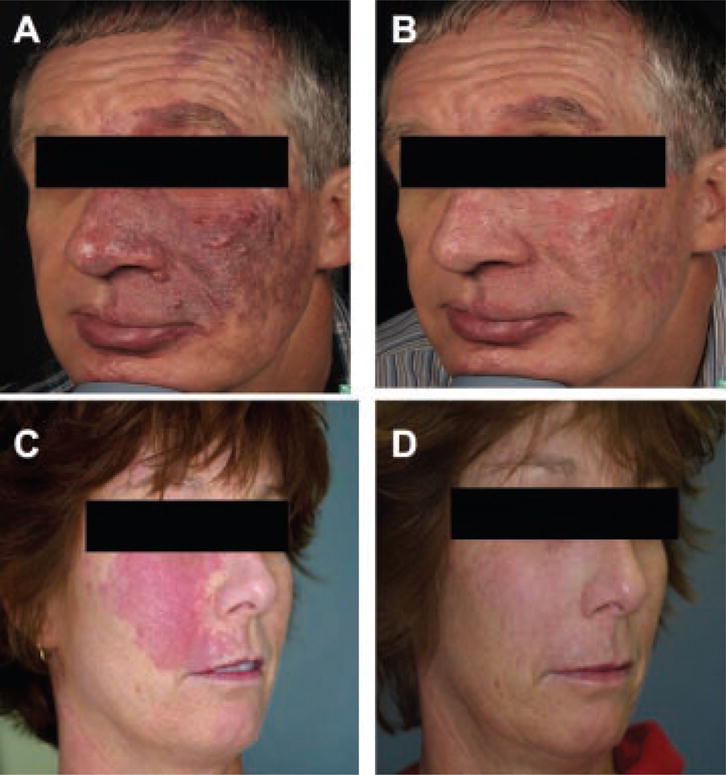
Hypertrophic PWS treated with Alexandrite laser with concurrent PDL (A,C: before; B,D: after treatment).
Twelve of the 17 patients with resistant PWS had moderate lightening after treatment with Alexandrite laser alone, or in combination with PDL (7 patients) (Fig. 3). Most treated patients showed a mild noticeable lightening of their PWS after each treatment session. The number of treatment sessions with Alexandrite laser varied from 3 to 10. Most of these lesions showed moderate to significant improvement with prior PDL treatments (2–45 sessions) before reaching a responsiveness plateau.
Fig. 3.
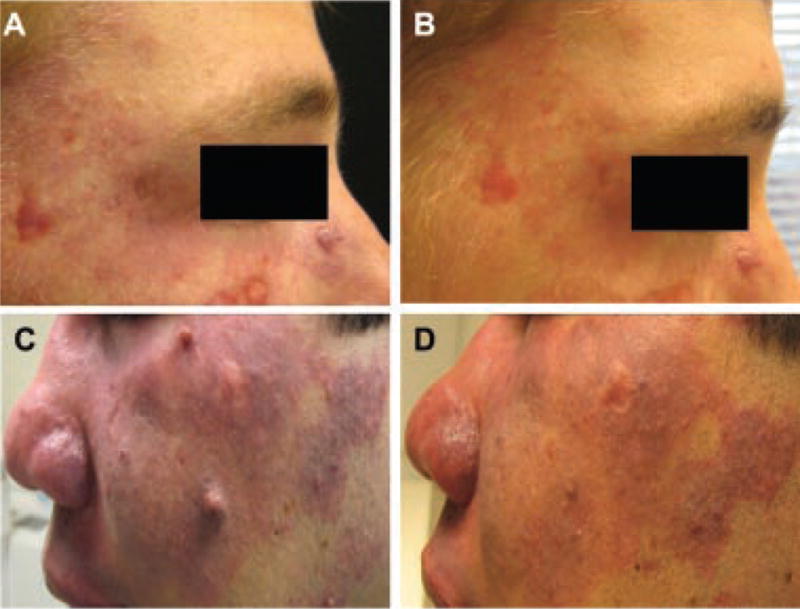
PDL-resistant PWS treated with Alexandrite laser with concurrent PDL (A,B), or fractional 1,550 nm photo-thermolysis (C,D) (A,C: before; B,D: after treatment).
Generally, Alexandrite laser treatments were well tolerated. Side effects were usually transient and predictable, including erythema, edema, purpura, and pain. All subsided by 10–14 days after treatment. Two patients developed isolated and small hypertrophic scars after blistering occurred in areas treated with the 755 nm laser. These scars improved with time. Scarred areas were not subsequently retreated with the 755 nm laser. Additionally, an area of hypopigmentation after treatment of a Fitzpatrick types 2 and 3 patient with a 755 nm laser also improved with time and sun avoidance, and was not retreated with the laser.
Three patients with resistant PWS had mild lightening with a 755 nm laser (two with Alexandrite alone, one with Alexandrite and concurrent PDL), and one showed no response (Alexandrite alone). All four of these patients had significant improvement from prior PDL treatments (8–20 sessions) before reaching a responsiveness plateau. Side effects were similarly predictable, as above, and transient. None of the PWS continued to darken or hypertrophy during the treatment course.
DISCUSSION
This retrospective series of clinical cases demonstrates that hypertrophic and PDL-resistant PWS in adult and pediatric patients can respond favorably to therapy with a 755 nm laser with relatively few complications. In particular, hypertrophic lesions in adults showed signifi-cant improvement which was most rapid and notable in patients treated with concurrent Alexandrite and PDL, with no increased complications. Most PDL-resistant lesions showed moderate improvement with transient and predictable side effects.
However, some PDL-resistant lesions remained resistant to Alexandrite laser therapy, improving only mildly or not at all after repeated sessions. The reasons for such heterogeneity in responses remain unknown. These may include the intrinsic heterogeneity of PWS microanatomy between patients and within each individual PWS, as well as differences in anatomic location, patient age, and genetics. It is also possible that PWS have more than one pathophysiologic origin.
Treatment with the 755 nm Alexandrite laser delivered near the clinical response threshold fluence, with cryogen spray skin cooling, produces relatively few serious complications. The most common sequelae of erythema, edema, purpura, pain, and permanent hair loss are expected, and should be discussed with the patient or parents prior to treatment. In case of extensive lesions, very young patients, or extreme anxiety or pain sensitivity, treatment can be done safely under general anesthesia, with the use of perioperative narcotic analgesics and sedatives. Extreme care should be exercised to protect the eye when using Alexandrite lasers, with the use of a stainless steel intraocular shield [10]. While treatment with a 755 nm wavelength laser carries a theoretical risk of heating a metal eyeshield [11], no associated complications have been observed to date. Although drug–laser interaction studies are lacking, drugs such aspirin that limit platelet function should be avoided for 1 week before and several weeks after treatment.
Potentially serious side effects, such as tissue necrosis, blistering, and scarring are rare after Alexandrite laser treatment. In this case series, there were two cases (10%) of local scarring and dyspigmentation, which improved gradually with time. Since the cohort of patients included only one infant and six children aged 5 years or younger, it is possible that the incidence of scarring in this population might be different from that in adolescents, teenagers, or adults. The risk of scarring with the Alexandrite laser appears to be greater than the 0–4.3% risk of scarring reported with the PDL [12–14], likely due to significantly greater tissue penetration of the 755 nm wavelength. Consequently, it is important to beware that aggressive and supratherapeutic treatment of PWS in any age group with this modality is likely to produce adverse outcomes, such as tissue necrosis and subsequent scarring of the treated area. Fractional photothermolysis may aid the resolution of scarring; for hypertrophic scars, treatment with PDL and intralesional steroid may be useful. Indeed, one of the patients in our study (Fig. 3) was treated successfully with the 755 nm laser for his resistant facial PWS, and concurrent non-ablative 1,550 nm fractional thermolysis for pre-existing hypertrophic scarring.
While most lesions and patients in our study responded favorably to a 755 nm laser treatment, none cleared completely. As with PDL therapy, it is logical to assume that these residual Alexandrite-treated lesions will follow the natural history of PWS, including gradual darkening and hypertrophy.
Based on our observations, the combination of Alexandrite and PDL treatment of the same lesional areas in the same session may be more efficacious for hypertrophic PWS than treatment with Alexandrite laser alone. This approach also might be more efficacious for PDL-resistant PWS, since targeting the deeper component first with a 755 nm laser, and targeting the more superficial component second with PDL, would be expected to produce a greater degree of vessel damage throughout the dermis. While this combined approach carries a greater chance of adverse events, we did not see any serious complications in the two patients with hypertrophic PWS treated in this manner. Indeed, further studies with a greater number of patients would be necessary to assess fully the safety of this method in hypertrophic as well as PDL-resistant PWS.
When treating with a 755 nm laser, it is important to be aware that this is a deeply penetrating near-IR laser that can produce a dermal burn. The full extent of the tissue response, which occurs mainly at a depth greater than 1 mm in the skin [1], is invisible to the observer. Hence, the visible response represents just the “tip of the iceberg.” From our clinical observations, the appropriate visible tissue response for effective and safe therapy is a subtle gray-blue darkening of the skin that evolves over several minutes into deeper purpura [15]. In our practice, the current approach is to determine the fluence threshold for such a response in the darkest PWS portion, where this threshold is the lowest. We then perform one pass, increase the fluence by 5–10 J/cm2, and subsequently treat the unresponsive areas.
Laser therapy of PWS remains a major challenge, despite decades of improvement. The use of multiple laser wavelengths, pulse durations, and fluences—delivered alone or in combination—should be evaluated to address this important problem. Selective photothermolysis may also be an intrinsically limited process for PWS clearing, because angiogenesis is part of the wound healing response after treatment [16]. Other emerging modalities include the use of topical or systemic angiogenesis inhibitors after laser treatment [16] and photodynamic therapy [17].
This was a series of cases performed in our clinical practice without rigorous controls. Although Alexandrite 755 nm approach is intriguing, clinical validation in large numbers of PWS patients is required. Prospective, comparative, and controlled clinical studies conducted by experienced investigators on a multi-center basis against accepted treatment regimens are required so that the role of 755 nm Alexandrite laser therapy of PWS may be fully defined.
Acknowledgments
This project was supported in part by research grants awarded from the National Institutes of Health (AR47551 and EB002495 to JSN).
References
- 1.Yang MU, Yaroslavsky AN, Farinelli WA, Flotte TJ, Rius-Diaz F, Tsao SS, Anderson RR. Long-pulsed neodymium: yttrium-aluminum-garnet laser treatment for port-wine stains. J Am Acad Dermatol. 2005;52(3 Pt 1):480–490. doi: 10.1016/j.jaad.2004.10.876. [DOI] [PubMed] [Google Scholar]
- 2.Chapas AM, Eickhorst K, Geronemus RG. Efficacy of early treatment of facial port wine stains in newborns: A review of 49 cases. Lasers Surg Med. 2007;39(7):563–568. doi: 10.1002/lsm.20529. [DOI] [PubMed] [Google Scholar]
- 3.Sakamoto FH, Wall T, Avram MM, Anderson RR. Wolff K, Goldsmith LA, Katz SI, Gilchrest B, Paller AS, Leffell DJ, editors. Chapter 240. Lasers and Flashlamps in Dermatology. Fitzpatrick’s Dermatology in General Medicine. (7) http://www.accessmedicine.com/content.aspx?aID=3005338.
- 4.Huikeshoven M, Koster PH, de Borgie CA, Beek JF, van Gemert MJ, van der Horst CM. Redarkening of port-wine stains 10 years after pulsed-dye-laser treatment. N Engl J Med. 2007;356(12):1235–1240. doi: 10.1056/NEJMoa064329. [DOI] [PubMed] [Google Scholar]
- 5.Garden JM. Laser removal of port wine stains: How close are we? A commentary. Lasers Surg Med. 2007;39(7):569–570. doi: 10.1002/lsm.20539. [DOI] [PubMed] [Google Scholar]
- 6.Jasim ZF, Handley JM. Treatment of pulsed dye laser-resistant port wine stain birthmarks. J Am Acad Dermatol. 2007;57(4):677–682. doi: 10.1016/j.jaad.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Li L, Kono T, Groff WF, Chan HH, Kitazawa Y, Nozaki M. Comparison study of a long-pulse pulsed dye laser and a long-pulse pulsed Alexandrite laser in the treatment of port wine stains. J Cosmet Laser Ther. 2008;10(1):12–15. doi: 10.1080/14764170701817023. [DOI] [PubMed] [Google Scholar]
- 8.McGill DJ, MacLaren W, Mackay IR. A direct comparison of pulsed dye, Alexandrite, KTP and Nd:YAG lasers and IPL in patients with previously treated capillary malformations. Lasers Surg Med. 2008;40(6):390–398. doi: 10.1002/lsm.20638. [DOI] [PubMed] [Google Scholar]
- 9.Black JF, Wade N, Barton JK. Mechanistic comparison of blood undergoing laser photocoagulation at 532 and 1,064 nm. Lasers Surg Med. 2005;36(2):155–165. doi: 10.1002/lsm.20134. [DOI] [PubMed] [Google Scholar]
- 10.Hammes S, Augustin A, Raulin C, Ockenfels HM, Fischer E. Pupil damage after periorbital laser treatment of a port-wine stain. Arch Dermatol. 2007;143(3):392–394. doi: 10.1001/archderm.143.3.392. [DOI] [PubMed] [Google Scholar]
- 11.Ries WR, Clymer MA, Reinisch L. Laser safety features of eye shields. Lasers Surg Med. 1996;18(3):309–315. doi: 10.1002/(SICI)1096-9101(1996)18:3<309::AID-LSM13>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Seukeran DC, Collins P, Sheehan-Dare RA. Adverse reactions following pulsed tunable dye laser treatment of port wine stains in 701 patients. Br J Dermatol. 1997;136(5):725–729. [PubMed] [Google Scholar]
- 13.Kelly KM, Nanda VS, Nelson JS. Treatment of port-wine stain birthmarks using the 1.5-msec pulsed dye laser at high fluences in conjunction with cryogen spray cooling. Dermatol Surg. 2002;28(4):309–313. doi: 10.1046/j.1524-4725.2002.02071.x-i1. [DOI] [PubMed] [Google Scholar]
- 14.Stier MF, Glick SA, Hirsch RJ. Laser treatment of pediatric vascular lesions: Port wine stains and hemangiomas. J Am Acad Dermatol. 2008;58(2):261–285. doi: 10.1016/j.jaad.2007.10.492. [DOI] [PubMed] [Google Scholar]
- 15.Izikson L, Anderson RR. Treatment endpoints with a 755 nm laser. J Cosmet Laser Ther. 2009;11(1):52–55. doi: 10.1080/14764170802524452. [DOI] [PubMed] [Google Scholar]
- 16.Phung TL, Oble DA, Jia W, Benjamin LE, Mihm MC, Jr, Nelson JS. Can the wound healing response of human skin be modulated after laser treatment and the effects of exposure extended? Implications on the combined use of the pulsed dye laser and a topical angiogenesis inhibitor for treatment of port wine stain birthmarks. Lasers Surg Med. 2008;40(1):1–5. doi: 10.1002/lsm.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu Y, Huang NY, Liang J, Pan YM, Liu FG. Clinical study of 1949 cases of port wine stains treated with vascular photo-dynamic therapy (Gu’s PDT) Ann Dermatol Venereol. 2007;134(3 Pt 1):241–244. doi: 10.1016/s0151-9638(07)91816-5. [DOI] [PubMed] [Google Scholar]


